Abstract
The entry of primate immunodeficiency viruses into cells is dependent on the interaction of the viral envelope glycoproteins with receptors, CD4, and specific members of the chemokine receptor family. Although in many cases the tropism of these viruses is explained by the qualitative pattern of coreceptor expression, several instances have been observed where the expression of a coreceptor on the cell surface is not sufficient to allow infection by a virus that successfully utilizes the coreceptor in a different context. For example, both the T-tropic simian immunodeficiency virus (SIV) SIVmac239 and the macrophagetropic (M-tropic) SIVmac316 can utilize CD4 and CCR5 as coreceptors, and both viruses can infect primary T lymphocytes, yet only SIVmac316 can efficiently infect CCR5-expressing primary macrophages from rhesus monkeys. Likewise, M-tropic strains of human immunodeficiency virus type 1 (HIV-1) do not infect primary rhesus monkey macrophages efficiently. Here we show that the basis of this restriction is the low level of CD4 on the surface of these cells. Overexpression of human or rhesus monkey CD4 in primary rhesus monkey macrophages allowed infection by both T-tropic and M-tropic SIV and by primary M-tropic HIV-1. By contrast, CCR5 overexpression did not specifically compensate for the inefficient infection of primary monkey macrophages by T-tropic SIV or M-tropic HIV-1. Apparently, the limited ability of these viruses to utilize a low density of CD4 for target cell entry accounts for the restriction of these viruses in primary rhesus monkey macrophages.
Infection with human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) causes AIDS in humans, which is characterized by a progressive loss of CD4-positive T lymphocytes and fatal opportunistic infections (7, 33, 35). A similar illness can be induced in Asian macaques by infection with various strains of simian immunodeficiency virus (SIV), a closely related lentivirus (23, 46). The similarities of the viruses, hosts, and pathological sequelae in HIV-infected humans and SIV-infected monkeys make the latter system an excellent model for understanding AIDS pathogenesis and for the evaluation of potential therapeutics and vaccines (24).
HIV and SIV infection is usually initiated by binding of the viral envelope glycoproteins, a heavily glycosylated, trimeric complex of noncovalently associated gp120 and gp41 subunits, to the CD4 receptor on the target cell (14, 20, 43). This interaction triggers conformational changes in gp120, creating or unmasking a high-affinity binding site for a second cellular receptor (62, 70, 73). The interaction with this coreceptor is believed to induce structural changes in the transmembrane glycoprotein gp41 that lead to the fusion of viral and target cell membranes (72, 74).
The predominant coreceptors used by HIV-1 are the chemokine receptors CCR5 and CXCR4 (2, 16, 18, 21, 27). All HIV-1 isolates studied to date utilize at least one of these coreceptors, and the expression pattern of the receptors usually explains the observed cell tropism of HIV-1 variants. CCR5 has been shown to be the major coreceptor for macrophagetropic (M-tropic) primary HIV-1 isolates, whereas CXCR4 serves as a coreceptor for primary T-cell-tropic (T-tropic) and T-cell line-adapted HIV-1 strains (11, 19, 38). One subject of ongoing controversy is the extent to which T-cell line-adapted HIV-1 strains can infect and replicate in CXCR4-positive, primary human macrophages (8, 45, 66, 71, 76). It has been suggested that cell-type-specific modulation of postentry events and/or the presence of functionally restricted CXCR4 forms may limit productive infection of these cells (26, 45, 64). Whatever the cause of the poor infectability of primary human macrophages by T-cell line-adapted HIV-1, this example points out that coreceptor use does not always explain the tropism of primate immunodeficiency viruses. In addition to CCR5 and CXCR4, some HIV-1 strains can utilize, at lower levels of efficiency, the alternative coreceptors CCR3, CCR2b, Apj, CCR8, and US28 (17, 27, 39, 58). HIV-2 is more closely related to SIV than to HIV-1, and this relationship is also mirrored in the preferences of HIV-2 for coreceptors (12, 22, 51). Most SIV strains can use very efficiently a number of coreceptors, including CCR5, STRL33, gpr15, gpr1, and ChemR23 (3, 17, 22, 31, 60, 61). This provides the means for SIV to replicate in peripheral blood mononuclear cells (PBMC) from individuals homozygous for a 32-bp deletion in CCR5 and in some CCR5-negative T-cell lines as well (16). However, the in vivo relevance of the usage of alternative coreceptors besides CCR5 by SIV is still uncertain, and with very few exceptions, all SIV isolates are able to use CCR5 (29).
SIV variants that differ in target cell tropism and the ability to induce particular pathological sequelae have been described previously (1, 25, 48, 68). Several of these variants arose in vivo from SIVmac239, a molecularly cloned virus that replicates efficiently in rhesus monkey T lymphocytes but not in primary macrophages (4, 6, 34, 40, 55, 59, 65). SIVmac239 typically causes AIDS-like disease in inoculated monkeys, but a subset of infected animals develop meningoencephalitis or granulomatous interstitial pneumonia. Central nervous system or pulmonary disease is associated with primary infection of microglia or tissue macrophages, respectively, and with the emergence of SIV variants that replicate efficiently in cultured macrophages. One such variant, SIVmac316, was isolated from the alveolar macrophages of a rhesus monkey infected with SIVmac239 (25). The macrophage tropism of SIVmac316 is determined by five of the sequence differences between the envelope glycoproteins of SIVmac316 and SIVmac239 (54). The tropism change is apparently not due to qualitative alterations in coreceptor use, as both SIVmac316 and SIVmac239 envelope glycoproteins support entry into cells expressing CD4 and either CCR5, gpr15, or STRL33 (63). Indeed, one report has suggested that this env-associated change in tropism is determined at a postentry step in SIV replication (53).
Here we examine the ability of primary rhesus monkey macrophages to be infected with recombinant viruses containing the envelope glycoproteins of SIVmac239, SIVmac316, and several HIV-1 strains. The basis for the restricted entry of SIVmac239 and primary M-tropic HIV-1 into these cells is investigated.
MATERIALS AND METHODS
Cells.
