Abstract
The conclusions of the European Food Safety Authority (EFSA) following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State Poland and co‐rapporteur Member State Hungary for the pesticide active substance triclopyr (variant triclopyr‐butotyl) and the assessment of applications for maximum residue levels (MRLs) are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative uses of triclopyr (variant triclopyr‐butotyl) as a herbicide on established pasture and non‐recreational amenity grassland (field use). MRLs were assessed in rice. The reliable end points, appropriate for use in regulatory risk assessment and the proposed MRLs, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are reported where identified.
Keywords: herbicide, MRL art 10, peer review, pesticide, risk assessment, triclopyr, triclopyr‐butotyl
SUMMARY
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012. Triclopyr is one of the active substances listed in Regulation (EU) No 686/2012.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), Poland, and co‐rapporteur Member State (co‐RMS), Hungary, received an application from Corteva Agriscience (previously Dow AgroSciences (DAS)) for the renewal of approval of the active substance triclopyr (variant triclopyr‐butotyl). In addition, the applicant submitted an application for maximum residue levels (MRLs), as referred to in Article 7 of Regulation (EC) No 396/2005.
An initial evaluation of the dossier on the active substance was provided by the RMS in the renewal assessment report (RAR) and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The uses of triclopyr (variant triclopyr‐butotyl) according to the representative uses as a herbicide on established pasture and non‐recreational amenity grassland, as proposed at EU level, result in a sufficient herbicidal efficacy against the target weeds.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to identity, physical/chemical properties and analytical methods.
In the section mammalian toxicology, the acceptability of the proposed maximum levels for the impurities cannot be concluded and the representativeness of the batches used in the toxicity studies with regard to the reference specifications cannot be considered fully demonstrated. With regard to the non‐dietary exposure estimates, it should be noted that the exposure estimates with the EFSA model (2014b), not applicable at the time of dossier submission, are above the AOEL while the use of previous models (e.g. German model) does not raise any concern.
In the section of residues, the finalisation of the consumer dietary risk assessment considering both the representative uses as well as the use of the MRL application is pending clarification of several data gaps. The MRL requests were supported by GAP compliant residue field trials but are subject to data gaps. Given the results of a recent ruminant feeding study, it is recommended to revisit the MRLs for animal commodities derived in the former MRL review according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2017) and implemented in the current legislation.
An update of the chronic consumer dietary risk assessment that was conducted in the framework of the review of MRLs, considering the residue values for triclopyr and metabolite 3,5,6‐TCP from the recent feeding study and the amended toxicological reference values for these two substances, resulted in an increased estimated chronic consumer exposure corresponding to 44% of the ADI at the maximum (NL, toddler) for triclopyr and 8% of the ADI at the maximum (NL, toddler) for 3,5,6‐TCP.
The data available on environmental fate and behaviour were sufficient to carry out the required environmental exposure assessments at the EU level for the representative uses with the notable exception that a data gap was identified for information on the effect of water treatment processes on the nature of residues of both the active substance and its identified metabolites potentially present in surface water, when surface water is abstracted for drinking water. This data gap leads to the consumer risk assessment from the consumption of drinking water being not finalised for all representative uses.
In the area of ecotoxicology, critical areas of concern were identified for wild mammals and non‐target arthropods. The risk assessment for aquatic organisms could not be finalised.
An assessment not finalised was identified as the formulation for the representative uses contains the active substance aminopyralid and a complete risk assessment for the mixture was not available for all groups of non‐target organisms and groundwater exposure.
Based on the available information, it can be concluded that triclopyr and its variant(s) do not meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
BACKGROUND
Commission Implementing Regulation (EU) No 844/2012, 1 as amended by Commission Implementing Regulation (EU) No 2018/1659 2 (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009. 3 This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS Poland and co‐RMS Hungary received an application from Corteva Agriscience (formerly Dow AgroSciences (DAS)) for the renewal of approval of the active substance triclopyr (variant triclopyr‐butotyl). In addition, the applicant submitted an application for maximum residue levels (MRLs) as referred to in Article 7 of Regulation (EC) No 396/2005. 4 Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Hungary), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier in the RAR, which was received by EFSA on 27 September 2018 (Poland, 2018). The RAR included a proposal to set MRLs, submitted under Article 7 of Regulation (EC) No 396/2005.
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, Corteva Agriscience (previously Dow AgroSciences (DAS)), for consultation and comments on 10 October 2018. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 15 February 2019. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of a reporting table. The applicant was invited to respond to the comments in column 3 of the reporting table. The comments and the applicant's response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS on 5 April 2019. On the basis of the comments received, the applicant's response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues and ecotoxicology.
In addition, following a consultation with Member States in the Pesticides Peer Review Experts' meetings PREV 11 and 14 (September 2019), it was considered necessary to apply an additional clock stop of 30 months in accordance with Commission Implementing Regulation (EU) No 2018/1659, to be able to conclude whether the approval criteria for endocrine disruption in line with the scientific criteria for the determination of endocrine disrupting properties, as laid down in Commission Regulation (EU) 2018/605, 5 are met.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment and on the proposed MRLs took place with Member States via a written procedure in June 2023 and in June/July 2024.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the formulation for representative uses, evaluated on the basis of the representative uses of triclopyr (variant triclopyr‐butotyl) as a herbicide on established pasture and non‐recreational amenity grassland (field use), as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion. MRLs were assessed in rice. A list of the relevant end points for the active substance and the formulation, and the proposed MRLs is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2023a), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting tables (8 April 2019 and 11 January 2023 6 );
the evaluation tables (July 2023, updated in July 2024);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (Poland, 2024), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulation for representative uses
Triclopyr is the ISO common name for [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetic acid (IUPAC). Triclopyr‐butotyl, a derivative of triclopyr is the modified ISO common name for 2‐butoxyethyl [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetate (IUPAC).
The formulation for representative uses supported for the evaluation was ‘GF‐1365’, an emulsion, oil in water (EW), containing 334 g/L triclopyr‐butotyl (equivalent to 240 g/L triclopyr) and 35.5 g/L aminopyralid potassium (equivalent to 30 g/L aminopyralid).
The representative uses evaluated were broadcast and spot foliar applications to control broadleaf weeds and woody plants (brush) in established pasture and non‐recreational amenity grassland in southern and central zones. Full details of the GAPs can be found in the list of end points in Appendix B.
Data were submitted to conclude that the use of triclopyr‐butotyl according to the representative uses proposed at EU level results in a sufficient herbicidal efficacy against the target weeds, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
Conclusions of the evaluation
1. IDENTITY, PHYSICAL/CHEMICAL/TECHNICAL PROPERTIES AND METHODS OF ANALYSIS
The following guidance documents were followed in the production of this conclusion: European Commission (2000a, 2000b, 2010).
The active substance is produced and used in the representative formulation as triclopyr‐butotyl. The proposed specification for triclopyr‐butotyl is based on batch data from industrial scale productions. An updated reference specification, with a minimum purity of the technical material of 960 g/kg, was proposed by the applicant and accepted by the RMS. EFSA notes that the proposed reference specification for some impurities is not in accordance with the data of the batches provided for the renewal. The assessment of the toxicological relevance of most impurities was not finalised (see Section 2), therefore it cannot be concluded whether the batches used in the toxicological assessment support the existing reference specification and/or the newly proposed one (See Section 2). The applicant has not provided a clear and complete assessment of the batches used in the ecotoxicity studies compared to the newly proposed, and existing, reference specification (see Section 5). Pending finalisation of the evaluation of the toxicological relevance of the impurities (see Section 2), EFSA proposes an update of the reference specification based on the data of the batches provided for the renewal. 7 There is no FAO specification available for triclopyr‐butotyl.
It should be noted that the majority of the data evaluated for the renewal belong to the variant triclopyr‐butotyl, unless otherwise specified.
The main data regarding the identity of triclopyr and triclopyr‐butotyl and their physical and chemical properties are given in Appendix B. A data gap for Log Kow for some of the components of the residue definition for risk assessment was identified (see Section 10).
Adequate methods are available for the generation of data required for the risk assessment. Methods of analysis are available for the determination of the active substance in the technical material and in the formulation for representative uses and the impurities in the technical material. Pending finalisation of the evaluation of the toxicological relevance of the impurities further data might be required.
Triclopyr residues in food and feed of plant origin can be monitored by liquid chromatography with tandem mass spectrometry (LC–MS/MS) with a limit of quantification (LOQ) of 0.01 mg/kg in all commodity groups. The efficiency of the extraction procedure used in the method was not demonstrated (data gap, see Section 10). Triclopyr and 3,5,6‐TCP and its base‐labile conjugates in food of animal origin can be determined by LC–MS/MS with a LOQ of 0.01 mg/kg in all animal matrices. The extraction efficiency of the monitoring method for eggs was not addressed (data gap, see Section 10).
Triclopyr residues in soil could be monitored by LC–MS/MS with a LOQ of 0.05 mg/kg. However, the LOQ of the method does not comply with the EC10‐value of the most sensitive crop, therefore a data gap for a more sensitive method was identified (see Section 10). Triclopyr residues could be analysed in water by LC–MS/MS with a LOQ of 0.1 μg/L. Pending finalisation of the residue definition for monitoring in surface water (aquatic risk assessment for metabolites is open, see Sections 5 and 9.1.1), new monitoring methods might be needed. Appropriate gas chromatography–mass spectrometry (GC–MS) method exists for monitoring of triclopyr‐butotyl in air with a LOQ of 0.8 μg/m3. Triclopyr residues in air could be determined by LC–MS/MS with a LOQ of 0.9 μg/m3.
LC–MS/MS method can be used for monitoring of triclopyr in body fluids (blood and urine) with a LOQ of 0.05 mg/L. Triclopyr residues in body tissues can be determined by using the monitoring methods for residues in food of animal origin.
2. MAMMALIAN TOXICITY
The following guidance documents were followed in the production of this conclusion: European Commission (2003, 2012), EFSA (2014b), EFSA PPR Panel (2012) and ECHA (2017).
Triclopyr was discussed at the Pesticides Peer Review Experts' Meeting 11 in September 2019 and at the Pesticides Peer Review Experts' TC 131 in March 2024.
Toxicity data package has been submitted for triclopyr and/or triclopyr‐butotyl (triclopyr variant, ester) and/or triclopyr triethylammonium (triclopyr variant, salt). All submitted data have been assessed.
In the reference specification for triclopyr‐butotyl, for most of the impurities, the toxicological relevance could not be concluded (data gap). 8 As a consequence, even though a mutagenicity study has been conducted with the new technical material, the acceptability of the proposed maximum levels for the impurities cannot be concluded and the representativeness of the toxicological batches with regard to the original and newly proposed reference specifications cannot be considered fully demonstrated (issue not finalised, see Section 9.1.1).
Triclopyr is rapidly and extensively absorbed. It is found to distribute mainly in kidneys and no potential for accumulation is observed, except at very high doses. Triclopyr and its variants triclopyr‐butotyl and triclopyr triethylammonium are toxicokinetically equivalent. Triclopyr and triclopyr‐butotyl have similar urinary metabolite profiles after dosing with equimolar doses. Blood data indicate that triclopyr‐butotyl is hydrolysed to triclopyr prior to absorption. Most of the administered triclopyr is excreted as parent compound (> 80%) (in all species tested) and only a small proportion is found in the rat urine as 3,5,6‐TCP, as well as its glucuronide and sulfate conjugates. The human absorption, metabolism and excretion are similar to rats and other mammalian species, and there are no unique human metabolites.
Triclopyr is proposed for residue definition in body fluids for monitoring purpose.
Triclopyr is acutely toxic when administered orally to rats, it causes eye damage and is a skin sensitiser. It is not acutely toxic following dermal administration and not skin irritant. Criteria for classification according to Regulation (EC) No 1272/2008 9 (ECHA, 2017) may be met for acute toxicity Cat 4, skin sensitisation Cat 1 and eye damage Cat 1. Triclopyr‐butotyl is also acutely toxic via the oral route and it is a skin sensitiser. It is not acutely toxic via the dermal and inhalation routes. It is non‐irritating to skin. Criteria for classification according to Regulation (EC) No 1272/2008 (ECHA, 2017) may be met for acute toxicity Cat 4 and skin sensitisation Cat 1. Triclopyr triethylammonium is not acutely toxic via the oral, dermal or inhalation routes. It is non‐irritating to skin but does cause irritation to the eye. It is not a skin sensitiser. Criteria for classification according to Regulation (EC) No 1272/2008 (ECHA, 2017) may be met for eye irritation Cat 2. The UVB absorption profile of triclopyr and the two variants (triclopyr‐butotyl and triclopyr triethylammonium) is similar, therefore the phototoxicity study conducted with triclopyr is considered valid also for triclopyr‐butotyl and triclopyr triethylammonium. Triclopyr is not phototoxic in the 3T3 Neutral Red Uptake (NRU) Phototoxicity (PT) test. However, triclopyr (as well as triclopyr‐butotyl and triclopyr triethylammonium) is mainly a UVB absorber and the 3T3 NRU‐PT test might not be suitable to test UVB absorbers. It is noted however phototoxicity testing applying the OECD TG 498 test would allow proper assessment of UVB absorbers (data gap, see Section 10).
The short‐term studies testing triclopyr and its variants (triclopyr triethylammonium and triclopyr‐butotyl) are considered equivalent in terms of doses, findings and target organs (i.e. kidney in rat and dog). The overall short‐term no observed adverse effect level (NOAEL) is 0.5 mg/kg bw per day, based on renal findings (changes in blood urea nitrogen (BUN), creatinine, phenolsulfonphthalein (PSP) excretion and histopathological findings) observed at 2.5 mg/kg bw per day in the 1‐year dog study conducted with triclopyr. This was subsequently confirmed 10 and the submitted toxicokinetic (TK) and in vitro data were assessed as not sufficient to dismiss the uncertainties and to consider the dog as a non‐reliable model for the risk assessment.
