Abstract
Background
There is substantial evidence that patients with COVID-19 were treated with sustained deep sedation during the pandemic. However, it is unknown whether such guideline-discordant care had spillover effects to patients without COVID-19.
Research Question
Did patterns of early deep sedation change during the pandemic for patients on mechanical ventilation without COVID-19?
Study Design and Methods
We used electronic health record data from 4,237 patients who were intubated without COVID-19. We compared sedation practices in the first 48 h after intubation across prepandemic (February 1, 2018, to January 31, 2020), pandemic (April 1, 2020, to March 31, 2021), and late pandemic (April 1, 2021, to March 31, 2022) periods.
Results
In the prepandemic period, patients spent an average of 13.0 h deeply sedated in the first 48 h after intubation. This increased 1.9 h (95% CI, 1.0-2.8) during the pandemic period and 2.9 h (95% CI, 2.0-3.8) in the late pandemic period. The proportion of patients that spent over one-half of the first 48 h deeply sedated was 18.9% in the prepandemic period, 22.3% during the pandemic period, and 25.9% during the late pandemic period. Ventilator-free days decreased during the pandemic, with a subdistribution hazard ratio of being alive without mechanical ventilation at 28 days of 0.87 (95% CI, 0.79-0.95) compared with the prepandemic period. Tracheostomy placement increased during the pandemic period compared with the prepandemic period (OR, 1.41; 95% CI, 1.08-1.82). In the medical ICU, early deep sedation increased 2.5 h (95% CI, 0.6-4.4) during the pandemic period and 4.9 h (95% CI, 3.0-6.9) during the late pandemic period, compared with the prepandemic period.
Interpretation
We found that among patients on mechanical ventilation without COVID-19, sedation use increased during the pandemic. In the subsequent year, these practices did not return to prepandemic standards.
Key Words: critical care, mechanical ventilation, sedation
Take-home Points.
Study Question: Did patterns of early deep sedation change during the pandemic for patients on mechanical ventilation without COVID-19?
Results: Among 4,237 patients without COVID-19 intubated from February 1, 2018, to March 31, 2022, the average duration of deep sedation in the first 48 h after intubation increased 1.9 h (95% CI, 1.0-2.8) during the pandemic period and 2.9 h (95% CI, 2.0-3.8) in the late pandemic period, compared with a prepandemic baseline of 13.0 h.
Interpretation: We found that among patients on mechanical ventilation without COVID-19, sedation use increased during the pandemic and did not return to prepandemic standards in the subsequent year.
The COVID-19 pandemic had an unprecedented impact on critical care delivery, completely upending routine ICU practices. Deviations from guideline-directed care to a new crisis mode standard of care were sudden and took place worldwide.1 The etiology was multifactorial, including high patient volume; increased severity of illness; shortages of labor, medication, and personal protective equipment; staff burnout; fear of infection transmission; and family visitation restrictions.2,3 These unique circumstances may also have adversely impacted routine ICU practices in patients without COVID-19 that are linked to patient outcomes.
One important practice impacted by the pandemic was sedation in patients receiving invasive mechanical ventilation. Critical care guidelines suggest targeting light levels of sedation for patients on mechanical ventilation, based on evidence that minimizing sedation is associated with improved patient outcomes (eg, shorter length of stay and duration of mechanical ventilation).4,5 Considerable efforts were made during the 2010s to decrease sedation utilization and bring bedside practices in line with guideline recommendations6; however, the COVID-19 pandemic may have brought this momentum to a halt. There is substantial evidence that patients with COVID-19 were treated with sustained deep levels of sedation during the pandemic,2,3,7, 8, 9 which may be one reason why they had worse outcomes.2 This shift in practice patterns occurred worldwide, where one large, multinational study found that > 80% of patients on mechanical ventilation with COVID-19 experiencing deep sedation had a median of 10 comatose days.8 However, it is not known whether guideline-discordant deep sedation use among patients with COVID-19 had a spillover effect to adult patients on mechanical ventilation without COVID-19.
To address this gap, we used detailed electronic health record data to evaluate sedation practices and clinical outcomes among patients on mechanical ventilation without COVID-19 from February 2018 to March 2022. This 4-year period allowed us to compare sedation practices in prepandemic, pandemic, and late pandemic cohorts. We hypothesized that the patients without COVID-19 would exhibit increased early deep sedation during the pandemic compared with their prepandemic counterparts. We also hypothesized that these spillover effects would be sustained during the second year of the pandemic.
Study Design and Methods
We performed an electronic health record-based retrospective cohort study of 4,237 adult mechanically ventilated, non-COVID-19 patient encounters across six ICUs within a 1,100-bed tertiary care academic institution between February 1, 2018, and March 31, 2022. Patients in the study received immediate postintubation care in the ED, operating or procedure room, or one of the facility’s six ICUs (medical, surgical, neurologic, trauma/burn, cardiac, and cardiac surgery). Patients who only received mechanical ventilation while in an operating or procedure room were not included in the study.
