Abstract
BACKGROUND
Recurrence of hepatocellular carcinoma (HCC) following liver transplantation (LT) has a devastating influence on recipients’ survival; however, the risk of recurrence is not routinely stratified. Risk stratification is vital with a long LT waiting time, as that could influence the recurrence despite strict listing criteria.
AIM
This study aims to identify predictors of recurrence and develop a novel risk prediction score to forecast HCC recurrence following LT.
METHODS
A retrospective review of LT for HCC recipients at University Hospitals Birmingham between July 2011 and February 2020. Univariate and multivariate analyses were performed to identify recurrence predictors, based on which the novel SIMAP500 (satellite nodules, increase in size, microvascular invasion, AFP > 500, poor differentiation) risk score was proposed.
RESULTS
234 LTs for HCC were performed with a median follow-up of 5.3 years. Recurrence developed in 25 patients (10.7%). On univariate analyses, RETREAT score > 3, α-fetoprotein (AFP) at listing 100-500 and > 500, bridging, increased tumour size between imaging at the listing time and explant histology, increase in the size of viable tumour between listing and explant, presence of satellite nodules, micro- and macrovascular invasion on explant and poor differentiation of tumours were significantly associated with recurrence, based on which, the SIMAP500 risk score is proposed. The SIMAP500 demonstrated an excellent predictive ability (c-index = 0.803) and outperformed the RETREAT score (c-index = 0.73). SIMAP500 is indicative of the time to disease recurrence.
CONCLUSION
SIMAP500 risk score identifies the LT recipients at risk of HCC recurrence. Risk stratification allows patient-centric post-transplant surveillance programs. Further validation of the score is recommended.
Keywords: Liver transplantation, Hepatocellular carcinoma, Recurrence, Survival
Core Tip: Hepatocellular carcinoma recurrence following liver transplantation has a devastating effect on recipients’ survival. Different scoring systems were proposed to identify recipients at higher risk of recurrence. We used univariate and multivariate analysis to build a new scoring system [SIMAP500 (satellite nodules, increase in size, microvascular invasion, AFP > 500, poor differentiation)] and validated the novel scoring system using our cohort of liver transplant recipients. SIMAP500 has an excellent predictive power of recurrence and can be used to tailor post-transplant surveillance programs.
INTRODUCTION
Liver transplantation (LT) is a feasible treatment option for patients with hepatocellular carcinoma (HCC) and underlying chronic liver disease. Approximately 20%-25% of LT procedures in Western transplant programs are specifically intended to treat HCC[1-3]. The benchmark selection criteria of HCC candidates for LT are based on the Milan Criteria (MC)[4], which have been associated with satisfactory post-LT survival[5,6]. Extended criteria have been proposed to offer LT for patients outside the MC or following downstaging locoregional treatments with acceptable outcomes[7-9]. Despite these cautious selection criteria, 6%-18% of LT recipients develop HCC recurrence[7,10-12].
Surveillance following surgical resection for HCC is the standard of care[13]. However, following LT, surveillance for HCC recurrence has been debated amongst healthcare providers. Some clinicians point to a lack of high-level evidence for routine surveillance following LT, given that there are limited curative options and life expectancy following recurrence. Furthermore, surveillance could be associated with significant anxiety for some patients. Although the standard treatment options and survival, unfortunately, remain limited for those patients with recurrence, a recent series reported survival benefit of further treatment in selected patients[14]. Therefore, other clinicians favour surveillance as it may offer earlier diagnosis and access to locoregional and systemic treatment options. A pragmatic choice could be to tailor the surveillance program to recipients at high risk for HCC recurrence[15,16].
Several post-transplant predictive models have been proposed, including the United States HCC Consortium[17], Post-MORAL[18], Decaens score[19], and the Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score[20]. The RETREAT score encompasses α-fetoprotein (AFP) and explant characteristics to predict individual risk for recurrence; the RETREAT score performed well in a recent study using a United Kingdom cohort[21]. The 5-year recurrence rate based on RETREAT is estimated to be 3% for a score of 0 vs 75% for a score of 5 or more. However, none of these scores consider the logistics of waiting time for liver transplantation, a factor that is mainly an issue in some of the LT programs.
This study aims to identify recurrence predictors, develop a novel risk score, and compare its performance with the RETREAT score as a post-LT recurrence risk prediction tool.
MATERIALS AND METHODS
This study was a retrospective review of all patients who underwent LT for an indication of HCC at the University Hospitals Birmingham NHS Foundation Trust between July 2011 and February 2020. The inclusion criteria were all LT recipients over 18 years who were transplanted with HCC as their listing indication. Clinical notes and electronic records were used to retrieve prospectively maintained donor and recipient data. All patients with HCC eligible for LT were discussed at the HCC multidisciplinary team meeting before listing. The United Kingdom criteria for eligibility for listing for LT was single HCC tumour ≤ 5 cm diameter, up to 5 tumours all ≤ 3 cm, or single tumour > 5 cm and ≤ 7 cm diameter, where there has been no evidence of tumour progression and no extrahepatic spread and no new nodule formation over six months, and AFP less than 1000[22]. Trans arterial chemoembolization (TACE) or ablative therapies were used as bridging treatment for patients on the waitlist.
All candidates had updated imaging performed at three monthly intervals while on the waiting list for transplantation. The choice of donation after brainstem death and donation after circulatory death grafts was made at the transplant listing multidisciplinary team meeting, and the on-call team decided on the organ suitability when offered.
Post-transplantation surveillance imaging was not part of the protocol. Patients were followed up in the long term by the unit’s hepatology team and the referring gastroenterology teams. Those recipients suspected of recurrence were investigated, and the imaging was reviewed at the HCC multi-disciplinary team.
