Abstract
Species of Pseudocercospora are commonly associated with leaf and fruit spots on diverse plant hosts in sub-tropical and tropical regions. Pseudocercospora spp. have mycosphaerella-like sexual morphs, but represent a distinct genus in Mycosphaerellaceae (Mycosphaerellales, Dothideomycetes). The present study adds a further 29 novel species of Pseudocercospora from 413 host species representing 297 host genera occurring in 60 countries and designates four epitypes and one lectotype for established names. This study recognises 329 species names, with an additional 69 phylogenetic lineages remaining unnamed due to difficulty in being able to unambiguously apply existing names to those lineages. To help elucidate the taxonomy of these species, a phylogenetic tree was generated from multi-locus DNA sequence data of the internal transcribed spacers and intervening 5.8S nuclear nrRNA gene (ITS), partial actin (actA), and partial translation elongation factor 1-alpha (tef1), as well as the partial DNA-directed RNA polymerase II second largest subunit (rpb2) gene sequences. Novel species described in this study include those from various countries as follows: Australia, Ps. acaciicola from leaf spots on Acacia sp., Ps. anopter from leaf spots on Anopterus glandulosus, Ps. asplenii from leaf spots on Asplenium dimorphum, Ps. australiensis from leaf spots on Eucalyptus gunnii, Ps. badjensis from leaf spots on Eucalyptus badjensis, Ps. erythrophloeicola from leaf spots on Erythrophleum chlorostachys, Ps. grevilleae from leaf spots on Grevillea sp., Ps. lophostemonigena from leaf spots on Lophostemon confertus, Ps. lophostemonis from leaf spots on Lophostemon lactifluus, Ps. paramacadamiae from leaf spots on Macadamia integrifolia, Ps. persooniae from leaf spots on Persoonia sp., Ps. pultenaeae from leaf spots on Pultenaea daphnoides, Ps. tristaniopsidis from leaf spots on Tristaniopsis collina, Ps. victoriae from leaf spots on Eucalyptus globoidea. Brazil, Ps. musigena from leaf spots on Musa sp. China, Ps. lonicerae-japonicae from leaf spots on Lonicera japonica, Ps. rubigena leaf spots on Rubus sp. France (Réunion), Ps. wingfieldii from leaf spots on Acacia heterophylla. Malaysia, Ps. musarum from leaf spots on Musa sp. Netherlands, Ps. rhododendri from leaf spots on Rhododendron sp. South Africa, Ps. balanitis from leaf spots on Balanites sp., Ps. dovyalidicola from leaf spots on Dovyalis zeyheri, Ps. encephalarticola from leaf spots on Encephalartos sp. South Korea, Ps. grewiana from leaf spots on Grewia biloba, Ps. parakaki from leaf spots on Diospyros kaki, Ps. pseudocydoniae from leaf spots on Chaenomeles lagenaria, Ps. paracydoniae from leaf spots on Chaenomeles speciosa. Thailand, Ps. acerigena from leaf spots on Acer sp., Ps. tectonigena from leaf spots on Tectona grandis. Epitypes are designated for Cercospora bonjeaneae-rectae, Cercospora halleriae, Ps. eucleae, and an epitype as well as a lectotype for Ps. macadamiae. Results obtained in the present study contribute to a better understanding of the host specificity and distribution in Pseudocercospora spp., many of which represent important pathogens of food or fibre crops, or organisms of quarantine concern.
Citation: Groenewald JZ, Chen YY, Zhang Y, Roux J, Shin H-D, Shivas RG, Summerell BA, Braun U, Alfenas AC, Ujat AH, Nakashima C, Crous PW (2024). Species diversity in Pseudocercospora. Fungal Systematics and Evolution 13: 29–89. doi: 10.3114/fuse.2024.13.03
Keywords: Multi-gene phylogeny Mycosphaerellaceae new taxa, plant pathogen taxonomy
INTRODUCTION
In his monograph, Chupp (1954) placed all cercosporoid species in the genus Cercospora that he treated in a very wide sense (Mycosphaerellaceae, Mycosphaerellales, Dothideomycetes). Subsequent morphological studies have shown however that several cercosporoid genera can be distinguished based on a combination of characteristics such as conidial pigmentation, and the structure of conidiogenous loci (scars) and hila ( Deighton 1976, 1979, 1983, 1987, 1990, Pons & Sutton 1988, Braun 1995, Crous & Braun 2003). The separation of these genera was further corroborated in molecular studies ( Crous et al. 2013a, Nakashima et al. 2016, Chen et al. 2022), which led to them being widely accepted among mycologists and plant pathologists ( Braun et al. 2013a, 2014, 2015a, b, 2016). With the end of dual nomenclature, several names were again reduced to synonymy, such as Mycosphaerella under Ramularia ( Videira et al. 2015, 2016), while genera with mycosphaerella-like sexual morphs, such as Passalora, Pseudocercospora and Zasmidium, were recognised as distinct ( Videira et al. 2017).
With several hundred species associated with leaf spot diseases on a wide global host range, Pseudocercospora (Ps.) is a well-known genus of cercosporoid fungi that contains numerous important plant pathogenic species. Well-known pathogens include Ps. angolensis causing fruit and leaf spot disease on Citrus ( Pretorius et al. 2003), Ps. pini-densiflorae causing brown spot needle blight of Pinus ( Quaedvlieg et al. 2012, Braun et al. 2013a), Ps. griseola causing angular leaf spot of Phaseolus ( Crous et al. 2006), Ps. ulei causing South American leaf blight of Hevea spp. ( Hora Júnior et al. 2014), and Ps. fijiensis, causing Black Sigatoka disease on Musa spp. ( Churchill 2011, Chang et al. 2016, Crous et al. 2021).
To place these taxa into a broader evolutionary context, Crous et al. (2013a) published a phylogenetic analysis of 146 Pseudocercospora spp. based on multi-locus sequence data of the nuclear ribosomal RNA (nrRNA) gene (LSU; 28S), the internal transcribed spacers and intervening 5.8S nuclear nrRNA gene (ITS), partial actin (actA), and translation elongation factor 1-alpha (tef1) gene regions. To further improve the backbone resolution of the genus, Nakashima et al. (2016) added data of the partial DNA-directed RNA polymerase II second largest subunit (rpb2) gene, which proved effective in delineating species and genera in the Mycosphaerellaceae ( Videira et al. 2015). Based on these results most species of Pseudocercospora were shown to be highly host or genus specific ( Bakhshi et al. 2014, Shivas et al. 2015, Guatimosim et al. 2016, Silva et al. 2016), while the genus Pseudocercospora was resolved as monophyletic ( Nakashima et al. 2016). Furthermore, these studies also concluded that Pseudocercospora spp. on the same host species or family that are morphologically similar, frequently represented distinct species on different continents, and that European or American names could not readily be applied to taxa from other continents. These previous studies were however hampered by the non-availability of sequence data from (ex-)type material or at least authentic material, a situation that has not greatly improved in recent years.
Given the huge species diversity within Pseudocercospora, the present study further expands the generic phylogeny generated by Crous et al. (2013a) and Nakashima et al. (2016), and treats 329 species of Pseudocercospora, introducing 29 novel species of Pseudocercospora from 413 host species representing 297 host genera occurring in 60 countries, and designates four epitypes and one lectotype. A further 69 phylogenetic lineages remain unnamed due to difficulty in being able to unambiguously apply existing names to those lineages, and an existing name is tentatively applied to 14 lineages pending future neo- or epitypification.
MATERIALS AND METHODS
Isolates
Isolations were made from conidiophores with conidia on leaves, twigs, and fruits (Suppl. Table S1). Some samples were incubated in moist chambers for 2–3 d to enhance sporulation before single conidial colonies were established on 2 % malt extract agar (MEA) ( Crous et al. 2019b). Leaf spots bearing ascomata were soaked in water for approximately 2 h, after which they were attached to the inner surface of Petri dish lids over plates containing MEA for 24–48 h ( Crous et al. 1991). Colonies were sub-cultured onto synthetic nutrient-poor agar (SNA), potato-dextrose agar (PDA), oatmeal agar (OA), and MEA ( Crous et al. 2019b), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Reference strains and specimens of the studied fungi are maintained in the culture collection and fungarium (CBS and CBS H) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands; the culture collection (MUCC) and the fungarium (TSU-MUMH) of the Phytopathology Lab., Mie University, Tsu, Mie, Japan; the research center of genetic resources (MAFF), National agriculture and food research organization, Tsukuba, Japan; and the fungarium (TFM), Forestry and Forest products research institute, Tsukuba, Japan.
DNA extraction, PCR amplification and sequencing
Fungal mycelium (Suppl. Table S1) was scraped from the surface of actively growing agar cultures with a sterile scalpel and the genomic DNA was extracted using either a DNeasy ® UltraClean® Microbial DNA isolation kit (Qiagen, Hilden, Germany) or the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA), following the manufacturer’s instructions. Four loci were amplified and sequenced as described in Nakashima et al. (2016), namely the internal transcribed spacers and intervening 5.8S nuclear nrRNA (ITS), the partial actin (actA), translation elongation factor 1-alpha (tef1), and DNA-directed RNA polymerase II second largest subunit (rpb2) gene regions. The resulting amplicons were sequenced in both directions using the respective PCR primers and the BigDyeTM Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems, Life Technologies, Carlsbad, CA, USA). DNA sequencing amplicons were subsequently purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA) and analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences were analysed and consensus sequences were computed using Geneious Prime v. 2022.0.2 (http://www.geneious.com, Kearse et al. 2012).