Based on the submitted in vitro and in vivo data, including one in vivo micronucleus study with proof of bone marrow exposure, a genotoxic potential is unlikely to be attributed to triclopyr and the two variants, triclopyr‐butotyl and triclopyr triethylammonium.
Long‐term/carcinogenicity studies have been conducted with triclopyr only. In the rat, the long‐term NOAEL is 3 mg triclopyr/kg bw per day, based on effects on kidney (increased absolute and relative weight with microscopic degenerative changes in the descending part of the proximal tubule) in males at 12 mg triclopyr/kg bw per day. The NOAEL for carcinogenicity is 3 mg/kg bw per day, based on increased incidence in mammary gland tumours (combined adenoma and carcinoma) at 12 mg triclopyr /kg bw per day. No increased incidence in tumours is observed in mouse up to 135 mg/kg bw per day, the highest tested dose. The NOAEL for systemic toxicity is 5 mg triclopyr/kg bw per day based on kidney toxicity (increased weight and functional effects (reduced urine specific gravity, increased urinary proteins and increased water consumption in males)) at 26.5 mg triclopyr/kg bw per day. 11 Based on effects observed in kidney, the criteria for classification according to Regulation (EC) No 1272/2008 (ECHA, 2017) may be met for STOT‐RE 2. The experts considered that a clear conclusion on classification for carcinogenicity Carc. Cat 2 based on findings in mammary glands cannot be reached. The RMS does not support proposal for classification of triclopyr as Carc. Cat 2.
In the multigenerational rat toxicity study, the parental NOAEL is 5 mg triclopyr/kg bw per day, based on nephrotoxicity ≥ 25 mg/kg bw per day; the reproductive and developmental NOAELs are 25 mg/kg bw per day, based on adverse effects on mating, conception and fertility indices (P2) and on adverse effects on litter size, pup body weight and pup survival (F1 and F2), respectively.
In the rat, the relevant developmental and maternal NOAEL is 5 mg triclopyr‐butotyl/kg bw per day (corresponding to 3.7 mg/kg bw per day of triclopyr equivalent), based on visceral and skeletal anomalies in litters and on decreased maternal body weight gain observed at 30 mg/kg bw per day, respectively. In the rabbit, the relevant developmental and maternal NOAEL is 30 mg triclopyr‐butotyl/kg bw per day (corresponding to 21.6 mg/kg bw per day of triclopyr equivalent), based on increased resorption, early embryonic death, post implantation loss, increased sternebral centres, decreased digital bone ossification and extra ribs, and on two maternal mortalities observed at 100 mg/kg bw per day, respectively.
Triclopyr has no potential for neurotoxicity or immunotoxicity.
Based on the available data (including two in vivo micronucleus studies in mouse, with proof of bone marrow exposure), a genotoxicity potential for 3,5,6‐TCP (common metabolite to chlorpyrifos and chlorpyrifos‐methyl) is unlikely and an acceptable daily intake (ADI) of 0.06 mg/kg bw per day (based on the NOAEL of 12 mg/kg bw per day from the 1‐year study in dogs and applying an uncertainty factor (UF) of 200) and an acute reference dose (ARfD) of 0.25 mg/kg bw (based on the NOAEL of 25 mg/kg bw from the rabbit developmental toxicity study and applying an UF of 100) apply (EFSA, 2019a, 2019b).
The ADI is set at 0.005 mg triclopyr/kg bw per day, based on the NOAEL of 0.5 mg/kg bw per day from the 1‐year dog study, using an UF of 100. 12 An ADI value of 0.03 mg triclopyr/kg bw per day was established in the previous assessment, based on the 2‐year rat study and using an UF of 100 (EFSA, 2006).
The acceptable operator exposure Level (AOEL) is 0.005 mg triclopyr/kg bw per day, on the same basis as for the ADI setting, using an UF of 100 (no correction for oral absorption is needed). An AOEL value of 0.05 mg triclopyr/kg bw per day was established in the previous assessment, based on the 13‐week study in rat and using an UF of 100 (EFSA, 2006).
The ARfD is 0.3 mg triclopyr‐butotyl/kg bw based on the rabbit developmental toxicity study with triclopyr‐butotyl, using an UF of 100. The same ARfD value was established in the previous assessment, on the same basis (EFSA, 2006).
The acute AOEL (AAOEL) is 0.3 mg triclopyr‐butotyl/kg bw, on the same basis as for the ARfD setting, using an UF of 100 (no correction for oral absorption is needed). No AAOEL value was established in the previous assessment (EFSA, 2006).
Dermal absorption values for the formulated triclopyr‐butotyl (‘GF‐1365’) are 0.9% for the concentrate (240 g/L), 28% for the spray dilution (1 g/L) for the representative use on established pasture, and 34% for the spray dilution (0.8 g/L) for the representative use on non‐recreational amenity grassland.
Dermal absorption values for formulated aminopyralid (‘GF‐1365’) are 1% for the concentrate (30 g/L), 2% for the spray dilution (0.12 g/L) for the representative use on established pasture, and 2.4% for the spray dilution (0.1 g/L) for the representative use on non‐recreational amenity grassland.
For the non‐dietary exposure assessment, predictions were also provided for aminopyralid, the second active substance contained in the formulation for representative uses ‘GF‐1365’.
With regard to operator exposure estimates for triclopyr‐butotyl, predictions were below the (A)AOEL for the tractor‐mounted broadcast application on pasture and amenity grassland with the use of personal protective equipment (PPE) (German model and EFSA model 13 ). For the spot application, considering the closest scenario of hand‐held application (both tractor‐mounted gun spraying and knapsack spraying), all exposure estimates were above the (A)AOEL (with UK POEM and EFSA model13) for both uses. However, based on higher tier field studies for spot application, the resulting exposure values were below the AOEL for these hand‐held applications with the use of PPE (gloves and coverall). For the combined exposure with aminopyralid, the sum of the exposure estimates (in % of respective AOEL for each compound) were all below the AOEL with the same protective equipment as for triclopyr alone.
With regard to the worker exposure estimates during crop inspection, predictions were below the AOEL for triclopyr and in case of combined exposure with aminopyralid, with the use of PPE with the German model. With the EFSA model,13 exposure for triclopyr is above the AOEL (and PPE are not applicable to this scenario).
With regard to the resident and bystander exposure estimates, predictions were below the AOEL for triclopyr and aminopyralid, except for residential children with the EFSA model.13 With the German approach, 14 estimates for residential children were below the AOEL.
3. RESIDUES
The assessment in the residue section is based on the following guidance documents: OECD (2009, 2011), European Commission (2011), JMPR (2004, 2007).
Triclopyr was discussed at the Pesticides Peer Review Experts' Meeting 16 in September 2019.
3.1. Representative use residues
All studies were performed with triclopyr except for a rotational crop confined metabolism study and studies with grass upon foliar application, which were performed with triclopyr‐butotyl.
Metabolism studies with triclopyr were available for apples (fruit crops) and radishes (root crops) with both foliar and soil applications, and with triclopyr‐butotyl with ryegrass (cereal/grass crops) with foliar application. An additional supplementary study with foliar application to ryegrass demonstrated the degradation of triclopyr‐butotyl to triclopyr. Despite some shortcomings with regard to missing information on storage stability data in the ryegrass study, the evidence is sufficient to elucidate the metabolic fate of triclopyr and triclopyr‐butotyl in plants. Overall, the metabolic pattern is similar, with triclopyr, free and conjugated, being the predominant residues in primary crops. 3,5,6‐TCP is only found in root crops (tuber and leaves), both after foliar and soil application up to 3% of the total radioactive residues (TRR). Residues observed in a recent rotational crops confined metabolism study with triclopyr‐butotyl confirm a similar pattern as in primary crops, with triclopyr in wheat grain and straw and radish tops and leaves, and 3,5,6‐TCP in radish roots and tops only being the predominant residues. Residues in leafy crops were low and resulted in four unidentified compounds with each of them being below 0.01 mg eq/kg.
The general plant residue definition after foliar and soil applications for risk assessment is set as triclopyr (free and conjugated) and for enforcement purposes as triclopyr. The current residue definitions for enforcement and for risk assessment as applied in the Article 12 MRL review (EFSA, 2017) do not explicitly include the conjugates, but it is noted that historical methods used for field trials included a hydrolysis step, therefore it is expected that the available results from the residue trials include conjugated triclopyr. The nominal change of the residue definition is not expected to result in a need to revise the available residue trials and MRLs as assessed in the framework of the Article 12 MRL review. The residue definitions are based on plant metabolism studies performed with triclopyr and triclopyr‐butotyl (see above). It is acknowledged that other variants might be on the international market, and that this may be considered by risk managers for MRL setting.
A sufficient number of GAP compliant independent residue field trials in grass were presented. However, a storage stability study with triclopyr covering the storage time for the samples analysed in the grass residue trials is requested to fully accept the results of these residue trials (data gap, see Section 9.1.1). It is noted that for the MRL application for the modification of existing maximum residue levels for triclopyr in animal commodities (EFSA, 2023b) an interim report of a storage stability study was presented. In this study stability for triclopyr was demonstrated for 8 months in lettuce and carrots, but not performed as requested with grass. Therefore, the following animal dietary burden calculation and the consumer risk assessment are provisional and subject to confirmation. Triclopyr was found to be stable when subjected to conditions of standard hydrolysis conditions and also under conditions of ensiling.
The use in grass triggers only a metabolism study with ruminants as grass is neither fed to poultry nor to fish. It is noteworthy that the representative use in grass is driving the animal burden for ruminants and that residues from the use in rice (MRL application) and apple (the only feed commodity from Art 12 review uses) do not lead to an increase of the dietary burden.
In a valid ruminant metabolism study triclopyr was found in milk (76% TRR or 0.162 mg eq/kg), fat (34% TRR or 0.01 mg eq/kg) and kidney (17% TRR or 0.07 mg eq/kg). 3,5,6‐TCP is the predominant metabolite in fat (49% TRR or 0.141 mg eq/kg), muscle (37% TRR or 0.023 mg eq/kg), kidney (59% TRR or 0.242 mg eq/kg) and liver (79% TRR or 0.244 mg eq/kg), but it was found in milk only in concentrations below 10% TRR or below 0.05 mg eq/kg. Although not required for the representative uses, metabolism data are also available from a valid study with poultry. The major extractable residue identified in kidney, liver and skin was triclopyr accounting for 90, 89 and 86% TRR, respectively. The non‐solvent extractable residue in the kidney was triclopyr. Residues in eggs, breast and thigh muscle were below 0.03 mg eq/kg, in fat below 0.05 mg eq/kg and no attempts for identification/characterisation were made. The available animal metabolism studies indicated that triclopyr and 3,5,6‐TCP should be considered for the residue definition. Since different toxicological reference values are derived for triclopyr and 3,5,6‐TCP (see Section 2), two separate residue definitions are proposed. The first animal residue definition, both for risk assessment and enforcement, is triclopyr, and is applicable to ruminants and poultry. The second residue definition for risk assessment is the sum of 3,5,6‐TCP and its conjugates, expressed as 3,5,6‐TCP, and applicable only to ruminants. This second residue definition was also proposed as optional for enforcement in the Pesticides Peer Review Experts' Meeting 16 and in the Article 12 MRL review (EFSA, 2017). It is noted that 3,5,6‐TCP is also a metabolite of chlorpyrifos and chlorpyrifos‐methyl. However, exposure from these substances is not expected as they are no longer authorised in the EU (Commission Implementing Regulations (EU) No 2020/17 15 and No 2020/18 16 ).
A recent valid cow feeding study with dairy cattle resulted in higher residues compared to the results from a previously evaluated non‐GLP study considered for MRL setting under the Article 12 process. This is now leading to higher MRL proposals for triclopyr. After the withdrawal of the authorisations of chlorpyrifos and chlorpyrifos‐methyl, it might be sufficient to monitor only triclopyr, which is found in the ruminant feeding study at 1X rate in all matrices. However, in case risk managers consider that enforcement of the metabolite 3,5,6‐TCP might be necessary, MRLs for 3,5,6‐TCP (free and conjugated) are derived for this purpose (see Appendix B). Clarification on discrepancies in reporting of % recovery versus mg/kg recovery in the storage stability of 3,5,6‐TCP in liver and kidney is pending and leads to a data gap (see Section 9.1.1). A feeding study with poultry is not required as grass is not a feed item for poultries.
Chronic and acute consumer dietary intake assessments were performed for residue intake from plant and animal commodities and considering the dietary burden contribution from the representative uses indicated in this peer review and using the revision 3.1 of the EFSA PRIMo (Pesticide Residue Intake Model). The assessments are provisional pending the data gaps related to storage stability data (for grass and liver and kidney) to demonstrate the acceptability of the residue trials on grass and the acceptability of the ruminant feeding study. The calculated theoretical maximum daily intake (TMDI) accounts for 13% and 1% of the ADI for triclopyr and 3,5,6‐TCP, respectively. Both TMDIs are attributed to NL toddlers from intake of milk. The international estimated short‐term intake (IESTI) reaches a maximum of 5% and 8% of the ARfD for triclopyr and 3,5,6‐TCP, respectively. Both IESTIs are attributed to the consumption of bovine kidney.
As the representative formulation contains the active substance aminopyralid besides triclopyr, intake calculation has been performed considering the European authorised uses for aminopyralid on rapeseeds, cereals and grass assessed in the framework of the Article 12 MRL review (EFSA, 2020) and also the residues of aminopyralid in animal matrices. Considering the ADI and ARfD of 0.26 mg/kg bw per day for aminopyralid (EFSA, 2013b, 2020), the TDMI accounted for 0.2% of the ADI (NL toddler) and the IESTI for 0.5% of the ARfD when using the EFSA PRIMo rev. 3.1 model. It is unlikely that the exposure of the consumers to residues from the two active substances will raise any consumer safety concern.
An update of the chronic consumer dietary risk assessment that was conducted in the review of MRLs according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2017), has been performed using the lower ADI of 0.005 mg/kg bw per day for triclopyr established in this peer review process (see Section 2) and a higher ADI of 0.06 mg/kg bw per day for 3,5,6‐TCP established during the peer review of chlorpyrifos (EFSA, 2019b). The updated consumer risk assessment considered also the residue values for triclopyr and 3,5,6‐TCP from the recent feeding study and resulted in an increased estimated chronic consumer exposure corresponding to 44% of the ADI at the maximum (NL, toddler) for triclopyr and 8% of the ADI at the maximum (NL, toddler) for 3,5,6‐TCP. The latter calculation is conservative as residue data for 3,5,6‐TCP were taken from the Art 12 evaluation which included still exposure through use of chlorpyrifos. However, given that the consumer exposure for 3,5,6‐TCP is low, a refinement was not deemed necessary.
The consumer risk assessment from the consumption of drinking water is also not finalised considering the lack of appropriate information to address the effect of water treatment processes on the nature of residues of the active substance and its possible metabolites, potentially present in surface water, when surface water is abstracted for drinking water (see Sections 4 and 9.1.1).
