Abstract
1. The cytosolic aspartate aminotransferase was purified from human liver. 2. The isoenzyme contains four cysteine residues, only one of which reacts with 5,5'-dithiobis-(2-nitrobenzoic acid) in the absence of denaturing agents. 3. The amino acid sequence of the isoenzyme is reported, as determined from peptides produced by digestion with trypsin and with CNBr, and from sub-digestion of some of these peptides with Staphylococcus aureus V8 proteinase. 4. The isoenzyme shares 48% identity of amino acid sequence with the mitochondrial form from human heart. 5. Comparisons of the amino acid sequences of all known mammalian cytosolic aspartate aminotransferases and of the same set of mitochondrial isoenzymes are reported. The results indicate that the cytosolic isoenzymes have evolved at about 1.3 times the rate of the mitochondrial forms. 6. The time elapsed since the cytosolic and mitochondrial isoenzymes diverged from a common ancestral protein is estimated to be 860 x 10(6) years. 7. Experimental details and confirmatory data for the results presented here are given in a supplementary paper that has been deposited as a Supplementary Publication SUP 50158 (25 pages) at the British Library Document Supply Centre, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1990) 265, 5.
Full text
PDF
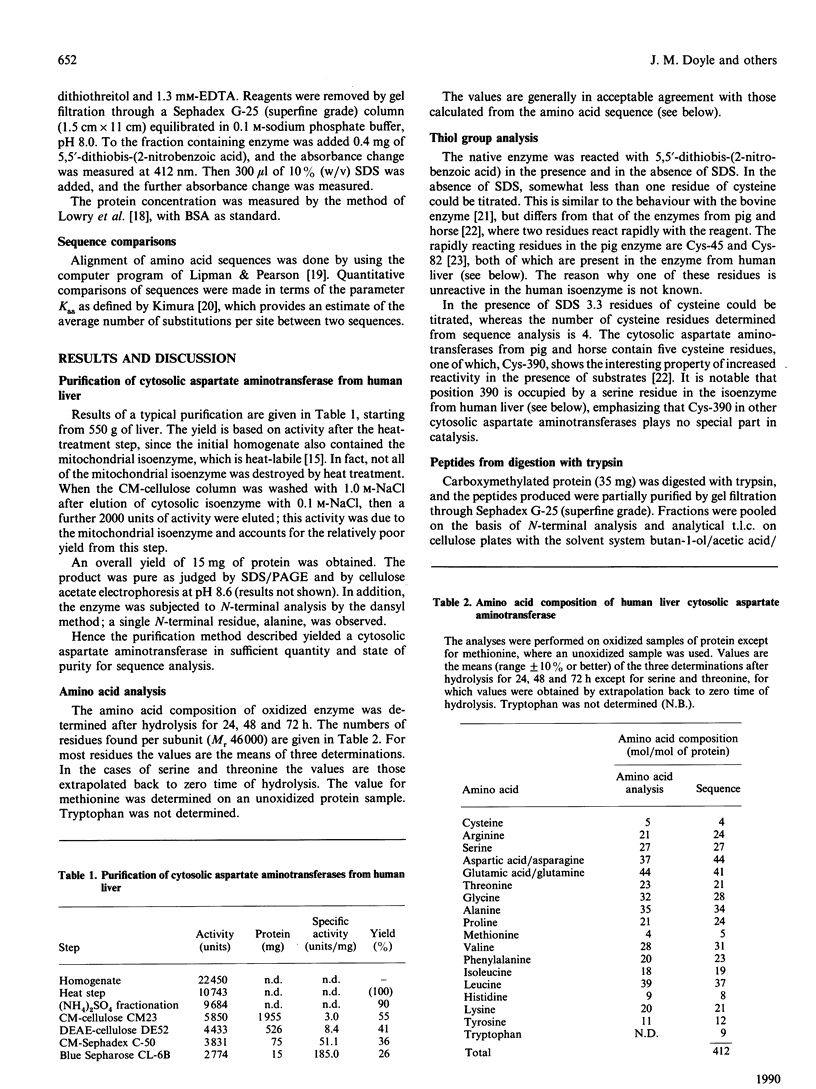

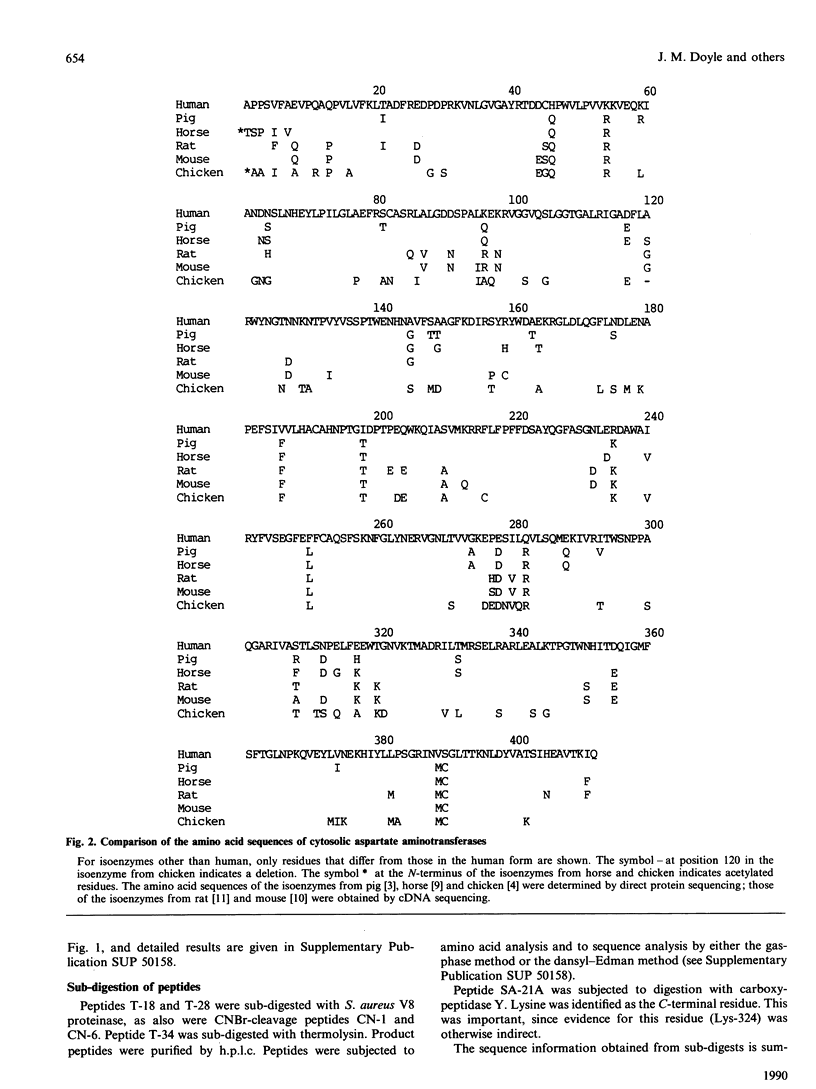



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra D., Bossa F., Doonan S., Fahmy H. M., Hughes G. J., Kakoz K. Y., Martini F., Petruzzelli R. The structure of mitochondrial asparate aminotransferase from pig heart and comparison with that of the cytoplasmic isozyme. FEBS Lett. 1977 Nov 15;83(2):241–244. doi: 10.1016/0014-5793(77)81013-2. [DOI] [PubMed] [Google Scholar]
- Barra D., Bossa F., Doonan S., Fahmy H. M., Hughes G. J., Martini F., Petruzzelli R., Wittmann-Liebold B. The cytosolic and mitochondrial aspartate aminotransferases from pig heart. A comparison of their primary structures, predicted secondary structures and some physical properties. Eur J Biochem. 1980 Jul;108(2):405–414. doi: 10.1111/j.1432-1033.1980.tb04736.x. [DOI] [PubMed] [Google Scholar]
- Critz W. J., Martinez-Carrion M. Sulphydryl group modification of aspartate aminotransferase with 3-bromo-1,1,1-trifluoropropanone during catalysis. Biochemistry. 1977 Apr 19;16(8):1554–1558. doi: 10.1021/bi00627a004. [DOI] [PubMed] [Google Scholar]