Phylogenetic analysis
The sequences for each gene region were subjected to megablast searches in the NCBI’s GenBank nucleotide database ( Zhang et al. 2000), and also supplemented with sequences of ex-type strains of more distant published species. Sequence alignments were generated per locus using the online version of MAFFT v. 7 (https://mafft.cbrc.jp/alignment/server/index.html; Katoh et al. 2019) with default settings. Leading and trailing gaps were removed as far as possible without removing too much potential phylogenetic signal, after which the alignments were concatenated using SequenceMatrix v. 1.9 ( Vaidya et al. 2011). An initial guide tree was constructed with IQ-TREE v. 2.1.3 ( Nguyen et al. 2015), after which the sequences in the alignment were sorted according to the tree topology using Mesquite v. 3.70 ( Maddison & Maddison 2023), and the local alignment of adjacent sequences improved by eye where necessary using Geneious Prime v. 2023.2.1. Maximum-likelihood (ML) phylogenetic trees were constructed using IQ-TREE v. 2.1.3 and branch support values were calculated with 1 000 non-parametric bootstrap replicates and optimal modelfinding using the TESTNEW option of ModelFinder ( Kalyaanamoorthy et al. 2017) as implemented in IQ-TREE. RAxML v. 8.0.0.0 ( Stamatakis 2014) was used with default parameters and 1 000 non-parametric bootstrap replicates to provide additional ML support values for the multigene phylogeny. All resulting trees were printed with Geneious Prime v. 2023.2.1 and layout of the tree was done with Adobe Illustrator 2024 v. 28.0. Sequences derived in this study were deposited in GenBank (Suppl. Table S1), and the alignment and phylogenetic trees in figshare.com (doi: 10.6084/m9.figshare.23447345).
Morphology
Slide preparations were mounted in clear lactic acid or Shear’s mounting fluid. Descriptions were chiefly based on fungarium specimens. In cases where this was not possible, descriptions were based on colonies sporulating on MEA, PDA, SNA or OA. Observations were made with a Nikon SMZ25 dissection microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and images recorded on a Nikon DS-Ri2 camera with associated software. Colony characters and pigment production were noted after 2–4 wk of growth on MEA, PDA and OA ( Crous et al. 2019b) incubated at 25 °C. Colony colours (surface and reverse) were scored using the colour charts of Rayner (1970). Taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004a).
RESULTS
Phylogeny
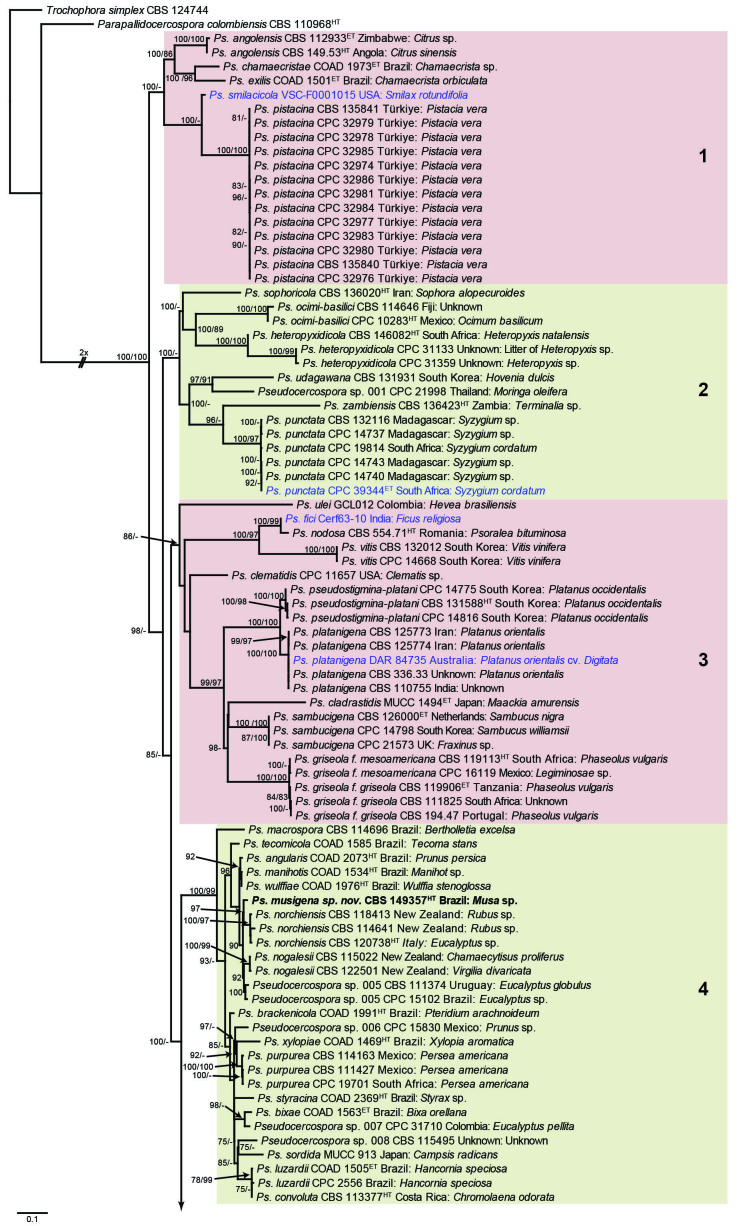