As no data were submitted, a data gap is set (see Section 10) to provide data or information on residue levels in pollen and in bee products for human consumption resulting from residues taken up by honeybees from the representative uses in established pasture and non‐recreational amenity grassland at blossom.
3.2. Maximum residue levels
To support the MRL application for rice a sufficient number of GAP‐compliant residue trials analysing for triclopyr (free and conjugates) and 3,5,6‐TCP in grain and straw was provided. The results of the residue trials in rice are considered provisional and pending the demonstration of stability of the residues of triclopyr in a crop of the high starch commodity group (cereal grain) and covering the maximum storage times for the samples analysed in the residue trials with rice (data gap, see Section 9.2.1). It is noted that for the MRL application for the modification of existing maximum residue levels for triclopyr in animal commodities (EFSA, 2023b), an interim report of a storage stability study was presented. In this study stability for triclopyr was demonstrated for 8 months in rice. Specimen from the residue field trials were stored for up to 17 months. Depending on the outcome of the study with respect to maximal storage stability, it could cover the requested data. However, the data gap is still pending the submission of the final study report. Pending on this confirmation, an increase of the MRL for rice of 0.4 mg/kg can be proposed with respect to the Article 12 review.
The dietary burden calculation from the use in rice only triggered both ruminant and poultry metabolism studies which were provided and discussed under Section 3.1. Based on the representative uses on established pasture and non‐recreational amenity grassland and the intended use on rice, MRLs for triclopyr in animal commodities can be derived from a recent feeding study with lactating dairy cattle. The acceptability of this study is pending clarification of storage stability data for liver and kidney (data gap, see Section 9.2.1). MRLs are derived for 3,5,6‐TCP in case risk managers consider the need for an enforcement for this compound. In contrast to those derived during the Article 12 review, the current MRLs take into account residues in livestock from the use of triclopyr only, and do not consider any longer the contribution of chlorpyrifos and chlorpyrifos‐methyl. The increase of the MRL values with respect to the Article 12 review is due to higher residue values observed in a recent feeding study with ruminants. A revision of the existing MRLs compared to those set in the former MRL review for animal commodities is therefore recommended.
A residue definition for poultry was also established that had not been derived under the Article 12 MRL review (see Section 3.1). From the overdosed metabolism study with triclopyr quantifiable residues in poultry commodities are not expected, hence no MRLs are required.
Although fat‐soluble, the dietary burden for triclopyr‐butotyl from the use in rice was below the trigger value of 0.1 mg/kg dry matter (DM) feed for a fish metabolism study.
Chronic and acute consumer dietary intake assessments were performed for residue intake from plant and animal commodities and considering the dietary burden contribution from the intended use on rice and using the revision 3.1 of the EFSA PRIMo model. The assessments are provisional pending the data gaps related to storage stability data (for rice and liver and kidney) to demonstrate the acceptability of the residue trials on rice and the acceptability of the ruminant feeding study. As animal commodities are the driver for the consumer risk assessment, the respective values for TMDI and IESTI (see Section 3.1) are identical for the representative uses alone and the intended use on rice.
Information on residues in pollen and bee products was not provided and is not requested since rice is not considered to have melliferous capacity (European Commission, 2016).
Confirmatory data following the Art 12 MRL review for triclopyr is assessed in the context of a separate output (EFSA‐Q‐2024‐00215) following a specific mandate from the European Commission.
4. ENVIRONMENTAL FATE AND BEHAVIOUR
The rates of dissipation and degradation in the environmental matrices investigated were estimated using FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, triclopyr‐butotyl (triclopyr‐BEE) rapidly degraded to triclopyr. Triclopyr exhibited low to moderate persistence, forming the major (> 10% applied radioactivity (AR)) metabolite 3,5,6‐trichloro‐2‐pyridinol (3,5,6‐TCP, max. 32.9% AR), which exhibited low to moderate persistence. Mineralisation of the pyridine ring 14C radiolabel of triclopyr‐butotyl and of triclopyr to carbon dioxide accounted for 8%–66% AR after 100 days and for 49%–56% AR after 90 days, respectively. The formation of unextractable residues (not extracted by acetonitrile / water) for this radiolabel accounted for 22%–46% AR after 100 days for triclopyr‐butotyl and for 15%–24% AR after 90 days for triclopyr.
Under anaerobic soil incubations, triclopyr formed the major degradation products 3,6‐dichloro‐2‐pyridinol (3,6‐DCP, max. 29.7% AR) and 3,5,6‐trichloro‐2‐methoxypyridine (TMP, max. 7.2% AR). Anaerobic conditions are unlikely to occur for the representative uses of triclopyr on established pasture and non‐recreational amenity grassland between March and August. However, Member States might need to perform the environmental risk assessment of the two major anaerobic metabolites for uses of triclopyr on other crops than the representative uses considered to address situations where prolonged soil anaerobic conditions are prevalent.
The contribution of photolytic transformation processes on soil surfaces to the dissipation of triclopyr from the soil environment is regarded as negligible.
The mobility of triclopyr‐butotyl was estimated via QSAR methods using the tool KOCWin ver. 2.00. Triclopyr‐butotyl resulted to be immobile. Based on batch adsorption studies triclopyr exhibited very high to high mobility in soil, and metabolite 3,5,6‐TCP exhibited high soil mobility. It was concluded that the adsorption of triclopyr was not pH dependent.
In satisfactory field dissipation studies carried out at 1 site in Germany, 2 sites in France, 1 site in Portugal and 1 in Italy (spray application to the soil surface on bare soil plots in early autumn) triclopyr exhibited low to moderate persistence. Sample analyses were carried out for the parent triclopyr and for metabolite 3,5,6‐TCP, which exhibited low to high persistence. Field study DegT50 values were derived following normalisation to FOCUS reference conditions (20°C and pF2 soil moisture) following the EFSA (2014a) DegT50 guidance. The field data endpoints were not combined with laboratory values to derive modelling endpoints for triclopyr, while for metabolite 3,5,6‐TCP field data endpoints were combined with laboratory values to derive modelling endpoints.
In laboratory incubations in dark aerobic natural sediment water systems, triclopyr‐butotyl exhibited very low persistence and triclopyr exhibited moderate persistence forming the major metabolites DCA (max. 8.7% AR in water, exhibiting moderate persistence) and 3,6‐DCP (max. 8.8% AR in water). The unextractable sediment fraction for the pyridine ring 14C radiolabel accounted for 8.4%–15% AR at study end (100 days). Mineralisation of this radiolabel accounted for 2.9%–13.8% AR at the end of the study.
Irradiation of pyridine‐labelled triclopyr in natural water resulted in formation of the major photodegradation products ((3‐chloro,5,6 dihydroxy‐2‐pyrindinyl)oxy)acetic acid (max. 10.7% AR), the mixtures of succinamic acid and succinic acid (max. 22.8% AR), and chloromaleamic acid and chlorofumaric amide (max. 31.1% AR). Irradiation of pyridine‐labelled triclopyr in buffer resulted in formation of the major photodegradation products maleamic acid (max. 10.3% AR) and ((3‐chloro,5,6 dihydroxy‐2‐pyrindinyl)oxy)acetic acid (max. 29.4% AR), and the mixture of chloromaleamic acid and chlorofumaric amide (max. 27.8% AR).
The surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for the metabolites DCA, 3,6‐DCP, maleamic acid, succinamic acid, succinic acid, ((3‐chloro,5,6 dihydroxy‐2‐pyrindinyl)oxy)acetic acid or isomer, and chloromaleamic acid, fumaric acid and chlorofumaric amide, using the FOCUS (FOCUS, 2001) step 1 and step 2 approach (version 3.2 of the Steps 1–2 in FOCUS calculator). For triclopyr‐butotyl, triclopyr and metabolite 3,5,6‐TCP, step 3 (FOCUS, 2001) calculations were available. 17 FOCUS step 4 calculations were available for triclopyr‐butotyl and triclopyr 18 The step 4 calculations appropriately followed the FOCUS (FOCUS, 2007) guidance, with no‐spray drift buffer zones of up to 20 m being implemented for the drainage scenarios (representing a 91%–93% spray drift reduction), and combined no‐spray buffer zones with vegetative buffer strips of up to 20 m (reducing solute flux in run‐off by 80% and erosion runoff of mass adsorbed to soil by 95%) being implemented for the run‐off scenarios. The SWAN tool (version 5.0.1) was appropriately used to implement these mitigation measures in the simulations. However, risk managers and others may wish to note that whilst run‐off mitigation is included in the step 4 calculations available, the FOCUS (FOCUS, 2007) report acknowledges that for substances with K Foc < 2000 mL/g (i.e. triclopyr), the general applicability and effectiveness of run‐off mitigation measures had been less clearly demonstrated in the available scientific literature, than for more strongly adsorbed compounds. No specific exposure assessment was provided for spot applications where the applicant has indicated that no more than 20% of the surface area will be treated, however, the exposure assessment for broadcast applications covers also the spot applications.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.4 19 for triclopyr‐butotyl, triclopyr and metabolite 3,5,6‐TCP. The potential for groundwater exposure from the representative uses by triclopyr above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all 9 FOCUS groundwater scenarios for triclopyr‐butotyl, triclopyr and metabolite 3,5,6‐TCP.
The applicant did not provide appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water, when surface water is abstracted for drinking water, with the exception of the potential formation of nitrosamines. This has led to the identification of a data gap and results in the consumer risk assessment not being finalised (see Section 9.1.1).
For metabolite 3,5,6‐TCP the estimated photochemical oxidation half‐life in the atmosphere of 60.5 days indicated the potential for long‐range atmospheric transport via air.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix B of this conclusion. A key to the persistence and mobility class wording used, relating these words to numerical DT and Koc endpoint values can be found in Appendix C. PEC for aminopyralid, included as a second active substance in the formulation for representative uses are not available, leading to an assessment not finalised (see Section 9.1.1). It should be noted that the RMS did not agree with this data gap.
5. ECOTOXICOLOGY
The risk assessment was based on the following documents: European Commission (2002), SETAC (2001), EFSA (2009), EFSA PPR Panel (2013) and EFSA (2013a).
Triclopyr was discussed at the Pesticides Peer Review Experts' Meeting 14 in September 2019 and at the Pesticides Peer Review Experts' TC 21 in July 2020.
As noted in Section 4, triclopyr‐butotyl rapidly transforms to triclopyr, following application in the environment. Acute risk assessments for non‐target organisms are needed for both triclopyr‐butotyl and triclopyr, whereas only triclopyr needs to be addressed for chronic assessments. The formulation for representative uses ‘GF‐1365’ contains a second active substance (30 g/L aminopyralid). It is acknowledged that a mixture risk assessment, considering the two active substances in the formulation, has not been presented for all groups of non‐target organisms. Therefore, a data gap, leading to an assessment not finalised, is identified (see Section 9.1.1). It may be noted that the RMS does not agree that a data gap for the mixture assessment should be identified.
The applicant has not provided a clear and complete assessment of the batches used in the ecotoxicity studies compared to the new, and existing, reference specification (data gap, see Section 10).
Acute toxicity studies with triclopyr‐butotyl, triclopyr, the formulation for representative uses (‘GF‐1365’) and metabolite 3,5,6‐TCP were available for both birds and mammals. Long‐term studies with triclopyr were available for birds and mammals. In addition, a long‐term study was also available for mammals for metabolite 3,5,6‐TCP.
The acute and long‐term risk to birds was assessed as low based on a screening level assessment. A high acute and long‐term risk to mammals was indicated both at the screening level and at tier 1. Several proposed refinements were discussed at the experts' meeting. 20 The experts did not agree with the refined deposition factors as it was not demonstrated that the factors were representative of the amount of substance reaching the plants below the treated weed when applied according to the representative uses (which include both broadcast and spot applications). Furthermore, it was noted that the representative uses include the treatment of broadleaf weeds which will be directly consumed by mammals. The experts did not consider that the scale of use for the spot applications could be accounted for in the refined acute risk assessment as mammals may feed exclusively in the treated area during a single feeding session. However, for the refined long‐term risk assessment, the experts considered that the scale of use for the spot applications should be accounted for in a qualitative manner. The refined residue per unit dose (RUD) values were not accepted by the experts. However, sufficient data were available to derive refined DT50 values for central and southern EU. Nevertheless, even accounting for the refined DT50 values, a high long‐term risk to mammals was concluded. No suitable refinements were available for the acute assessment and therefore a high acute risk is also concluded (critical area of concern, see Section 9.1.2). The experts at the meeting agreed that insufficient information was available to account for a quantitative reduction in exposure for the representative uses via spot applications where the applicant has indicated that no more that 20% of the surface area will be treated. No reliable qualitative risk assessment was available which was able to exclude a high long‐term risk to mammals for the spot applications.
A low risk to birds and mammals from plant metabolites, from secondary poisoning and via consumption of contaminated water was concluded.
Aquatic toxicity data for triclopyr‐butotyl, triclopyr, the formulation for representative uses (‘GF‐1365’) and metabolites 3,5,6‐TCP, DCA and 3,6‐DCP were available. The reliability of the aquatic toxicity studies was discussed at the expert meeting, 21 where it was agreed that a number of studies were not suitable to derive reliable endpoints. Reliable toxicity data for algae and aquatic plants with triclopyr‐butotyl were not available (data gap, see Section 9.1.1). The aquatic toxicity data for the pertinent surface water metabolites were incomplete and did not allow for a comprehensive risk assessment for aquatic organisms (see Table 1 and the list of data gaps given in Section 9.1.1). Consequently, the risk assessment for aquatic organisms could not be finalised. It should be noted that chronic toxicity data for triclopyr‐butotyl are not triggered since the substance rapidly hydrolyses. However, since a chronic study with triclopyr was not available for fish, it was decided that a risk assessment should be performed with the available endpoint for triclopyr‐butotyl (expressed in terms of triclopyr) which would cover the chronic risk to both the acid and the ester. Other than for aquatic plants, reliable toxicity data were not available for the formulated product for representative uses (data gap, see Section 9.1.1).
TABLE 1.
Summary of the outcome of the aquatic risk assessment for triclopyr‐butyl, triclopyr, the formulation for representative uses (‘GF‐1365’) and the pertinent surface water metabolites.
| Triclopyr‐butotyl | Triclopyr | 3,5,6‐TCP | DCA | 3,6‐DCP | Maleamic acid | Succinamic acid | Succinic acid | (3‐chloro,5,6 dihydroxy − 2‐pyrindinyl)oxy)acetic acid or isomer | Chloro‐maleamic acid | Fumaric acid | Chlorofumaric amide | GF‐1365 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute fish (rainbow trout) | Low risk (7/7 FOCUS SW scenarios at step 4 considering mitigation) | NA | Low risk (FOCUS SW step 2) | High risk not excluded based on screening assessment at FOCUS step 2 | High risk not excluded based on screening assessment at FOCUS step 2 | NA | NA | NA | NA | NA | NA | NA | NA |