Cf2Th, HeLa, and HEK293 cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, 100 U of penicillin/ml, and 100 U of streptomycin/ml. A Cf2Th cell line stably expressing high levels of human CCR5 has been described previously (52). Cf2Th cells stably expressing low levels of human CCR5 were obtained from a population of cells expressing a broad range of CCR5 by fluorescence-activated cell sorting (FACS) on a Vantage flow cytometer (Becton Dickinson) using dialyzed anti-CCR5 phycoerythrin (PE)-conjugated antibody (2D7; Pharmingen) for staining.
PBMC from SIV-seronegative rhesus macaques were purified by Ficoll-Paque density gradient centrifugation of heparinized blood obtained from the New England Regional Primate Research Center. Cells were washed three times with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, antibiotics, 5 μg of concanavalin A/ml, and 100 U of interleukin-2/ml. After 2 days of stimulation, the medium was changed and cells were cultured in medium without concanavalin A for another 3 days. Human PBMC were purified in the same way from leucopacs obtained from the Dana-Farber Blood Center.
Monocyte-derived macrophages were obtained as previously described (67). Briefly, approximately 3 × 106 mononuclear cells, isolated on a Ficoll-Paque gradient and washed three times with PBS, were seeded in each well of a 12-well plate. Macrophages were obtained by culture in macrophage driving medium (75% RPMI, 10% human AB serum, 15% conditioned medium from L929 fibroblasts, 10 mM HEPES, antibiotics, 55 μM β-mercaptoethanol, 50 U of granulocyte-macrophage colony-stimulating factor/ml, and 5 U of macrophage colony-stimulating factor/ml. Cultures were washed three times every 3 days for 2 weeks to remove nonadherent cells, and fresh medium was added to the adherent cells.
Plasmids and recombinant viruses.
The SIVmac-based retroviral vector pSHIVΔenvCAT, described previously (13), was generated by inserting an env-deleted, HIV-1 HXBc2 fragment containing tat and rev sequences into a plasmid containing an SIVmac239 provirus. The chloramphenicol acetyltransferase (CAT) gene was cloned into the BamHI site, thus inactivating the rev gene. Thus, CAT is expressed in a Rev-independent manner from a multiply spliced mRNA. Cloning of pSIvec1ΔenvGFP has been described elsewhere (37). This vector is similar to pSHIVΔenvCAT but contains the green fluorescent protein (GFP) gene in place of the CAT gene. Plasmids pSIvec1ΔenvhuCD4, pSIvec1ΔenvrhCD4, pSIvec1ΔenvrhCCR5, and pSIvec1ΔenvhuCXCR4 were generated by replacing the AgeI-NotI fragment containing the GFP gene in pSIvec1ΔenvGFP with the cDNA sequences of human and rhesus monkey CD4, rhesus monkey CCR5, and human codon-optimized CXCR4, respectively. The CD4 and coreceptor sequences were amplified from previously described plasmids (32) using the Pfu DNA polymerase and primers with introduced restriction sites for cloning. The AgeI site in human and rhesus CD4 (position 1346) has been removed by introduction of a silent mutation changing the CGG encoding arginine to CGA. The HIV-1-based retroviral vector pHXBH10ΔenvCAT contains an HIV-1 provirus with a deletion in env and the CAT gene replacing the nef open reading frame (69). Plasmids pSIVΔgpv239 and pSIVΔgpv316 (50) were used to express the envelope glycoproteins of SIVmac239 and SIVmac316, respectively. Envelope glycoproteins of HIV-1 strains YU2 and JR-FL were expressed using the previously published pSVIIIenv vectors (18, 36). The similar plasmid pSVIIIΔKS contains a deletion in env and was used as a control. Plasmid pHCM-VSV-G encodes the vesicular stomatitis virus envelope glycoprotein (VSV G) (75). The previously described pcDNA3 plasmids (18, 50, 50a) were used for expression of human or rhesus monkey CD4 and CCR5 in Cf2Th cells.
Recombinant viruses capable of a single round of infection were generated by cotransfecting human cell lines by the calcium phosphate method with 20 μg of an env-defective retroviral vector and 5 μg of a plasmid expressing the envelope glycoproteins of interest. For efficient virus production, 2.5 μg of the HIV-1 Rev-expressing plasmid psrev was included (37). Virions made with pHXBH10ΔenvCAT were generated in HeLa cells; all other recombinant viruses were generated in 293T cells, and the concentration of virus was normalized based on reverse transcriptase activity.
Transduction of macrophages with CD4- or chemokine receptor-expressing viral vectors.
Eleven-day-old monocyte-derived rhesus monkey macrophages were transduced with VSV G-pseudotyped recombinant viruses encoding human CD4, rhesus CD4, rhesus CCR5, human CXCR4, or GFP. Transductions were performed overnight with 90,000 reverse transcriptase units (RT units) for the CD4- and GFP-expressing vectors and 20,000 RT units for the CCR5- and CXCR4-expressing vectors. Three days later, the transduction efficiency was evaluated by FACS as described below. The transduction efficiency of the GFP-expressing vector was >90% (data not shown). Both transduced and native macrophages from the same donor preparation were infected overnight with 50,000 RT units of CAT reporter viruses containing the envelope glycoproteins of SIVmac239, SIVmac316, and the M-tropic HIV-1 strains YU2 and JR-FL as described below.
env complementation assay.
The efficiency of a single round of infection by CAT-expressing recombinant virions was determined by using the previously published env complementation assay (36). The target cells were 3 × 106 PBMC, rhesus macrophages derived from 3 × 106 PBMC, or 2 × 105 Cf2Th cells. PBMC and macrophages were cultured in 12-well plates and incubated with 50,000 RT units of virus in 1 ml for 12 h at 37°C. Cf2Th cells were incubated in six-well plates with 10,000 RT units of virus at 37°C for 12 h or, for the experiments with TAK-779, for 1 h.
Cytofluorometric analyses.
Macrophages were detached by treatment with 25 mM EDTA-PBS, followed by gentle scraping of already rounded cells. CD4 expression was determined by staining with the anti-CD4 monoclonal antibody (MAb) OKT4 conjugated with fluorescein isothiocyanate (FITC) (Ortho Diagnostics). For determination of CCR5 expression on rhesus macrophages, a biotin-conjugated anti-CCR5 MAb (clone 45531.111; R&D Systems) and PE-conjugated streptavidin were used. Human CCR5 expression on Cf2Th cells and CXCR4 expression on rhesus macrophages were measured by staining with PE-conjugated MAbs 2D7 (Pharmingen) and 12G5, respectively. Nonrelated mouse antibodies of corresponding isotype and conjugation were used as negative controls. Cells were fixed in 2% formaldehyde and analyzed on a FACscan cytometer (Becton Dickinson).