- Doonan S., Barra D., Bossa F. Structural and genetic relationships between cytosolic and mitochondrial isoenzymes. Int J Biochem. 1984;16(12):1193–1199. doi: 10.1016/0020-711x(84)90216-7. [DOI] [PubMed] [Google Scholar]
- Doonan S., Martini F., Angelaccio S., Pascarella S., Barra D., Bossa F. The complete amino acid sequences of cytosolic and mitochondrial aspartate aminotransferases from horse heart, and inferences on evolution of the isoenzymes. J Mol Evol. 1986;23(4):328–335. doi: 10.1007/BF02100642. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Graf-Hausner U., Wilson K. J., Christen P. The covalent structure of mitochondrial aspartate aminotransferase from chicken. Identification of segments of the polypeptide chain invariant specifically in the mitochondrial isoenzyme. J Biol Chem. 1983 Jul 25;258(14):8813–8826. [PubMed] [Google Scholar]
- Horio Y., Tanaka T., Taketoshi M., Nagashima F., Tanase S., Morino Y., Wada H. Rat cytosolic aspartate aminotransferase: molecular cloning of cDNA and expression in Escherichia coli. J Biochem. 1988 May;103(5):797–804. doi: 10.1093/oxfordjournals.jbchem.a122349. [DOI] [PubMed] [Google Scholar]
- Huynh Q. K., Sakakibara R., Watanabe T., Wada M. Primary structure of mitochondrial glutamic oxaloacetic transaminase from rat liver : comparison with that of the pig heart isozyme. Biochem Biophys Res Commun. 1980 Nov 28;97(2):474–479. doi: 10.1016/0006-291x(80)90287-9. [DOI] [PubMed] [Google Scholar]
- Kagamiyama H., Sakakibara R., Wada H., Tanase S., Morino Y. The complete amino acid seqeunce of mitochondrial asparatate aminotransferase from pig heart. J Biochem. 1977 Jul;82(1):291–294. doi: 10.1093/oxfordjournals.jbchem.a131682. [DOI] [PubMed] [Google Scholar]
- Kondo K., Wakabayashi S., Yagi T., Kagamiyama H. The complete amino acid sequence of aspartate aminotransferase from Escherichia coli: sequence comparison with pig isoenzymes. Biochem Biophys Res Commun. 1984 Jul 18;122(1):62–67. doi: 10.1016/0006-291x(84)90439-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leung F. Y., Henderson A. R. Isolation and purification of aspartate aminotransferase isoenzymes from human liver by chromatography and isoelectric focusing. Clin Chem. 1981 Feb;27(2):232–238. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Martini F., Angelaccio S., Barra D., Doonan S., Bossa F. Partial amino-acid sequence and cysteine reactivities of cytosolic aspartate aminotransferase from horse heart. Biochim Biophys Acta. 1984 Aug 28;789(1):51–56. doi: 10.1016/0167-4838(84)90059-1. [DOI] [PubMed] [Google Scholar]
- Martini F., Angelaccio S., Barra D., Pascarella S., Maras B., Doonan S., Bossa F. The primary structure of mitochondrial aspartate aminotransferase from human heart. Biochim Biophys Acta. 1985 Nov 8;832(1):46–51. doi: 10.1016/0167-4838(85)90172-4. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Obaru K., Nomiyama H., Shimada K., Nagashima F., Morino Y. Cloning and sequence analysis of mRNA for mouse aspartate aminotransferase isoenzymes. J Biol Chem. 1986 Dec 25;261(36):16976–16983. [PubMed] [Google Scholar]
- Petrilli P., Pucci P., Garzillo A. M., Sannia G., Marino G. Reactivity of sulphydryl groups of cytosolic and mitochondrial bovine aspartate aminotransferases. Mol Cell Biochem. 1981 Mar 13;35(2):121–128. doi: 10.1007/BF02354826. [DOI] [PubMed] [Google Scholar]
- Pol S., Bousquet-Lemercier B., Pave-Preux M., Pawlak A., Nalpas B., Berthelot P., Hanoune J., Barouki R. Nucleotide sequence and tissue distribution of the human mitochondrial aspartate aminotransferase mRNA. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1309–1315. doi: 10.1016/s0006-291x(88)81017-9. [DOI] [PubMed] [Google Scholar]
- Shlyapnikov S. V., Myasnikov A. N., Severin E. S., Myagkova M. A., Torchinsky Y. M., Braunstein A. E. Primary structure of cytoplasmic aspartate aminotransferase from chicken heart and its homology with pig heart isoenzymes. FEBS Lett. 1979 Oct 15;106(2):385–388. doi: 10.1016/0014-5793(79)80537-2. [DOI] [PubMed] [Google Scholar]
- Sonderegger P., Christen P. Comparison of the evolution rates of cytosolic and mitochondrial aspartate aminotransferase. Nature. 1978 Sep 14;275(5676):157–159. doi: 10.1038/275157a0. [DOI] [PubMed] [Google Scholar]
- Teranishi H., Kagamiyama H., Teranishi K., Wada H., Yamano T., Morino Y. Cytosolic and mitochondrial isoenzymes of glutamic-oxalacetic transaminase from human heart. Structural comparison with the isoenzymes from pig heart. J Biol Chem. 1978 Dec 25;253(24):8842–8847. [PubMed] [Google Scholar]


