Abstract
Despite a record setting number of heart transplants performed annually, the national donor shortage continues to plague transplant teams across the United States. Here we describe the barriers to adaptation of numerous “non-traditional” orthotopic heart transplant donor characteristics including donors with hepatitis C virus, those meeting criteria for donation after cardiac death, donors with coronavirus disease 19 infection, donors with the human immunodeficiency virus, and grafts with left ventricular systolic dysfunction. Our center’s objective was to increase our transplant volume by expanding our donor pool from “traditional” donors to these “non-traditional” donors. We detail how medical advances such as certain laboratory studies, pharmacologic interventions, and organ care systems have allowed our center to expand the donor pool thereby increasing transplantation volume without adverse effects on outcomes.
Keywords: Orthotopic heart transplantation, Hepatitis C virus, COVID-19, Left ventricular systolic dysfunction, Human immunodeficiency virus, Donation after cardiac death
Core Tip: Since the turn of the 21st century, advances in certain laboratory studies, pharmacologic interventions, and organ care systems have led to the expansion of the donor pool in the field of orthotopic heart transplantation. This includes: (1) Nucleic acid testing and direct acting antivirals for donors with hepatitis C virus; (2) Organ care systems for donors who meet criteria for donation after cardiac death; (3) Anti-viral therapies for donors with coronavirus disease 19; (4) Improved donor management for grafts with left ventricular systolic dysfunction; and (5) Better understanding of immunosuppression and medication interaction in patients with human immunodeficiency virus. We detail how these advances have allowed our center to expand the donor pool thereby increasing transplantation volume without adverse effects on outcomes.
INTRODUCTION
In 2022 the United States set a record for the number of orthotopic heart transplantations performed annually, exceeding four-thousand transplants for the first time. This record pace of transplantation has, in part, been accomplished by the changing landscape of donor eligibility over the past decade from “traditional” donors to “non-traditional” donors. However, despite this, the national donor shortage continues to plague transplant teams and eager recipients, while waitlist mortality remains high[1].
For the purposes of this paper, we define a “traditional” donor as one who is: (1) Free of pre-existing communicable disease; (2) Meets criteria for donation after brain death; and (3) Has normal left ventricular systolic function. “Non-traditional” donors include donors with hepatitis C virus (HCV), those meeting criteria for donation after cardiac death (DCD), donors with coronavirus disease 2019 (COVID-19) infection, donors with the human immunodeficiency virus (HIV), and grafts with left ventricular systolic dysfunction (LVSD).
Since the turn of the 21st century, laboratory studies, pharmacologic interventions, and organ care systems (OCS) have allowed for better management of “non-traditional” donors and their recipients. In the wake of these advances, our center’s objective was to increase our transplant volume by expanding our donor pool to “non-traditional” donors. Here we discuss the brief history, barriers to adaptation, implementation, and challenges associated with the transplantation of various “non-traditional” donors, while detailing how the inclusion of these donors has now eclipsed the percentage of “traditional” donors at our center without adversely affecting outcomes.
HCV DONORS
Barriers to adaptation
For most of the 21st century, the number of transplantations utilizing organs from HCV donors in the United States did not exceed more than two dozen annually. Poor outcomes in HCV transplantation greatly deterred centers from adaptation. The development of coronary artery vasculopathy and overall mortality was nearly three times higher in HCV negative recipients who received an HCV positive donor organ, fulminant hepatitis was common, and the use of certain immunosuppressive agents was associated with lower survival[2,3]. These poor outcomes in the early 2000’s can be attributed to two main barriers: Lack of timely detection and poorly efficacious treatment.
Prior to 2015 when nucleic acid testing (NAT) was introduced to the routine workup of donors, HCV antibody testing was the primary tool centers had to screen for the virus. This was problematic as the latency from infection to seroconversion can range from 1 month to > 4 years and left centers performing transplants of HCV antibody positive (HCV Ab+) donors despite not knowing the donors viremic status[4]. As a result, HCV transmission rates from positive donors to naïve recipients was shown to be as high as 82%[5]. Moreover, the levels of immunosuppression in these recipients often hindered seroconversion, making the detection of a new HCV infection in the recipient difficult. With the introduction of NAT to the routine workup of donors in 2015, centers were now able to detect HCV RNA within 1 week of infection with 99% sensitivity and specificity[6].