Virus replication assay.
SIVmac239 and SIVmac316 viruses were provided by Ronald Desrosiers, New England Regional Primate Research Center. Macrophages cultured as described above were infected with 50,000 RT units of replication-competent virus for 12 h, washed three times, and refed with 2.5 ml of fresh medium. Cell-free supernatants (0.5 ml) were collected every second day and replaced with fresh medium. The collected supernatants were assayed for the presence of SIV p27 core protein by enzyme-linked immunoassay (Coulter).
TAK-779 and soluble CD4.
TAK-779 was kindly provided by Masahiko Fujino (Takeda Chemical Industries, Osaka, Japan). Human soluble CD4 (sCD4) was donated by Raymond Sweet (SmithKline Beecham, King of Prussia, Pa.).
RESULTS
Restriction of SIVmac239 and M-tropic HIV-1 infection in rhesus macrophages.
The pathogenic molecular clone SIVmac239 is able to replicate in rhesus monkey T lymphocytes but is restricted for replication in rhesus monkey macrophages. This restriction is determined by the env sequences (54). The reported inability of a simian-human immunodeficiency virus chimera (SHIV) carrying an envelope glycoprotein from the M-tropic HIV-1 JR-FL to replicate in rhesus macrophages (15) prompted us to include M-tropic HIV-1 envelope glycoproteins in our studies. To study the extent and nature of these tropism restrictions, we examined the ability of recombinant SHIV virions carrying different SIV or HIV-1 envelope glycoproteins to infect various target cell types. A defective SHIV provirus with a deleted env gene and the nef gene replaced by a CAT gene (37) was cotransfected with plasmids expressing the envelope glycoproteins of interest. The recombinant viruses produced were normalized based on reverse transcriptase activity and incubated with primary target cells from rhesus monkeys. The level of CAT expression in the target cells reflects the efficiency of a single round of the retroviral infection cycle. Similar levels of CAT activity were found in lysates of stimulated rhesus PBMC infected with SHIVs pseudotyped with the SIVmac239 and SIVmac316 envelope glycoproteins (Fig. 1A), a result consistent with previous reports (42, 54). In sharp contrast, only the virus with the SIVmac316 envelope glycoproteins could efficiently infect rhesus monkey macrophages (Fig. 1B). Viruses with the M-tropic HIV-1 envelope glycoproteins efficiently infected rhesus monkey PBMC (Fig. 1A and data not shown) but did not infect rhesus monkey macrophages (Fig. 1B). These results demonstrate that efficient infection of rhesus monkey macrophages by SIVmac239 and M-tropic HIV-1 strains is impaired at a step that is determined by the envelope glycoproteins on the infecting virus. As these M-tropic HIV-1 envelope glycoproteins support efficient infection of primary human macrophages, the results also point to a difference in the susceptibility of rhesus monkey and human macrophages to particular HIV-1 isolates, consistent with previous results (15).
FIG. 1.
Infectability of primary rhesus monkey cells by viruses with different envelope glycoproteins. Recombinant CAT-expressing viruses containing the envelope glycoproteins of SIVmac316, SIVmac239, or HIV-1 strain YU2, or lacking functional envelope glycoproteins (the ΔKS control), were incubated overnight with either rhesus monkey PBMC (A) or rhesus monkey monocyte-derived macrophages (B). Three days later, CAT activity was measured in cell lysates. Samples in which the conversion of chloramphenicol to acetylated forms was greater than 70% were diluted and reassayed to bring the conversion value within range.
Coreceptor usage of SIVmac316 on rhesus monkey macrophages.
In addition to CCR5, SIVmac strains are able to use a broad variety of coreceptors for entry. SIVmac316 has been shown to use some of these other receptors much more efficiently than SIVmac239 or M-tropic HIV-1 strains (17, 61). To determine whether CCR5 serves as the principal coreceptor for SIVmac316 entry into rhesus macrophages, we used a previously published (5) nonpeptide compound, TAK-779, in our single-round infection assay. It has been shown that TAK-779 specifically binds to human CCR5 and inhibits infection of M-tropic HIV-1 in vitro. TAK-779 also exhibits a low affinity for CCR2b, but as CCR2b does not support entry of SIVmac316 (17), this is not likely to be a problem in our assays. At a concentration of 100 nM, TAK-779 significantly inhibited infection of Cf2Th cells expressing either human or rhesus monkey CD4 and CCR5 by a virus containing the SIVmac316 envelope glycoproteins (Fig. 2A and data not shown). Thus, TAK-779 blocks virus infection mediated by human and rhesus monkey CCR5. No inhibition was detected on Cf2Th cells expressing CD4 together with gpr15, STRL33, Apj, or gpr1 (data not shown), alternative coreceptors used by SIVmac316 in in vitro assays (17, 31). On rhesus monkey macrophages, TAK-779 inhibited infection by viruses with the SIVmac316 envelope glycoproteins in a concentration-dependent manner, with an estimated 50% inhibitory concentration of ∼4 nM (Fig. 2B). No significant inhibition of infection by a virus pseudotyped with VSV G was observed (Fig. 2C). This result indicates that CCR5 is the major coreceptor used by SIVmac316 for entry into rhesus monkey macrophages.
FIG. 2.
Inhibition of SIVmac316 entry by TAK-779. Cf2Th cells transiently expressing rhesus monkey CD4 and CCR5 proteins (A) or primary rhesus monkey macrophages (B and C) were infected with recombinant CAT-expressing viruses with the envelope glycoproteins of SIVmac316 (A and B) or with VSV G (C) in the presence of increasing TAK-779 concentrations. The results are reported as percentages of chloramphenicol conversion to acetylated forms and represent the means and standard deviations of three samples for each TAK-779 concentration. A representative experiment of three is shown.
Effect of CD4 and CCR5 overexpression on entry into rhesus macrophages.