| Acute Daphnia | NA | NA | Low risk (FOCUS SW step 2) | NA | Low risk (FOCUS SW step 1) | NA | NA | NA | NA | NA | NA | NA | NA |
| Acute aquatic invertebrates (Crassostrea virginica) | Low risk (7/7 FOCUS SW scenarios at step 3) | Low risk (FOCUS SW step 1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chronic fish | NA | High risk (3/7 FOCUS SW scenarios at step 4) | High risk (1/7 FOCUS SW scenarios at step 3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chronic aquatic invertebrate | NA | Low risk (FOCUS SW step 1) | High risk (1/7 FOCUS SW scenarios at step 3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Green algae | NA | No data | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Other algae | NA | Low risk (FOCUS SW step 1) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lemna spp. | NA | No data | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Other aquatic plant | NA | High risk (2/7 FOCUS SW a at step 3) | NA | Low risk at FOCUS SW Step 2 | Low risk (FOCUS SW step 1) | NA | NA | NA | NA | NA | NA | NA | Low risk |
Note: No reliable toxicity data available. In the case of metabolites, a screening assessment was not performed.
Abbreviation: NA, not available.
High risk in D1 and D2 FOCUS surface water scenarios.
Where suitable data were available, the risk assessment was performed. This led to a high risk in the following:
A high acute risk to fish from triclopyr‐butotyl in 7/7 FOCUS surface water scenarios at step 3. A low risk was concluded using FOCUS step 4 exposure estimates, considering risk mitigation, for all scenarios.
A high chronic risk to fish from triclopyr in 7/7 FOCUS surface water scenarios at step 3. A low risk was concluded using FOCUS step 4 exposure estimates, considering risk mitigation, in 4/7 FOCUS surface water scenarios.
A high risk to aquatic plants from triclopyr in 2/7 FOCUS surface water scenarios at step 3. FOCUS step 4 exposure estimates, considering risk mitigation, were available but did not reduce the exposure.
A high chronic risk to fish and aquatic invertebrates from metabolite 3,5,6‐TCP in 1/7 FOCUS surface water scenarios at step 3. FOCUS step 4 exposure estimates, considering risk mitigation, were not available.
It should be noted that no specific surface water exposure assessment was available for the representative uses via spot applications where the applicant has indicated that no more than 20% of the surface area will be treated (see Section 4).
There are 10 pertinent surface water metabolites which warranted an aquatic risk assessment. Toxicity data were available for aquatic invertebrates for two metabolites (metabolites 3,5,6‐TCP and 3,6‐DCP) and for aquatic plants for two metabolites (DCA and 3,6‐DCP). An acute fish screening assessment, assuming the metabolite was of ten times greater toxicity than triclopyr‐butotyl was presented, using FOCUS step 2 exposure estimates for metabolites 3,6‐DCP and DCA but did not exclude a risk. Overall, the risk assessment for aquatic metabolites was incomplete (refer to Table 1) and data gaps were identified (see Section 9.1.1).
Owing to the complexity of the aquatic risk assessment, the outcome is presented in Table 1 below.
Data demonstrating the acute toxicity of the formulation for representative uses, ‘GF‐1365’, to honeybees were available. Additional acute toxicity studies with another formulation (‘Garlon 4’) containing only triclopyr‐butotyl were also available. No reliable acute toxicity data were available with either triclopyr‐butotyl or triclopyr. A chronic toxicity study with adult honeybees and honey bee larvae was available for triclopyr but not for the formulation for representative uses, ‘GF‐1365’ (data gap, see Section 10). No toxicity data for sublethal effects (data gap, see Section 10) or accumulative effects to honeybees were available. Furthermore, no toxicity data for bumblebees and solitary bees were available.
An acute risk assessment for honeybees for the formulation for representative uses, performed in accordance with both European Commission (2002) and EFSA (2013a) guidance documents, were available and indicated a low risk. As no reliable toxicity studies were available for triclopyr‐butotyl or triclopyr, a quantitative risk assessment for honeybees could not be performed. However, given that a low risk was indicated to the formulated product and the available supportive studies indicated low toxicity, it is considered that a low acute risk to honeybees can be concluded with the information available. This conclusion is not in line with that of the RMS who considered that a data gap was needed for acute toxicity studies for honeybees with triclopyr‐butotyl and triclopyr. 22
No chronic risk assessment for adult honeybees and honey bee larvae, in accordance with EFSA (2013a), was available (data gap, see Section 10). No plant metabolites triggering the need for a risk assessment for bees were identified.
The risk assessment for non‐target arthropods was discussed at the Pesticides Peer Review Experts' TC 21. 23 No tier 1 toxicity studies were available to address the risk to non‐target arthropods. Tier 2 studies, with the formulation for representative uses, ‘GF‐1365’, for three species were available. Additional toxicity studies with another formulation (‘Garlon 4’) containing only triclopyr‐butotyl were also available. The risk assessment indicated a high in‐field risk to two of these species (Typhlodromus pyri and Coccinella septempunctata). A high off‐field risk was indicated to only C. septempunctata. To refine the risk to Typhlodromus pyri, it was suggested to use toxicity data from another formulated product (‘Garlon 4’) containing only triclopyr‐butotyl. However, the experts at the meeting did not agree with this approach. More importantly, there was a greater than 50% effect on mortality which the experts agreed should be accounted for in the risk assessment. To address the risk to C. septempunctata, reference was made to a study performed at higher rates. At the higher rate, there was less than 50% effect. However, without a sound scientific justification this is not considered to override the observed effect at the lower application rate. Overall, the experts agreed that the available data and assessment indicated a high in‐field risk to C. septempunctata. Furthermore, data were lacking for a fourth species for the off‐field risk assessment. The experts also considered that there was insufficient information to indicate a low off‐field risk to non‐target arthropods. Overall, with the data available, a high risk to non‐target arthropods is concluded (critical area of concern, see Section 9.1.2). It should be further noted that no risk assessment for non‐target arthropods was available for the representative uses via spot applications where the applicant has indicated that no more than 20% of the surface area will be treated.
Toxicity data investigating the chronic effects of triclopyr and metabolite 3,5,6‐TCP to earthworms and other soil macroorganisms were available. No reliable toxicity study was available for the formulation for representative uses ‘GF‐1365’ to earthworms (data gap, see Section 10). However, data were available for other soil macroorganisms. Only a single metabolite, 3,5,6‐TCP, was identified in soil as to require a risk assessment for soil organisms. The available risk assessments, for triclopyr and metabolite 3,5,6‐TCP, for earthworms and soil macroorganisms indicated a low risk. A low risk to soil macroorganisms (other than earthworms) for the formulation for representative uses was also concluded. A risk assessment for soil macroorganisms was not considered necessary for triclopyr‐butotyl since it will rapidly transform to triclopyr in soil.
On the basis of the available studies, a low risk to soil microorganisms from triclopyr, metabolite 3,5,6‐TCP and the formulation for representative uses ‘GF‐1365’ was concluded. A risk assessment for soil microorganisms was not considered necessary for triclopyr‐butotyl since it will rapidly transform to triclopyr in soil.
Toxicity data for non‐target plants were available for the formulation for representative uses ‘GF‐1365’. In accordance with the current guidance document (European Commission, 2002), a low risk to non‐target terrestrial plants was concluded provided that risk mitigation measures, equivalent to 50% drift reduction, are used (see Section 8.1). However, Member States may wish to note that an uncertainty was raised during the peer review that the studies did not derive quantified endpoints for phytotoxic effects which was noted to be the most sensitive parameter. 24 Furthermore, an additional uncertainty was raised as to whether the risk assessment is protective of potentially more sensitive species. 25
A low risk to organisms involved in biological sewage treatment processes was concluded for the representative uses.
6. ENDOCRINE DISRUPTION PROPERTIES
Triclopyr has been discussed at the Pesticides Peer Review Experts' Meeting 11 (Mammalian toxicology), and at the Pesticides Peer Review Experts' Meeting 14 (Ecotoxicology) in September 2019. For non‐target organisms other than wild mammals, further data were considered necessary 26 and a long‐term stop of the clock was applied to complete the data package as regards the endocrine disruption (ED) potential as laid down in Article 13(3a) of Regulation 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The newly submitted data were evaluated leading to the conclusions as indicated below.
With regard to the assessment of the ED potential of triclopyr for humans according to the ECHA/EFSA guidance (2018), in determining whether triclopyr interacts with the oestrogen, androgen and steroidogenesis (EAS) and thyroid (T) mediated pathways, the number and type of effects induced, and the magnitude and pattern of responses observed across studies were considered. Additionally, the conditions under which effects occur were considered; in particular, whether or not endocrine‐related responses occurred at dose(s) that also resulted in overt toxicity. The assessment is therefore providing a weight‐of‐evidence analysis of the potential interaction of triclopyr with the EAS and T signalling pathways using the available evidence in the dataset.
For the EATS modalities the data set is complete, and no adversity has been observed. Therefore, in line with the ECHA/EFSA guidance (2018), scenario 1a is applicable and triclopyr is not considered to meet the ED criteria as laid down in point 3.6.5 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605. Triclopyr and its variants triclopyr‐butotyl and triclopyr triethylammonium are toxicokinetically equivalent. Triclopyr and triclopyr‐butotyl have similar urinary metabolite profiles after dosing with equimolar doses. Blood data indicate that triclopyr‐butotyl is hydrolysed to triclopyr prior to absorption. Therefore, the conclusions on the ED properties of triclopyr are applicable also to the variants.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms. For non‐target organisms other than mammals, an amphibian metamorphosis assay (AMA) and fish short‐term reproduction assay (FSTRA) in line with OECD TG 231 and 229 respectively, were available. There was no evidence of EATS‐mediated endocrine activity in those studies, therefore, further testing is not necessary for non‐target organisms. The conclusions on the ED properties of triclopyr apply also to the variant triclopyr‐butotyl.
Based on the available information, it can be concluded that triclopyr and its variant(s) do not meet the ED criteria for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
7. OVERVIEW OF THE RISK ASSESSMENT OF COMPOUNDS LISTED IN RESIDUE DEFINITIONS TRIGGERING ASSESSMENT OF EFFECTS DATA FOR THE ENVIRONMENTAL COMPARTMENTS (TABLES 2, 3, 4–5)
TABLE 2.
Soil.
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Triclopyr‐butotyl | Risk assessment not considered necessary considering the rapid transformation in soil |
| Triclopyr | Low risk to soil organisms |
| 3,5,6‐TCP | Low risk to soil organisms |
TABLE 3.
Groundwater. a
| Compound (name and/or code) | > 0.1 μg/L at 1 m depth for the representative uses b Step 2 | Biological (pesticidal) activity/relevance Step 3a | Hazard identified Steps 3b. and 3c | Consumer RA triggered Steps 4 and 5 | Human health relevance |
|---|---|---|---|---|---|
| Triclopyr‐butotyl (Triclopyr‐BEE) | No | Yes | – | – | Yes |
| Triclopyr 3,5,6‐TCP | No | Assessment not triggered | Assessment not triggered | Assessment not triggered | Assessment not triggered |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or relevant lysimeter. Ranges indicated for FOCUS scenarios include the result from the model giving the highest concentration at each scenario, as needed to comply with European Commission (2014a) guidance.
TABLE 4.
Surface water and sediment.
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Triclopyr butotyl (Triclopyr‐BEE) | Where suitable data were available for risk assessment, a low risk was indicated with suitable risk mitigation. Data gap for additional taxa |
| Triclopyr | High chronic risk to fish for 3/7 FOCUS SW scenarios (step 4) |
| High risk to aquatic plants for 2/7 FOCUS SW scenarios (step 4) | |
| 3,5,6‐TCP (soil, water/sediment) | High risk to fish and aquatic invertebrates (1/7 FOCUS step 3 scenarios). Data gap for additional taxa |
| DCA (water/sediment) | Data gap |
| 3,6‐DCP (water/sediment) | Data gap |
| Maleamic acid (direct aqueous photolysis) | Data gap |
| Succinamic acid (indirect aqueous photolysis) | Data gap |
| Succinic acid (indirect aqueous photolysis) | Data gap |
| (3‐chloro,5,6 dihydroxy − 2‐pyrindinyl)oxy)acetic acid or isomer (direct and indirect aqueous photolysis) | Data gap |
| Chloromaleamic acid (direct and indirect aqueous photolysis) | Data gap |
| Fumaric acid (direct and indirect aqueous photolysis) | Data gap |
| Chlorofumaric amide (direct and indirect aqueous photolysis) | Data gap |
TABLE 5.
Air.
| Compound (name and/or code) | Toxicology |
|---|---|
| Triclopyr butotyl (Triclopyr‐BEE) |
|
| Triclopyr | No data available |
8. PARTICULAR CONDITIONS PROPOSED TO BE TAKEN INTO ACCOUNT BY RISK MANAGERS
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant's proposal(s) during the peer review, if any, are presented in this section (Table 6). These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level).
8.1. Particular conditions proposed for the representative uses evaluated
TABLE 6.
Risk mitigation measures proposed for the representative uses assessed.
| Representative use | Established pasture | Non‐recreational amenity grassland | |
|---|---|---|---|
| F | F | ||
| Broadcast application Spot application: Max 20% of the surface treated | Broadcast application Spot application: Max 20% of the surface treated | ||
| Operator exposure | Triclopyr |
Use of gloves during mixing/loading and application (ML&A) for tractor‐mounted application (EFSA, 2014b); Use of gloves during ML&A, protective garment, sturdy footwear, hood and visor during A (German model); Use of gloves and coverall for spot application (hand‐held) (field studies) |
Use of gloves during ML&A for tractor‐mounted application (EFSA, 2014b); Use of gloves during ML&A, protective garment, sturdy footwear, hood and visor during A (German model); Use of gloves and coverall for spot application (hand‐held) (field studies) |
| Combined exposure with aminopyralid | Same RMM as for triclopyr | Same RMM as for triclopyr | |
| Worker exposure | Triclopyr | Use of gloves (German model) | Use of gloves (German model) |
| Combined exposure with aminopyralid | Same RMM as for triclopyr | Same RMM as for triclopyr | |
| Resident/bystander exposure | Triclopyr | Buffer 5m, drift reduction (German approach*) | Buffer 5m, drift reduction (German approach*) |
| Combined exposure with aminopyralid | Buffer 5m, drift reduction (German approach*) | Buffer 5m, drift reduction (German approach*) | |
| Aquatic organisms | Triclopyr a |
No spray buffer zone of 20 m in areas represented by D3, D4 and D5 FOCUS surface water scenarios No spray buffer zone of 20 m and vegetated buffer strip in areas represented by R2 FOCUS surface water scenario |
No spray buffer zone of 20 m in areas represented by D3, D4 and D5 FOCUS surface water scenarios No spray buffer zone of 20 m and vegetated buffer strip in areas represented by R2 FOCUS surface water scenario |
| Non target terrestrial plants | Formulation for representative uses (‘GF‐1365’) | RMMs equivalent to 50% drift reduction are needed | RMMs equivalent to 50% drift reduction are needed |
The German approach (Martin et al., 2008) is no longer scientifically supported, since limited data were included for three‐dimensional exposure to spray drift and no estimates are provided for exposure to vapour from low volatility compounds. Accordingly, the predictions are considered underestimated.