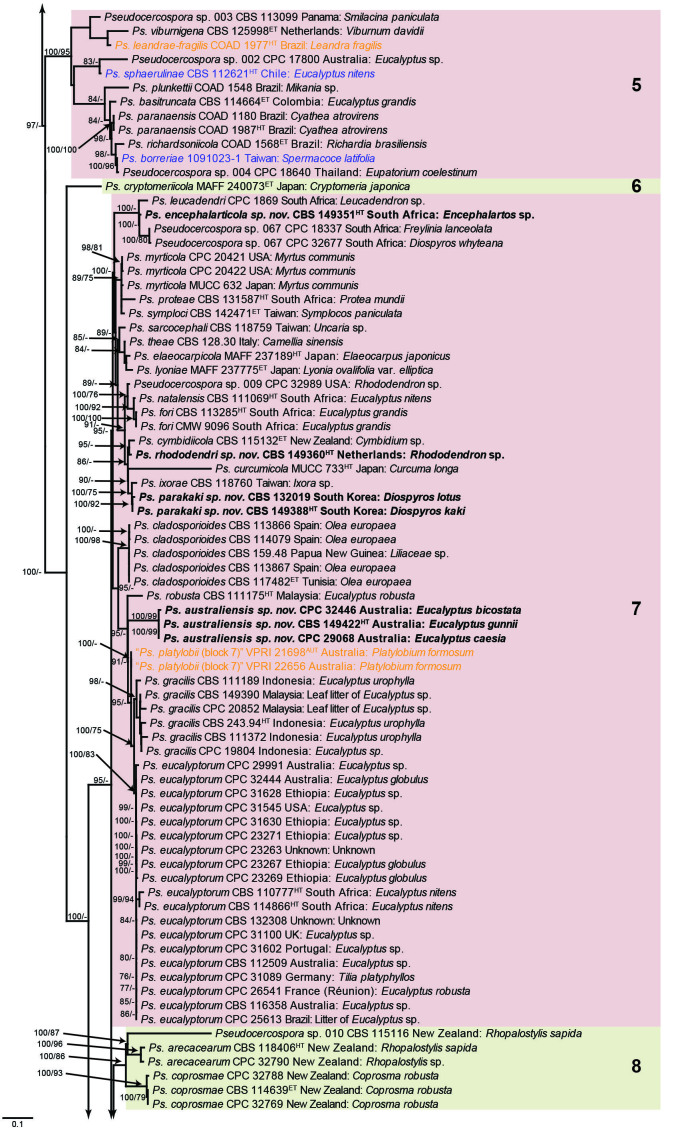
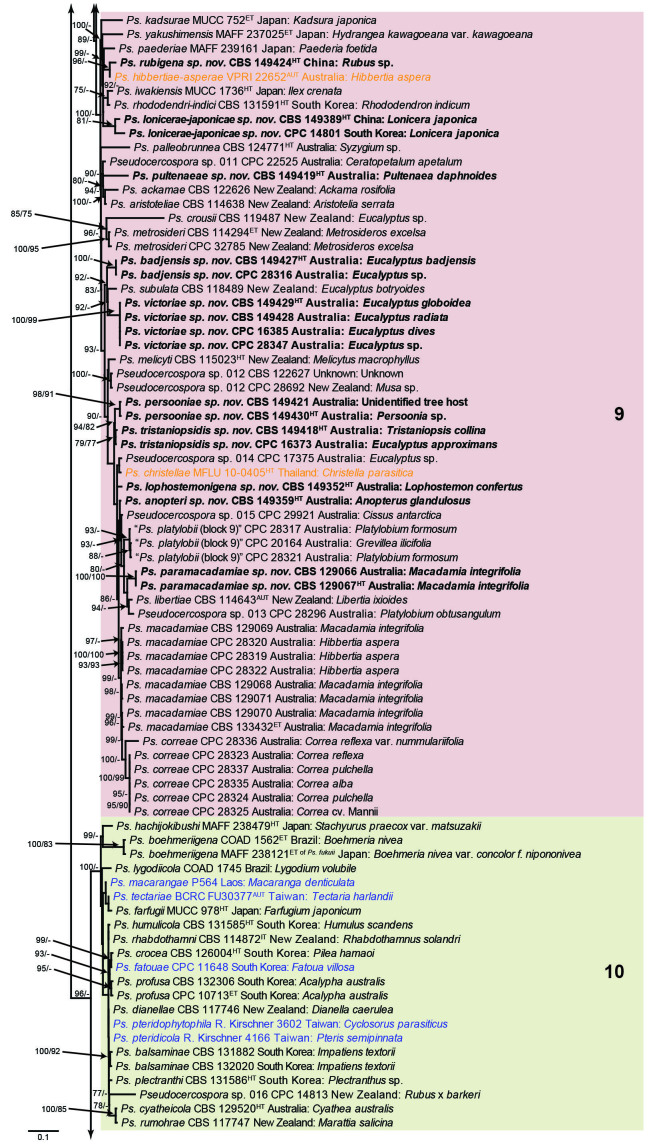
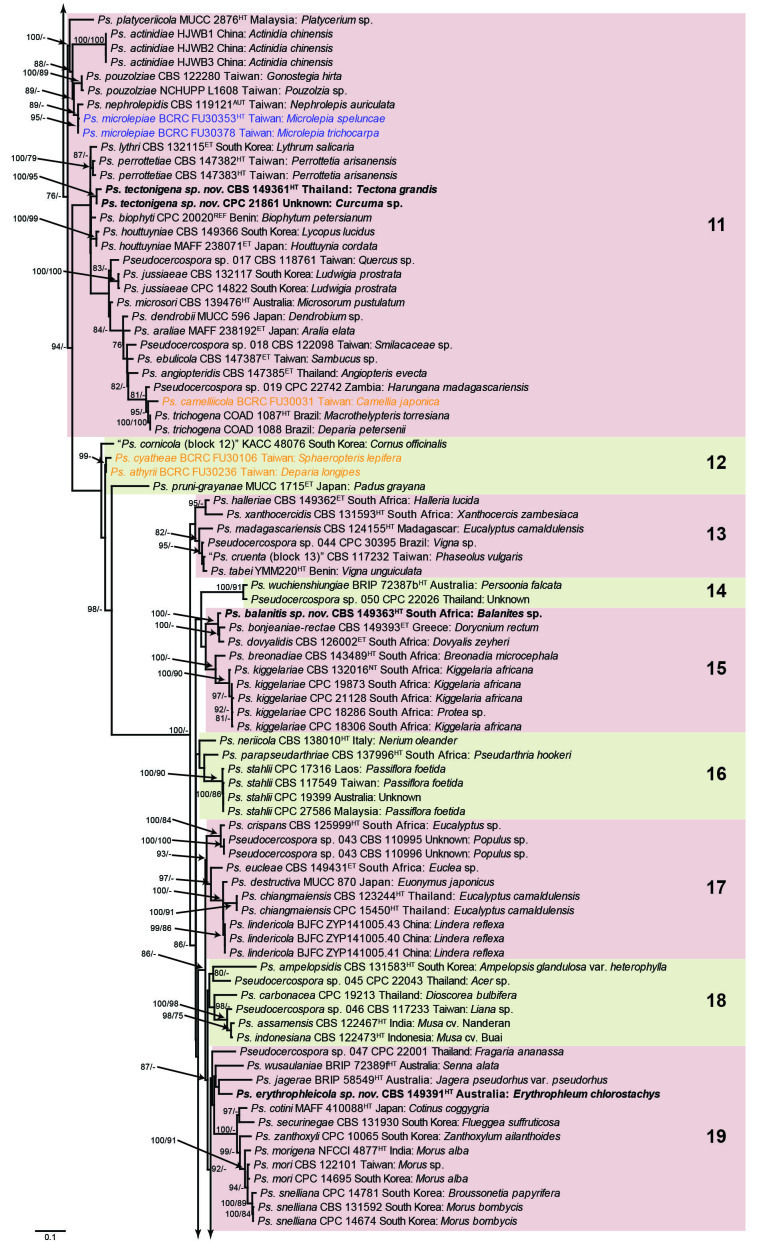
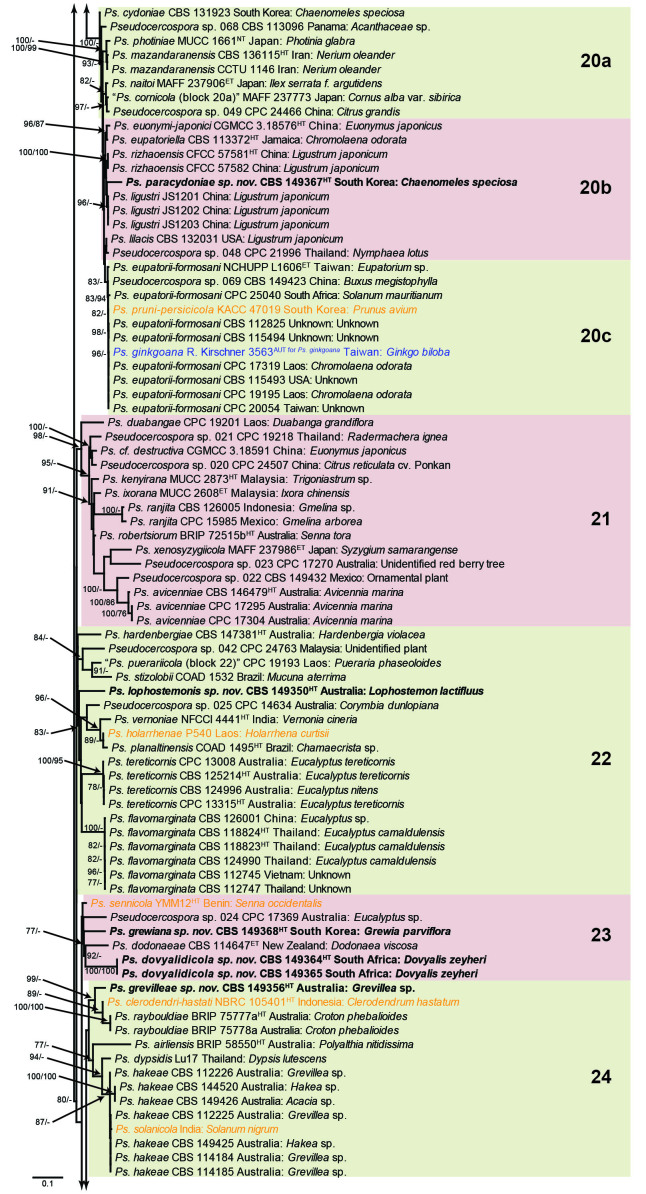
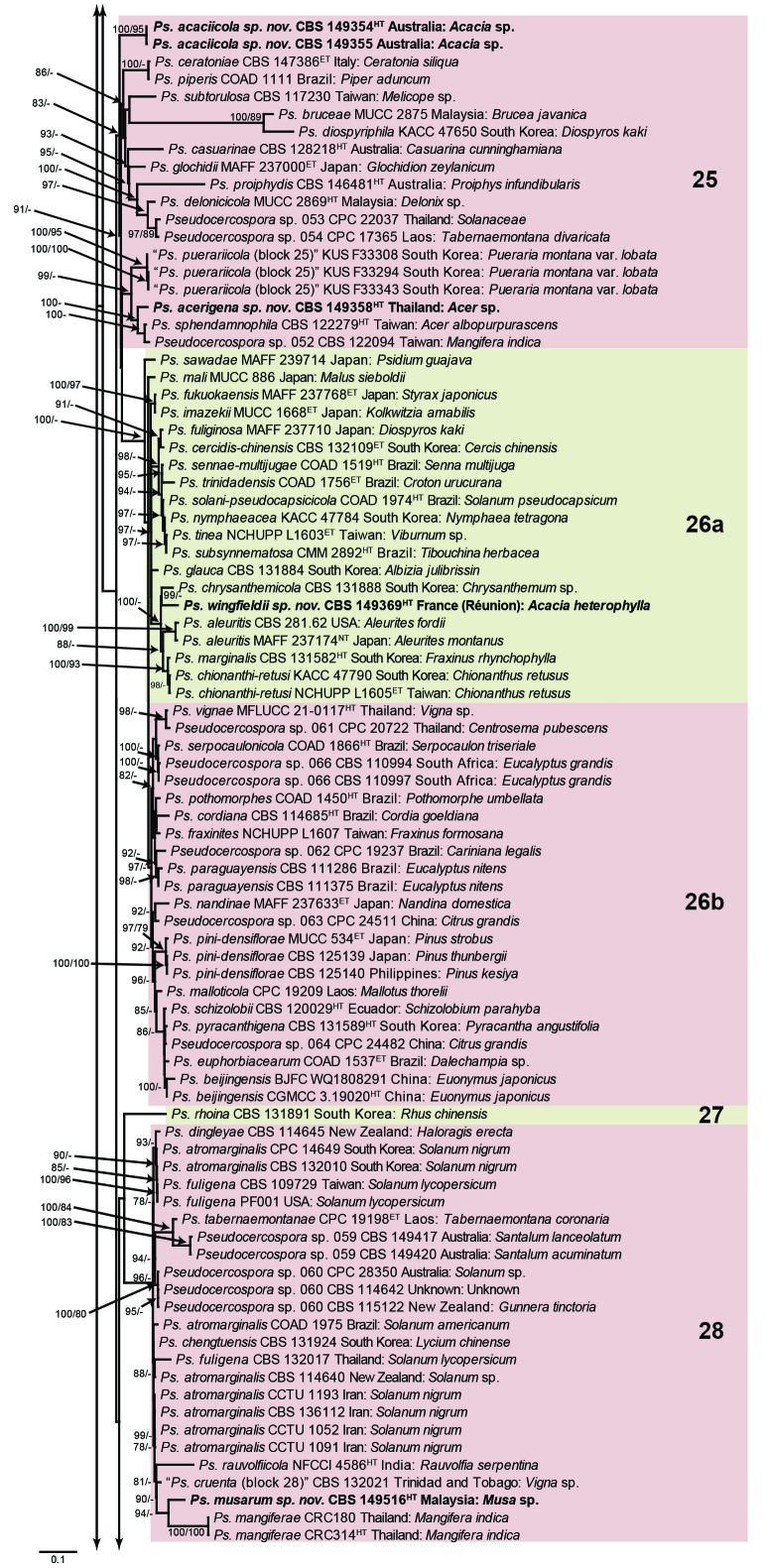
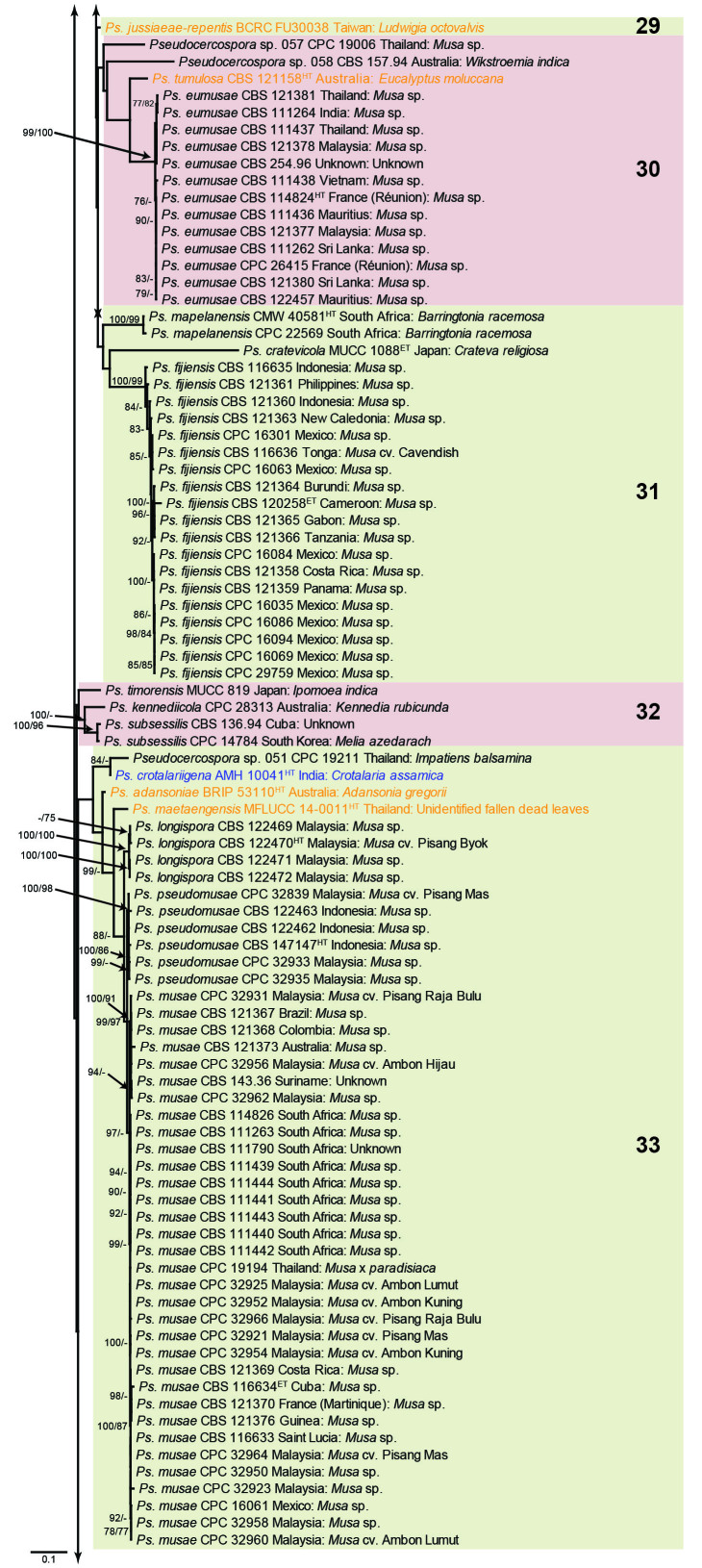
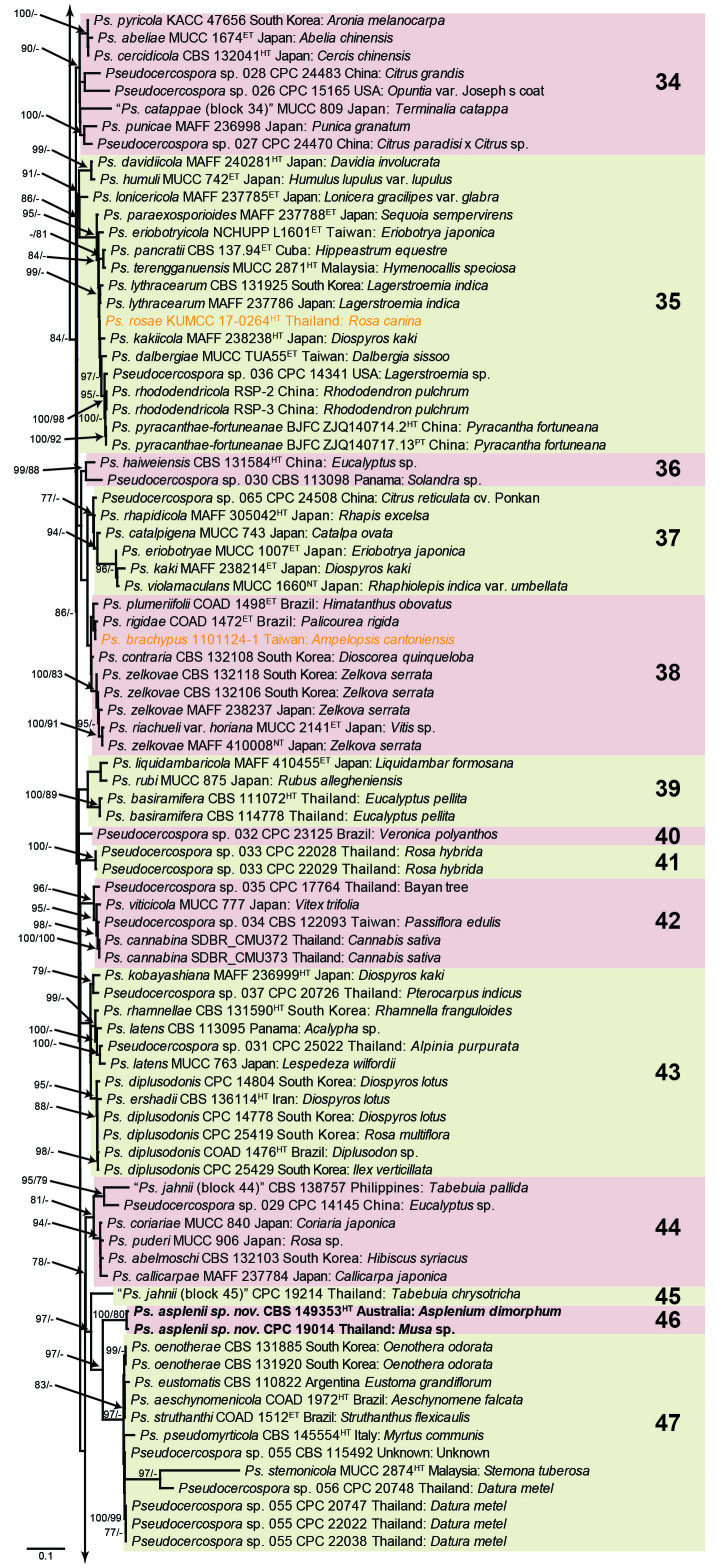
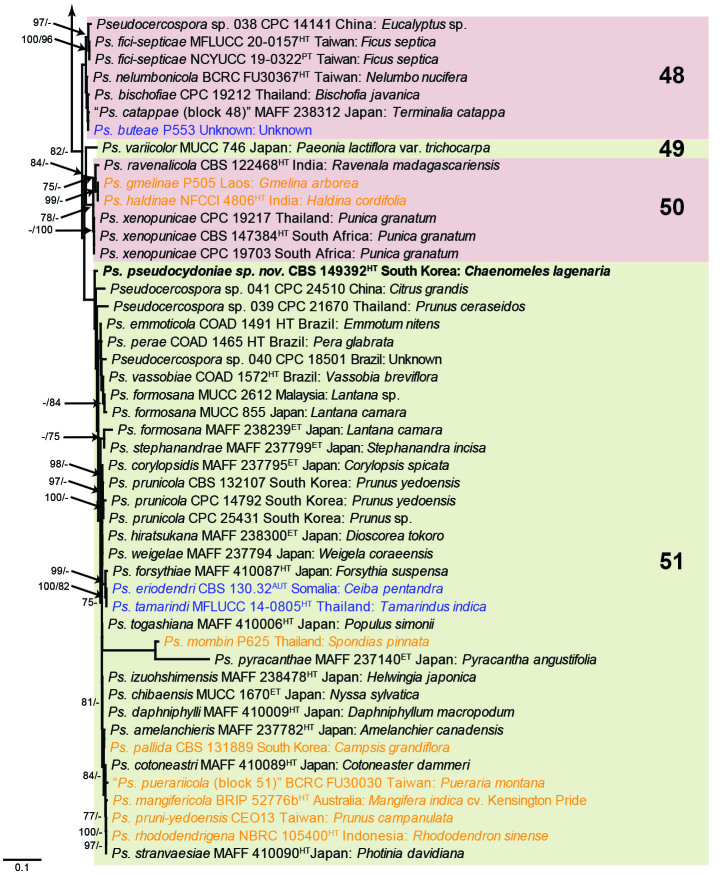
The concatenated alignment consists of 723 strains, including the outgroup (Trochophora simplex) and the basal relative (Parapallidocercospora colombiensis). The alignment contained a total of 2 489 characters with the following partitions: 1–520 (ITS), 521–760 (actA), 761–1 813 (tef1) and 1 814–2 489 (rpb2). The phylogeny resulting from the IQ-TREE maximum likelihood analysis is presented in Fig. 1, with the bootstrap support values from both the IQ-TREE and RAxML analyses plotted on the branches. Statistics for the IQ-TREE analysis are 1 842 distinct patterns, 1 242 parsimony-informative, 277 singleton, and 970 constant sites; additional statistical measures for the different analyses are provided in Suppl. Table S2. Blocks (loosely applied to correspond to phylogenetic clades or groups of clades for most instances) were numbered to facilitate referencing to the position of a species in the phylogenetic tree in the Taxonomy section below.