The donor, recipient, and explant tumour parameters were evaluated. Outcomes assessed were time to recurrence, sites of recurrence, disease-free survival, and overall survival. Univariate and multivariate analysis was performed to identify predictors of HCC recurrence.
Statistical analysis
The median (range) of continuous parameters was used as comparison points. Some continuous variables (year of transplant, RETREAT score, date of listing to transplant, largest tumour size, number of tumours, AFP at listing, explant viable tumour number) were organized into clinically relevant intervals. Data were analysed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). The Kolmogorov-Smirnov was used to verify the normality of the distribution of variables. Categorical variables were assessed using the χ2 test. Any missing data were taken into account during statistical analysis. Kaplan-Meier Survival curve was used, and Cox regression analysis was done to identify the factors associated with a significant relation to HCC recurrence. Results were statistically significant if P < 0.05. The new SIMAP500 (satellite nodules, increase in size, microvascular invasion, AFP > 500, poor differentiation) scoring system was developed depending on factors positive in the univariate analysis and using the B-coefficient to weight of each factor in the novel score.
RESULTS
Patients and donor characteristics
Between July 2011 and February 2020, 234 LTs for HCC were performed with a median follow-up period of 5.3 years. Donor and tumour characteristics are summarized in Table 1. The time between listing and LT was less than one month in 23.1% of the recipients, between 1 and 3 months in 37.6%, and more than three months in 39.3% of the recipients. The median of the Model for End-Stage Liver Disease (MELD) score at listing was 9 (4-24), while the median of the United Kingdom Model for End-Stage Liver Disease (UKELD) was 49 (41-65). 95.3% of the recipients were within the MC at the listing time, while 98% were within the extended UK criteria. On pre-LT imaging, the median number of tumours was 1 and the median size of the largest tumour was 2.4 cm. The median AFP at listing was 7, and 64.1% received pre-LT TACE or ablation.
Table 1.
Pretransplant donor and tumour characteristics
|
|
No. (%)
|
| Donor age (/years) | |
| Median (Min.-Max.) | 54 (12-83) |
| Donor sex | |
| Male | 146 (62.4) |
| Female | 88 (37.6) |
| Donor type | |
| DBD | 126 (53.8) |
| DCD | 108 (46.2) |
| Tumour largest size (cm) | |
| ≤ 5 | 226 (98.3) |
| > 5 | 4 (1.7) |
| Median (range) | 2.4 (1-6.7) |
| Number of tumours | |
| ≤ 3 | 225 (97) |
| > 3 | 7 (3) |
| Median | 1 (1-6) |
| AFP-listing | |
| < 100 | 205 (88.8) |
| 100-500 | 21 (9) |
| 500-1000 | 1 (0.4) |
| > 1000 | 4 (1.7) |
| Median (range) | 7 (1-2634) |
| TACE | 93 (39.7) |
| Ablation | 57 (24.4) |
| MELD | |
| Median (range) | 9 (4-24) |
| UKELD | |
| Median (range) | 49 (41-65) |
AFP: Alpha fetoprotein; DCD: Donation after circulatory death; DBD: Donation after brain stem death; TACE: Transarterial chemoembolization; MELD: Model for End-Stage Liver Disease; UKELD: United Kingdom Model for End-Stage Liver Disease.
On the explant histology, the median size of the largest viable tumour was 2 cm (0.5-13), and the median number of viable tumours was one tumour. The microvascular invasion was documented in 119 (50.9%) explants; macrovascular invasion was recorded in 14 (6%), and satellite nodules in 17 (7.3%) of the explants. On analysis of the explanted tumours, 50 (21.2%) were well differentiated, 123 (53%) were moderately differentiated, 27 (11.6%) were poorly differentiated and 32 (13.8%) showed complete necrosis. Between listing and LT, the tumour size decreased in 101 (49.8%), remained stable in 22 (10.8%) and increased in size in 80 (39.4%). While on the waitlist, 61 (27.6%) had a declining AFP, 105 (47.5%) had a stable AFP, and 55 (24.9%) showed a rise in AFP.
Recurrence patterns and survival
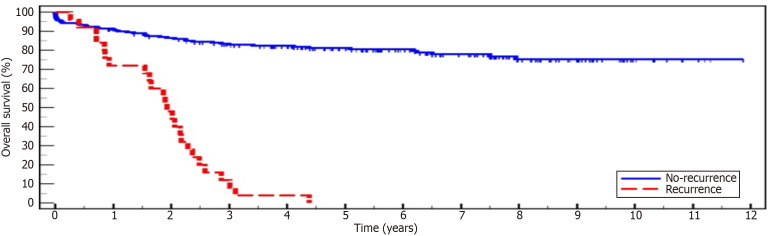
Recurrence of HCC developed in 25 patients (10.7%). The median time between LT and recurrence was 1.1 years (0.2-2.9). Twenty patients (80%) had recurrence at a single site, and 5 (20%) experienced multiple site recurrence. The most common site of recurrence was in the liver in 17 (68%) recipients, followed by extrahepatic abdominal recurrence in 9 recipients (36%), bone in 7 (28%), and lung in 5 (20%). There was a significant difference in survival between the recurrence and non-recurrence groups (P < 0.001), with none among the recurrence groups achieving 5 years of survival compared to 81.2% in the non-recurrence group (Table 2, Figure 1).
Table 2.
Survival with and without recurrence post-liver transplantation for hepatocellular carcinoma
|
|
Total No.
|
No. of dead
|
Mean (years)
|
1 year (%)
|
3 years (%)
|
5 years (%)
|
End of study (%)
|
Log rank
|
|
|
χ
2
|
P value
|
||||||||
| Non-recurrence | 209 | 44 | 9.59 | 90.9 | 83.0 | 81.2 | 75.3 | 96.401 | < 0.001 |
| Recurrence | 25 | 25 | 1.87 | 72.0 | 12.0 | 0.0 | 0.0 | ||
Figure 1.
