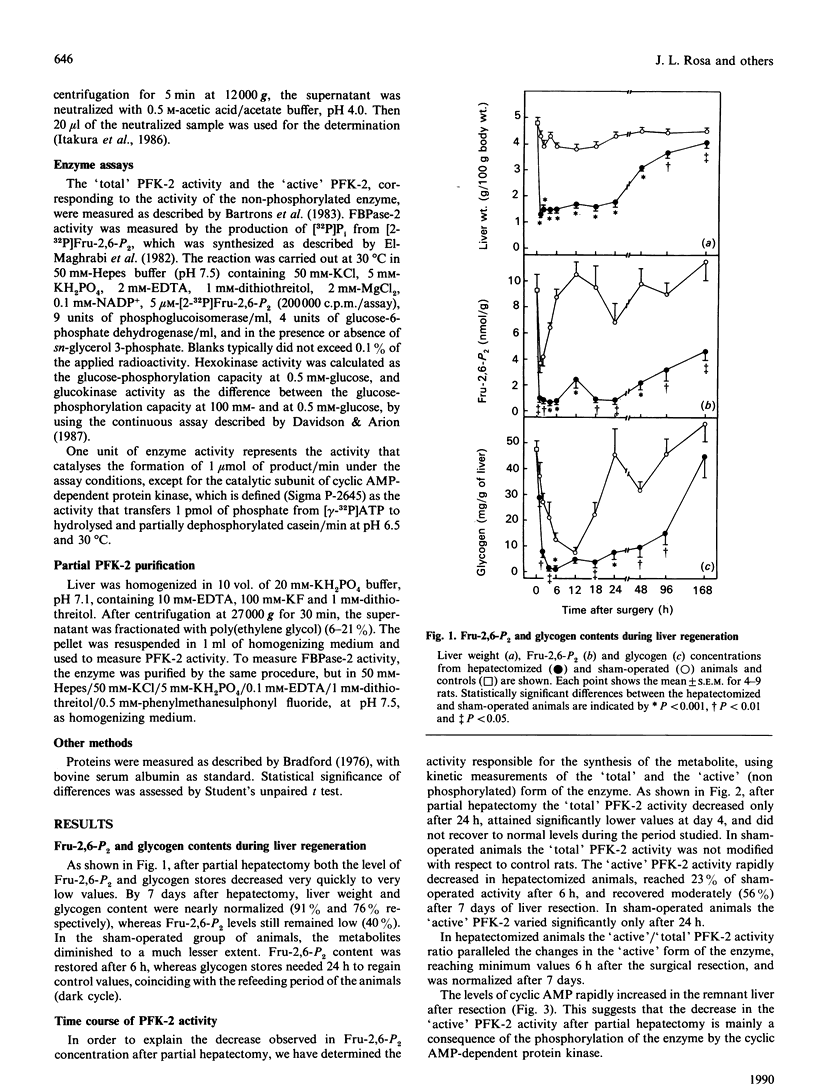
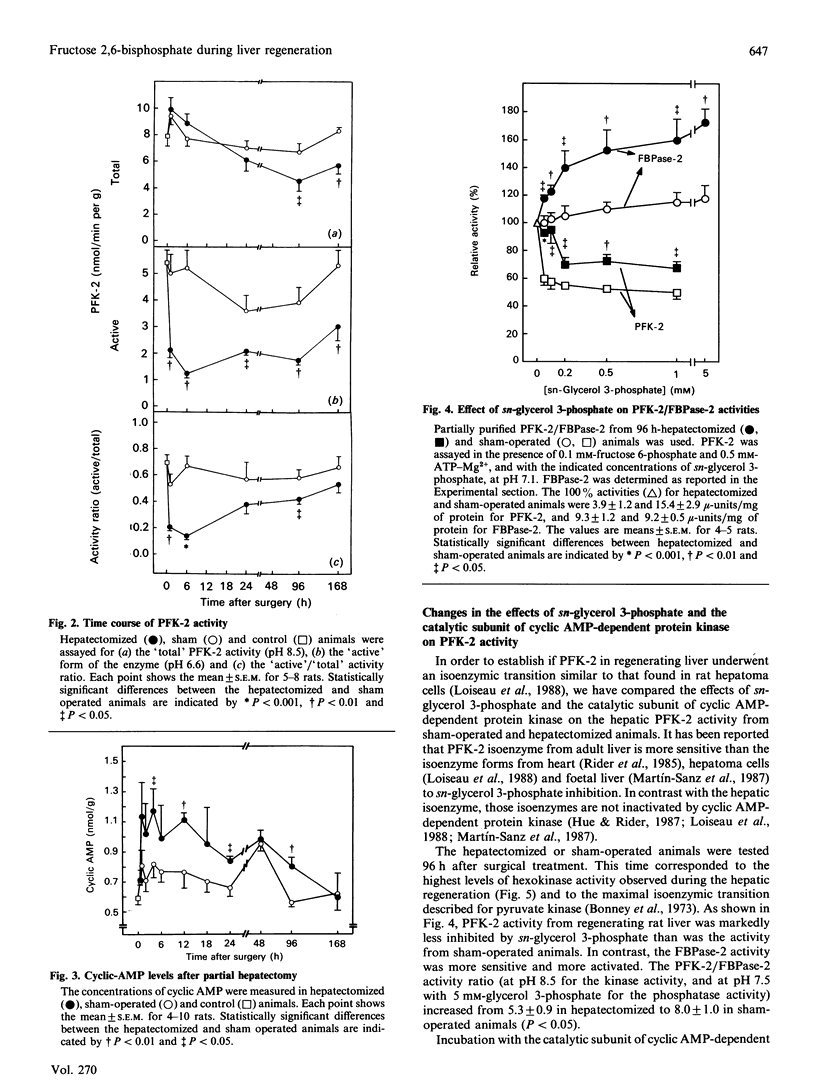
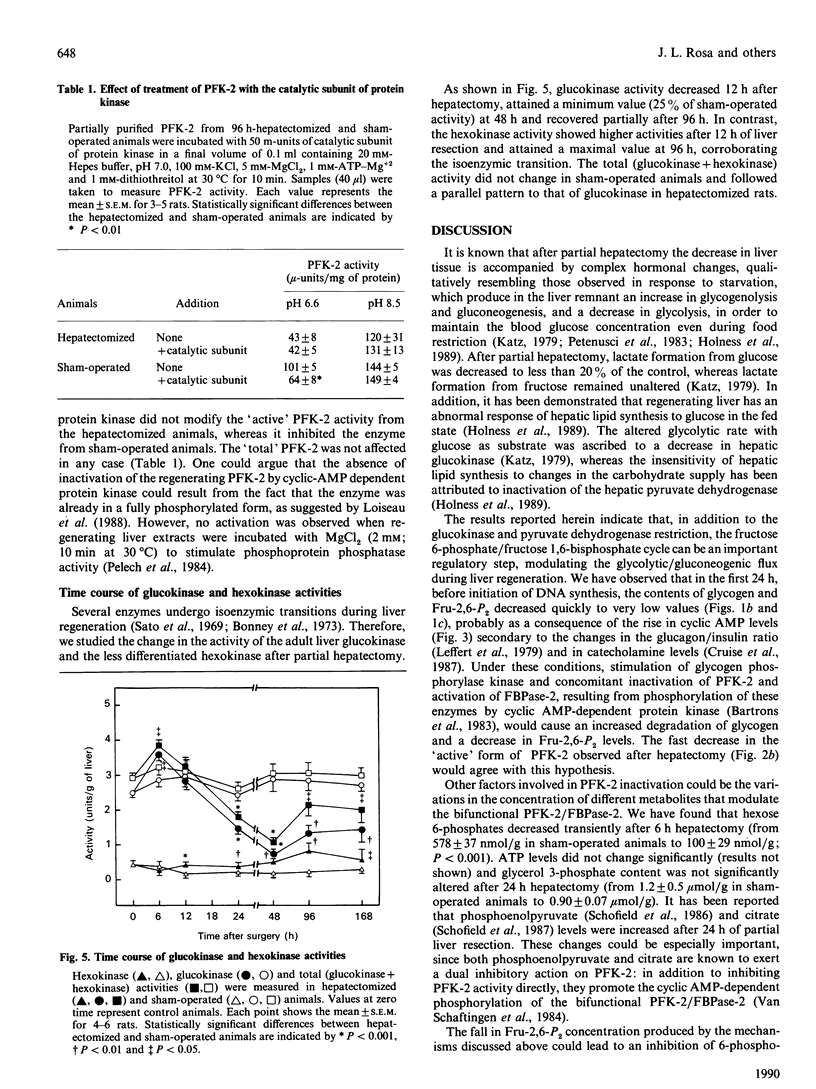
Abstract
Glycogen and fructose 2,6-bisphosphate levels in rat liver decreased quickly after partial hepatectomy. After 7 days the glycogen level was normalized and fructose 2,6-bisphosphate concentration still remained low. The 'active' (non-phosphorylated) form of 6-phosphofructo-2-kinase varied in parallel with fructose 2,6-bisphosphate levels, whereas the 'total' activity of the enzyme decreased only after 24 h, similarly to glucokinase. The response of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase from hepatectomized rats (96 h) to sn-glycerol 3-phosphate and to cyclic AMP-dependent protein kinase was different from that of the enzyme from control animals and similar to that of the foetal isoenzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartrons R., Hue L., Van Schaftingen E., Hers H. G. Hormonal control of fructose 2,6-bisphosphate concentration in isolated rat hepatocytes. Biochem J. 1983 Sep 15;214(3):829–837. doi: 10.1042/bj2140829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Hopkins H. A., Walker P. R., Potter V. R. Glycolytic isoenzymes and glycogen metabolism in regenerating liver from rats on controlled feeding schedules. Biochem J. 1973 Sep;136(1):115–124. doi: 10.1042/bj1360115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bucher N. L., Weir G. C. Insulin, glucagon, liver regeneration, and DNA synthesis. Metabolism. 1976 Nov;25(11 Suppl 1):1423–1425. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- CARROLL N. V., LONGLEY R. W., ROE J. H. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956 Jun;220(2):583–593. [PubMed] [Google Scholar]