We included patients first intubated between February 1, 2018, and January 31, 2020 (prepandemic), April 1, 2020, and March 31, 2021 (pandemic), and April 1, 2021, and March 31, 2022 (late pandemic). We excluded patients intubated from February 2020 to March 2020, during the initial rise in COVID-19 cases, when COVID-19 testing was less readily available. We selected April 2021 as the cutoff between the pandemic and late pandemic periods because all adults in Michigan were eligible for the COVID-19 vaccine by April 2021.
Consistent with prior studies, we limited the analysis to patients who were mechanically ventilated at least 24 h.10, 11, 12 We assumed that shorter periods of mechanical ventilation were unlikely to include enough sedation time to impact outcomes. These patients are also likely different than those receiving longer durations of mechanical ventilation, reflecting more elective surgeries, younger patients with lower baseline reintubation risks, and patients who transitioned to comfort care or otherwise died shortly after intubation.13, 14, 15
Patients were excluded if they were COVID-19 positive, had a laryngectomy, had an existing tracheostomy on hospital presentation, had a tracheostomy placed within 24 h of intubation, received chronic home ventilation, had no Richmond Agitation Sedation Scale (RASS) data recorded while mechanically ventilated, were ventilated after death for organ donation, or were transferred while mechanically ventilated from another hospital to a non-ED location (e-Fig 1). Patients were also excluded if they received any continuous neuromuscular blockade infusion outside of an operating or procedure room in the first 48 h postintubation. COVID-19 status was determined based on the results of all COVID-19 polymerase chain reaction testing during the admission or any outpatient testing during the week prior to admission. By April 2020, COVID-19 testing was routinely performed on all patients on mechanical ventilation at our institution at least once during admission; therefore, patients without a positive test were considered negative for COVID-19.
We abstracted electronic health record data related to age; sex; race; ethnicity; BMI; hospital location; admission and discharge timing; vasopressor, neuromuscular blockade, and sedative use; extracorporeal membrane oxygenation utilization; tracheostomy placement; procedural timing; and date of death. The Acute Physiology and Chronic Health Evaluation (APACHE) IV score was calculated based on data obtained during the first 24 h of ICU admission.16 This study was approved by the University of Michigan institutional review board, and informed consent was waived among study participants.
Quantification of Sedation
The primary outcome was the total hours of early deep sedation, defined as the first 48 h after initial intubation. Forty-eight hours was selected based on evidence from prior observational studies that increased deep sedation during this period is associated with patient-centered outcomes, including fewer ventilator-free days (VFDs), increased length of stay, and increased mortality.9,12,17 Deep sedation was defined by a RASS score of −3, −4, or −5, consistent with other studies of early deep sedation in the literature.12,18 RASS score −2 was considered light sedation, according to the original RASS study definition.19 We measured the total hours of deep sedation, rather than a representative measure (eg, median, mean), because such measures fail to capture sedation duration and the variability of RASS scores over short periods of time. This is especially relevant in the first 48 h postintubation, when patients often require brief targeted periods of deep sedation immediately after intubation or for imaging studies and procedures.
RASS score was assumed to be −5 at the time of mechanical ventilation initiation. For postintubation measurements, RASS score was obtained from nursing documentation in the electronic health record. We assumed that any reported RASS score was maintained at that level until a new RASS score was documented.
To visualize exposure to deep sedation over time, we grouped patients by the time-weighted average of their RASS score in each 12-h window after intubation (0-12, 12-24, 24-36, and 36-48 h) and generated an alluvial plot. Patients were categorized into deep sedation (weighted average RASS score −3, −4, and −5), target sedation (RASS score −2 to 1), and agitated (RASS score ≥ 2) groups. Transitions from one category to another that corresponded to < 1% of the data, which included transitions to all agitated states, were not visualized.
Statistical Analyses
We compared the duration of time patients spent in deep sedation in the prepandemic period (February 1, 2018, to January 31, 2020) to the pandemic (April 1, 2020, to March 31, 2021) and late pandemic (April 1, 2021, to March 31, 2022) periods. The primary research question was whether the duration of early deep sedation was different between the prepandemic and pandemic periods, with the hypothesis that patients intubated during the pandemic would be deeply sedated longer than those intubated in the prepandemic period. A linear regression model was fit to evaluate the association between time period and hours spent in early deep sedation, adjusted for age, sex, APACHE score, BMI, and ICU location (e-Table 1). As a secondary analysis, we evaluated whether the duration of early deep sedation was different between the late pandemic and prepandemic periods.