Kaplan-Meier survival curve for recurrence vs non-recurrence groups.
Predictors of HCC recurrence post-LT
The following variables were significantly associated with HCC recurrence post-LT on univariate analyses: RETREAT score > 3, AFP at listing 100-500 and > 500, bridging, increased tumour size between imaging at the listing time and explant histology, increase in the size of viable tumour between listing and explant, presence of satellite nodules, micro- and macrovascular invasion on explant and poor differentiation of tumours (Table 3). AFP at listing > 500, poor differentiation, and the presence of tumour satellites on the explant histology were the only statistically significant variables associated with HCC recurrence following multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analysis of predictors of hepatocellular carcinoma recurrence following liver transplantation, n (%)
|
|
No-recurrence (n = 209)
|
Recurrence (n = 25)
|
Univariate
|
Multivariate
|
||
|
HR (LL-UL 95%CI)
|
P value
|
HR (LL-UL 95%CI)
|
P value
|
|||
| LT era | ||||||
| 2010-2013 | 64 (30.6) | 7 (28.0) | 2.146 (0.446-10.330) | 0.341 | ||
| 2014-2017 | 106 (50.7) | 16 (64.0) | 2.700 (0.621-11.745) | 0.185 | ||
| 2018-2020 | 39 (18.7) | 2 (8.0) | 1.000 | |||
| RETREAT score | ||||||
| ≤ 3 | 135 (68.2) | 8 (32.0) | 1.000 | 1.000 | ||
| > 3 | 63 (31.8) | 17 (68.0) | 4.403 (1.900-10.206) | 0.001 | 1.011 (0.315-3.238) | 0.986 |
| Donor type | ||||||
| DBD | 110 (52.6) | 16 (64.0) | 1.475 (0.652-3.337) | 0.351 | ||
| DCD | 99 (47.4) | 9 (36.0) | 1.000 | |||
| Listing to transplant interval (months) | ||||||
| < 1 | 45 (21.5) | 9 (36.0) | 1.000 | |||
| 1-3 | 82 (39.2) | 6 (24.0) | 0.414 (0.147-1.164) | 0.095 | ||
| > 3 | 82 (39.2) | 10 (40.0) | 0.692 (0.281-1.704) | 0.423 | ||
| Within Milan criteria | 199 (95.2) | 24 (96.0) | 1.359 (0.184-10.046) | 0.764 | ||
| Within United Kingdom criteria | 204 (97.6) | 25 (100.0) | 0.048 (7.721 × 10-7–2965.3) | 0.589 | ||
| Tumour largest size (cm) | ||||||
| ≤ 5 | 203 (98.5) | 23 (95.8) | 1.000 | |||
| > 5 | 3 (1.5) | 1 (4.2) | 2.380 (0.321-17.629) | 0.396 | ||
| Number of tumours | ||||||
| ≤ 3 | 200 (96.6) | 25 (100.0) | 1.000 | |||
| > 3 | 7 (3.4) | 0 (0.0) | 0.047 (0.000-519.138) | 0.520 | ||
| AFP-listing | ||||||
| < 100 | 185 (89.8) | 20 (80.0) | 1.000 | 1.000 | ||
| 100-500 | 18 (8.7) | 3 (12.0) | 1.468 (0.436-4.941) | 0.536 | 0.723 (0.201-2.596) | 0.619 |
| > 500 | 3 (1.5) | 2 (8.0) | 5.170 (1.207-22.138) | 0.027 | 8.958 (1.689-47.503) | 0.010 |
| Bridging | 117 (56.0) | 21 (84.0) | 3.707 (1.272-10.801) | 0.016 | ||
| The trend of size between listing and LT on imaging | ||||||
| Decrease | 94 (52.5) | 7 (29.2) | 1.000 | 1.000 | ||
| Same | 20 (11.2) | 2 (8.3) | 1.243 (0.258-5.986) | 0.786 | 0.851 (0.153-4.738) | 0.854 |
| Increase | 65 (36.3) | 15 (62.5) | 3.097 (1.263-7.598) | 0.014 | 1.180 (0.392-3.554) | 0.768 |
| The trend of AFP between listing and LT | ||||||
| Decrease | 57 (29.1) | 4 (16.0) | 1.000 | |||
| Same | 94 (48.0) | 11 (44.0) | 1.697 (0.540-5.329) | 0.365 | ||
| Increase | 45 (23.0) | 10 (40.0) | 2.912 (0.913-9.284) | 0.071 | ||
| Size change between explant viable largest tumour and listing largest tumour | ||||||
| Decrease | 119 (57.5) | 7 (28.0) | 1.000 | |||
| Same | 22 (10.6) | 3 (12.0) | 2.090 (0.540-8.082) | 0.285 | ||
| Increase | 66 (31.9) | 15 (60.0) | 3.842 (1.566-9.424) | 0.003 | ||
| Explant viable tumour number | ||||||
| ≤ 3 | 152 (84.4) | 18 (75.0) | 1.000 | |||
| > 3 | 28 (15.6) | 6 (25.0) | 1.889 (0.750-4.759) | 0.177 | ||
| Microvascular invasion | 98 (46.9) | 21 (84.0) | 6.087 (2.089-17.735) | 0.001 | 3.014 (0.697-13.032) | 0.140 |
| Macrovascular invasion | 8 (3.8) | 6 (24.0) | 5.320 (2.121-13.348) | < 0.001 | 2.888 (0.907-9.198) | 0.073 |
| Satellite nodules | 11 (5.3) | 6 (24.0) | 4.728 (1.887-11.845) | 0.001 | 3.673 (1.204-11.210) | 0.022 |
| Tumor grade | ||||||
| Complete necrosis | 31 (15.0) | 1 (4.0) | 0.235 (0.032-1.736) | 0.156 | ||
| Well-differentiated | 48 (23.2) | 2 (8.0) | 0.311 (0.073-1.320) | 0.113 | ||
| Moderately differentiated | 112 (54.1) | 11 (44.0) | 0.673 (0.305-1.481) | 0.325 | ||
| Poorly differentiated | 16 (7.7) | 11 (44.0) | 7.805 (3.536-17.230) | < 0.001 | 3.727 (1.428-9.730) | 0.007 |
AFP: Alpha fetoprotein; DCD: Donation after circulatory death; DBD: Donation after brain stem death; RETREAT: Risk Estimation of Tumor Recurrence After Transplant; LT: Liver transplantation.