Fig. 1.
Consensus phylogram (50 % majority rule) obtained from the maximum likelihood analysis with IQ-TREE v. 2.1.3 of the concatenated nucleotide alignment. Bootstrap support values (> 74 %) from 1 000 non-parametric bootstrap replicates are shown at the nodes, followed by RAxML 1 000 replicate bootstrap support values (> 74 %). Culture collection or voucher numbers are followed by the country and host information where available. Sequences derived from material with a type status are indicated with a superscript HT (from (ex-)type), IT (from (ex-)isotype), PT (from (ex-)paratype), NT (from (ex-)neotype, AUT (from authentic), REF (from reference) and ET (from (ex-)epitype). Strains in dark blue font represent strains with only an ITS sequence in the dataset but with no conflict in their positions between the IQ-TREE and RAxML analyses, whereas an orange font indicates such a strain with a conflict in its position between the two analyses. Numbered coloured blocks are provided to facilitate referencing to the position of a species in the phylogenetic tree. Taxa for which it was not possible to assign the correct name due to lack of type material are indicated together with their block number between parentheses in the phylogenetic tree, Suppl. Tables S1 and S3 and the Taxonomy section. Novel species described in this study are highlighted with bold font. The tree was rooted to Trochophora simplex with Parapallidocercospora colombiensis as internal distant genus. The scale bar indicates the expected number of changes per site.
The majority of terminal (species) clades are virtually the same between the two analyses. In many instances where a single strain moved to a different position between the analyses, this was due to the fact that the given strain has only an ITS sequence available. Forty-five strains representing 43 species are represented by ITS sequences only in this dataset (Suppl. Table S3). In Suppl. Table S3, the two strains each for Ps. microlepiae and “Ps. platylobii (block 7)” were collapsed to a single row as each set of two strains functioned as a unit in the phylogenetic trees. Two other strains represent species for which additional strains are available with complete datasets (Ps. platanigena and Ps. punctata); these two strains also clusters with the other strains in both the IQ-TREE and RAxML analyses. Of the 45 strains, 18 did not have conflicting positions in the phylogenetic trees obtained from the IQ-TREE (Fig. 1) and RAxML (see Figshare) analyses. The remaining 27 strains did cluster differently between the phylogenetic trees obtained from the IQ-TREE and RAxML analyses and these differences are highlighted in Suppl. Table S3 and in the species notes below. For example, Ps. camelliicola (BCRC FU30031) is located towards the bottom of block 11 in Fig. 1 as close relative of Ps. trichogena, but in the RAxML phylogeny (see Figshare) it is located on a long branch between the two strains of Ps. lonicerae-japonicae, which is located in block 9 of Fig. 1 based on the IQ-TREE phylogeny. Strains represented by only an ITS sequence in the dataset are shown in Fig. 1 in dark blue font if there are no conflicts in their position between the IQ-TREE and RAxML analyses, and in an orange font if there are conflicts in their position. Only the bootstrap support values from the RAxML analysis are plotted on Fig. 1; the obtained RAxML phylogenetic tree is deposited in Figshare. Overall, the obtained RAxML bootstrap support values were much lower than the obtained IQ-TREE bootstrap support values. This resulted in many branches not having a RAxML bootstrap support value as the value was below the threshold value chosen for plotting even though the clustering of the isolates was the same between the two analyses.
TAXONOMY
Pseudocercospora Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires, Ser. 3, 20: 437. 1910.
Synonyms: See Crous & Braun (2003), Braun et al. (2013a), Crous et al. (2013a), Videira et al. (2017).
Foliicolous, chiefly phytopathogenic, but also endophytic; commonly associated with leaf spots, but also occurring on fruits and twigs. Mycelium internal and external, consisting of smooth, septate, subhyaline to brown branched hyphae. Stroma absent to well-developed. Conidiophores in vivo arranged in loose to dense fascicles, sometimes forming distinct synnemata or sporodochia, emerging through stomata or erumpent through the cuticle, often arising from substomatal or subcuticular to intraepidermal stromata, or occurring singly on superficial hyphae, short to long, septate or continuous, i.e., conidiophores may be reduced to conidiogenous cells, simple to branched and straight to geniculate-sinuous, subhyaline, pale to dark olivaceous to brown, smooth to finely verruculose. Conidiogenous cells integrated, terminal, occasionally intercalary, polyblastic, sympodial, or monoblastic, proliferating percurrently via inconspicuous or darkened, irregular annellations, subhyaline, olivaceous, pale to dark brown, with inconspicuous, or only thickened along the rim, or flat, and unthickened or almost so but refractive or even slightly darkened-refractive loci, but never pronounced. Conidia solitary, rarely in simple chains or disarticulating, subhyaline, olivaceous, pale to dark brown, usually scolecosporous, i.e., obclavate-cylindrical, filiform, acicular, and transversely multi-euseptate, occasionally also with oblique to longitudinal septa, conidia rarely amero- to phragmosporous, short subcylindrical or ellipsoidal-ovoid, aseptate or only with few septa, apex subacute to obtuse, base obconically truncate to truncate, or bluntly rounded, with or without a minute marginal frill, straight to curved, rarely sigmoid, smooth to finely verruculose; hila usually unthickened, not darkened, at most somewhat refractive, occasionally slightly thickened along the rim, or rarely flat, unthickened or almost so, but slightly refractive or even slightly darkened-refractive, but never pronounced (from Crous et al. 2013a). Ascomata pseudothecial, single or aggregated, black, immersed becoming erumpent, globose, with apical ostiole; wall of 3–4 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, ovoid to obclavate, straight or incurved, 8-spored. Ascospores tri- to multiseriate, overlapping, hyaline, guttulate, thin-walled, slightly curved to straight, fusoid-ellipsoid to obovoid, 1(–3)-septate, tapering toward both ends (from Crous 1998).
Type species: Pseudocercospora vitis (Lév.) Speg.