The primary analysis was conducted both hospital-wide and stratified by ICU type and race/ethnicity. As an exploratory analysis, we also evaluated the duration of early deep sedation by race/ethnicity in a multivariable model adjusting for time period, age, sex, BMI, APACHE score, and ICU.
We conducted several sensitivity analyses to assess the robustness of the primary outcome results. We evaluated whether results were robust to inclusion of patients receiving neuromuscular blockade in the first 48 h (e-Appendix 1). An additional analysis was performed to evaluate potential cluster effects of ICU location using a mixed-effects model (e-Table 2). We also used a hurdle regression model (e-Table 3) to account for left and right boundary values of sedation data inherent to the 48-h period of interest.
We conducted additional secondary analyses comparing the duration of deep sedation and other outcomes between the prepandemic, pandemic, and late pandemic periods. Secondary outcomes were considered supportive and exploratory. Each was guided by a hypothesis and not adjusted for multiple comparisons. We analyzed the proportion of patients spending at least 24 of the first 48 h deeply sedated, tracheostomy placement, 90-day mortality, and the number of VFDs during the first 28 days after intubation.
Multivariable logistic regression models were fit to evaluate the association between time period and three binary outcomes: spending over 24 of the first 48 h after intubation deeply sedated (category of interest; reference category 0-24 h deeply sedated), tracheostomy placement, and 90-day mortality. All logistic analyses were adjusted for age, sex, APACHE score, BMI, and ICU. We plotted a time-to-event curve to compare VFDs across time periods (e-appendix 2). We also fit a Fine-Gray competing risk survival model to compare time to extubation, with death as a competing risk, using the cmprsk package in R. We reported the subdistribution hazard ratio (SHR), adjusted for age, sex, APACHE score, BMI, and ICU.
We were also interested in whether the duration of early deep sedation was associated with clinical outcomes, which has been variable across studies.20 We fit multivariable logistic regression models to evaluate the association between the duration of early deep sedation with tracheostomy placement and 90-day mortality, respectively. Both analyses were adjusted for age, sex, APACHE score, BMI, and ICU. Average marginal effects were calculated for tracheostomy and 90-day mortality outcomes.
The primary and secondary outcomes were specified a priori. Pearson χ2 test and Welch t test were used for statistical testing, with P < .05 signifying statistical significance. All analyses were conducted in R (4.2.1; The R Foundation), with the exception of hurdle regression modeling conducted in Stata (15.1; StataCorp).
Results
The study included 4,237 non-COVID-19 patient encounters, divided into prepandemic, pandemic, and late pandemic cohorts (Table 1). The cohorts were similar, without differences in demographic characteristics of age, sex, race/ethnicity, and BMI. There was also no difference in several markers of illness severity, including APACHE IV score (mean, 64.6 overall) and the proportion of patients on extracorporeal membrane oxygenation (3.1%). Compared with the prepandemic cohort, the pandemic and late pandemic cohorts had higher vasopressor usage, a higher proportion of patients that underwent a procedure in an operating or procedure room during their admission, and a different composition of postintubation ICU locations that shifted away from general medical and surgical ICUs toward more specialized units. The late pandemic cohort also had different presenting locations than the prepandemic cohort. The pandemic and late pandemic cohorts had higher proportions of patients on propofol and fentanyl infusions during the first 48 h after intubation, compared with the prepandemic cohort, whereas differences in midazolam infusions were not significant (Table 1).
Table 1.
Characteristics of Prepandemic, Pandemic, and Late Pandemic Cohorts of Adult Patients Without COVID-19 Who Received Mechanical Ventilation
| Characteristic | Prepandemic (n = 2,126) | Pandemic (n = 1,046) | Late Pandemic (n = 1,065) |
|---|---|---|---|
| Age, y | 59.3 ± 16.1 | 59.1 ± 15.3 | 60.0 ± 15.6 |
| Sex, female | 41.6 | 40.2 | 40.0 |
| Race and ethnicity | |||
| Non-Hispanic White | 79.4 | 79.5 | 79.8 |
| Non-Hispanic Black | 14.8 | 13.3 | 14.3 |
| Hispanic | 2.6 | 2.3 | 2.4 |
| Other (Non-Hispanic American Indian, Alaska Native, Native Hawaiian, Other Pacific Islander, Asian, or self-reported other) | 3.1 | 4.9 | 3.5 |
| APACHE IV score | 64.8 ± 24.5 | 65.0 ± 25.8 | 63.9 ± 24.3 |
| BMI, kg/m2 | 29.2 ± 8.1 | 28.9 ± 8.2 | 29.0 ± 8.5 |
| Time from admission to first intubation, d | 3.0 ± 8.2 | 3.1 ± 6.7 | 3.2 ± 5.9 |
| Any vasopressor use | 74.9 | 80.9a | 82.3a |
| Any continuous fentanyl use in first 48 h | 67.6 | 78.3a | 79.5a |
| Any continuous propofol use in first 48 h | 91.6 | 95.0a | 96.6a |
| Any continuous midazolam use in first 48 h | 9.6 | 10.1 | 10.2 |
| Extracorporeal membrane oxygenation | 3.2 | 3.0 | 3.1 |
| Total hospital length of stay, d | 19.9 ± 20.0 | 22.0 ± 24.1b | 21.4 ± 21.1 |
| Presenting hospital location | |||
| ED | 63.5 | 61.9 | 60.7b |
| Operating/procedure room | 16.0 | 17.0 | 20.0 |
| ICU | 7.0 | 7.6 | 6.2 |
| Floor | 13.5 | 13.6 | 13.1 |
| Any off-unit procedure during admission | 52.8 | 58.9c | 62.3a |
| First ICU after intubation | |||
| Medical | 31.2 | 27.7c | 25.1c |
| Surgical | 15.1 | 12.2 | 14.2 |
| Neurologic | 12.4 | 12.2 | 15.5 |
| Trauma/burn | 7.6 | 10.7 | 8.8 |
| Cardiac (medical) | 5.1 | 5.8 | 5.4 |
| Cardiac (surgical) | 28.6 | 31.3 | 31.0 |
Values are reported as mean ± SD or %. The prepandemic cohort was used as the reference group for all comparisons. APACHE = Acute Physiology and Chronic Health Evaluation.