SIMAP500 risk score
SIMAP500 score risk score is proposed based on the factors that were significantly associated with HCC recurrence on univariate and multivariate analysis of our cohort. The score for each factor is weighed based on the B coefficient (rounded to the nearest integer) (Table 4).
Table 4.
SIMAP500 score
|
Factor score
|
|
| Satellite nodules | 2 |
| Increased viable tumour size from listing to LT | 1 |
| Microvascular invasion | 3 |
| AFP at listing > 500 | 4 |
| Poorly differentiated HCC grade | 3 |
LT: Liver transplantation; AFP: Alpha fetoprotein; HCC: Hepatocellular carcinoma.
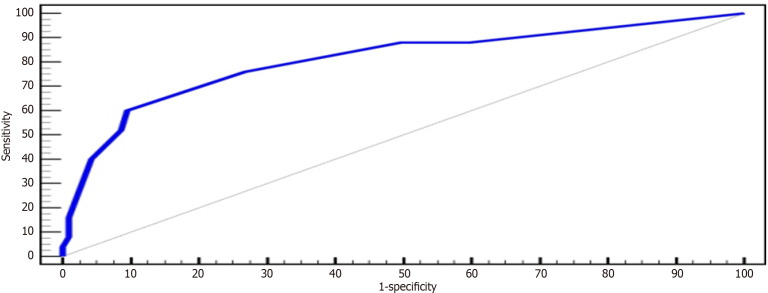
At a cut-off point score of 5 (using the Youden index), the SIMAP500 scoring system has a positive predictive value of 42.9% and a negative predictive value of 95%. The area under the curve (AUC) of the proposed new score is 0.807 (Figure 2) and has outperformed the RETREAT score (AUC = 0.703) in our cohort, indicating its superiority. Unlike RETREAT scoring system, the novel SIMAP500 scoring system consider the increased tumour size on the waiting list, presence of satellite nodules and poorly differentiated HCC on explant histology (Table 5). In addition to predicting risk of recurrence, SIMAP500 indicates the time to HCC recurrence (Table 6), the higher the score, the shorter the time to recurrence.
Figure 2.
Receiver operating characteristic curve for SIMAP500 scoring system. ROC: Receiver operating characteristic curve.
Table 5.
Comparison between SIMAP500 and RETREAT scoring systems
|
Characteristic
|
SIMAP500 score
|
RETREAT score
|
| Consider the presence of satellite nodules on explant histology | Yes | No |
| Consider the tumour differentiation grade on explant histology | Yes | No |
| AFP capture | At listing with a cutoff point of 500 | At LT with AFP divided into four intervals (very wide range between 100-999 receiving 2 points) |
| Consider the increase in the size of the tumour while on the waiting list | Yes | No |
| Consider microvascular invasion on explant histology | Yes | Yes |
| AUC | 0.807 | 0.703 |
RETREAT: Risk Estimation of Tumor Recurrence After Transplant; SIMAP500: Satellite nodules, Increase in size, Microvascular invasion, AFP > 500, Poor differentiation; LT: Liver transplantation; AFP: Alpha fetoprotein; AUC: Area under the curve.
Table 6.
Relation between SIMAP500 score and recurrence rate, time to recurrence
|
SIMAP500 score
|
Recurrence No. (%)
|
Median time between LT and recurrence (years)
|
| 0 | 3 (12.0) | 1.44 (1.16-2.18) |
| 1-3 | 3 (12.0) | 1.68 (0.90-1.78) |
| 4 | 4 (16.0) | 1.56 (1.32-2.19) |
| 5 | 2 (8.0) | 0.94 (0.74-1.14) |
| ≥ 6 | 13 (52.0) | 0.70 (0.21-2.95) |
SIMAP500: Satellite nodules, increase in size, microvascular invasion, AFP > 500, poor differentiation; LT: Liver transplantation.
DISCUSSION
The purpose of the current study is to explore the frequency of HCC recurrence following LT, identify potential predictive factors associated with recurrence, and propose a novel risk score that can help to predict the probability of HCC recurrence post-LT. The novel SIMAP500 score captures AFP at a single point of listing, counts the size increase during the waiting period for suitable grafts, and incorporates the explant histology findings. With an AUC of 0.8, the new suggested score has an excellent potential of identifying recipients at higher risk of HCC recurrence. Such risk stratification will enable us to tailor surveillance strategies to patients at higher risk of disease recurrence.
The present report corresponds to data from one of the largest LT centres in Europe where a routine radiological post-LT surveillance program for HCC candidates is yet to be implemented. The overall post-LT recurrence rate in this cohort was 10.7%, similar to reported HCC recurrence rates of 6%-18% in the literature[7,10-12]. The wide disparity of recurrence rates might reflect variations in inclusion criteria, and pre-operative and post-operative surveillance protocols in the reported centres. The median time between LT and HCC recurrence in the current study was 1.15 years, perhaps sooner than the 2-3 years reported in the published literature[23,24].