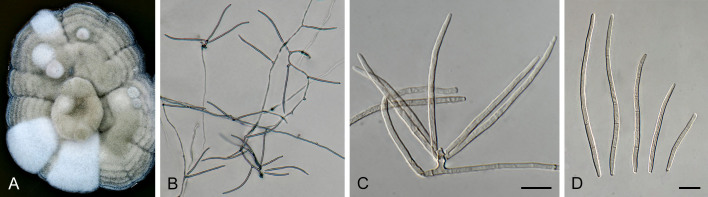
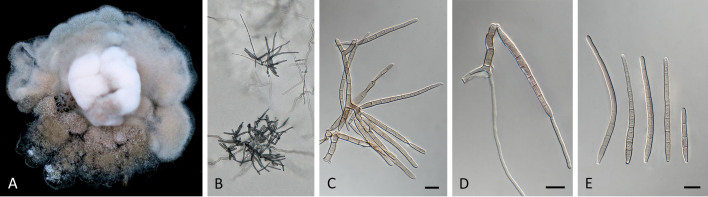
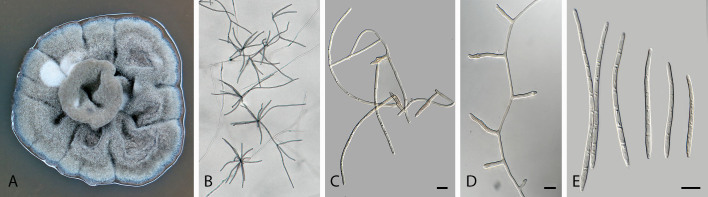
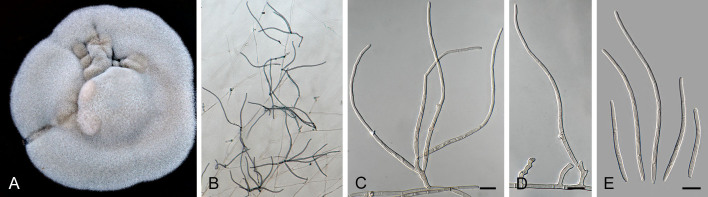
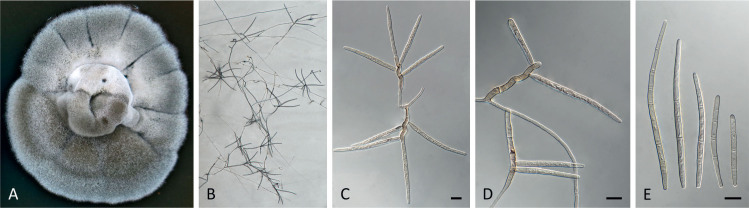
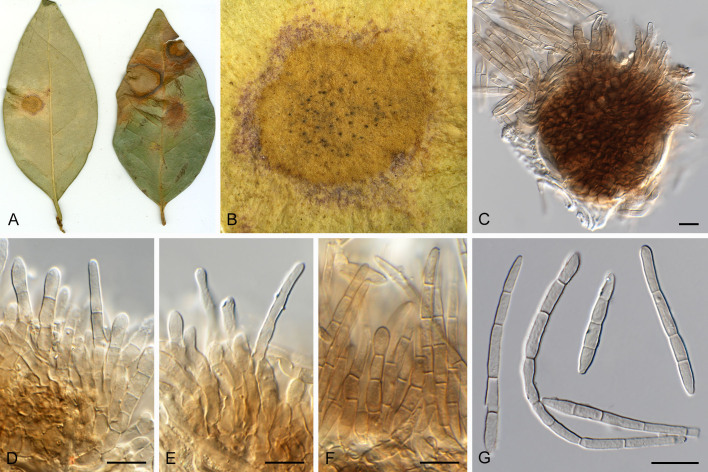
Pseudocercospora acaciicola Crous, R.G. Shivas & Yuan Yuan Chen, sp. nov. MycoBank MB 852260. Figs 2–4.
Fig. 2.
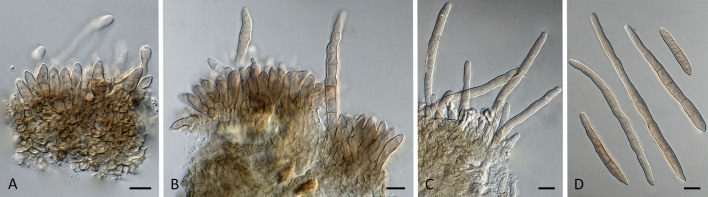
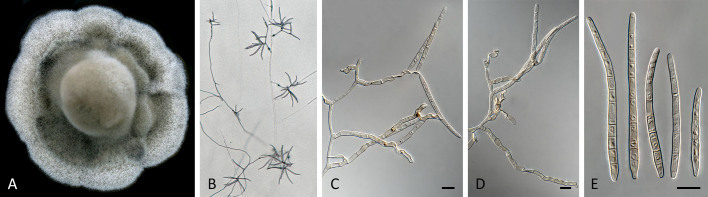
Pseudocercospora acaciicola (CPC 17039, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C–H. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 4.
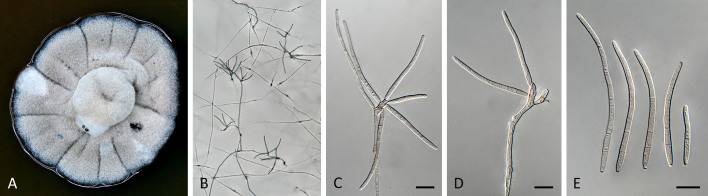
Pseudocercospora acaciicola (CPC 17121). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Acacia.
Leaf spots amphigenous, sub-circular to circular, 2–8 mm diam, brown with raised border and dark brown margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, amphigenous, brown on leaves, up to 70 μm diam and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 30 μm diam and 50 μm high; conidiophores pale brown, smooth, 1–6-septate, subcylindrical, straight to geniculate-sinuous, branched below or above, 30–60 × 4–5 μm. Conidiogenous cells terminal and intercalary, branched or not, pale brown, smooth, tapering to flat-tipped apical loci, 1.5–2 μm diam, proliferating sympodially, 7–20 × 2.5–3 μm; scars inconspicuous, 1.5–2 μm diam. Conidia solitary, pale brown, smooth, guttulate, narrowly obclavate to subcylindrical, apex obtuse, base tapering slightly to a truncate hilum, straight to slightly curved, (1–)3-septate, (40–)50–70(–85) × (2.5–)3 μm; hila neither thickened nor darkened-refractive, 1.5–2 μm diam.
Description in vitro (SNA; CPC 17121): Mycelium pale brown, smooth, delicate, 1.3–3 μm diam. Conidiophores macronematous, emerging from hyphae, pale brown to brown, solitary, smooth, unbranched, subcylindrical to obovoid, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–2-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 12.5–24 × 5–9 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to medium brown, mono- or polyblastic, proliferating sympodially, unbranched, conidiogenous loci inconspicuous, apex conically truncate, 4.8–15 × 3.8–9 μm; scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, narrowly obclavate, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, (30–)40–110 × 2.5–4.5 μm, 2–11-septate; hila neither thickened nor darkenedrefractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, aerial mycelium absent, with smooth, lobate margins. Surface irregularly folded, pale mouse grey, with patches of cinnamon; reverse vinaceous buff, becoming pale mouse grey at margin, colonies reaching 26 mm diam.
Typus: Australia, Queensland, Brisbane, Raven Street Reserve, from leaf spots on Acacia sp. (Fabaceae), 12 Jul. 2009, P.W. Crous (holotype CBS H-25032, culture ex-type CPC 17039 = CBS 149354).
Additional material examined: Australia, Queensland, Brisbane, from leaf spots on Acacia sp. (Fabaceae), 12 Jul. 2009, P.W. Crous, CBS H-25033, culture CPC 17121 = CBS 149355.
Notes: Several species of Pseudocercospora have been described from Acacia ( Crous et al. 2004b), most of which have since been allocated to allied cercosporoid genera ( Videira et al. 2017). Pseudocercospora acaciicola (Fig. 1, block 25) needs to be compared to Ps. acaciae (Acacia concinna, Uttar Pradesh), but the latter is distinct in that it has conidiophores up to 270 μm long, and obclavate conidia that are much wider than those of Ps. acaciicola (21.5–70 × 7–11 μm; Kamal & Singh 1980). A further species common on Acacia spp. in Asia, Ps. acaciae-confusae, is distinct in that it has shorter conidiophores (0–1-septate, 10–20 × 3.5–4 μm), and wider conidia (40–70 × 3.5–4 μm; Hsieh & Goh 1990).
Pseudocercospora acaciicola is presently known from two Australian collections, with the second specimen (CPC 17121) being rather depauperate, with conidiogenous cells that proliferate sympodially and percurrently near apex (annellations irregular), 10–22 × 3.5–4.5 μm, and conidia that are pale olivaceous, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight, 1–3-septate, (15–)20–30 × (2.5–)3(–4) μm.
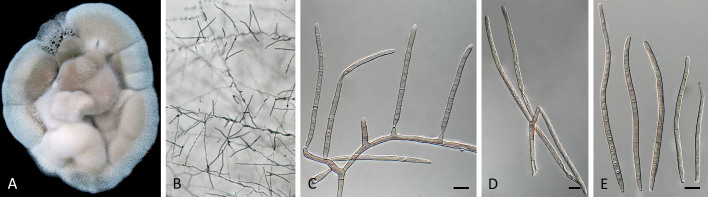
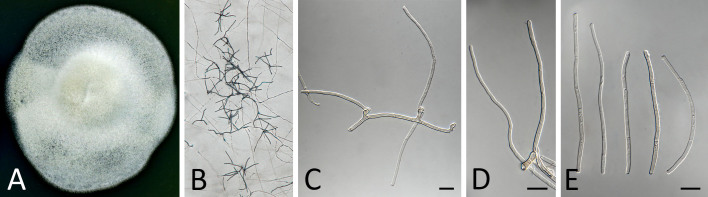
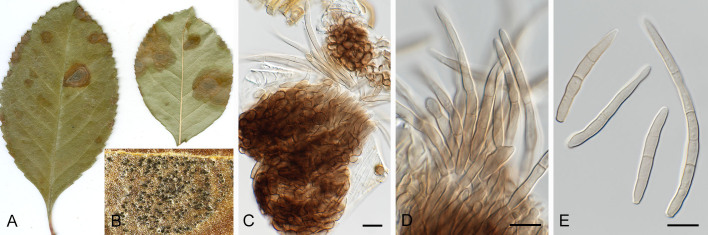
Pseudocercospora acerigena Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852261. Fig. 5.