P < .001.
P < .05.
P < .01.
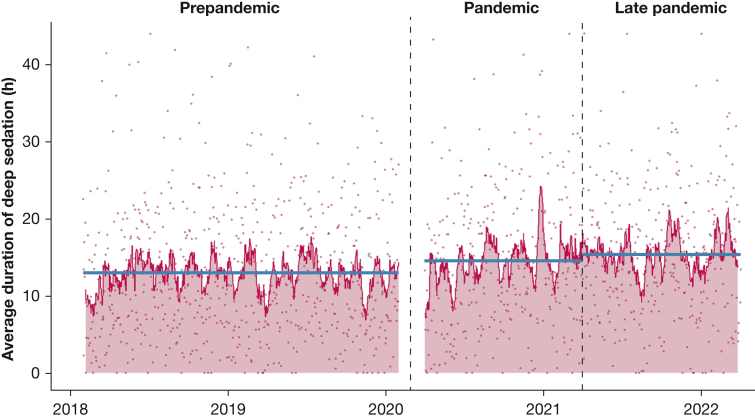
The average time that patients without COVID-19 spent deeply sedated during the first 48 h after intubation increased from 13.0 h in the prepandemic period to 14.6 h during the pandemic period and 15.4 h in the late pandemic period (Fig 1). These associations remained significant when adjusting for age, sex, APACHE score, BMI, and ICU, with an increase of 1.9 h (95% CI, 1.0-2.8) in the pandemic period and 2.9 h (95% CI, 2.0-3.8) in the late pandemic period compared with the prepandemic period, respectively.
Figure 1.
Deep sedation use during the prepandemic, pandemic, and late pandemic periods. Dots represent the average duration of deep sedation for all patients intubated on a given day. Red line represents the 14-d moving average of average durations of deep sedation for all patients intubated on a given day. Blue lines represent average duration of deep sedation utilization for each period.
The proportion of patients who spent over one-half of the first 48 h deeply sedated was 18.9% in the prepandemic period, 22.3% during the pandemic period, and 25.9% in the late pandemic period (e-Table 4). The late pandemic proportion was significantly higher than both prepandemic and pandemic levels after adjusting for age, sex, APACHE score, BMI, and ICU (Table 2). The adjusted odds of spending at least 24 of the first 48 h deeply sedated was 1.72 (95% CI, 1.43-2.07) in the late pandemic period compared with the prepandemic period and 1.35 (95% CI, 1.09-1.68) in the late pandemic period compared with the pandemic period.
Table 2.
Results of Secondary Outcomes
| Result | Pandemic | Late Pandemic |
|---|---|---|
| Proportion with 24 of first 48 h deeply sedated | 1.27 (1.05-1.55) | 1.72 (1.43-2.07) |
| Tracheostomy placement | 1.41 (1.08-1.82) | 1.16 (0.89-1.51) |
| 90-d mortality | 1.16 (0.97-1.40) | 1.18 (0.99-1.42) |
| Total ventilator-free days, SHR (95% CI) | 0.87 (0.79-0.95) | 0.88 (0.80-0.96) |
Values are OR (95% CI) or as otherwise indicated. The prepandemic cohort is the reference standard for all comparisons. All models are adjusted for age, sex, Acute Physiology and Chronic Health Evaluation IV score, BMI, and ICU location. SHR = subdistribution hazard ratio.