Poor differentiation of tumours, high AFP at listing, and the presence of satellite lesions in explants were the other factors significantly associated with HCC recurrence. This observation is also consistent with previous literature[25-28], which raises the question of whether pre-LT biopsy needs performing routinely to identify those with poor differentiation and if it should qualify for patient delisting. However, this is unlikely to be clinically applicable as pre-LT biopsy results have shown a considerable discrepancy with the explant histology[29,30] and the risks of tumour seeding and bleeding[31].
Given the limited donor pool[32], tools to predict the high risk of HCC recurrence are essential. Patients at increased risk of recurrence could then either be managed by treatments alternative to LT, reassessed after a period of observation following bridging therapy, or counselled about their higher chances of post-LT recurrence. The currently available pre-LT HCC recurrence risk calculation tools are radiographic tumour burden, AFP levels, and tumour biopsy features. The majority of the aforementioned factors contributing to the risk of recurrence can only be diagnosed postoperatively through the evaluation of parameters determined in pathomorphological examination. Consequently, their applicability in determining the suitability of patients for transplant procedures is constrained[33].
The discrepancy between radiological assessment and explant histology has been reported previously[34,35]. Radiological evaluation of HCC tends to be under-staged, compared to explant histology, in up to 25%-30% of recipients[36-39]. In this study, 95.3% of recipients were within MC on pretransplant radiological imaging. However, explant analyses showed 24.8% were outside the MC. Luca et al[40] retrospectively examined 125 cirrhotic candidates imaged by multidetector computed tomography (MDCT) before LT; 117 out of 131 HCC identified in the explanted livers were detected on MDCT, with an overall sensitivity of 89%. The missed HCC lesions were mainly less than 20 mm in diameter. MDCT reached a precise staging of HCC in only 46% of the candidates, with 2% overestimated and 52% underestimated compared to explant histology. This area requires future research where radiomics and related machine learning models may enable improved pre-LT tumour characterization and more accurate radiological prognostic assessment.
Cuadrado et al[41] conducted a retrospective review at a tertiary centre in Spain between January 2010 and December 2019. Utilizing data from 66 LTs for HCC, the study aimed to ascertain predictors of HCC recurrence. Notably, the study suggested a significant correlation between obesity and HCC recurrence, a finding of particular significance given the escalating prevalence of metabolic-associated fatty liver disease and its association with obesity. However, the study failed to validate the association with other indices such as post-Moral, Combo-Moral, or RETREAT, either due to their lack of statistical significance in the univariable analysis or their exclusion from the multivariable model to mitigate collinearity and redundancies. It is worth noting that the limited sample size, absence of high-risk cases, and the consideration of only viable nodules in the explant due to the use of bridging therapy may have impacted these results.
AFP was used as a valuable pre-LT biological marker and has been incorporated in most predictive models of post-LT HCC recurrence. Elevated AFP levels can serve as a prognostic indicator and provide valuable insight into the biological aggressiveness of the tumor, particularly during vascular microinvasion[33]. Exceptionally high AFP levels are associated with a higher risk of HCC recurrence as shown in this multivariate analysis. However, lower AFP levels are only partially innocent as candidates transplanted with AFP below 20 could still experience poor post-LT outcomes[5,42]. In our study, 10 recipients (40%) from the recurrence group had AFP < 20 pre-LT. It should also be noted that factors other than HCC occasionally contribute to the raised AFP levels as in viral hepatitis.
Different post-transplant prognostic models have used various combinations of tumour burden (tumour number and size), biomarkers (AFP and neutrophil/lymphocyte ratio), and explant findings (degree of differentiation and microvascular invasion) to quantify the risk of HCC recurrence post-LT[17-20]. A systematic review of risk prediction models of HCC recurrence conducted by Al-Ameri et al[43] has recommended utilizing the RETREAT score to define surveillance intervals and stratify recurrence risk. The current study also found the RETREAT score to have a reasonable predictive ability with AUC = 0.73 while the SIMAP500 scoring system has outperformed RETREAT with AUC = 0.83.
SIMAP500 also demonstrated superior performance compared to the published literature on REcurrent Liver cAncer Prediction ScorE (RELAPSE). The RELAPSE model was developed based on the analysis of clinical, radiologic, and pathologic data from the United States Multicentre HCC Transplant Consortium and validated in the European Hepatocellular Cancer Liver Transplant study group. The external validation of RELAPSE consistently demonstrated 2- and 5-year recurrence risk discrimination, with AUCs of 0.77 and 0.75, respectively. However, these values remain lower than the AUC of 0.83 for SIMAP500 in our cohort, which is slated for external validation in the future[44].
A higher SIMAP500 score was associated with an increased risk of recurrence and early recurrence, with candidates scoring 5 or more having a median time to recurrence of less than one year from LT. According to UNOS data[45], early recurrence within 1 year was associated with an overall survival of 30%, vs 60% if recurrence occurred after the first year[46].
No consensus exists on the ideal surveillance protocol for HCC recurrence following LT. Some centres like ours do not have routine surveillance programs, while others follow a structured program. Mehta et al[20,45] proposed a potential screening programme based on the RETREAT score: RETREAT score 0 - no screening (based on estimated recurrence hazard < 3%), score 1-3, screening every six months for two years; score of 4, surveillance extended to 5 years with a yearly scan for additional 3 years; score greater than 5, 3 to 4 monthly scan for two years followed by every six months until year 5. However, such a surveillance protocol would be resource intensive. The majority of experts and investigators recommend undergoing CT or MRI scans with contrast for the abdominal cavity and chest, as well as AFP testing every 3-6 months for 2-3 years. After this period, the interval between tests is extended to 6-12 months. When recurrence is diagnosed, bone scintigraphy is usually performed[12,46-50].