Until 2011, interferon-based treatment was the mainstay for HCV infection, which had a high adverse event profile with a low rate of sustained virologic response - approximately 5%-6%[7]. Studies have since shown that with the introduction of direct acting antivirals (DAAs), sustained virologic response rates have increased over 20-fold, preventing post-transplant seroconversion of the recipient in nearly all cases[8]. Indeed, in one study, a four week course of a pangenotypic antiviral regimen led to all of patients having an undetectable viral load at 6 months and excellent graph function, while additional studies showed that 1-year survival was equivalent to those without hepatitis[9,10].
Implementation and challenges
Our center organized an interdisciplinary meeting consisting of advanced heart failure and transplant cardiologists, infectious disease physicians, and hepatology experts. There was unanimous decision that, considering the developments of HCV detection and treatment, the use of HCV antibody-positive (HCV Ab+/NAT-) donors did not differ from those donors who met standard Public Health Services increased risk criteria. Thus, our center expanded our donor pool and carried out its first HCV Ab+ transplant in 2017, two years after the introduction of NAT[11]. By the end of the year, 15 of 49 (30.6%) of our transplants were from HCV Ab+ donors. This was in stark contrast to national data, based on the United Network for Organ Sharing (UNOS) database, where only 103 of 3307 (3.1%) of transplants nationally were HCV Ab+.
In the years that followed, viremic NAT+ donors comprised more of our total share of HCV donors than those who were antibody positive alone. Since HCV genotype may not be known, we routinely start glecaprevir/pibrentasvir (Mavyret) as pangenotypic therapy immediately following transplant. However, with the introduction of DAAs came concern over interaction with immunosuppressants and how to choose the proper regimen for a given recipient. A prior study from our center showed that this regimen induces a reversible change in calcineurin-inhibitor metabolism, warranting up to a 50% reduction in immunosuppression dosing[12]. As such, our center always initiates DAA treatment while inpatient with close monitoring of immunosuppression levels and liver function tests. However, despite these challenges, we have observed the elimination of the virus and absence of seroconversion in all 25 HCV NAT+ patients to date.
DCD DONORS
Barriers to adaptation
DCD, despite being utilized for the very first heart transplant in 1967 by Christiaan Barnard[13], also did not gain popularity until two decades into the 21st century. In 2014, the first procurement of a distant donor took place with the use of OCS (TransMedics, Andover, MA, United States)[14]. These devices, comprised of a perfusion module, oxygenator and pulsatile pump, utilize normothermic machine perfusion to circulate a blood and nutrient-rich solution at near-physiologic temperatures[15].
Unlike the standard of care, donation after brain death, which allows for the possibility to evaluate the functioning donor organ immediately before harvest, DCD donors must have circulatory arrest prior to organ evaluation and harvest. After withdrawal of life support the donor enters the agonal phase, followed by circulatory arrest, and finally a formal declaration of death. During this time the organ is subject to a functional-warm ischemic injury. This is until either the donor heart is re-perfused via normothermic regional perfusion for evaluation in vivo, or until there is direct procurement of the organ and placement into the OCS for further evaluation ex vivo[16].
These intricacies of DCD donation highlight numerous barriers to adaptation. First, the aforementioned process resulting in ischemic injury was long thought to cause irreversible harm to the non-beating donor organ[17]. Second, the potential donor may not meet circulatory death criteria after withdrawal of support and the transplant may be cancelled. Third, there exist some ethical controversies regarding normothermic regional perfusion and the restoration of circulation in the donor[18]. Lastly, the cost of a single use of the OCS in 2019 was estimated at $40000 which can strain transplant team funding[19].
Implementation and challenges
When considering expansion of our center’s donor pool to include DCD donors, the primary concern was that the irreversible ischemic injury endured by the organ would lead to poor recipient outcomes. To determine if this was the case in practice, we performed a UNOS database analysis which showed no significant difference in 30-day or 6-month mortality amongst DCD recipients[16]. This study gave us the confidence to expand our donor pool to DCD donors and in 2022 our center carried out its first DCD transplant. By the end of 2022, 16 of 41 (39%) of our transplants were from DCD donors as compared to national trends where 372 of 4226 (8.8%) of transplants were from DCD donors.