Risk assessment for the mixture of the two active substances in the formulation for representative uses was not available (see Section 5). Therefore, the necessary risk mitigation measures could not be identified.
8.2. Particular conditions proposed for the maximum residue level applications
No particular conditions are proposed for the MRL application.
9. CONCERNS AND RELATED DATA GAPS
9.1. Concerns and related data gaps for the representative uses evaluated
9.1.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related.
- The acceptability of the proposed maximum levels of the impurities and representativeness of the toxicological batches with regard to the original and the newly proposed reference specifications cannot be finalised (see Section 2).
- Assessment of the toxicological relevance of most of the impurities included in the reference specification should be provided (relevant for all representative uses evaluated; see Section 2).
- The consumer risk assessment is provisional pending the data gaps related to storage stability data (for grass and liver and kidney) to demonstrate the acceptability of the residue trials on grass and the acceptability of the ruminant feeding study (see Section 3.1).
- A storage stability study with triclopyr covering the storage time for the samples analysed in the grass residue trials is required (relevant for all representative uses evaluated; see Section 3.1);
- Clarification needed on the discrepancies between recoveries for 3,5,6‐TCP expressed in % and mg/kg observed in the storage stability study in the context of the ruminant feeding study for the matrix liver (all 3 time points) and for kidney (except time point 105 days) (relevant for all representative uses evaluated; see Section 3.1).
- The consumer risk assessment through drinking water could not be finalised considering the lack of appropriate information to address the effect of water treatment processes on the nature of residues of the active substance and its possible metabolites, potentially present in surface water, when surface water is abstracted for drinking water (see Sections 3 and 4).
- The effect of water treatment processes on the nature of residues present in surface water, when surface water is abstracted for drinking water (Article 4 (approval criteria for active substances) 3. (b) of Regulation (EC) No 1107/2009) has not been assessed, with the exception of the potential formation of nitrosamines. Probably, in the first instance, a consideration of the processes of ozonation and chlorination would appear appropriate. If an argumentation is made that concentrations at the point of abstraction for drinking water purposes will be low, this argumentation should cover metabolites predicted to be in surface water, as well as the active substance. Should this consideration indicate novel compounds might be expected to be formed from water treatment, the risk to human or animal health through the consumption of drinking water containing them would need to be addressed (relevant for all representative uses evaluated; see Section 4).
| Substance | Missing assessment |
|---|---|
| 3,5,6‐TCP | Algae and aquatic plants |
| DCA | Fish, aquatic invertebrates, algae |
| 3,6‐DCP | Fish and algae |
| Maleamic acid | Fish, aquatic invertebrates, algae and aquatic plants |
| Succinamic acid | Fish, aquatic invertebrates, algae and aquatic plants |
| Succinic acid | Fish, aquatic invertebrates, algae and aquatic plants |
| (3‐chloro,5,6 dihydroxy −2‐pyrindinyl)oxy)acetic acid or isomer | Fish, aquatic invertebrates, algae and aquatic plants |
| Chloro‐maleamic acid | Fish, aquatic invertebrates, algae and aquatic plants |
| Fumaric acid | Fish, aquatic invertebrates, algae and aquatic plants |
| Chlorofumaric amide | Fish, aquatic invertebrates, algae and aquatic plants |
-
c
Acute toxicity data for aquatic organisms (fish, aquatic invertebrates, algae) and a suitable risk assessment for the formulation for representative uses ‘GF‐1365’ were not available (relevant for all representative uses evaluated; see Section 5).
-
5An exposure assessment for aminopyralid is not available. Furthermore, risk assessments for the mixture of the two active substances present in the formulation for representative uses was not available for all groups of non‐target organisms (see Sections 4 and 5).
- Predicted environmental concentrations (PEC) in soil, groundwater, surface water and sediment for aminopyralid were not available (see Section 4).
- A mixture risk assessment for the formulation for representative uses which includes the second active substance aminopyralid was not available for all non‐target organisms (see Section 5).
9.1.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related.
9.1.3. Overview of the concerns identified for each representative use considered (Table 7)
TABLE 7.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios.
| Representative use | Established pasture | Non‐recreational amenity grassland | |
|---|---|---|---|
| F | F | ||
|
Broadcast application Spot application: Max 20% of the surface treated |
Broadcast application Spot application: Max 20% of the surface treated |
||
| Operator risk | Risk identified | ||
| Assessment not finalised | |||
| Worker risk | Risk identified | X d | X d |
| Assessment not finalised | |||
| Resident/bystander risk | Risk identified | X d | X d |
| Assessment not finalised | |||
| Consumer risk | Risk identified | ||
| Assessment not finalised | X2,3 | X2,3 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X6, c | X6, c |
| Assessment not finalised | X5 | X5 | |
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X7, c | X7, c |
| Assessment not finalised | X5 | X5 | |
| Risk to aquatic organisms | Risk identified | 3/7 FOCUS surface water scenarios e | 3/7 FOCUS surface water scenarios e |
| Assessment not finalised | X4,5 | X4,5 | |
| Groundwater exposure to active substance | Legal parametric value breached | ||
| Assessment not finalised | X5 | X5 | |
| Groundwater exposure to metabolites | Legal parametric value breached a | ||
| Parametric value of 10 μg/L b breached | |||
| Assessment not finalised | |||
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
It should be noted that the available assessments for terrestrial vertebrates and non‐target arthropods have assumed a broadcast application to the full field. No quantified risk assessment was available for the spot application where applications are indicated not to exceed 20% of the treated surface area.
It should be noted that the exposure estimates with the EFSA model (2014b), not applicable at the time of dossier submission, are above the AOEL, while the use of previous models (e.g. German model) does not raise any concern.
High chronic risk to fish in areas represented by D1, D2 and R3 FOCUS surface water scenarios.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8.1, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 7.)
9.2. Issues related to the maximum residue level applications
9.2.1. Issues not finalised under the maximum residue level applications
- The consumer risk assessment is provisional pending the data gaps related to storage stability data (for rice and liver and kidney) to demonstrate the acceptability of the residue trials on rice and the acceptability of the ruminant feeding study (see Section 3.2).
- Storage stability study for triclopyr in a crop of the high starch commodity group (cereal grain) and covering the maximum storage time for the samples analysed in the residue trials with rice is required (relevant for the use in rice; see Section 3.2);
- Clarification needed on the discrepancies between recoveries for 3,5,6‐TCP expressed in % and mg/kg observed in the storage stability study in the context of the ruminant feeding study for the matrix liver (all three time points) and for kidney (except time point 105 days) (relevant for the use in rice; see Section 3.2).
9.2.2. Consumer risk identified under the maximum residue level applications
None identified.
10. LIST OF OTHER OUTSTANDING ISSUES
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections:
Log Kow for some of the components (3,6‐DCP, maleamic acid, succinamic acid, succinic acid, chloromaleamic acid, fumaric acid, chlorofumaric amide) of the residue definition for risk assessment should be provided (relevant for all representative uses evaluated; see Section 1).
Verification of the efficiency of the extraction procedure used in the analytical method for determination of residues in food/feed of plant origin (relevant for all representative uses evaluated; see Section 1).
Verification of the efficiency of the extraction procedure used in the analytical method for determination of residues in eggs (relevant for all representative uses evaluated; see Section 1).
A monitoring method for soil with a LOQ in compliance with the EC10‐value of the most sensitive crop is needed (relevant for all representative uses evaluated; see Section 1).
Triclopyr was not phototoxic in the OECD 3T3 NRU‐PT test. However, the OECD 3T3 NRU‐PT might not allow concluding properly on the phototoxicity potential of triclopyr since it is a UVB absorber and the 3T3 NRU‐PT test might not be an appropriate test for UVB absorbers. It is noted however that phototoxicity testing applying the OECD TG 498 test would allow for proper assessment of UVB absorbers (relevant for all representative uses evaluated; see Section 2).
Data or information on residue levels in pollen and in bee products for human consumption resulting from residues taken up by honeybees are needed (relevant for all representative uses evaluated; see Section 3.1).
More details (e.g. the history at the collection site, the collection procedure, the measurement of the microbial biomass, the use of a control) on the studies CA B.8.1.1.1.1/1, CA B.8.1.1.1.1/2 and CA B.8.1.1.1.1/4 in RAR Vol. 3 B.8 (AS) were not provided (relevant for all representative uses evaluated; see Evaluation Table Section 4, data requirements 4.1, 4.2 and 4.3 (EFSA, 2023a)).
The analysis of the Unknown 1 and the relevant chromatograms for soils Breirlow and South Witham in the study CA B.8.1.1.1.3/3 in RAR Vol. 3, B.8 (AS) were not provided (relevant for all representative uses evaluated; see Evaluation Table Section 4, data requirement 4.4 (EFSA, 2023a)).
The source of the equation used for calculating DT50sun in the study CA B.8.1.1.2.2/1 in RAR Vol. 3, B.8 (AS) and its correlation to the equations in the OECD Guideline 316 were not provided (relevant for all representative uses evaluated; see Evaluation Table Section 4, data requirement 4.9 (EFSA, 2023a)).
Chronic toxicity studies with adult honeybees and honeybee larvae are needed for ‘GF‐1365’ (relevant for all representative uses evaluated; see Section 5).
Information to address sublethal effects to honeybees is needed (relevant for all representative uses evaluated; see Section 5).
A chronic risk assessment for adult honeybees and honey bee larvae, in accordance with EFSA (2013a), is needed (relevant for all representative uses evaluated; see Section 5).
A reliable chronic earthworm toxicity study with the formulation for representative uses should be made available (relevant for all representative uses evaluated; see Section 5).
A clear and complete assessment of the batches used in the ecotoxicity studies compared to the new, and existing, technical specification was missing (relevant for all representative uses evaluated; see Section 5).
ABBREVIATIONS
- ADE
actual dermal exposure
- ADI
acceptable daily intake
- AF
assessment factor
- AAOEL
acute acceptable operator exposure level
- AhR
aryl hydrocarbon receptor
- AOEL
acceptable operator exposure level
- AOP
adverse outcome pathway
- AP
alkaline phosphatase
- AR
applied radioactivity
- AR
androgen receptor
- ARfD
acute reference dose
- AST
aspartate aminotransferase (SGOT)
- AUC
area under the blood concentration/time curve
- AV
avoidance factor
- BCF
bioconcentration factor
- BUN
blood urea nitrogen
- bw
body weight
- CL
confidence limits
- DAA
days after application
- DAR
draft assessment report
- DAT
days after treatment
- DDD
daily dietary dose
- DM
dry matter
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- dw
dry weight
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- FIR
food intake rate
- GAP
Good Agricultural Practice
- GC
gas chromatography
- HR
hazard rate
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ISO
International Organization for
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LC50
lethal concentration, median
- LC–MS
liquid chromatography–mass spectrometry
- LC–MS‐MS
liquid chromatography with tandem mass spectrometry
- LDH
lactate dehydrogenase
- LH
luteinizing hormone
- LOAEL
lowest observable adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- MCH
mean corpuscular haemoglobin
- MCHC
mean corpuscular haemoglobin concentration
- MCV
mean corpuscular volume
- MOA
mode of action
- MRL
maximum residue level
- MS
mass spectrometry
- MSDS
material safety data sheet
- MTD
maximum tolerated dose
- MWHC
maximum water‐holding capacity
- NESTI
national estimated short‐term intake
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- RUD
residue per unit dose
- SPG
specific protection goal
- STMR
supervised trials median residue
- TLV
threshold limit value
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- WHO
World Health Organization
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBERS
EFSA‐Q‐2016‐00226; EFSA‐Q‐2018‐01005
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Supporting information
List of end points for the active substance and the formulation for representative uses
ACKNOWLEDGEMENTS
EFSA wishes to thank the rapporteur Member State Poland for the preparatory work provided to this scientific output.
APPENDIX A. Consideration of cut‐off criteria for triclopyr according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
A.1.
| Properties | Conclusion a | |
|---|---|---|
| CMR | Carcinogenicity (C) | Triclopyr is not considered to be a carcinogen according to point 3.6.3 of Annex II of Regulation (EC) No 1107/2009 |
| Mutagenicity (M) | Triclopyr is not considered to be a mutagen according to point 3.6.2 of Annex II of Regulation (EC) No 1107/2009 | |
| Toxic for Reproduction (R) | Triclopyr is not considered to be toxic for reproduction according to point 3.6.4 of Annex II of Regulation (EC) No 1107/2009 | |
| Endocrine disrupting properties | Triclopyr does not meet the ED criteria for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605 | |
| POP | Persistence | Triclopyr is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009 |
| Bioaccumulation | ||
| Long‐range transport | ||
| PBT | Persistence | Triclopyr is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009 |
| Bioaccumulation | ||
| Toxicity | ||
| vPvB | Persistence | Triclopyr is not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009 |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
APPENDIX B. List of end points for the active substance and the formulation for representative uses
B.1.
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.8177.
APPENDIX C. Wording EFSA used in Section 4 of this conclusion, in relation to DT and Koc ‘classes’ exhibited by each compound assessed
C.1.
| Wording | DT50 normalised to 20°C for laboratory incubationsa or not normalised DT50 for field studies (SFO equivalent, when biphasic, the DT90 was divided by 3.32 to estimate the DT50 when deciding on the wording to use) |
|---|---|
| Very low persistence | < 1 day |
| Low persistence | 1 to < 10 days |
| Moderate persistence | 10 to < 60 days |
| Medium persistence | 60 to < 100 days |
| High persistence | 100 days to < 1 year |
| Very high persistence | A year or more |
Note: These classes and descriptions are unrelated to any persistence class associated with the active substance cut‐off criteria in Annex II of Regulation (EC) No 1107/2009. For consideration made in relation to Annex II, see Appendix A.
For laboratory soil incubations normalisation was also to field capacity soil moisture (pF2/10 kPa). For laboratory sediment water system incubations, the whole system DT values were used.
| Wording | K oc (either KFoc or Kdoc) mL/g |
|---|---|
| Very high mobility | 0–50 |
| High mobility | 51–150 |
| Medium mobility | 151–500 |
| Low mobility | 501–2000 |
| Slight mobility | 2001–5000 |
| Immobile | > 5000 |
Note: Based on McCall et al. (1980).
APPENDIX D. Used compound codes
D.1.
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula b |
|---|---|---|
| Triclopyr |
[(3,5,6‐trichloro‐2‐pyridyl)oxy]acetic acid O=C(O)COC1 = NC(Cl) = C(Cl)C=C1Cl REEQLXCGVXDJSQ‐UHFFFAOYSA‐N |
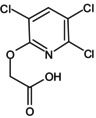
|
|
Triclopyr‐butotyl (Triclopyr‐BEE) (Triclopyr butoxyethyl ester) (BEE) |
2‐butoxyethyl [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetate O=C(OCCOCCCC)COC1 = NC(Cl) = C(Cl)C=C1Cl IVDRCZNHVGQBHZ‐UHFFFAOYSA‐N |