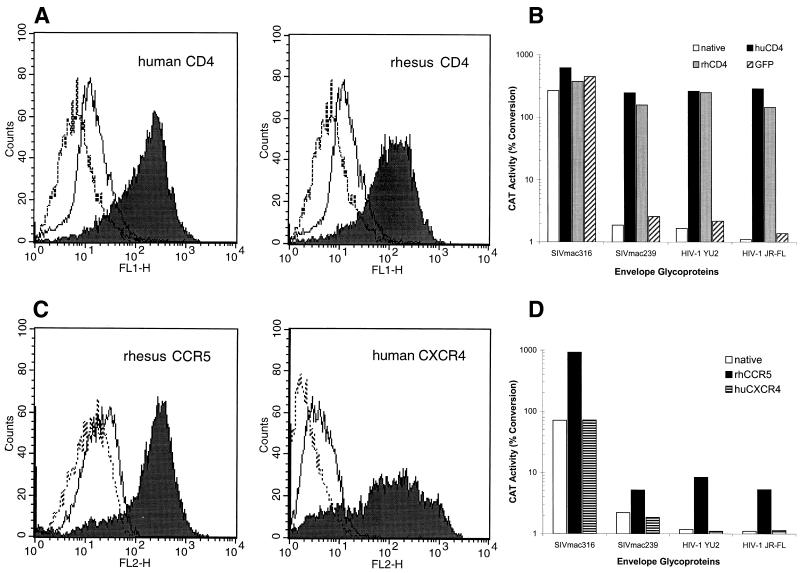
The expression of CD4 on the surface of macrophages is several times lower than the expression level on CD4-positive lymphocytes (47, 56). It is therefore conceivable that the efficiency of CD4 utilization by different SIV strains influences the ability to infect particular cell types. To evaluate whether CD4 overexpression in rhesus monkey macrophages can overcome the block to infection of SIVmac239 and M-tropic HIV-1 strains, we created lentiviral vectors expressing human and rhesus monkey CD4. Replication-incompetent, VSV G-pseudotyped viruses encoding CD4 were produced in 293T cells and used for infection of rhesus monkey macrophages. The transduction efficiency was greater than 90% (Fig. 3A). A similar construct expressing GFP was used as a control and exhibited a similar transduction efficiency (data not shown). Three days after transduction, the macrophages were incubated with CAT reporter viruses containing either SIV or HIV-1 envelope glycoproteins. The overexpression of human or rhesus monkey CD4 in the macrophages resulted in a three- to fourfold enhancement of infection by viruses with the SIVmac316 envelope glycoproteins (Fig. 3B). By contrast, infection by the viruses with SIVmac239, YU2, or JR-FL envelope glycoproteins was dramatically (100- to 300-fold) stimulated by overexpression of either human or rhesus monkey CD4 and almost reached the level attained by viruses with the SIVmac316 envelope glycoproteins. Truncation of the cytoplasmic tails of human and rhesus monkey CD4 at position 401 (9) did not decrease the observed enhancement of infection, indicating that signal transduction events mediated by CD4 are not responsible for the increase (data not shown). A slight (∼2-fold) increase in the CAT activity in control macrophages expressing GFP was observed after infection with all of the viruses. This nonspecific activation could be explained by the production of Tat by the CD4-expressing lentivirus vector. These results demonstrate that the low level of CD4 expression on rhesus monkey macrophages is a major factor limiting efficient infection by SIVmac239 and M-tropic HIV-1 strains.
FIG. 3.
Effect of CD4 and CCR5 overexpression on infection of rhesus monkey macrophages with recombinant viruses. Primary rhesus monkey macrophages were transduced with retroviral vectors encoding either CD4 (A and B) or chemokine receptors (C and D). As a control, cells were transduced in parallel with a similar vector encoding GFP. (A) FACS profile of cells transduced with the human CD4 (left) and rhesus monkey CD4 (right) genes. The anti-CD4 antibody OKT4 was used to generate the unbroken curve (untransduced macrophages) and the filled curve (transduced macrophages). The broken curve was generated using a control antibody of the same isotype. (B) CAT activity in native rhesus monkey macrophages and in macrophages transduced with a vector expressing either human or rhesus monkey CD4 (huCD4 or rhCD4) or GFP after infection with CAT reporter viruses containing the indicated envelope glycoproteins. (C) FACS profiles of untransduced macrophages and macrophages transduced with rhesus monkey CCR5 (left) or human CXCR4 (right). The broken curves were obtained using isotype-matched irrelevant antibodies. In the left panel, the anti-CCR5 antibody 45531.111 was used to stain untransduced macrophages (unbroken curve) or macrophages transduced with the rhesus CCR5 gene (filled curve). In the right panel, the 12G5 anti-CXCR4 antibody was used to stain untransduced macrophages (unbroken curve) or macrophages transduced with the human CXCR4 gene (filled curve). (D) CAT activity in native rhesus monkey macrophages and in macrophages transduced with the gene for either rhesus monkey CCR5 or human CXCR4 following infection with CAT reporter viruses containing the indicated envelope glycoproteins.
The high level of infection of rhesus monkey macrophages overexpressing CD4 by viruses with SIVmac239, YU2, and JR-FL envelope glycoproteins enabled us to utilize the TAK-779 inhibitor to address whether the CCR5 coreceptor contributes to infection. At a TAK-779 concentration of 100 nM, infection by viruses with SIVmac316, SIVmac239, YU2, and JR-FL envelope glycoproteins was more than 90% inhibited (data not shown). These findings demonstrate that CCR5 on rhesus monkey macrophages is used by SIVmac239 and M-tropic HIV-1 strains, if the expression of CD4 is sufficient.
To assess the effect of CCR5 overexpression on the infection of rhesus monkey macrophages, we created a recombinant lentivirus carrying the rhesus monkey CCR5 gene. A substantial increase in CCR5 expression was observed in rhesus monkey macrophages infected by this virus (Fig. 3C). Infection of the transduced cells by viruses with the SIVmac239, YU2, and JR-FL envelope glycoproteins was increased 3- to 7-fold, and infection by viruses with the SIVmac316 envelope glycoproteins was increased approximately 10- to 20-fold, compared with that of untransduced macrophages (Fig. 3D). Thus, infection of all of the CCR5-using viruses was moderately enhanced by CCR5 overexpression. However, the native level of CCR5 surface expression on primary rhesus monkey macrophages does not appear to be a factor specifically limiting infection by SIVmac239 or the M-tropic HIV-1.