- Cruise J. L., Knechtle S. J., Bollinger R. R., Kuhn C., Michalopoulos G. Alpha 1-adrenergic effects and liver regeneration. Hepatology. 1987 Nov-Dec;7(6):1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- Davidson A. L., Arion W. J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987 Feb 15;253(1):156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- Garnett M. E., Dyson R. D., Dost F. N. Pyruvate kinase isozyme changes in parenchymal cells of regenerating rat liver. J Biol Chem. 1974 Aug 25;249(16):5222–5226. [PubMed] [Google Scholar]
- Holness M. J., Schofield P. S., Sugden M. C. Altered interactions between glycogenesis and lipid synthesis after liver resection: specific effects of liver regeneration, coupled with non-specific effects of surgery. Clin Sci (Lond) 1989 Mar;76(3):317–322. doi: 10.1042/cs0760317. [DOI] [PubMed] [Google Scholar]
- Hue L., Rider M. H. Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem J. 1987 Jul 15;245(2):313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura M., Maeda N., Tsuchiya M., Yamashita K. Increased rate of de novo purine synthesis and its mechanism in regenerating rat liver. Am J Physiol. 1986 Nov;251(5 Pt 1):G585–G590. doi: 10.1152/ajpgi.1986.251.5.G585. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz N. Correlation between rates and enzyme levels of increased gluconeogenesis in rat liver and kidney after partial hepatectomy. Eur J Biochem. 1979 Aug 1;98(2):535–542. doi: 10.1111/j.1432-1033.1979.tb13214.x. [DOI] [PubMed] [Google Scholar]
- Koide Y., Earp H. S., Ong S. H., Steiner A. L. Alterations in the intracellular distribution of cGMP and guanylate cyclase activity during rat liver regeneration. J Biol Chem. 1978 Jun 25;253(12):4439–4445. [PubMed] [Google Scholar]
- Kurland I. J., Pilkis S. J. Indirect versus direct routes of hepatic glycogen synthesis. FASEB J. 1989 Sep;3(11):2277–2281. doi: 10.1096/fasebj.3.11.2673899. [DOI] [PubMed] [Google Scholar]
- Kuwajima M., Golden S., Katz J., Unger R. H., Foster D. W., McGarry J. D. Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate. J Biol Chem. 1986 Feb 25;261(6):2632–2637. [PubMed] [Google Scholar]