|
|
triclopyr triethylammonium (TEA) (Triclopyr‐TEA) |
triethylammonium [(3,5,6‐trichloro‐2‐pyridyl)oxy]acetate O=C([O‐])COC1 = NC(Cl) = C(Cl)C=C1Cl.CC[NH+](CC)CC ROKVVMOXSZIDEG‐UHFFFAOYSA‐N |
|
|
3,5,6‐TCP (3,5,6‐trichloro‐2‐pyridinol) (TCP) |
3,5,6‐trichloropyridin‐2‐ol OC1 = NC(Cl) = C(Cl)C=C1Cl WCYYAQFQZQEUEN‐UHFFFAOYSA‐N |

|
|
3,5,6‐TCP Glucuronide |
3,5,6‐trichloropyridin‐2‐yl D‐glucopyranosiduronic acid ClC1 = C(Cl)C=C(Cl)C(OC2[C@@H]([C@H]([C@@H]([C@@H](C(O) = O)O2)O)O)O) = N1 XIPKXSPRDJUGPJ‐OVSONBGKSA‐N |

|
|
3,5,6‐TCP Sulfate |
3,5,6‐trichloropyridin‐2‐yl hydrogen sulfate ClC1 = C(Cl)C=C(Cl)C(OS(=O)(O) = O) = N1 VTTUBUMMKMLULX‐UHFFFAOYSA‐N |