While there are many challenges prior to DCD transplantation, there are also challenges in the post-transplant period. The main challenge faced after DCD transplantation relative to traditional donation after brain death donors is transient right ventricular (RV) dysfunction. D’Alessandro et al[20] demonstrated that DCD recipients experienced greater right atrial pressure (RAP), lower pulmonary artery pulsatility index and higher (right atrial pressure)/(pulmonary capillary wedge pressure) relative to traditional donors. The etiologies of the RV dysfunction are likely multifactorial including the greater ischemic time and the catecholaminergic surge experienced during brain death[21]. Our center has mitigated this challenge with careful interdisciplinary team monitoring of post-transplant hemodynamics and extended use of inotropes and pulmonary vasodilators when necessary.
COVID+ DONORS
Barriers to adaptation
Hepatitis was not the only infectious disease transplant centers had to adapt to over the past decade, as COVID quickly dominated the health care landscape in 2020. The COVID pandemic immediately presented an enormous risk towards post-transplant immunocompromised recipients. For those still on the waitlist, the demands of the pandemic led to cancelled surgeries and reduced transplant volume; at our center volume in 2020 dropped by nearly 50% from 2019 levels. Most importantly, the COVID pandemic added yet another barrier to donor eligibility. Great concern was given to the notion that a COVID+ donor may lead to COVID infection in the newly immunocompromised recipient. At the time, this was not a risk that transplant centers were willing to take, as one study showed that COVID infection in a transplant recipient was associated with a 25% case fatality rate[22]. Nevertheless, certain transplant centers began to move forward with COVID+ kidney, liver and eventually heart transplantation. Outcomes of these transplants was published rapidly and demonstrated that the risk of a recipient developing symptomatic COVID from a positive donor organ was minimal to none[23,24].
Implementation and challenges
Before fully expanding our donor pool, our center began vaccinating patients on our waiting list. Shortly after, as the severity of the pandemic began to wean with vaccinations becoming increasingly available and adopted with greater confidence, we elected to move forward with transplantation of COVID+ donors in late 2021. At our center, since the pandemic began, 6 of 66 (9.1%) otherwise ‘traditional’ donors were COVID+ at the time of transplantation. Using those donors, we did not observe COVID infection in any of the recipients in the acute post-transplant phase.
However, as with other “non-traditional” donors, we found it prudent to conduct a UNOS analysis to examine outcomes in these recipients. We found that transplants from donors with active COVID infection (defined as COVID NAT+ within 2 days of procurement) may have increased mortality at 6-months and 1-year relative to those with recently recovered COVID infection (defined as COVID NAT+ initially but became negative prior to procurement) and non-COVID donors[25]. These results prompted the use of anti-viral therapies such as remdesivir in our patients receiving COVID+ donor hearts. Since knowledge on the use of COVID+ donors continues to evolve, we urge a careful risk-benefit analysis and possible consideration of peri-transplant antiviral therapies when using donors who remain COVID+ at the time of procurement and continue to encourage vaccination with COVID boosters for those on the waitlist.
HIV+ DONORS
Barriers to adaptation
In 1988, at the peak of the acquired immunodeficiency syndrome epidemic, the harvesting of donor organs from patients with known or suspected HIV was federally prohibited. Over the two decades that followed, the development of highly active anti-retroviral therapy (HAART) turned what was once a death sentence into a manageable chronic illness. In 2013, the United States HIV Organ Policy Equity Act was signed into law. While the act had many components, one mandate was to rescind the ban on HIV+ donor organs, which was later accomplished in 2015[26].
Since 2015, centers across the country have paved with way with HIV+ liver and kidney transplantations[27,28], demonstrating the feasibility of a successful HIV+ heart transplantation and extinguishing any fears over increased immunosuppression in a patient whom, by infection with HIV, is already immunosuppressed. However, despite these successes, the attitudes held towards HIV+ transplantation remain a barrier to increased adoption. Within just the last decade, over half of transplant centers surveyed believed that immunosuppression may induce progression to acquired immunodeficiency syndrome and thus considered HIV a contraindication to orthotropic heart transplantation[29]. In another survey, 50% of centers believed that only half of their HIV+ waitlisted patients would be willing to accept an HIV+ donor organ. On the contrary, centers who held more positive attitudes regarding their waitlisted patients willingness to accept HIV+ donor organs were more likely to perform an HIV+ transplant[30].