Effect of CD4 overexpression on the replication of infectious viruses.
To determine the ability of SIVmac239 to replicate in rhesus monkey macrophages expressing elevated CD4 levels, we transduced 11-day-old macrophages with the human CD4 gene and then infected them 72 h later with 50,000 RT units each of replication-competent SIVmac239 and SIVmac316. After 12 h, the cells were washed and returned to medium; supernatant samples were collected at 2-day intervals to assay for viral p27 concentration. Consistent with previous reports showing a 100- to 1,000-fold-higher replication efficiency of SIVmac316 compared with SIVmac239 in rhesus monkey macrophages (42, 53), the replication of SIVmac239 on untransduced cells was either undetectable or very poor, depending on the donor animal. Very low p27 levels (<0.2 ng/ml) were measured in transduced control cells expressing GFP and infected with SIVmac239 (Fig. 4). A similar p27 level was detected in the supernatants of all transduced uninfected macrophages (data not shown) and probably resulted from gag expression by the env-deficient vector used to express GFP or overexpress CD4. The expression of gag from this vector is impaired by the lack of the HIV-1 Rev protein in the transduced cells and the fact that SIV Rev, provided by the replication-competent SIV after infection, does not function on the HIV-1 Rev-responsive element present in our SHIV vectors (10). SIVmac239 infection of rhesus macrophages overexpressing human or rhesus CD4 resulted in a considerable level of virus replication, with peak p27 levels greater than 1 ng/ml (Fig. 4). CD4 overexpression did not significantly enhance the replication of SIVmac316, which was still fivefold higher than that of SIVmac239. These results indicate that the replication efficiency of SIVmac239 can be strongly enhanced by overexpressing CD4 on rhesus monkey macrophages, but it did not reach the replication level of SIVmac316 under the conditions present in our system.
FIG. 4.
Replication of SIVmac239 and SIVmac316 in rhesus monkey macrophages overexpressing human or monkey CD4. Primary rhesus monkey macrophages were transduced with VSV G-pseudotyped vectors containing the gene for human (hu) CD4, rhesus monkey (rh) CD4, or GFP. Three days after transduction, the 14-day-old cells were incubated with wild-type SIVmac316 or SIVmac239 (50,000 RT units/ml) for 12 h. Culture supernatants were harvested and assayed for p27 protein concentration every 2 days thereafter. The experiment was repeated four times with macrophages obtained from different rhesus monkeys, and the results were similar to those shown here.
CD4 dependence of SIV and HIV-1 entry into Cf2Th cells in the presence of high and low CCR5 levels.
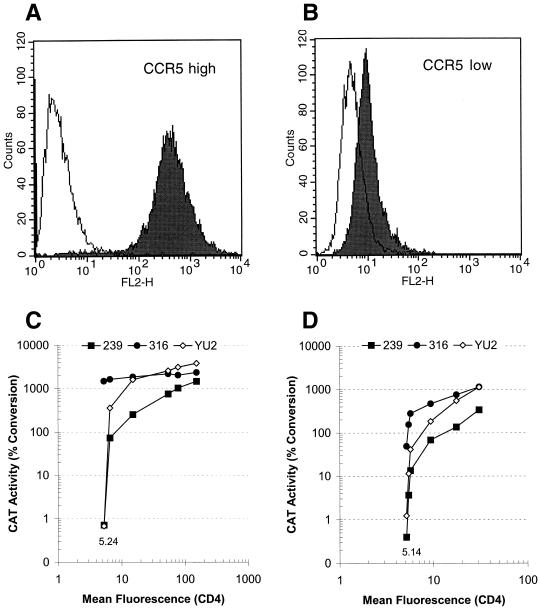
A remarkable feature of many SIV strains is their ability to bind CCR5 and to enter CCR5-expressing cells independently of CD4 (28, 30, 63). The results presented above suggest that the CD4 and CCR5 levels on rhesus monkey macrophages support entry of SIVmac316 but restrict efficient entry of SIVmac239. To study the CD4 and CCR5 requirements for entry in more detail, we generated Cf2Th canine thymocytes stably expressing high and low levels of human CCR5 (Fig. 5A and B). These cells were then transiently transfected with increasing amounts of a CD4-expressing plasmid, keeping the total DNA amount constant by addition of an irrelevant plasmid. Two days after transfection, the cells were infected with CAT reporter viruses containing different envelope glycoproteins; in parallel, the CD4 expression level was determined by FACS. The efficiency with which viruses with the SIVmac316 envelope glycoproteins infected CD4-negative Cf2Th cells was strongly dependent on the level of CCR5 expression. Viruses with SIVmac316 envelope glycoproteins efficiently infected cells with a high level of CCR5 regardless of the presence or level of expression of CD4 (Fig. 5C). By contrast, in cells expressing low CCR5 levels, infection by viruses with the SIVmac316 envelope glycoproteins was significantly enhanced by coexpression of CD4 (Fig. 5D). Detectable infection of cells expressing either high or low levels of CCR5 by viruses with the SIVmac239 and YU2 envelope glycoproteins was strictly CD4 dependent. The efficiency of infection by viruses with the SIVmac239 envelope glycoproteins exhibited the greatest dependence on CD4 levels. These data demonstrate that, depending on the level of CCR5 expressed on the target cell surface, the level of CD4 expressed can give rise to differences in the efficiency of SIVmac316 and SIVmac239 infection that range from 3- to 1,000-fold. As the CCR5 expression level on primary rhesus monkey macrophages more closely resembles that on our Cf2Th cells expressing low levels of CCR5, the low levels of CD4 on primary macrophages could readily account for the observed differences in the efficiency with which SIVmac316 and SIVmac239 infect these cells.
FIG. 5.