Given the limited resources in many healthcare delivery systems and the increased risk of recurrence in the first two years post-LT, we recommend that surveillance should cover at least the first 2-3 years post-LT.
Patients with recurrent HCC post-LT have a poorer prognosis but can increasingly be considered for treatment options that include surgical resection, locoregional therapy, and systemic therapy, It is imperative to note that undergoing multiple liver transplantations is not recommended[33]. Surgical resection of isolated HCC recurrence was reported as a forecaster of long-term survival[51-53], with 4-year survival rates significantly higher in the patients who underwent surgical resection of recurrent HCC compared to those who did not[54]. Caution should be exercised when analysing this data as most candidates who qualify for resection are selected based on limited disease recurrence.
Goldaracena et al[47] confirmed previous findings by Sapisochin et al[46], identifying three crucial factors for poor prognosis in liver transplant patients with hepatocellular carcinoma: the inability to undergo radical treatment (resection or thermoablation), elevated AFP levels (≥ 100 ng/mL) at the time of diagnosis, and early recurrence (< 12 months after liver transplantation). Patients who do not meet any of these criteria (with potential for tumor resection, low AFP levels, or late recurrence) are classified as low-risk. Patients meeting one or two criteria fall into the average-risk group, while those meeting all three criteria are classified as high-risk. The one-year, three-year, and five-year survival rates, based on the risk group, were 73% vs 55% vs 17%, 41% vs 19% vs 0%, and 34% vs 6% vs 0%, respectively[47].
Limited data are available regarding locoregional and chemotherapy, and that is undoubtedly a subject that is worth further exploration in the future. Newer systemic therapies are also emerging and will need further investigation to assess if they could improve outcomes for recurrent HCC. Some of the recent patients in our cohort received systemic treatment, but it is early to judge its efficacy.
Strategies to mitigate the high risk of recurrence include immunosuppression modification and adjuvant systemic therapy. Relying on the mammalian target of rapamycin inhibitors, given their antiproliferative properties against HCC, and reducing calcineurin inhibitors have been proposed as an immunosuppression strategy in high-recurrence risk group[42]. There is no current evidence to support the utilization of adjuvant systemic therapy to lessen HCC recurrence risk. Still, there is some promising data regarding utilizing agents such as Licartin as adjuvant chemotherapy in patients with advanced HCC[43]. High-quality studies are required to test its effectiveness in reducing the risk of recurrence post-LT.
The current study proposes a minimum surveillance duration in high-risk patients, allowing further prospective studies on larger multicentre cohorts to identify and select patients with recurrent disease for various treatment modalities so that the cost-effectiveness and clinical benefit of routine surveillance could be evaluated.
The present study encompasses data from one of the largest centres in the United Kingdom with a prospectively maintained database. Nonetheless, there are few limitations. The first is the retrospective nature of the study. Second is the study duration of 10 years over which the pre-transplant imaging modalities, the graft choices and post-LT care have evolved. The score needs external validation. Lastly, it would be more beneficial to risk stratify patients in relation to recurrence before LT so the organs could be utilised more efficiently among those with favourable tumour biology, but unfortunately, tools to forecast recurrence pre-LT are limited, including SIMAP500, and exploring these factors in greater detail through future research could yield valuable insights.
CONCLUSION
The SIMAP500 score is a useful risk score that can help stratify the patients at risk of recurrence following LT for HCC, and surveillance programmes could be considered for the high-risk group.
Footnotes
Institutional review board statement: The study has been approved and registered on the clinical audit registration and management system of the trust under the code CARMS-18313.
Informed consent statement: Participants' consent was not obtained but the presented data are anonymized and risk of identification is low.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade B, Grade C, Grade E
Novelty: Grade B, Grade C, Grade D
Creativity or Innovation: Grade B, Grade C, Grade D
Scientific Significance: Grade B, Grade B, Grade D
P-Reviewer: Ergenç M; Jiang L; Manrai M S-Editor: Gong ZM L-Editor: A P-Editor: Wang WB
Contributor Information
Amr Alnagar, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Nekisa Zakeri, Centre for Liver Research, Institute of Biomedical Research, Birmingham B15 2TT, United Kingdom.
Konstantinos Koilias, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Rosemary E Faulkes, Department of Hepatology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Rachel Brown, Department of Pathology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
Owen Cain, Department of Pathology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2GW, United Kingdom.
M Thamara P R Perera, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Keith J Roberts, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Rebeca Sanabria-Mateos, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
David C Bartlett, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Yuk Ting Ma, Department of Oncology, Queen Elizabeth Hospital, University Hospitals of Birmingham, Birmingham B15 2GW, United Kingdom.
Shivan Sivakumar, Department of Oncology, Queen Elizabeth Hospital, University Hospitals of Birmingham, Birmingham B15 2GW, United Kingdom.
Shishir Shetty, Centre for Liver Research, Institute of Biomedical Research, Birmingham B15 2TT, United Kingdom.
Tahir Shah, Department of Hepatology, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom.
Bobby V M Dasari, Department of HBP and Liver Transplantation Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2GW, United Kingdom. b.dasari@bham.ac.uk.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author.