Implementation and challenges
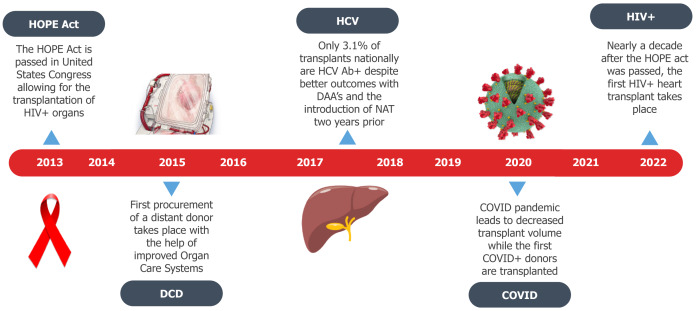
Increased adaptation of HIV+ heart transplantation is vital as studies have demonstrated that those who are HIV+ are at greater risk of developing heart failure relative to uninfected individuals[31]. Between 2018 and 2022 the rate of new HIV diagnoses per 100000 people was highest in the Bronx, NY, where our center is located, relative to all other boroughs and New York City as a whole[32]. This high burden of disease translated to a higher prevalence of HIV in our recipient pool, and a great need to expand donor eligibility. As a result, in 2022, we expanded eligibility to HIV+ donors, matched with HIV+ recipients, and performed the first ever intentional HIV+ donor to HIV+ recipient heart transplant[33] (Figure 1).
Figure 1.
Key events that have shaped 21st century donor characteristics. Despite the changing landscape of donor characteristics, national volume of non-traditional donors remains low. HOPE: HIV Organ Policy Equity; HCV Ab: Hepatitis C virus antibody; HIV: Human immunodeficiency virus; DAA: Direct acting antiviral; NAT: Nucleic acid testing; DCD: Donation after cardiac death; COVID: Coronavirus disease.
The biggest challenge our center faced after HIV+ organ transplantation was the use of HAART and immunosuppressive agents. Increased levels of immunosuppression were not the primary concern, rather that HAART agents and immunosuppressive agents are metabolized by or eliminated via the cytochrome P450 enzyme system. This leads to changes in serum concentration of both agents and necessitates dosage reduction and careful monitoring for toxicity[34]. Drug-drug interactions with immunosuppressive agents are not limited to HAART agents but also may interfere with statins needed for coronary artery vasculopathy prophylaxis and antiarrhythmic agents.
DONORS WITH LVSD
Barriers to adaptation
While systemic donor characteristics such as infectious disease carrier status often determine organ eligibility, the innate function of the donor organ is paramount in determining if the organ is a suitable fit for a recipient. Oftentimes the left ventricular systolic function of the organ in question is reduced, and consequently one in four donor hearts are rejected due to LVSD[35]. Similar to the RV dysfunction experienced in DCD donors, it is believed that this LVSD is multifactorial and may be related to the catecholamine surge during the dying process which results in myocardial stunning[36]. In fact, it has been shown that nearly half of patients who are declared dead secondary to brain injury experience LVSD in the absence of prior cardiac pathology[37]. However, despite this, transplant centers remain hesitant to accept these organs out of concern for sustained or worsening graft function in the recipient.
Implementation and challenges
Unlike the other “non-traditional” donors discussed, our center expanded the donor pool to those with LVSD as of 2010, seven years before expanding to an additional “non-traditional” donor (Supplementary Figure 1). Since 2010, 20 of 382 (5.2%) otherwise ‘traditional’ donors had LVSD as defined as an initial ejection fraction (EF) < 50%. Despite this early adoption, as with prior “non-traditional” donors, our center conducted a UNOS analysis by reviewing echocardiograms of donors with reduced EF (< 40%) that resolved (to an EF > 50%) with donor management. The study demonstrated that there was no difference in mortality, cardiac allograph vasculopathy, or primary graft failure between donors with an initial low EF that recovered, and those with a normal EF at the outset. Relative to those with a normal EF, those with an improved EF had significantly greater use, and received higher doses of, dobutamine, epinephrine and norepinephrine[38].