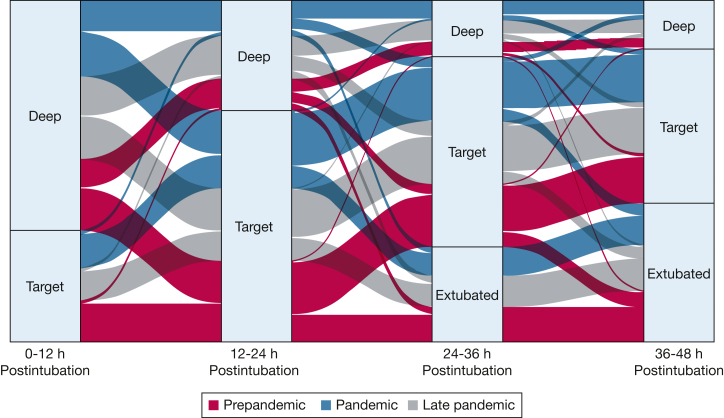
We created an alluvial plot to visualize exposure to deep sedation during the first 48 h after intubation (Fig 2). A time-weighted average RASS score corresponding to deep sedation was common among all groups during the first 12 h after intubation, occurring in 62.2% of patients (n = 1,322) in the prepandemic period, 65.0% of patients (n = 608) in the pandemic period, and 68.7% of patients (n = 731) in the late pandemic period. The proportion of patients with deeply sedated average RASS scores decreased as the time receiving mechanical ventilation progressed, down to 22.7% in the prepandemic period (n = 273), 29.3% in the pandemic period (n = 197), and 27.5% in the late pandemic period (n = 179) by 36 to 48 h postintubation. A nontrivial subset of patients was deeply sedated on average throughout all four 12-h periods, including 6.1% in the prepandemic period (n = 130), 9.9% in the pandemic period (n = 104), and 9.4% in the late pandemic period (n = 100).
Figure 2.
Exposure to deep sedation during the first 48 h after intubation. Patients are categorized at each interval based on the weighted average Richmond Agitation Sedation Scale (RASS) score during the prior 12-h period. An alluvial plot illustrates the proportions of patients in each cohort with a deep (RASS score −3, −4, or −5) or target (RASS score −2 to 1) weighted average RASS score during the prior 12-h period. Transitions from one category to another that correspond to < 1% of the data, which included transitions to all agitated states, are not visualized.
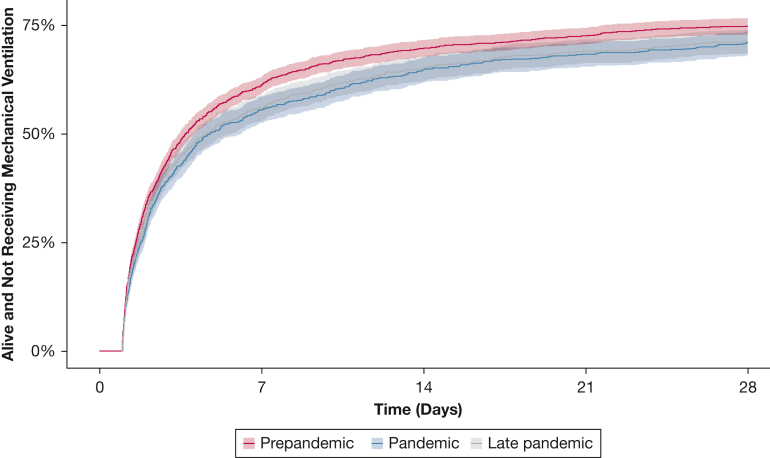
The mean number of VFDs was 17.7 days in the prepandemic period, 16.4 days during the pandemic period, and 16.5 days in the late pandemic period. In the competing risk time to event analysis, patients hospitalized in the pandemic and late pandemic periods were less likely to be alive and breathing without mechanical ventilation compared with the prepandemic period, when accounting for death as a competing risk (e-Table 4, Fig 3). These differences remained when adjusting for age, sex, APACHE score, BMI, and ICU with a pandemic to prepandemic SHR of 0.87 (95% CI, 0.79-0.95) and late pandemic to prepandemic SHR of 0.88 (95% CI, 0.80-0.96). There was no significant difference in 90-day mortality across time periods (Table 2), with a 90-day mortality of 28.4% in the prepandemic period, 30.5% during the pandemic period, and 30.1% mortality in the late pandemic period.
Figure 3.
Time to breathing without mechanical ventilation among patients who were intubated in the prepandemic, pandemic, and late pandemic periods.
Tracheostomy placement rose from the prepandemic (7.9%) to the pandemic period (11.0%) (e-Table 4). The increase remained when adjusting for age, sex, APACHE score, BMI, and ICU. The adjusted OR of requiring tracheostomy was 1.41 (95% CI, 1.08-1.82) (Table 2) during the pandemic period compared with the prepandemic period.