References
- 1.Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:179–187. doi: 10.1080/17474124.2019.1549989. [DOI] [PubMed] [Google Scholar]
- 2.Ivanics T, Shwaartz C, Claasen MPAW, Patel MS, Yoon P, Raschzok N, Wallace D, Muaddi H, Murillo Perez CF, Hansen BE, Selzner N, Sapisochin G. Trends in indications and outcomes of liver transplantation in Canada: A multicenter retrospective study. Transpl Int. 2021;34:1444–1454. doi: 10.1111/tri.13903. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Larson JJ, Watt KD, Allen AM, Wiesner RH, Gores GJ, Roberts LR, Heimbach JA, Leise MD. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol. 2017;15:767–775.e3. doi: 10.1016/j.cgh.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutcliffe JR, McKenna HP. Establishing the credibility of qualitative research findings: the plot thickens. J Adv Nurs. 1999;30:374–380. doi: 10.1046/j.1365-2648.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Burra P, Ghobrial M, Hibi T, Metselaar H, Sapisochin G, Bhoori S, Kwan Man N, Mas V, Ohira M, Sangro B, van der Laan LJW. Posttransplant Management of Recipients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Working Group Report From the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1143–1149. doi: 10.1097/TP.0000000000003196. [DOI] [PubMed] [Google Scholar]
- 8.Lingiah VA, Niazi M, Olivo R, Paterno F, Guarrera JV, Pyrsopoulos NT. Liver Transplantation Beyond Milan Criteria. J Clin Transl Hepatol. 2020;8:69–75. doi: 10.14218/JCTH.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947–956. doi: 10.1016/S1470-2045(20)30224-2. [DOI] [PubMed] [Google Scholar]
- 10.Scortegagna E Jr, Karam AR, Sioshansi S, Bozorgzadeh A, Barry C, Hussain S. Hepatocellular carcinoma recurrence pattern following liver transplantation and a suggested surveillance algorithm. Clin Imaging. 2016;40:1131–1134. doi: 10.1016/j.clinimag.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–217. doi: 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- 12.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Lee DD, Sapisochin G, Mehta N, Gorgen A, Musto KR, Hajda H, Yao FY, Hodge DO, Carter RE, Harnois DM. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation. 2020;104:2105–2112. doi: 10.1097/TP.0000000000003117. [DOI] [PubMed] [Google Scholar]
- 15.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, Roberts J, Reich DJ, Schwartz ME, Mieles L, Lee FT, Florman S, Yao F, Harper A, Edwards E, Freeman R, Lake J. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 16.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS Jr. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557–564. doi: 10.1097/SLA.0000000000001966. [DOI] [PubMed] [Google Scholar]
- 19.Decaens T, Roudot-Thoraval F, Badran H, Wolf P, Durand F, Adam R, Boillot O, Vanlemmens C, Gugenheim J, Dharancy S, Bernard PH, Boudjema K, Calmus Y, Hardwigsen J, Ducerf C, Pageaux GP, Hilleret MN, Chazouillères O, Cherqui D, Mallat A, Duvoux C. Impact of tumour differentiation to select patients before liver transplantation for hepatocellular carcinoma. Liver Int. 2011;31:792–801. doi: 10.1111/j.1478-3231.2010.02425.x. [DOI] [PubMed] [Google Scholar]
- 20.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy SHS, Mehta N, Dodge JL, Hakeem AR, Khorsandi SE, Jassem W, Vilca-Melendez H, Cortes-Cerisuelo M, Srinivasan P, Prachalias A, Heneghan MA, Aluvihare V, Suddle A, Miquel R, Rela M, Heaton ND, Menon KV. Liver transplantation for HCC: validation of prognostic power of the RETREAT score for recurrence in a UK cohort. HPB (Oxford) 2022;24:596–605. doi: 10.1016/j.hpb.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Millson C, Considine A, Cramp ME, Holt A, Hubscher S, Hutchinson J, Jones K, Leithead J, Masson S, Menon K, Mirza D, Neuberger J, Prasad R, Pratt A, Prentice W, Shepherd L, Simpson K, Thorburn D, Westbrook R, Tripathi D. Adult liver transplantation: A UK clinical guideline - part 1: pre-operation. Frontline Gastroenterol. 2020;11:375–384. doi: 10.1136/flgastro-2019-101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croome KP, Lee DD, Burns JM, Musto K, Paz D, Nguyen JH, Perry DK, Harnois DM, Taner CB. The Use of Donation After Cardiac Death Allografts Does Not Increase Recurrence of Hepatocellular Carcinoma. Am J Transplant. 2015;15:2704–2711. doi: 10.1111/ajt.13306. [DOI] [PubMed] [Google Scholar]
- 24.Azoulay D, Audureau E, Bhangui P, Belghiti J, Boillot O, Andreani P, Castaing D, Cherqui D, Irtan S, Calmus Y, Chazouillères O, Soubrane O, Luciani A, Feray C. Living or Brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma: A Multicenter, Western, Intent-to-treat Cohort Study. Ann Surg. 2017;266:1035–1044. doi: 10.1097/SLA.0000000000001986. [DOI] [PubMed] [Google Scholar]
- 25.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 26.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. doi: 10.1097/00000658-199810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, Tzakis AG. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30; discussion 31. [PubMed] [Google Scholar]
- 28.Grasso A, Stigliano R, Morisco F, Martines H, Quaglia A, Dhillon AP, Patch D, Davidson BR, Rolles K, Burroughs AK. Liver transplantation and recurrent hepatocellular carcinoma: predictive value of nodule size in a retrospective and explant study. Transplantation. 2006;81:1532–1541. doi: 10.1097/01.tp.0000209641.88912.15. [DOI] [PubMed] [Google Scholar]
- 29.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, Abdalla EK. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 30.Court CM, Harlander-Locke MP, Markovic D, French SW, Naini BV, Lu DS, Raman SS, Kaldas FM, Zarrinpar A, Farmer DG, Finn RS, Sadeghi S, Tomlinson JS, Busuttil RW, Agopian VG. Determination of hepatocellular carcinoma grade by needle biopsy is unreliable for liver transplant candidate selection. Liver Transpl. 2017;23:1123–1132. doi: 10.1002/lt.24811. [DOI] [PubMed] [Google Scholar]