This prompted our center to be more judicious when reviewing a donor with LVSD. First, we would request that the donor be placed on inotropes, often at higher doses. Second, we would request that a repeat transthoracic echocardiogram is obtained after a specified amount of time on inotropes had elapsed. This improvement in donor management would often lead to significant recovery of LVEF, and an organ acceptable for transplantation.
CONCLUSION
Over the past decade, improved detection methods and anti-viral therapy for HCV+ and COVID+ donors, OCS for DCD donors, pharmacologic management of low EF donors, and highly active anti-retroviral therapy for HIV+ donors have allowed for positive outcomes in these “non-traditional” donor recipients. Since we expanded to additional “non-traditional” donors in 2017, we have carried out 178 successful “traditional” donor transplants and 84 successful “non-traditional” donor transplants. One-year unadjusted survival of traditional donors was 167 of 178 (93.8%) while one-year unadjusted survival of non-traditional donors was 77 of 84 (91.7%). Overall one-year survival in the last Scientific Registry of Transplant Recipients report for our center was 96.3% (January 2024).
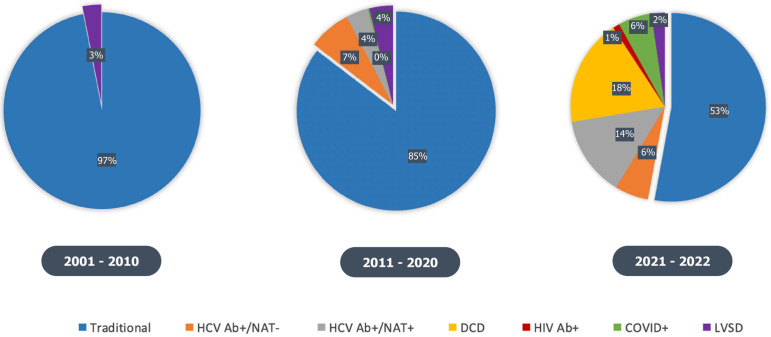
More than half of the transplants performed by our center in 2022 were from “non-traditional” donors (Figure 2). However, we have detailed how this is not the case in national trends, where despite a donor shortage, transplant centers have been somewhat reluctant to expand donor eligibility. This reluctance is likely secondary to: (1) Significant stigma and poor attitudes towards donors with certain communicable diseases; (2) Lack of exposure to more recent data demonstrating the improvement in outcomes in these “non-traditional” donors; and (3) Limited interdisciplinary team support or funding for “non-traditional” donor management strategies.
Figure 2.
Evolving single center donor characteristics during the 21st century. As of 2022, more than half of the transplants performed were from non-traditional donors. HCV Ab: Hepatitis C virus antibody; NAT: Nucleic acid testing; DCD: Donation after cardiac death; LVSD: Left ventricular systolic dysfunction; HIV: Human immunodeficiency virus; COVID: Coronavirus disease.
The ongoing donor organ shortage, increasing number of patients with advanced heart failure, and increasing prevalence of communicable diseases, underscore the importance of expanding donor eligibility to these “non-traditional” donors to increase equity and reduce waitlist times. While we have encountered numerous challenges as we have expanded our donor pool, our experience illustrates the dramatic evolution of the “suitable donor” profile over the past decade and suggests that other centers can safely expand their eligibility criteria from the “traditional” metrics.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country of origin: United States
Peer-review report’s classification
Scientific Quality: Grade C, Grade D
Novelty: Grade B, Grade B
Creativity or Innovation: Grade A, Grade C
Scientific Significance: Grade A, Grade A
P-Reviewer: Oliveira AP, Portugal S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
Contributor Information
Alexander M Spring, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Christiana Gjelaj, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Shivank Madan, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Snehal R Patel, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Omar Saeed, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Sandhya Murthy, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Yogita Rochlani, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Daniel B Sims, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Sasha Vukelic, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Stephen J Forest, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Jamil F Borgi, Division of Cardiothoracic Surgery, Tulane University, New Orleans, LA 70112, United States.