Influence of CD4 expression in Cf2Th canine thymocytes expressing high or low levels of CCR5. FACS profiles of Cf2Th cells stably expressing high (A) or low (B) levels of human CCR5 were determined using the anti-CCR5 antibody 2D7 or an isotype-matched control antibody. These cells were transfected with increasing amounts of a human CD4-expressing plasmid (pcDNA/CD4). The total DNA was kept constant in each transfection by the addition of empty vector DNA. After 48 h, the cells were infected with CAT reporter viruses containing the envelope glycoproteins of SIVmac316, SIVmac239, or HIV-1 strain YU2. In parallel, an aliquot of the same cells was analyzed for CD4 surface expression using the FITC-conjugated monoclonal antibody OKT4. The mean channel fluorescence of CD4-negative CF2Th cells was set at 5.24 for high (C) and 5.14 for low (D) CCR5 expressors. The CAT activity in the infected cells is plotted against the mean fluorescence intensity.
CD4 requirement of SIVmac316 for infection of rhesus macrophages.
To evaluate whether SIVmac316 requires CD4 for infection of rhesus monkey macrophages, we used the anti-CD4 antibody OKT4a to inhibit the entry of recombinant viruses with the SIVmac316 envelope glycoproteins. A concentration-dependent inhibition of infection by OKT4a was observed; at the highest concentration of antibody tested, infection was almost completely inhibited (Fig. 6A). An isotype-matched antitrinitrophenol antibody used as a control had no effect on the infection of viruses with the SIVmac316 envelope glycoproteins (data not shown). The effects of the OKT4a antibody were specific, as this antibody did not inhibit a virus pseudotyped with VSV G (Fig. 6B).
FIG. 6.
Effect of an anti-CD4 MAb and sCD4 on infection of rhesus monkey macrophages. Rhesus monkey macrophages were incubated with recombinant CAT reporter viruses (50,000 RT units/ml) with the envelope glycoproteins of SIVmac316 in the presence of increasing concentrations of the anti-CD4 antibody OKT4a (A) or sCD4 (C). VSV G-pseudotyped reporter virus was used as a control for any nonspecific effects of the OKT4a antibody (B). A representative experiment is shown. The data are means and standard deviations of three samples for each concentration.
The addition of sCD4 to viruses with the SIVmac316 envelope glycoproteins can result in enhanced efficiency of infection of Cf2Th cells transiently expressing CCR5 (63). We therefore examined whether the presence of sCD4 could enhance the infection of rhesus monkey macrophages by these viruses. In contrast to our findings on Cf2Th cells, sCD4 caused an overall inhibition of macrophage infection by viruses with the SIVmac316 envelope glycoproteins (Fig. 6C). These results indicate that SIVmac316 requires CD4 for efficient entry into rhesus monkey macrophages. The infection of rhesus monkey macrophages by viruses with the SIVmac239 envelope glycoproteins was not enhanced by sCD4 at concentrations ranging from 0.04 to 25 μg/ml (data not shown).
DISCUSSION
In this study, we examined the target cell variables that govern SIV cell tropism. As representative strains for our study, we used SIVmac239, the best-characterized T-tropic SIV isolate, and SIVmac316, an M-tropic strain isolated from the alveolar macrophages of a rhesus monkey infected with SIVmac239 (41, 44, 55). Previous studies suggested that the SIVmac239 envelope glycoproteins determined a postentry replication block in rhesus monkey macrophages (53). We also examined the basis of the previously reported inability of a SHIV bearing the envelope glycoproteins of the M-tropic HIV-1 isolate JR-FL to replicate on rhesus monkey macrophages (15). The use of recombinant viruses pseudotyped with the envelope glycoproteins of these viruses allowed us to examine events in the infection process specifically modulated in trans by viral env sequences. Our results indicate that a single round of infection of primary rhesus macrophages was mediated by the SIVmac239 envelope glycoproteins 30 to 100 times less efficiently than by the SIVmac316 envelope glycoproteins. Likewise, reporter viruses containing the envelope glycoproteins of several M-tropic HIV-1 strains were extremely inefficient at infecting primary rhesus monkey macrophages. As SIVmac239 and the M-tropic HIV-1 utilize monkey CD4 and CCR5 (16, 50a), which are expressed on the primary macrophages, the observed blocks could not be explained by the qualitative absence of appropriate receptors.
We examined whether quantitative increases in the expression of CD4, CCR5, or CXCR4 could influence the infectability of the primary rhesus monkey macrophages by the recombinant viruses. Our results show that only CD4 overexpression specifically overcame the restriction observed for viruses bearing the SIVmac239 or M-tropic HIV-1 envelope glycoproteins. Similar results were obtained by overexpressing a truncated CD4 glycoprotein lacking the cytoplasmic tail, indicating that signal transduction through CD4 is not necessary for overcoming the replication block. This result strongly suggests that a major restriction against infection of primary rhesus monkey macrophages by SIVmac239 and M-tropic HIV-1 occurs at the level of virus entry. Once this restriction is overcome, all of the steps in the virus replication cycle monitored by the single-round, env complementation assay occur efficiently. Indeed, overexpression of full-length CD4 in rhesus monkey macrophages allowed the replication of infectious SIVmac239. The level of replication observed was still lower than that of SIVmac316, possibly due to insufficient CD4 expression or to other factors, such as the previously described inefficiency of SIVmac239 envelope glycoprotein precursor processing in primary macrophages (67).
Our results imply that a major variable governing SIV macrophage tropism is the efficiency with which low levels of CD4 on the target cell surface can be utilized to support entry. The ability to utilize low levels of CD4 may reflect a higher affinity of the SIVmac316 envelope glycoproteins for CD4, relative to the affinities of the SIVmac239 and M-tropic HIV-1 envelope glycoproteins. Indeed, the binding affinity of monomeric gp120 from SIVmac316 for sCD4 is higher than that of SIVmac239 gp120 (63). Differences in affinity or cooperativity in CD4 binding might be even more apparent in the context of a trimeric envelope glycoprotein spike. The CD4 binding affinity of the assembled envelope glycoprotein complex of primary, M-tropic HIV-1 strains has been suggested to be low (52a). This could account for the observed restriction in primary rhesus macrophages against viruses with M-tropic HIV-1 envelope glycoproteins.