- 31.Cabibbo G, Craxì A. Needle track seeding following percutaneous procedures for hepatocellular carcinoma. World J Hepatol. 2009;1:62–66. doi: 10.4254/wjh.v1.i1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alnagar A, Daradka K, Kyrana E, Mtegha M, Palaniswamy K, Rajwal S, Mulla J, O'meara M, Karam M, Shawky A, Hakeem AR, Upasani V, Dhakshinamoorthy V, Prasad R, Attia M. Predictors of patient and graft survival following pediatric liver transplantation: Long-term analysis of more than 300 cases from single centre. Pediatr Transplant. 2022;26:e14139. doi: 10.1111/petr.14139. [DOI] [PubMed] [Google Scholar]
- 33.Straś WA, Wasiak D, Łągiewska B, Tronina O, Hreńczuk M, Gotlib J, Lisik W, Małkowski P. Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann Transplant. 2022;27:e934924. doi: 10.12659/AOT.934924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman RB, Mithoefer A, Ruthazer R, Nguyen K, Schore A, Harper A, Edwards E. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 35.Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Misdiagnosis of hepatocellular carcinoma in patients receiving no local-regional therapy prior to liver transplant: An analysis of the Organ Procurement and Transplantation Network explant pathology form. Clin Transplant. 2017;31 doi: 10.1111/ctr.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper AM, Edwards E, Washburn WK, Heimbach J. An early look at the Organ Procurement and Transplantation Network explant pathology form data. Liver Transpl. 2016;22:757–764. doi: 10.1002/lt.24441. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz ME. Liver transplantation for hepatocellular carcinoma: the best treatment, but for which patient? Hepatology. 1996;24:1539–1541. doi: 10.1002/hep.510240640. [DOI] [PubMed] [Google Scholar]
- 39.Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, Rosen M, Soulen M, Shaked A, Reddy KR, Olthoff KM. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911–918. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 40.Luca A, Caruso S, Milazzo M, Mamone G, Marrone G, Miraglia R, Maruzzelli L, Carollo V, Minervini MI, Vizzini G, Gruttadauria S, Gridelli B. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: prevalence of radiological vascular patterns and histological correlation with liver explants. Eur Radiol. 2010;20:898–907. doi: 10.1007/s00330-009-1622-0. [DOI] [PubMed] [Google Scholar]
- 41.Cuadrado A, Fortea JI, Rodríguez-Lope C, Puente Á, Fernández-Vilchez V, Echavarria VJ, Castillo Suescun FJ, Fernández R, Echeverri JA, Achalandabaso M, Toledo E, Pellón R, Rodríguez Sanjuan JC, Crespo J, Fábrega E. Risk of Recurrence of Hepatocarcinoma after Liver Transplantation: Performance of Recurrence Predictive Models in a Cohort of Transplant Patients. J Clin Med. 2023;12 doi: 10.3390/jcm12175457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 43.Al-Ameri AAM, Wei X, Wen X, Wei Q, Guo H, Zheng S, Xu X. Systematic review: risk prediction models for recurrence of hepatocellular carcinoma after liver transplantation. Transpl Int. 2020;33:697–712. doi: 10.1111/tri.13585. [DOI] [PubMed] [Google Scholar]
- 44.Tran BV, Moris D, Markovic D, Zaribafzadeh H, Henao R, Lai Q, Florman SS, Tabrizian P, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, Taner CB, Hoteit M, Levine MH, Cillo U, Vitale A, Verna EC, Halazun KJ, Tevar AD, Humar A, Chapman WC, Vachharajani N, Aucejo F, Lerut J, Ciccarelli O, Nguyen MH, Melcher ML, Viveiros A, Schaefer B, Hoppe-Lotichius M, Mittler J, Nydam TL, Markmann JF, Rossi M, Mobley C, Ghobrial M, Langnas AN, Carney CA, Berumen J, Schnickel GT, Sudan DL, Hong JC, Rana A, Jones CM, Fishbein TM, Busuttil RW, Barbas AS, Agopian VG. Development and validation of a REcurrent Liver cAncer Prediction ScorE (RELAPSE) following liver transplantation in patients with hepatocellular carcinoma: Analysis of the US Multicenter HCC Transplant Consortium. Liver Transpl. 2023;29:683–697. doi: 10.1097/LVT.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 45.Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant. 2018;18:1206–1213. doi: 10.1111/ajt.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lázaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 47.Goldaracena N, Mehta N, Scalera I, Sposito C, Atenafu EG, Yao FY, Muiesan P, Mazzaferro V, Sapisochin G. Multicenter validation of a score to predict prognosis after the development of HCC recurrence following liver transplantation. HPB (Oxford) 2019;21:731–738. doi: 10.1016/j.hpb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal A, Te HS, Verna EC, Desai AP. A National Survey of Hepatocellular Carcinoma Surveillance Practices Following Liver Transplantation. Transplant Direct. 2021;7:e638. doi: 10.1097/TXD.0000000000001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verna EC, Patel YA, Aggarwal A, Desai AP, Frenette C, Pillai AA, Salgia R, Seetharam A, Sharma P, Sherman C, Tsoulfas G, Yao FY. Liver transplantation for hepatocellular carcinoma: Management after the transplant. Am J Transplant. 2020;20:333–347. doi: 10.1111/ajt.15697. [DOI] [PubMed] [Google Scholar]
- 50.Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G, Kneteman NM. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342–347. doi: 10.1007/s00534-012-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440–447. doi: 10.1002/lt.24742. [DOI] [PubMed] [Google Scholar]
- 53.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, Montalto F, Coppa J, Mazzaferro V. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29–34. doi: 10.1007/pl00009947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author.