We performed subgroup analyses of the duration of early deep sedation and proportion of patients deeply sedated for > 24 of the first 48 h across time periods by ICU location and race/ethnicity. The increase in use of deep sedation was most notable in the medical ICU (e-Table 5). In the prepandemic period, patients in the medical ICU spent an average of 16.4 of the first 48 h deeply sedated. This duration increased by 2.5 h (95% CI, 0.6-4.4) during the pandemic period and 4.9 h (95% CI, 3.0-6.9) in the late pandemic period, adjusted for age, sex, BMI, and APACHE score. This corresponds to a continued worsening in early deep sedation between the pandemic and late pandemic periods, with an increase in the adjusted duration of deep sedation of 2.5 h (95% CI, 0.2-4.8) when comparing these periods directly. The proportion of patients in the medical ICU who were deeply sedated for > 24 of the first 48 h also increased from 29.8% in the prepandemic period to 43.7% in the late pandemic period. When stratifying by race and ethnicity, we found that both non-Hispanic White and non-Hispanic Black patients experienced an increase in the duration of early deep sedation in the late pandemic period compared with the prepandemic period (e-Table 6). There was no significant difference in the duration of early deep sedation for non-Hispanic Black patients compared with non-Hispanic White patients when adjusting for time period, age, sex, BMI, APACHE score, and ICU.
Finally, we also analyzed the association between early deep sedation and both tracheostomy placement and 90-day mortality. After adjusting for age, sex, BMI, APACHE score, and ICU, the probability of tracheostomy placement increased by 1% for every additional 6 h of deep sedation patients experienced in the first 48 h after intubation (95% CI, 0.4%-1.2%). After adjusting for age, sex, BMI, APACHE score, and ICU, the probability of death in 90 days increased by 3% for every additional 6 h of deep sedation in the first 48 h after intubation (95% CI, 2.5%-3.6%).
Discussion
We analyzed sedation practices among adult patients on mechanical ventilation without COVID-19 before and during the COVID-19 pandemic and found a significant increase in early deep sedation during the pandemic and late pandemic periods. Tracheostomy placement increased and VFDs decreased during the pandemic period. The proportion of patients spending > 24 of the first 48 h deeply sedated increased in the late pandemic period. Sedation use continued to worsen in the medical ICU during the late pandemic period.
Our finding that sedation practices among patients with COVID-19 may have had harmful spillover effects to a population without COVID-19 is consistent with work by Pumphrey et al,3 studying PICU patients. For 3 months in 2020, their PICU was repurposed to an adult respiratory ICU, and pediatric intensivists and nurses cared exclusively for adult patients on mechanical ventilation, with high sedation usage. After the ICU was converted back to a pediatrics unit, they found an increase in benzodiazepine use among their pediatric patients without COVID-19.
This study was not designed to investigate causality, however, two possible reasons for increased sedation usage include staff turnover and high burnout.21, 22, 23, 24 Nurses with less ICU-level experience and in settings of higher staffing ratios give sedation more readily than experienced nurses and well-staffed units.25,26 Burnout is an additional strain and was reported at record high levels during the COVID-19 pandemic.23,24 It is associated with increased health care-associated infections and major surgical errors, lower quality of care, and decreased patient satisfaction.27, 28, 29, 30
Prior prepandemic implementation studies identified several successful interventions to reduce sedation use. Common themes are that they are implemented at the bedside, multidisciplinary, and highly reliant on nonphysician participants.31, 32, 33, 34 A large cluster randomized trial of 118 ICUs lowered deep sedation use by using a nursing-led rounding checklist. This checklist included just two sedation-related questions, followed by a daily scheduled nursing reminder to physicians if intervention goals had not been met.31 Similarly, a randomized trial of 18 PICUs increased awake and calm days by implementing a nursing-led sedation algorithm.32 Interventions to bring sedation use back to pre-COVID-19 standards should similarly aim to empower a wide range of stakeholders involved in bedside care.
Although the goal of this study was not to evaluate a causal relationship between deep sedation and clinical outcomes, our results align with numerous other observational studies demonstrating associations between early deep sedation and worse clinical outcomes, including pre-COVID-1912,17,35 and COVID-19-era work.9 We found that tracheostomies increased during the pandemic and that early deep sedation was associated with increased tracheostomy placement. Tracheostomies are reported to occur more frequently among patients with COVID-19 than pre-COVID-19 patients with other types of ARDS.36 The current study’s pre-COVID-19 tracheostomy rate of 7.8% is very similar to prior reports.37 The subsequent pandemic-era rise in tracheostomies in our study is not surprising. We hypothesize that it is related to both the increased use of deep sedation during the pandemic and a spillover effect from the practice of high tracheostomy placement among patients with COVID-19.