Daniel J Goldstein, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Ulrich P Jorde, Montefiore-Einstein Center for Heart and Vascular Care, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States. ujorde@montefiore.org.
References
- 1.Dharmavaram N, Hess T, Jaeger H, Smith J, Hermsen J, Murray D, Dhingra R. National Trends in Heart Donor Usage Rates: Are We Efficiently Transplanting More Hearts? J Am Heart Assoc. 2021;10:e019655. doi: 10.1161/JAHA.120.019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochlani Y, Diab K, Jorde UP. Hepatitis C-Positive Donors in Cardiac Transplantation: Problems and Opportunities. Curr Heart Fail Rep. 2020;17:106–115. doi: 10.1007/s11897-020-00466-y. [DOI] [PubMed] [Google Scholar]
- 3.Haji SA, Starling RC, Avery RK, Mawhorter S, Tuzcu EM, Schoenhagen P, Cook DJ, Ratliff NB, McCarthy PM, Young JB, Yamani MH. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23:277–283. doi: 10.1016/S1053-2498(03)00148-7. [DOI] [PubMed] [Google Scholar]
- 4.Maple PA, McKee T, Desselberger U, Wreghitt TG. Hepatitis C virus infections in transplant patients: serological and virological investigations. J Med Virol. 1994;44:43–48. doi: 10.1002/jmv.1890440109. [DOI] [PubMed] [Google Scholar]
- 5.Ong JP, Barnes DS, Younossi ZM, Gramlich T, Yen-Lieberman B, Goormastic M, Sheffield C, Hoercher K, Starling R, Young J, Smedira N, McCarthy P. Outcome of de novo hepatitis C virus infection in heart transplant recipients. Hepatology. 1999;30:1293–1298. doi: 10.1002/hep.510300519. [DOI] [PubMed] [Google Scholar]
- 6.Gupta E, Bajpai M, Choudhary A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J Transfus Sci. 2014;8:19–25. doi: 10.4103/0973-6247.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon AM, Green PK, Berry K, Ioannou GN. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Aliment Pharmacol Ther. 2017;45:1201–1212. doi: 10.1111/apt.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, Coppolino A, Kusztos AE, Johnson ME, Chen K, Haddad EA, Fanikos J, Harrington DP, Camp PC, Baden LR DONATE HCV Trial Team. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019;380:1606–1617. doi: 10.1056/NEJMoa1812406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlendorf KH, Zalawadiya S, Shah AS, Wigger M, Chung CY, Smith S, Danter M, Choi CW, Keebler ME, Brinkley DM, Sacks SB, Ooi H, Perri R, Awad JA, Lewis S, Hayes R, O'Dell H, Darragh C, Carver A, Edmonds C, Ruzevich-Scholl S, Lindenfeld J. Early outcomes using hepatitis C-positive donors for cardiac transplantation in the era of effective direct-acting anti-viral therapies. J Heart Lung Transplant. 2018;37:763–769. doi: 10.1016/j.healun.2018.01.1293. [DOI] [PubMed] [Google Scholar]
- 11.Patel SR, Madan S, Saeed O, Sims DB, Shin JJ, Nucci C, Borukhov E, Goldstein DY, Jakobleff W, Forest S, Vukelic S, Murthy S, Reinus J, Puius Y, Goldstein DJ, Jorde UP. Cardiac transplantation from non-viremic hepatitis C donors. J Heart Lung Transplant. 2018;37:1254–1260. doi: 10.1016/j.healun.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Nnani DU, Campbell A, Ajaimy M, Saeed O, Patel SR, Ahmed S, Graham JA, Jorde UP. Effect of glecaprevir/pibrentasvir on weight-adjusted tacrolimus trough/dose ratios in heart and kidney transplant recipients. Transpl Infect Dis. 2021;23:e13716. doi: 10.1111/tid.13716. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DK. Christiaan Barnard and his contributions to heart transplantation. J Heart Lung Transplant. 2001;20:599–610. doi: 10.1016/s1053-2498(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 14.Dhital KK, Iyer A, Connellan M, Chew HC, Gao L, Doyle A, Hicks M, Kumarasinghe G, Soto C, Dinale A, Cartwright B, Nair P, Granger E, Jansz P, Jabbour A, Kotlyar E, Keogh A, Hayward C, Graham R, Spratt P, Macdonald P. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 15.Pinnelas R, Kobashigawa JA. Ex vivo normothermic perfusion in heart transplantation: a review of the TransMedics(®) Organ Care System. Future Cardiol. 2022;18:5–15. doi: 10.2217/fca-2021-0030. [DOI] [PubMed] [Google Scholar]