Our results demonstrate that CCR5 is the major coreceptor used by SIV and M-tropic HIV-1 isolates to infect primary rhesus monkey macrophages. mRNAs for other SIV receptors, such as gpr1 and gpr15 (31), have been found in macrophages, but the inhibition experiments using TAK-779 clearly indicate the dominant use of CCR5 by the viruses studied. The level of CCR5 expression can influence the dependency of SIV or M-tropic HIV-1 infection on CD4 expression levels, consistent with previous results (57). At the low CCR5 levels on the surface of rhesus monkey macrophages, SIVmac316 requires surface CD4 for efficient entry. By contrast, at high levels of CCR5 expression, efficient SIVmac316 entry can occur in the complete absence of CD4 on the target cell. It is noteworthy that other SIV variants that are M-tropic exhibit some measure of CD4 independence. For example, SIV/17E-Fr, which was derived from the brain of an SIVmac239-infected monkey, readily infects CD4-negative brain capillary endothelial cells (30, 49). CD4 independence in the presence of high surface levels of CCR5 may be a manifestation of the properties of M-tropic SIV envelope glycoproteins that allow the low levels of CD4 on primary monkey macrophages to be efficiently utilized. In addition to the increases in CD4 binding discussed above, these properties may include the more efficient attainment of a trimeric envelope glycoprotein conformation able to bind CCR5 at a lower state of occupancy by CD4.
The extremely inefficient infection of rhesus monkey macrophages by viruses with M-tropic HIV-1 envelope glycoproteins stands in contrast to the efficient infection of primary human macrophages by these viruses. This observation implies that differences, perhaps in levels of surface CD4 expression, exist between primary macrophages derived from humans and rhesus monkeys. These species-dependent distinctions have apparently shaped the evolution of M-tropic SIV and HIV-1 differently. The ability of the viral envelope glycoproteins to mediate fusion with a cell membrane containing sparse CD4, a property critical for infection of monkey macrophages, appears not to be essential for HIV-1 to enter human macrophages. Further investigation of this coincidence of viral and host cell characteristics may lead to a better understanding of the importance of macrophage tropism in the biology of the primary immunodeficiency viruses.
ACKNOWLEDGMENTS
We acknowledge Ronald Desrosiers, Raymond Sweet, and M. Fujino for reagents. We thank Maris Handley at the Dana-Farber Cancer Institute flow cytometry core facility for excellent technical support and Yvette McLaughlin and Sheri Farnum for manuscript preparation.
This work was supported by National Institutes of Health grants AI24755 and AI41851 and by Center for AIDS Research grant AI28691. Additional support was provided by the G. Harold and Leila Y. Mathers Foundation, the late William F. McCarty-Cooper, the Friends 10, and Douglas and Judith Krupp. N. Bannert was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG).
REFERENCES
- 1.Adamson D C, Dawson T M, Zink M C, Clements J E, Dawson V L. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M G, Hauer D, Sharma D P, Joag S V, Narayan O, Zink M C, Clements J E. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology. 1993;195:616–626. doi: 10.1006/viro.1993.1413. [DOI] [PubMed] [Google Scholar]
- 5.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banapour B, Marthas M L, Ramos R A, Lohman B L, Unger R E, Gardner M B, Pedersen N C, Luciw P A. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991;65:5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 8.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benkirane M, Jeang K T, Devaux C. The cytoplasmic domain of CD4 plays a critical role during the early stages of HIV infection in T-cells. EMBO J. 1994;13:5559–5569. doi: 10.1002/j.1460-2075.1994.tb06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berchtold S, Ries J, Hornung U, Aepinus C. Exchange of functional domains between Rev proteins of HIV-1 and SIVmac239 results in a dominant negative phenotype. Virology. 1994;204:436–441. doi: 10.1006/viro.1994.1550. [DOI] [PubMed] [Google Scholar]
- 11.Berger E A. Introduction: HIV co-receptors solve old questions and raise many new ones. Semin Immunol. 1998;10:165–168. doi: 10.1006/smim.1998.0126. [DOI] [PubMed] [Google Scholar]
- 12.Bron R, Klasse P J, Wilkinson D, Clapham P R, Pelchen-Matthews A, Power C, Wells T N, Kim J, Peiper S C, Hoxie J A, Marsh M. Promiscuous use of CC and CXC chemokine receptors in cell-to-cell fusion mediated by a human immunodeficiency virus type 2 envelope protein. J Virol. 1997;71:8405–8415. doi: 10.1128/jvi.71.11.8405-8415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cayabyab M, Karlsson G B, Etemad-Moghadam B A, Hofmann W, Steenbeke T, Halloran M, Fanton J W, Axthelm M K, Letvin N L, Sodroski J G. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–984. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Ido E, Jin M, Kuwata T, Igarashi T, Mizuno A, Koyanagi Y, Hayami M. Replication of human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus strain mac (SIVmac) and chimeric HIV-1/SIVmac viruses having env genes derived from macrophage-tropic viruses: an indication of different mechanisms of macrophage-tropism in human and monkey cells. J Gen Virol. 1998;79:741–745. doi: 10.1099/0022-1317-79-4-741. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 19.Choe H, Martin K A, Farzan M, Sodroski J, Gerard N P, Gerard C. Structural interactions between chemokine receptors, gp120 Env and CD4. Semin Immunol. 1998;10:249–257. doi: 10.1006/smim.1998.0127. [DOI] [PubMed] [Google Scholar]
- 20.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 21.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 22.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 23.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 24.Desrosiers R C. Non-human primate models for AIDS vaccines. AIDS. 1995;9(Suppl. A):S137–S141. [PubMed] [Google Scholar]
- 25.Desrosiers R C, Hansen-Moosa A, Mori K, Bouvier D P, King N W, Daniel M D, Ringler D J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991;139:29–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Dimitrov D S, Norwood D, Stantchev T S, Feng Y, Xiao X, Broder C C. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology. 1999;259:1–6. doi: 10.1006/viro.1999.9747. [DOI] [PubMed] [Google Scholar]
- 27.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 28.Edinger A L, Blanpain C, Kunstman K J, Wolinsky S M, Parmentier M, Doms R W. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J Virol. 1999;73:4062–4073. doi: 10.1128/jvi.73.5.4062-4073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edinger A L, Clements J E, Doms R W. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology. 1999;260:211–221. doi: 10.1006/viro.1999.9819. [DOI] [PubMed] [Google Scholar]