- Leffert H. L., Koch K. S., Moran T., Rubalcava B. Hormonal control of rat liver regeneration. Gastroenterology. 1979 Jun;76(6):1470–1482. [PubMed] [Google Scholar]
- Loiseau A. M., Rider M. H., Foret D., Rousseau G. G., Hue L. Rat hepatoma (HTC) cell 6-phosphofructo-2-kinase differs from that in liver and can be separated from fructose-2,6-bisphosphatase. Eur J Biochem. 1988 Jul 15;175(1):27–32. doi: 10.1111/j.1432-1033.1988.tb14161.x. [DOI] [PubMed] [Google Scholar]
- Martin-Sanz P., Cascales M., Boscá L. Glucagon-induced changes in fructose 2,6-bisphosphate and 6-phosphofructo-2-kinase in cultured rat foetal hepatocytes. Biochem J. 1989 Feb 1;257(3):795–799. doi: 10.1042/bj2570795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Sanz P., Cascales M., Boscá L. Fructose 2,6-bisphosphate in isolated foetal hepatocytes. FEBS Lett. 1987 Dec 10;225(1-2):37–42. doi: 10.1016/0014-5793(87)81127-4. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- Morley C. G., Kuku S., Rubenstein A. H., Boyer J. L. Serum hormone levels following partial hepatectomy in the rat. Biochem Biophys Res Commun. 1975 Nov 17;67(2):653–661. doi: 10.1016/0006-291x(75)90862-1. [DOI] [PubMed] [Google Scholar]
- Pelech S., Cohen P., Fisher M. J., Pogson C. I., El-Maghrabi M. R., Pilkis S. J. The protein phosphatases involved in cellular regulation. Glycolysis, gluconeogenesis and aromatic amino acid breakdown in rat liver. Eur J Biochem. 1984 Nov 15;145(1):39–49. doi: 10.1111/j.1432-1033.1984.tb08519.x. [DOI] [PubMed] [Google Scholar]