Our study has some limitations. It was conducted at a single tertiary care academic center; however, it analyzed six medical and surgical ICUs that were differentially affected by the pandemic and included a range of attending provider specialties, patient populations, and unit cultures. This study was also limited by the quality of data in the health record. Much of the data related to cohort selection and study outcomes were based on nursing and respiratory therapist documentation. Whenever possible, we sought to incorporate multiple data points to improve the reliability of measurements. Ultimately, using electronic health record data allowed for a level of granularity that is not practically obtainable in more traditional cohort studies.
Interpretation
We found that sedation practices among adult patients on mechanical ventilation without COVID-19 worsened during the pandemic and after 2 years had still not returned to prepandemic standards. Concerted efforts to improve guideline-based sedation strategies are needed in the post-COVID-19 era.
Funding/Support
This study was supported by the National Institutes of Health [Grant R01 HL 158626 to M. W. S., Grant R01 HL 157361 to T. S. V.].
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: A. K. B. and M. W. S. had full access to all the data in the study and take responsibility for the content of the manuscript, including the data and analysis. A. K. B. and M. W. S. contributed to the conception and design of the study. A. K. B., T. S. V., M. T. K., and M. W. S. contributed to data acquisition, analysis, or interpretation and drafting and revision of the manuscript. All authors approved the final version of the manuscript.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figure, and e-Tables are available online under "Supplementary Data."
Supplementary Data
References
- 1.Aziz S., Arabi Y.M., Alhazzani W., et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46(7):1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wongtangman K., Santer P., Wachtendorf L.J., et al. Association of sedation, coma, and in-hospital mortality in mechanically ventilated patients with coronavirus disease 2019–related acute respiratory distress syndrome: a retrospective cohort study. Crit Care Med. 2021;49(9):1524–1534. doi: 10.1097/CCM.0000000000005053. [DOI] [PubMed] [Google Scholar]
- 3.Pumphrey K., Bouzaher A., Achuff B.-J., Traube C. Sedation practices in the PICU: an unexpected casualty of COVID-19. Crit Care Explor. 2022;4(6) doi: 10.1097/CCE.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 5.Halpern S.D., Becker D., Curtis J.R., et al. An Official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine Policy Statement: The Choosing Wisely® Top 5 List in Critical Care Medicine. Am J Respir Crit Care Med. 2014;190(7):818–826. doi: 10.1164/rccm.201407-1317ST. [DOI] [PubMed] [Google Scholar]
- 6.Stollings J.L., Balas M.C., Chanques G. Evolution of sedation management in the intensive care unit (ICU) Intensive Care Med. 2022;48(11):1625–1628. doi: 10.1007/s00134-022-06806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flinspach A.N., Booke H., Zacharowski K., Balaban Ü., Herrmann E., Adam E.H. High sedation needs of critically ill COVID-19 ARDS patients—a monocentric observational study. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0253778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pun B.T., Badenes R., Calle G.H.L., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephens R.J., Evans E.M., Pajor M.J., et al. A dual-center cohort study on the association between early deep sedation and clinical outcomes in mechanically ventilated patients during the COVID-19 pandemic: the COVID-SED study. Crit Care. 2022;26(1):179. doi: 10.1186/s13054-022-04042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller B.M., Roberts B.W., Mohr N.M., et al. The ED-SED Study: a multicenter, prospective cohort study of practice patterns and clinical outcomes associated with Emergency Department SEDation for mechanically ventilated patients. Crit Care Med. 2019;47(11):1539–1548. doi: 10.1097/CCM.0000000000003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephens R.J., Ablordeppey E., Drewry A.M., et al. Analgosedation practices and the impact of sedation depth on clinical outcomes among patients requiring mechanical ventilation in the ED: a cohort study. Chest. 2017;152(5):963–971. doi: 10.1016/j.chest.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shehabi Y., Bellomo R., Reade M.C., et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 13.Kotfis K., Szylińska A., Listewnik M., et al. Balancing intubation time with postoperative risk in cardiac surgery patients – a retrospective cohort analysis. Ther Clin Risk Manag. 2018;14:2203–2212. doi: 10.2147/TCRM.S182333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford T.C., Magruder J.T., Grimm J.C., et al. Early extubation: a proposed new metric. Semin Thorac Cardiovasc Surg. 2016;28(2):290–299. doi: 10.1053/j.semtcvs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Richey M., Mann A., He J., et al. Implementation of an early extubation protocol in cardiac surgical patients decreased ventilator time but not intensive care unit or hospital length of stay. J Cardiothorac Vasc Anesth. 2018;32(2):739–744. doi: 10.1053/j.jvca.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman J.E., Kramer A.A., McNair D.S., Malila F.M. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34(5):1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 17.Shehabi Y., Bellomo R., Kadiman S., et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. 2018;46(6):850. doi: 10.1097/CCM.0000000000003071. [DOI] [PubMed] [Google Scholar]