- 16.Madan S, Saeed O, Forest SJ, Goldstein DJ, Jorde UP, Patel SR. Feasibility and Potential Impact of Heart Transplantation From Adult Donors After Circulatory Death. J Am Coll Cardiol. 2022;79:148–162. doi: 10.1016/j.jacc.2021.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Scheuer SE, Jansz PC, Macdonald PS. Heart transplantation following donation after circulatory death: Expanding the donor pool. J Heart Lung Transplant. 2021;40:882–889. doi: 10.1016/j.healun.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Entwistle JW, Drake DH, Fenton KN, Smith MA, Sade RM Cardiothoracic Ethics Forum. Normothermic regional perfusion: Ethical issues in thoracic organ donation. J Thorac Cardiovasc Surg. 2022;164:147–154. doi: 10.1016/j.jtcvs.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Pettit SJ, Petrie MC. Transplantation of Hearts Donated After Circulatory-Determined Death. Circ Heart Fail. 2019;12:e005991. doi: 10.1161/CIRCHEARTFAILURE.119.005991. [DOI] [PubMed] [Google Scholar]
- 20.D'Alessandro DA, Wolfe SB, Osho AA, Drezek K, Prario MN, Rabi SA, Michel E, Tsao L, Coglianese E, Doucette M, Zlotoff DA, Newton-Cheh C, Thomas SS, Ton VK, Sutaria N, Schoenike MW, Christ AM, Paneitz DC, Madsen JC, Pierson R, Lewis GD. Hemodynamic and Clinical Performance of Hearts Donated After Circulatory Death. J Am Coll Cardiol. 2022;80:1314–1326. doi: 10.1016/j.jacc.2022.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Bittner HB, Chen EP, Biswas SS, Van Trigt P 3rd, Davis RD. Right ventricular dysfunction after cardiac transplantation: primarily related to status of donor heart. Ann Thorac Surg. 1999;68:1605–1611. doi: 10.1016/s0003-4975(99)00987-x. [DOI] [PubMed] [Google Scholar]
- 22.Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y, Restaino S, Sayer G, Uriel N. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenberger EM, Kaul DR, Wolfe CR. The pandemic provides a pathway: What we know and what we need to know about using COVID positive donors. Transpl Infect Dis. 2021;23:e13727. doi: 10.1111/tid.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanaan CN, Iskandar JP, Gad MM, Kondoleon NP, Mirzai S, Hsich EM, Estep JD, Fares MA. Outcomes Associated With COVID-19 Hospitalization in Heart Transplantation Patients. Transplant Proc. 2022;54:2688–2691. doi: 10.1016/j.transproceed.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madan S, Chan MAG, Saeed O, Hemmige V, Sims DB, Forest SJ, Goldstein DJ, Patel SR, Jorde UP. Early Outcomes of Adult Heart Transplantation From COVID-19 Infected Donors. J Am Coll Cardiol. 2023;81:2344–2357. doi: 10.1016/j.jacc.2023.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durand CM, Segev D, Sugarman J. Realizing HOPE: The Ethics of Organ Transplantation From HIV-Positive Donors. Ann Intern Med. 2016;165:138–142. doi: 10.7326/M16-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durand CM, Florman S, Motter JD, Brown D, Ostrander D, Yu S, Liang T, Werbel WA, Cameron A, Ottmann S, Hamilton JP, Redd AD, Bowring MG, Eby Y, Fernandez RE, Doby B, Labo N, Whitby D, Miley W, Friedman-Moraco R, Turgeon N, Price JC, Chin-Hong P, Stock P, Stosor V, Kirchner VA, Pruett T, Wojciechowski D, Elias N, Wolfe C, Quinn TC, Odim J, Morsheimer M, Mehta SA, Rana MM, Huprikar S, Massie A, Tobian AAR, Segev DL HOPE in Action Investigators. HOPE in action: A prospective multicenter pilot study of liver transplantation from donors with HIV to recipients with HIV. Am J Transplant. 