|
|
3,6‐DCP (3,6‐dichloro‐2‐pyridinol) |
3,6‐dichloropyridin‐2‐ol ClC1 = CC=C(N=C1O)Cl UGPDKBDRRLFGFD‐UHFFFAOYSA‐N |

|
|
6‐MCP (6‐chloro‐2‐pyridinol) (MCP) (Monochloro‐2‐pyridinol) |
6‐chloropyridin‐2‐ol OC1 = NC(Cl) = CC=C1 CLNNBQDAAGDAHI‐UHFFFAOYSA‐N |

|
| TMP (3,5,6‐trichloro‐2‐methoxypyridine) |
2,3,5‐trichloro‐6‐methoxypyridine ClC1 = CC(Cl) = C(N=C1OC)Cl RLIVUWLXZBDMBL‐UHFFFAOYSA‐N |

|
| DCA ([(3,6‐Dichloropyridin‐2‐yl)oxy] acetic acid) |
2‐((3,6‐dichloropyridin‐2‐yl)oxy)acetic acid O=C(O)COC1 = NC(Cl) = CC=C1Cl KOSARFJJBUXYNE‐UHFFFAOYSA‐N |

|
| maleamic acid |
(Z)‐4‐amino‐4‐oxobut‐2‐enoic acid O=C(O)/C=C\C(N) = O FSQQTNAZHBEJLS‐UPHRSURJSA‐N |

|
| fumaric amide |
(E)‐4‐amino‐4‐oxobut‐2‐enoic acid O=C(O)/C=C/C(N) = O FSQQTNAZHBEJLS‐OWOJBTEDSA‐N |

|
| succinamic acid |
4‐amino‐4‐oxobutanoic acid O=C(O)CCC(N) = O JDVPQXZIJDEHAN‐UHFFFAOYSA‐N |

|
| succinic acid |
butanedioic acid O=C(O)CCC(O) = O KDYFGRWQOYBRFD‐UHFFFAOYSA‐N |