- 18.Shehabi Y., Howe B.D., Bellomo R., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380(26):2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 19.Sessler C.N., Gosnell M.S., Grap M.J., et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 20.Aitken L.M., Kydonaki K., Blackwood B., et al. Inconsistent relationship between depth of sedation and intensive care outcome: systematic review and meta-analysis. Thorax. 2021;76(11):1089–1098. doi: 10.1136/thoraxjnl-2020-216098. [DOI] [PubMed] [Google Scholar]
- 21.Aiken L.H., Sloane D.M., McHugh M.D., Pogue C.A., Lasater K.B. A repeated cross-sectional study of nurses immediately before and during the COVID-19 pandemic: implications for action. Nurs Outlook. 2023;71(1) doi: 10.1016/j.outlook.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmo S., Milner K.A. From open to closed: COVID-19 restrictions on previously unrestricted visitation policies in adult intensive care units. Am J Crit Care. 2023;32(1):31–41. doi: 10.4037/ajcc2023365. [DOI] [PubMed] [Google Scholar]
- 23.Kok N., Gurp J van, Teerenstra S., et al. Coronavirus disease 2019 immediately increases burnout symptoms in ICU professionals: a longitudinal cohort study. Crit Care Med. 2021;49(3):419. doi: 10.1097/CCM.0000000000004865. [DOI] [PubMed] [Google Scholar]
- 24.Shanafelt T.D., West C.P., Dyrbye L.N., et al. Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clin Proc. 2022;97(12):2248–2258. doi: 10.1016/j.mayocp.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guttormson J.L., Chlan L., Weinert C., Savik K. Factors influencing nurse sedation practices with mechanically ventilated patients: a U.S. national survey. Intensive Crit Care Nurs. 2010;26(1):44–50. doi: 10.1016/j.iccn.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Egerod I. Uncertain terms of sedation in ICU. How nurses and physicians manage and describe sedation for mechanically ventilated patients. J Clin Nurs. 2002;11(6):831–840. doi: 10.1046/j.1365-2702.2002.00725.x. [DOI] [PubMed] [Google Scholar]
- 27.Cimiotti J.P., Aiken L.H., Sloane D.M., Wu E.S. Nurse staffing, burnout, and health care–associated infection. Am J Infect Control. 2012;40(6):486–490. doi: 10.1016/j.ajic.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanafelt T.D., Balch C.M., Bechamps G., et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995. doi: 10.1097/SLA.0b013e3181bfdab3. [DOI] [PubMed] [Google Scholar]
- 29.Poghosyan L., Clarke S.P., Finlayson M., Aiken L.H. Nurse burnout and quality of care: cross-national investigation in six countries. Res Nurs Health. 2010;33(4):288–298. doi: 10.1002/nur.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahey D.C., Aiken L.H., Sloane D.M., Clarke S.P., Vargas D. Nurse burnout and patient satisfaction. Med Care. 2004;42(2 suppl):II57–II66. doi: 10.1097/01.mlr.0000109126.50398.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Writing Group for the CHECKLIST-ICU Investigators and the Brazilian Research in Intensive Care Network (BRICNet). Effect of a quality improvement intervention with daily round checklists, goal setting, and clinician prompting on mortality of critically ill patients: a randomized clinical trial. JAMA. 2016;315(14):1480–1490. doi: 10.1001/jama.2016.3463. [DOI] [PubMed] [Google Scholar]
- 32.Curley M.A.Q., Wypij D., Watson R.S., et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313(4):379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall J., Finn C.A., Theodore A.C. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36(2):427. doi: 10.1097/01.CCM.0000300275.63811.B3. [DOI] [PubMed] [Google Scholar]
- 34.Walsh T.S., Kydonaki K., Antonelli J., et al. Staff education, regular sedation and analgesia quality feedback, and a sedation monitoring technology for improving sedation and analgesia quality for critically ill, mechanically ventilated patients: a cluster randomised trial. Lancet Respir Med. 2016;4(10):807–817. doi: 10.1016/S2213-2600(16)30178-3. [DOI] [PubMed] [Google Scholar]
- 35.Balzer F., Weiß B., Kumpf O., et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. 2015;19(1):197. doi: 10.1186/s13054-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsonas A.M., Botta M., Horn J., et al. Practice of tracheostomy in patients with acute respiratory failure related to COVID–19 – insights from the PRoVENT–COVID study. Pulmonology. 2022;28(1):18–27. doi: 10.1016/j.pulmoe.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abril M.K., Berkowitz D.M., Chen Y., Waller L.A., Martin G.S., Kempker J.A. The epidemiology of adult tracheostomy in the United States 2002-2017: a serial cross-sectional study. Crit Care Explor. 2021;3(9) doi: 10.1097/CCE.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.