- Petenusci S. O., Freitas T. C., Roselino E. S., Migliorini R. H. Glucose homeostasis during the early stages of liver regeneration in fasted rats. Can J Physiol Pharmacol. 1983 Mar;61(3):222–228. doi: 10.1139/y83-034. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., el-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem. 1988;57:755–783. doi: 10.1146/annurev.bi.57.070188.003543. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Foret D., Hue L. Comparison of purified bovine heart and rat liver 6-phosphofructo-2-kinase. Evidence for distinct isoenzymes. Biochem J. 1985 Oct 1;231(1):193–196. doi: 10.1042/bj2310193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Matsushima T., Sugimura T. Hexokinase isozyme patterns of experimental hepatomas of rats. Cancer Res. 1969 Jul;29(7):1437–1446. [PubMed] [Google Scholar]
- Schofield P. S., Kerbey A. L., Sugden M. C. Hepatic pyruvate metabolism during liver regeneration after partial hepatectomy in the rat. Int J Biochem. 1986;18(5):453–458. doi: 10.1016/0020-711x(86)90188-6. [DOI] [PubMed] [Google Scholar]
- Schofield P. S., Sugden M. C., Corstorphine C. G., Zammit V. A. Altered interactions between lipogenesis and fatty acid oxidation in regenerating rat liver. Biochem J. 1987 Jan 15;241(2):469–474. doi: 10.1042/bj2410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek J., Chmelar V., Melka J., Pazderka, Charvát Z. Influence of protracted infusion of glucose and insulin on the composition and regeneration activity of liver after partial hepatectomy in rats. Nature. 1967 Mar 4;213(5079):910–911. doi: 10.1038/213910a0. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Bartrons R., Hers H. G. The mechanism by which ethanol decreases the concentration of fructose 2,6-bisphosphate in the liver. Biochem J. 1984 Sep 1;222(2):511–518. doi: 10.1042/bj2220511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv Enzymol Relat Areas Mol Biol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Weinhouse S. Isozyme alterations, gene regulation and the neoplastic transformation. Adv Enzyme Regul. 1983;21:369–386. doi: 10.1016/0065-2571(83)90024-9. [DOI] [PubMed] [Google Scholar]