|
| ((3‐chloro,5,6 dihydroxy − 2‐pyridinyl)oxy)acetic acid |
2‐((3‐chloro‐5,6‐dihydroxypyridin‐2‐yl)oxy)acetic acid O=C(O)COC1 = NC(O) = C(O)C=C1Cl SALNNZHUWLJLBD‐UHFFFAOYSA‐N |
|
| chloromaleamic acid |
(E)‐4‐amino‐3‐chloro‐4‐oxobut‐2‐enoic acid O=C(O)/C=C(Cl)\C(N) = O ZJSYFBCDJYEWPY‐OWOJBTEDSA‐N |

|
| fumaric acid |
(2E)‐but‐2‐enedioic acid O=C(O)/C=C/C(O) = O VZCYOOQTPOCHFL‐OWOJBTEDSA‐N |

|
| chlorofumaric amide |
(Z)‐4‐amino‐2‐chloro‐4‐oxobut‐2‐enoic acid NC(/C=C(Cl)/C(O) = O) = O LAZUBSHZGQELHK‐UPHRSURJSA‐N |

|
| deschloro TCP sulfonic acid |
2,5‐dichloro‐6‐hydroxypyridine‐3‐sulfonic acid OS(=O)(=O)c1cc(Cl)c(O)nc1Cl UFXGVNDRVPSLTR‐UHFFFAOYSA‐N |
or isomer |
| deschloro TMP sulfonic acid |
2,5‐dichloro‐6‐methoxypyridine‐3‐sulfonic acid OS(=O)(=O)c1cc(Cl)c(OC)nc1Cl ITRDYWURDCYPBJ‐UHFFFAOYSA‐N |
or isomer |
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
EFSA (European Food Safety Authority) , Álvarez, F. , Arena, M. , Auteri, D. , Leite, S. B. , Binaglia, M. , Castoldi, A. F. , Chiusolo, A. , Colagiorgi, A. , Colas, M. , Crivellente, F. , De Lentdecker, C. , De Magistris, I. , Egsmose, M. , Fait, G. , Ferilli, F. , Gouliarmou, V. , Halling, K. , Nogareda, L. H. , … Villamar‐Bouza, L. (2024). Peer review of the pesticide risk assessment of the active substance triclopyr (variant triclopyr‐butotyl). EFSA Journal, 22 (8), e8177. 10.2903/j.efsa.2024.8177
Approved: 17 July 2024
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine disrupting properties introduced by Regulation (EU) 2018/605
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
Reporting Table following consultation on the revised RAR on the assessment of the endocrine disrupting properties made available after the 30‐month clock stop applied in accordance with Commission Regulation (EU) No 2018/1659.
See reporting table point 2(154) and Evaluation Table Section 1 data requirement 1.13 and open point 1.14 (EFSA, 2023a).
See experts' consultation point 2.14 in the Report of the Pesticides Peer Review Experts' TC 131 (EFSA, 2023a).
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, pp. 1–1355.
See experts' consultation point 2.12 in the report of the Pesticides Peer Review Experts' TC 131 (EFSA, 2023a).
See experts' consultation point 2.5 in the report of the Pesticides Peer Review Experts' Meeting PREV 11 (EFSA, 2023a).
See experts' consultation point 2.9 in the Report of the Pesticides Peer Review Experts' Meeting PREV 11 (EFSA, 2023a) and point 2.13 in the Report of the Pesticides Peer Review Experts' TC 131 (EFSA, 2023a).
Not yet implemented at the time of dossier submission of triclopyr.
The Martin et al. (2008) is no longer scientifically supported, since limited data were included for three‐dimensional exposure to spray drift and no estimates are provided for exposure to vapour from low volatility compounds. Accordingly, the predictions are considered underestimated and are given for informative purpose.
Commission Implementing Regulation (EU) 2020/17 of 10 January 2020 concerning the non‐renewal of the approval of the active substance chlorpyrifos‐methyl, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 (Text with EEA relevance) OJ L 7, 13.1.2020, p. 11–13.
Commission Implementing Regulation (EU) 2020/18 of 10 January 2020 concerning the non‐renewal of the approval of the active substance chlorpyrifos, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 (Text with EEA relevance) OJ L 7, 13.1.2020, p. 11–13.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
See expert consultation point 5.1 in the Report of the Pesticides Peer Review Experts' Meeting 14 (EFSA, 2023a).
For full details of the experts' discussion, please refer to experts' consultation point 5.1 of the Pesticides Peer Review Experts' TC 21 in July 2020 (EFSA, 2023a).
For further details, please refer to the response to data requirement 5.39 in the Evaluation Table (EFSA, 2023a).
For full details of the expert discussion, please refer to expert consultation point 5.2 of the Pesticides Peer Review Experts' TC 21 in July 2020 (EFSA, 2023a).
For further details, please refer to the response to data requirement 5.49 in the Evaluation Table (EFSA, 2023a).
For further details, please refer to the response to data requirement 5.50 in the Evaluation Table (EFSA, 2023a).
See experts' consultation 5.3. in the Report of the Pesticide Peer Review Experts' Meeting 14 (September 2019).
After the completion of the peer review, the applicant provided additional argumentation to address a number of the aquatic metabolites. Considering the details of the argumentation it would need to be evaluated by the RMS and peer reviewed by the Member State experts. Consequently, the data gap was maintained.
It should be noted that the available assessments for terrestrial vertebrates and non‐target arthropods have assumed a broadcast application to the full field. No quantified risk assessment was available for the spot application where applications are indicated not to exceed 20% of the treated surface area (see Section 5).
It should be noted that the available assessments for terrestrial vertebrates and non‐target arthropods have assumed a broadcast application to the full field. No quantified risk assessment was available for the spot application where applications are indicated not to exceed 20% of the treated surface area (see Section 5).
REFERENCES
- ECHA (European Chemicals Agency) . (2017). Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0, July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978–92–9020‐050‐5. https://echa.europa.eu/guidance‐documents/guidance‐on‐clp
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC) , Andersson, N. , Arena, M. , Auteri, D. , Barmaz, S. , Grignard, E. , Kienzler, A. , Lepper, P. , Lostia, A. M. , Munn, S. , Parra Morte, J. M. , Pellizzato, F. , Tarazona, J. , Terron, A. , & Van der Linden, S. (2018). Guidance for the identification of endocrine disruptors in the context of regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal, 16(6), 5311. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2006). Conclusion regarding the peer review of the pesticide risk assessment of the active substance triclopyr. EFSA Scientific Report, 56, 56r. 10.2903/j.efsa.2006.56r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2008). Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal, 6(1), 622. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2009). Guidance on risk assessment for birds and mammals on request from EFSA. EFSA Journal, 7(12), 1438. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2013a). EFSA Guidance document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal, 11(7), 3295. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2013b). Conclusion regarding the peer review of the pesticide risk assessment of the active substance aminopyralid. EFSA Journal, 11(9), 3352. 10.2903/j.efsa.2013.3352 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014a). EFSA Guidance document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal, 12(5), 3662. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2014b). Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal, 12(10), 3874. 10.2903/j.efsa.2014.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , De Lentdecker, C. , Erdos, Z. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nougadere, A. , Pedersen, R. , Reich, H. , Sacchi, A. , Santos, M. , … Villamar‐Bouza, L. (2017). Reasoned opinion on the review of the existing maximum residue levels for triclopyr according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 15(3), 4735. 10.2903/j.efsa.2017.4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) . (2019a). Updated statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos‐methyl. EFSA Journal, 17(11), 5908. 10.2903/j.efsa.2019.5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2019b). Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos. EFSA Journal, 17(5), 5809. 10.2903/j.efsa.2019.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Bernasconi, G. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Rojas, A. , Sacchi, A. , Santos, M. , Stanek, A. , … Verani, A. (2020). Reasoned opinion on the review of the existing maximum residue levels for aminopyralid according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 18(8), 6229. 10.2903/j.efsa.2020.6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) . (2023a). Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance triclopyr, updated in July 2024. www.efsa.europa.eu
- EFSA (European Food Safety Authority) , Bellisai, G. , Bernasconi, G. , Brancato, A. , Cabrera, L. C. , Castellan, I. , Del Aguila, M. , Ferreira, L. , Santonja, G. G. , Greco, L. , Jarrah, S. , Leuschner, R. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Ruocco, S. , Santos, M. , Scarlato, A. P. , … Verani, A. (2023b). Reasoned Opinion on the modification of the existing maximum residue levels for triclopyr in animal commodities. EFSA Journal, 21(5), 8007. 10.2903/j.efsa.2023.8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) . (2012). Guidance on dermal absorption. EFSA Journal, 10(4), 2665. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) . (2013). Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal, 11(7), 3290. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission . (2000a). Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission . (2000b). Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission . (2002). Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission . (2003). Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission . (2010). Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission . (2011). Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9. March 2011. pp. 1–46.
- European Commission . (2012). Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission . (2014a). Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp. as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2, May 2014.
- European Commission . (2014b). Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission . (2016). Technical guidelines for determining the magnitude of pesticide residues in honey and setting Maximum Residue Levels in honey. SANTE/11956/2016 rev. 9.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) . (2001). FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp. as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) . (2006). Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp. as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1, December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) . (2007). Landscape and mitigation factors in aquatic risk assessment. Volume 1. Extended summary and recommendations. Report of the FOCUS Working Group on Landscape and Mitigation Factors in Ecological Risk Assessment. EC Document Reference SANCO/10422/2005 v. 2.0, 169 pp.
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO Core assessment group on pesticide residues, Rome, Italy, 20–29 September 2004, 383 pp. https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Reports_1991‐2006/report2004jmpr.pdf [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues) . (2007). Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO Core assessment group on pesticide residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- Martin, S. , Westphal, D. , Erdtmann‐Vourliotis, M. , Dechet, F. , Schulze‐Rosario, C. , Stauber, F. , Wicke, H. , & Chester, G. (2008). Guidance for Exposure and Risk Evaluation for Bystanders and Residents exposed to Plant Protection Products during and after Application. Journal of Verbraucherschutz und Lebensmittelsicherhit, 3, 272–281. 10.1007/s00003-008-0361-5 [DOI] [Google Scholar]
- McCall, P. J. , Laskowski, D. A. , Swann, R. L. , & Dishburger, H. J. (1980). Measurements of sorption coefficients of organic chemicals and their use in environmental fate analysis. In: Test Protocols for Environmental Fate and Movement of Toxicants. In: Proceedings of the 94th annual meeting of the American Association of Official Analytical Chemists (AOAC). Oct 21–22, Washington, DC. pp. 89–109.
- OECD (Organisation for Economic Co‐operation and Development) . (2009). Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development) . (2011). OECD MRL calculator: Spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. www.oecd.org
- Poland . (2018). Renewal Assessment Report (RAR) on the active substance triclopyr prepared by the rapporteur Member State Poland in the framework of Commission Implementing Regulation (EU) No 844/2012, June 2018. www.efsa.europa.eu
- Poland . (2024). Revised Renewal Assessment Report (RAR) on triclopyr prepared by the rapporteur Member State Poland in the framework of Commission Implementing Regulation (EU) No 844/2012, June 2024. www.efsa.europa.eu
- SETAC (Society of Environmental Toxicology and Chemistry) . (2001). Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the formulation for representative uses