Fig. 5.
Pseudocercospora acerigena (CPC 18629, ex-type culture). A. Leaf spots on lower leaf surface. B. Close-up of lesion. C, D. Spermatogonia. E, F. Conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Acer, + Latin adjectival ending -genus (produced in a certain place).
Leaf spots amphigenous, irregular to sub-circular, 2–8 mm diam, pale to medium brown with indistinct margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Conidiophores arising from hypophyllous brown spermatogonia, 50–80 μm diam, reduced to conidiogenous cells, medium brown, smooth, subcylindrical, holoblastic, 8–12 × 4–5 μm; scars inconspicuous, 2 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate to subcylindrical, apex obtuse, base truncate, straight to curved, 3–7-septate, (40–) 55–60(–70) × 3(–3.5) μm; hila neither thickened nor darkenedrefractive, 2–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with moderate/sparse to absent aerial mycelium, and smooth, lobate margins. Surface irregularly folded, pale olivaceous-grey; reverse olivaceous grey. Colonies reaching 25 mm diam.
Typus: Thailand, Chiang Mai, Koowang, Mae Wang, from leaf spots on Acer sp. (Sapindaceae), 5 Oct. 2010, P.W. Crous (holotype CBS H-25037, culture ex-type CPC 18629 = CBS 149358).
Notes: Pseudocercospora acerigena (Fig. 1, block 25) needs to be compared to Ps. acericola (CBS 122279; Fig. 1, block 25) and Ps. sphendamnophila ( Kirschner et al. 2009; Fig. 1, block 25). Pseudocercospora acericola differs from Ps. acerigena in the absence of stromata, the presence of external mycelium, and larger conidia [3–12-septate, 35–145 × 4–6 μm ( Guo et al. 1998); 3–9-septate, 30–120 × 3.5–5 μm ( Chupp 1954)], while Ps. sphendamnophila differs in having fascicles of well-developed conidiophores, and narrower conidia [1–6-septate, (12–)30–57(–67) × 2(–2.5) μm; Kirschner et al. 2009].
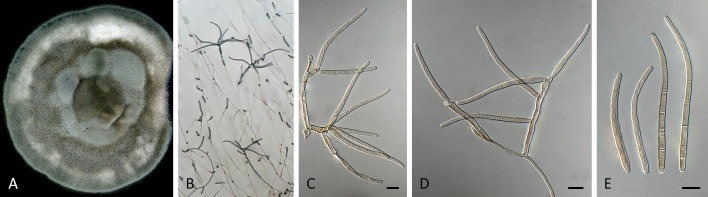
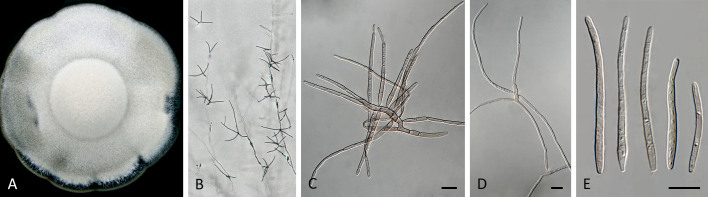
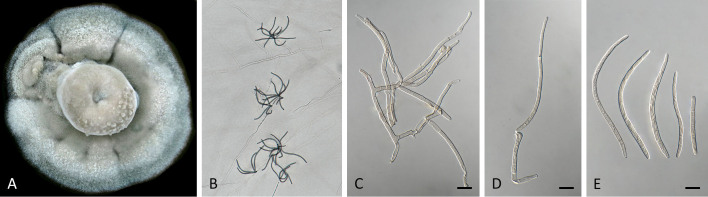
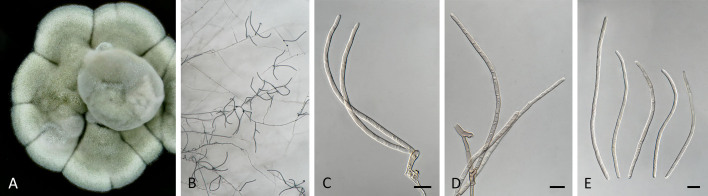
Pseudocercospora anopteri Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852262. Fig. 6.
Fig. 6.
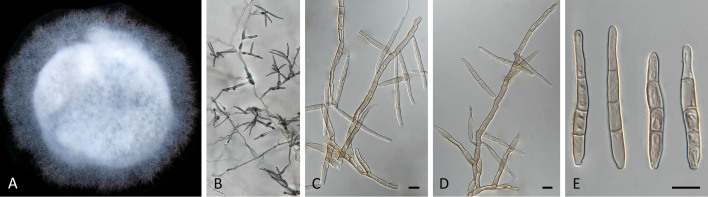
Pseudocercospora anopteri (CPC 20152, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus it was isolated from, Anopterus.
Description in vitro (SNA; CPC 20152): Mycelium subhyaline, smooth, uniform in width, 1–3 μm. Conidiophores macronematous, emerging from hyphae, pale brown, solitary, smooth or finely roughened, unbranched, cylindrical, straight to sinuous, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 8–42.5 × 2.5–3.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 8–22 × 2–3.5 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, narrowly cylindrical to filiform, apex obtuse to subobtuse, base truncate, slightly to strongly curved, 60–130(–147) × 2–3.5 μm, 6–14-septate; hila neither thickened nor darkened-refractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, lobate margins. Surface pale greyish yellow green to white; reverse olivaceous, Colonies reaching 34 mm diam.
Typus: Australia, Tasmania, from leaf spots on Anopterus glandulosus (Escalloniaceace), 11 Dec. 2011, W. Quaedvlieg (holotype CBS H-25038, culture ex-type CPC 20152 = CBS 149359).
Note: Pseudocercospora anopteri (Fig. 1, block 9) is introduced here as a new species, and represents the only species of Pseudocercospora known from the genus Anopterus, and family Escalloniaceace.
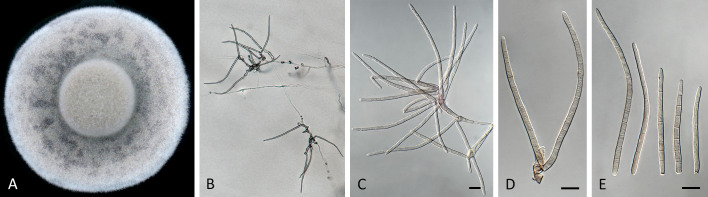
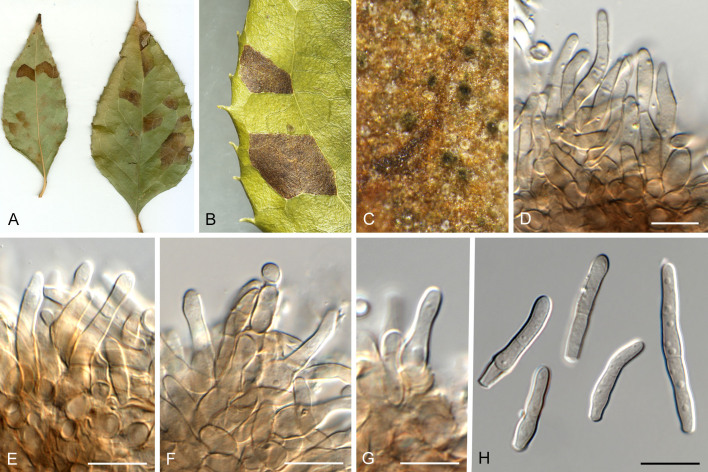
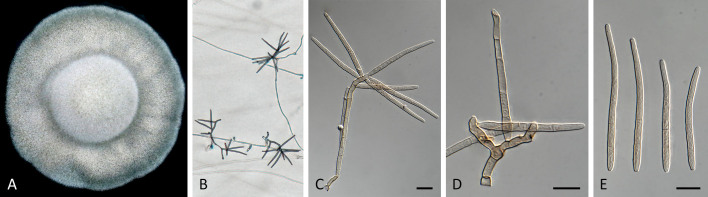
Pseudocercospora asplenii Crous, R.G. Shivas & Yuan Yuan Chen, sp. nov. MycoBank MB 852263. Fig. 7.
Fig. 7.
Pseudocercospora asplenii (CPC 17011, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus it was collected from, Asplenium.
Description in vitro (SNA; CPC 17011): Mycelium subhyaline, smooth, delicate, uniform in width, 1.5–2.5 μm. Conidiophores micro- to macronematous, emerging from hyphae or conidia, pale brown, solitary, smooth or finely roughened, unbranched, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, 1–2-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 3.8–25 × 2.3–4 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, 2.8–10 × 2.3–4 μm, scars inconspicuous, 0.5–1.5 μm diam. Conidia solitary, pale brown, guttulate, narrowly obclavate, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, (24–)30–80 × (1.5–)2–3 μm, (1–)3–6-septate; hila neither thickened nor darkened-refractive, 0.5–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, grey olivaceous to smoke grey with patches of white; reverse mouse grey to vinaceous buff, Colonies reaching 37 mm diam.
