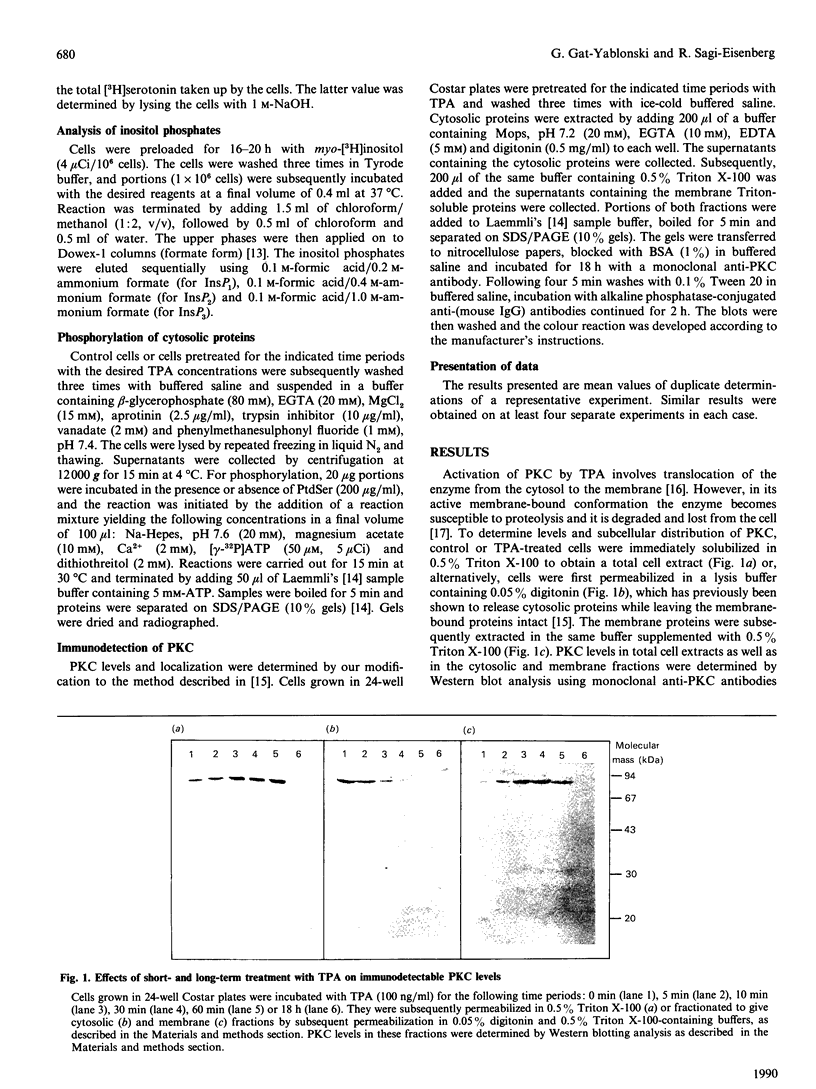
Abstract
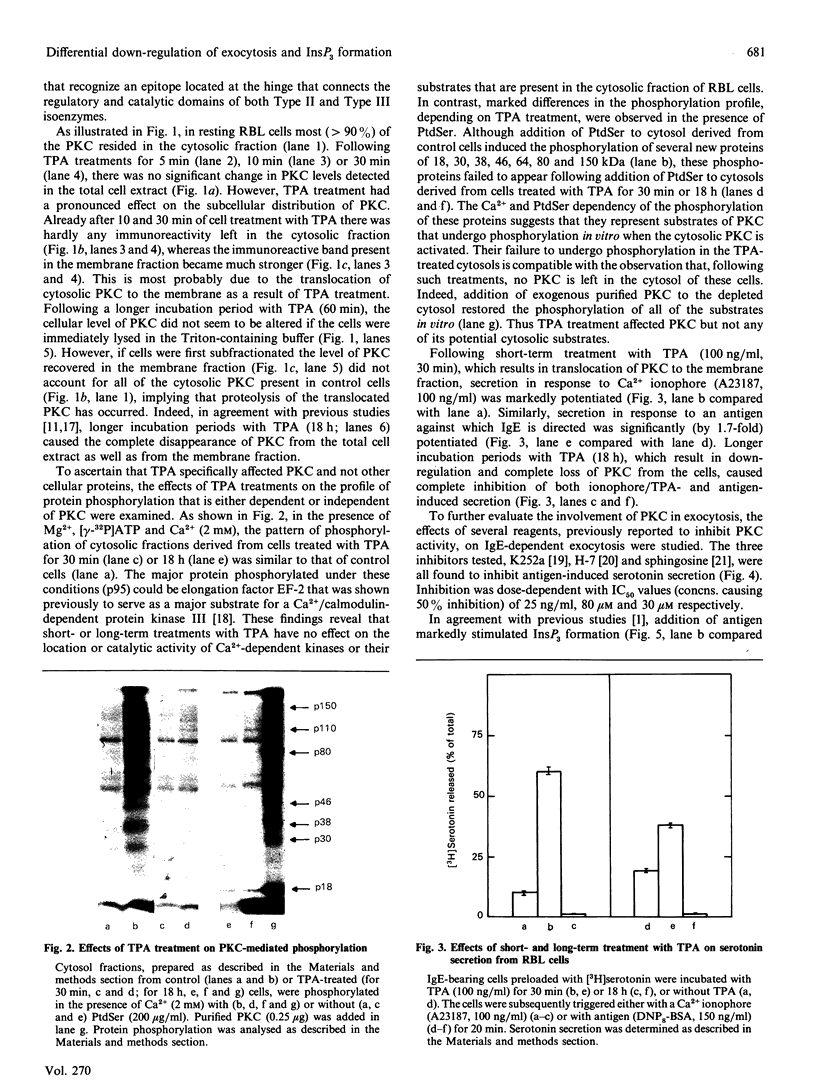
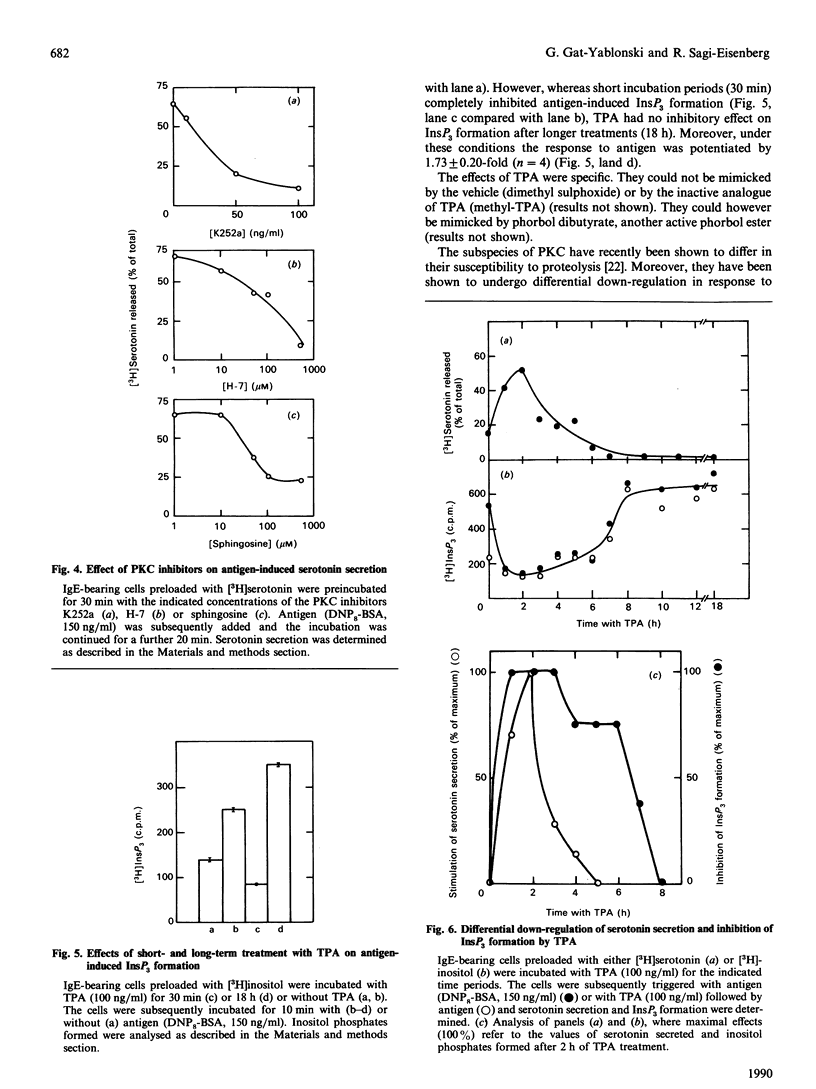
Short-term treatment of rat basophilic leukaemia (RBL-2H3) cells with the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) activates protein kinase C (PKC) and results in the inhibition of the IgE-dependent formation of inositol phosphates, but in the potentiation of serotonin secretion. Long-term treatment with TPA, which depletes the cells of their endogenous PKC, eliminates both Ca2(+)-ionophore- and TPA- as well as IgE-dependent secretion, but it potentiates by 1.7-fold IgE-induced inositol phosphate formation. Taken together, these observations strongly suggest that the dual actions of TPA on IgE-dependent responses are both mediated by PKC. The opposing effects of TPA are differentially down-regulated. Following TPA treatment, the rate by which the cells lose their ability to undergo exocytosis is faster than the rate at which inhibition of inositol phosphates formation is relieved and their production potentiated. In addition, both processes show different sensitivities to inhibitors of PKC action. Whereas IgE-dependent secretion is completely blocked by the PKC inhibitors K252a, H-7 and sphingosine [concns. causing 50% inhibition (IC50 values) = 25 ng/ml 80 microns and 30 microns respectively], these inhibitors do not relieve inhibition of inositol phosphate formation by TPA, nor do they potentiate this response. These results may imply that the bidirectional control exerted by PKC on IgE-dependent responses is mediated by its different isoenzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ase K., Berry N., Kikkawa U., Kishimoto A., Nishizuka Y. Differential down-regulation of protein kinase C subspecies in KM3 cells. FEBS Lett. 1988 Aug 29;236(2):396–400. doi: 10.1016/0014-5793(88)80064-4. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Guthrie D. F., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Synergistic signals in the mechanism of antigen-induced exocytosis in 2H3 cells: evidence for an unidentified signal required for histamine release. J Cell Biol. 1987 Sep;105(3):1129–1136. doi: 10.1083/jcb.105.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Purification and characterization of a phosphoinositide-specific phospholipase C from guinea pig uterus. Phosphorylation by protein kinase C in vivo. J Biol Chem. 1987 Oct 5;262(28):13789–13797. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARAH F. S., KERN M., EISEN H. N. The preparation and some properties of purified antibody specific for the 2,4-dinitrophenyl group. J Exp Med. 1960 Dec 1;112:1195–1210. doi: 10.1084/jem.112.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Yablonski G., Sagi-Eisenberg R. Evaluation of the role of inositol trisphosphate in IgE-dependent exocytosis. Biochem J. 1990 Sep 15;270(3):685–689. doi: 10.1042/bj2700685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Cunha-Melo J. R., Beaven M. A., Huang K. P. Differential down-regulation of protein kinase C isozymes. J Biol Chem. 1989 Mar 5;264(7):4238–4243. [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Matsuda Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J Antibiot (Tokyo) 1986 Aug;39(8):1059–1065. doi: 10.7164/antibiotics.39.1059. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Katakami Y., Kaibuchi K., Sawamura M., Takai Y., Nishizuka Y. Synergistic action of protein kinase C and calcium for histamine release from rat peritoneal mast cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):573–578. doi: 10.1016/0006-291x(84)90220-1. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989 Mar 5;264(7):4088–4092. [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Meier K. E., Krebs E. G. Rapid microassay for protein kinase C translocation in Swiss 3T3 cells. Biochemistry. 1986 Dec 30;25(26):8348–8353. doi: 10.1021/bi00374a002. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Lieman H., Pecht I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukaemia cells. Nature. 1985 Jan 3;313(5997):59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Pecht I. Protein kinase C, a coupling element between stimulus and secretion of basophils. Immunol Lett. 1984;8(5):237–241. doi: 10.1016/0165-2478(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Teshima R., Ikebuchi H., Terao T. Ca2+-dependent and phorbol ester activating phosphorylation of a 36K-dalton protein of rat basophilic leukemia cell membranes and immunoprecipitation of the phosphorylated protein with IgE-anti IgE system. Biochem Biophys Res Commun. 1984 Dec 28;125(3):867–874. doi: 10.1016/0006-291x(84)91363-9. [DOI] [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Sagi-Eisenberg R., Pines M., Gierschik P., Spiegel A. M. Multisite phosphorylation of the alpha subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]