- 30.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 33.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 34.Flaherty M T, Hauer D A, Mankowski J L, Zink M C, Clements J E. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 36.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann W, Schubert D, LaBonte J, Munson L, Gibson S, Scammell J, Ferrigno P, Sodroski J. Species-specific, postentry barriers to primate immunodeficiency virus infection. J Virol. 1999;73:10020–10028. doi: 10.1128/jvi.73.12.10020-10028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horuk R. Chemokine receptors and HIV-1: the fusion of two major research fields. Immunol Today. 1999;20:89–94. doi: 10.1016/s0167-5699(98)01396-6. [DOI] [PubMed] [Google Scholar]
- 39.Jinno A, Shimizu N, Soda Y, Haraguchi Y, Kitamura T, Hoshino H. Identification of the chemokine receptor TER1/CCR8 expressed in brain-derived cells and T cells as a new coreceptor for HIV-1 infection. Biochem Biophys Res Commun. 1998;243:497–502. doi: 10.1006/bbrc.1998.8130. [DOI] [PubMed] [Google Scholar]
- 40.Joag S V, Stephens E B, Galbreath D, Zhu G W, Li Z, Foresman L, Zhao L J, Pinson D M, Narayan O. Simian immunodeficiency virus SIVmac chimeric virus whose env gene was derived from SIV-encephalitic brain is macrophage-tropic but not neurovirulent. J Virol. 1995;69:1367–1369. doi: 10.1128/jvi.69.2.1367-1369.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 42.Kirchhoff F, Pohlmann S, Hamacher M, Means R E, Kraus T, Uberla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 44.Kodama T, Mori K, Kawahara T, Ringler D J, Desrosiers R C. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993;67:6522–6534. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapham C K, Zaitseva M B, Lee S, Romanstseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. . (Erratum, 5:590.) [DOI] [PubMed] [Google Scholar]
- 46.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 47.Lewin S R, Sonza S, Irving L B, McDonald C F, Mills J, Crowe S M. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retroviruses. 1996;12:877–883. doi: 10.1089/aid.1996.12.877. [DOI] [PubMed] [Google Scholar]
- 48.Mankowski J L, Flaherty M T, Spelman J P, Hauer D A, Didier P J, Amedee A M, Murphey-Corb M, Kirstein L M, Munoz A, Clements J E, Zink M C. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mankowski J L, Spelman J P, Ressetar H G, Strandberg J D, Laterra J, Carter D L, Clements J E, Zink M C. Neurovirulent simian immunodeficiency virus replicates productively in endothelial cells of the central nervous system in vivo and in vitro. J Virol. 1994;68:8202–8208. doi: 10.1128/jvi.68.12.8202-8208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcon L, Sodroski J. High degree of sensitivity of the simian immunodeficiency virus (SIVmac) envelope glycoprotein subunit association to amino acid changes in the glycoprotein 41 ectodomain. AIDS Res Hum Retroviruses. 1997;13:441–447. doi: 10.1089/aid.1997.13.441. [DOI] [PubMed] [Google Scholar]
- 50a.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus (SIVmac239) J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKnight A, Dittmar M T, Moniz-Periera J, Ariyoshi K, Reeves J D, Hibbitts S, Whitby D, Aarons E, Proudfoot A E, Whittle H, Clapham P R. A broad range of chemokine receptors are used by primary isolates of human immunodeficiency virus type 2 as coreceptors with CD4. J Virol. 1998;72:4065–4071. doi: 10.1128/jvi.72.5.4065-4071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu L, Wyatt R, Sodroski J. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J Biol Chem. 1999;274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 52a.Moore J P, McKeating J A, Huang Y X, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori K, Ringler D J, Kodama T, Desrosiers R C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naidu Y M, Kestler H W d, Li Y, Butler C V, Silva D P, Schmidt D K, Troup C D, Sehgal P K, Sonigo P, Daniel M D, et al. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988;62:4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ometto L, Zanchetta M, Cabrelle A, Esposito G, Mainardi M, Chieco-Bianchi L, De Rossi A. Restriction of HIV type 1 infection in macrophages heterozygous for a deletion in the CC-chemokine receptor 5 gene. AIDS Res Hum Retroviruses. 1999;15:1441–1452. doi: 10.1089/088922299309955. [DOI] [PubMed] [Google Scholar]
- 57.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 59.Ringler D J, Wyand M S, Walsh D G, MacKey J J, Sehgal P K, Daniel M D, Desrosiers R C, King N W. The productive infection of alveolar macrophages by simian immunodeficiency virus. J Med Primatol. 1989;18:217–226. [PubMed] [Google Scholar]
- 60.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samson M, Edinger A L, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms R W, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 62.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schenten D, Marcon L, Karlsson G B, Parolin C, Kodama T, Gerard N, Sodroski J. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol. 1999;73:5373–5380. doi: 10.1128/jvi.73.7.5373-5380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma D P, Zink M C, Anderson M, Adams R, Clements J E, Joag S V, Narayan O. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytotropic parental virus: pathogenesis of infection in macaques. J Virol. 1992;66:3550–3556. doi: 10.1128/jvi.66.6.3550-3556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simmons G, Reeves J D, McKnight A, Dejucq N, Hibbitts S, Power C A, Aarons E, Schols D, De Clercq E, Proudfoot A E, Clapham P R. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–8457. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephens E B, McClure H M, Narayan O. The proteins of lymphocyte- and macrophage-tropic strains of simian immunodeficiency virus are processed differently in macrophages. Virology. 1995;206:535–544. doi: 10.1016/s0042-6822(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 68.Stephens E B, Tian C, Li Z, Narayan O, Gattone V H., II Rhesus macaques infected with macrophage-tropic simian immunodeficiency virus (SIVmacR71/17E) exhibit extensive focal segmental and global glomerulosclerosis. J Virol. 1998;72:8820–8832. doi: 10.1128/jvi.72.11.8820-8832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thali M, Charles M, Furman C, Cavacini L, Posner M, Robinson J, Sodroski J. Resistance to neutralization by broadly reactive antibodies to the human immunodeficiency virus type 1 gp120 glycoprotein conferred by a gp41 amino acid change. J Virol. 1994;68:674–680. doi: 10.1128/jvi.68.2.674-680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 71.Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi A G, Vercelli D. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J Immunol. 1998;161:2084–2088. [PubMed] [Google Scholar]
- 72.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 73.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardosa A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interactions of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;184:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 74.Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 75.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]