2022;22:853–864. doi: 10.1111/ajt.16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durand CM, Zhang W, Brown DM, Yu S, Desai N, Redd AD, Bagnasco SM, Naqvi FF, Seaman S, Doby BL, Ostrander D, Bowring MG, Eby Y, Fernandez RE, Friedman-Moraco R, Turgeon N, Stock P, Chin-Hong P, Mehta S, Stosor V, Small CB, Gupta G, Mehta SA, Wolfe CR, Husson J, Gilbert A, Cooper M, Adebiyi O, Agarwal A, Muller E, Quinn TC, Odim J, Huprikar S, Florman S, Massie AB, Tobian AAR, Segev DL HOPE in Action Investigators. A prospective multicenter pilot study of HIV-positive deceased donor to HIV-positive recipient kidney transplantation: HOPE in action. Am J Transplant. 2021;21:1754–1764. doi: 10.1111/ajt.16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uriel N, Nahumi N, Colombo PC, Yuzefpolskaya M, Restaino SW, Han J, Thomas SS, Garan AR, Takayama H, Mancini DM, Naka Y, Jorde UP. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant. 2014;33:924–930. doi: 10.1016/j.healun.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Van Pilsum Rasmussen SE, Bowring MG, Shaffer AA, Henderson ML, Massie A, Tobian AAR, Segev DL, Durand CM. Knowledge, attitudes, and planned practice of HIV-positive to HIV-positive transplantation in US transplant centers. Clin Transplant. 2018;32:e13365. doi: 10.1111/ctr.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NYC Health. HIV in the Bronx, 2022. [cited 12 December 2023]. Available from: https://home.nyc.gov/assets/doh/downloads/pdf/dires/hiv-in-the-bronx-2022.pdf .
- 33.Hemmige V, Saeed O, Puius YA, Azzi Y, Colovai A, Borgi J, Goldstein DJ, Rahmanian M, Carlese A, Jorde UP, Patel S. HIV D+/R+ heart/kidney transplantation: First case report. J Heart Lung Transplant. 2023;42:406–408. doi: 10.1016/j.healun.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Izzedine H, Launay-Vacher V, Baumelou A, Deray G. Antiretroviral and immunosuppressive drug-drug interactions: an update. Kidney Int. 2004;66:532–541. doi: 10.1111/j.1523-1755.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 35.Zaroff JG, Babcock WD, Shiboski SC. The impact of left ventricular dysfunction on cardiac donor transplant rates. J Heart Lung Transplant. 2003;22:334–337. doi: 10.1016/s1053-2498(02)00554-5. [DOI] [PubMed] [Google Scholar]
- 36.Khush KK, Malinoski D, Luikart H, Wayda B, Groat T, Nguyen J, Belcher J, Nieto J, Neidlinger N, Salehi A, Geraghty PJ, Nicely B, Jendrisak M, Pearson T, Patrick Wood R, Zhang S, Weng Y, Zaroff J. Left Ventricular Dysfunction Associated With Brain Death: Results From the Donor Heart Study. Circulation. 2023;148:822–833. doi: 10.1161/CIRCULATIONAHA.122.063400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dujardin KS, McCully RB, Wijdicks EF, Tazelaar HD, Seward JB, McGregor CG, Olson LJ. Myocardial dysfunction associated with brain death: clinical, echocardiographic, and pathologic features. J Heart Lung Transplant. 2001;20:350–357. doi: 10.1016/s1053-2498(00)00193-5. [DOI] [PubMed] [Google Scholar]
- 38.Madan S, Saeed O, Vlismas P, Katsa I, Patel SR, Shin JJ, Jakobleff WA, Goldstein DJ, Sims DB, Jorde UP. Outcomes After Transplantation of Donor Hearts With Improving Left Ventricular Systolic Dysfunction. J Am Coll Cardiol. 2017;70:1248–1258. doi: 10.1016/j.jacc.2017.07.728. [DOI] [PubMed] [Google Scholar]