Typus: Australia, Queensland, Noosa, from leaf spots on Asplenium dimorphum (Aspleniaceae), 14 Jul. 2007, P.W. Crous (holotype CBS H-25031, culture ex-type CPC 17011 = CBS 149353).
Additional material examined: Thailand, Chiang Rai, on Musa sp., 4 Dec. 2010, R. Cheewangkoon, culture CPC 19014.
Notes: Pseudocercospora asplenii (Fig. 1, block 46) is introduced here as a new species, and represents the only species of Pseudocercospora known from the genus Asplenium, and family Aspleniaceae. For notes on Cercospora asplenii, see Braun et al. (2013a).
Pseudocercospora australiensis Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852264. Fig. 8.
Fig. 8.
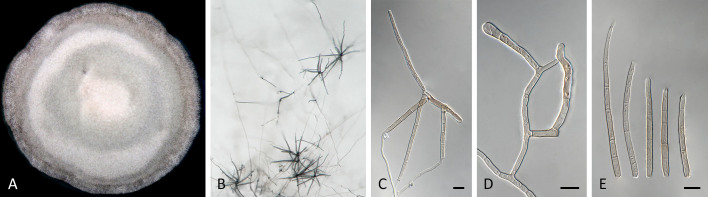
Pseudocercospora australiensis (CPC 29218, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the country where it was collected, Australia.
Leaf spots amphigenous, angular, delimited by leaf veins, 1–5 mm diam, medium brown with raised pale brown border.
Mycelium internal and external, pale brown, consisting of septate, branched, smooth hyphae, 2–3 μm diam. Caespituli fasciculate, hypophyllous, grey brown on leaves, up to 100 μm diam and 60 μm high. Conidiophores aggregated in loose to dense fascicles arising from the upper cells of a brown stroma, 40–70 μm diam; conidiophores medium brown, smooth, 2–4-septate, subcylindrical, straight to geniculate-sinuous, unbranched, 30–45 × 4–6 μm. Conidiogenous cells terminal, unbranched, medium brown, smooth, tapering to flat-tipped apical loci, 2.5–4 μm diam, proliferating sympodially or percurrently near apex, 12–20 × 3–5 μm; scars inconspicuous, 3–3.5 μm diam. Conidia solitary, pale to medium brown, smooth, guttulate, subcylindrical, apex subobtuse, base truncate, straight to geniculate, 3–6-septate, (55–)58–65(–75) × (3.5–)4(–5) μm; hila neither thickened nor darkened-refractive with marginal frill, 3.5–4 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; Surface irregularly folded, with a prominent network of ridges, mouse grey to white; reverse dark mouse grey to pale mouse grey, Colonies reaching 11 mm diam.
Typus: Australia, Western Australia, Mount Barker, from leaf spots on Eucalyptus gunnii (Myrtaceae), 23 Aug. 2015, P.W. Crous, HPC 606 (holotype CBS H-25053, culture ex-type CPC 29218 = CBS 149422).
Additional material examined: Australia, Western Australia, Mount Barker, from leaf spots on E. caesia, 23 Aug. 2015, P.W. Crous, HPC 607, culture CPC 29068; Victoria, La Trobe State Forest, on leaves and twig cankers of E. bicostata, Nov. 2017, P.W. Crous, HPC 1874, culture CPC 32446.
Notes: Pseudocercospora australiensis (Fig. 1, block 7) is phylogenetically distinct from all Pseudocercospora spp. occurring on Eucalyptus ( Crous et al. 2019c), including those not known from culture ( Braun & Dick 2002). It is presently known from three different eucalypt host species and was observed to also cause twig cankers on E. bicostata, which is a rarely observed disease symptom in the genus Pseudocercospora. Morphologically, it is characterised by loose to dense fascicles that give rise to short, wide, 3–6-septate, subcylindrical conidia.
Pseudocercospora badjensis Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852265. Figs 9, 10.
Fig. 9.
Pseudocercospora badjensis (CPC 32376, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C–H. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 10.
Pseudocercospora badjensis (CPC 32376, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host species on which it occurs, Eucalyptus badjensis.
Leaf spots amphigenous, sub-circular to angular, 3–5 mm diam, medium brown with raised dark brown border. Mycelium internal and external, pale to medium brown, consisting of septate, branched, smooth to finely verruculose hyphae, 2.5–3.5 μm diam. Caespituli fasciculate, hypophyllous, grey brown on leaves, up to 50 μm diam and 220 μm high. Conidiophores arising singly from superficial mycelium, or aggregated in loose fascicles arising from the upper cells of a brown stroma up to 40 μm diam and 30 μm high; conidiophores medium brown, finely verruculose, 4–10-septate, subcylindrical, straight to slightly curved, unbranched or branched above, 80–200 × 5–6 μm. Conidiogenous cells terminal or intercalary, unbranched, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 25–35 × 4–5 μm; scars inconspicuous, 2–3 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate, apex subobtuse, base long obconically truncate, straight to slightly curved, 4–10-septate, (45–)75–100(–120) × (3.5–)4(–5) μm; hila neither thickened nor darkened-refractive, 2.5–3 μm diam.
Description in vitro (SNA; CPC 32376): Mycelium subhyaline to pale brown, smooth, delicate, 1.5–2 μm diam. Conidiophores macronematous, emerging from hyphae or conidia, pale brown to medium brown, solitary, smooth or roughened, unbranched to branched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–4-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 8.5–78 × 3–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale to medium brown, mono- or polyblastic, proliferating sympodially, unbranched, conidiogenous loci at the apex and shoulders, apex conically truncate, 8.5–31.5 × 2.5–5 μm; scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse, base obconically truncate, straight to slightly curved, 34–87 × 3–4 μm, 3–8-septate; hila neither thickened nor darkened-refractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, with sparse to absent aerial mycelium, and smooth, rounded margins. Surface mouse grey to olivaceous grey with patches of dirty white; reverse dark mouse grey with olivaceous grey at centre. Colonies reaching 20 mm diam.
Typus: Australia, Victoria, Mount Best, Tin Mine road, from leaf spots on Eucalyptus badjensis (Myrtaceae), 2015, P.W. Crous, HPC 1799 (holotype CBS H-25060, culture ex-type CPC 32376 = CBS 149427).
Additional material examined: Australia, Victoria, inland from head of Wingan Inlet, Croajingolong National Park, on leaf spots of Eucalyptus sp. in natural habitat, 8 Nov. 2001, V. Beilharz, culture CPC 28316.
Notes: Pseudocercospora badjensis (Fig. 1, block 9) is closely related to Ps. subulata and Ps. victoriae. It is morphologically distinct from species known from Eucalyptus in having superficial mycelium with solitary, long conidiophores, and narrowly obclavate, 4–10-septate conidia that can be up to 120 μm long ( Braun & Dick 2002, Crous et al. 2019c).
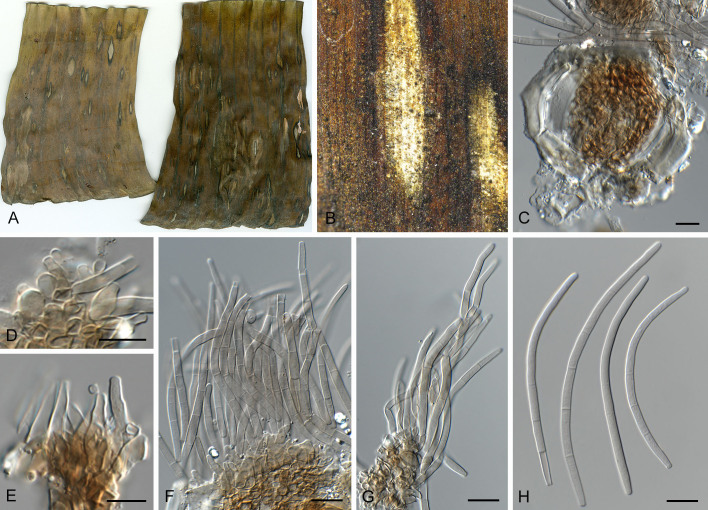
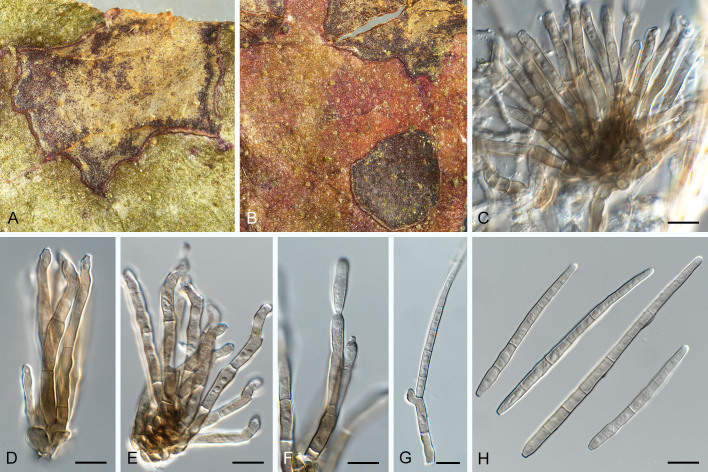
Pseudocercospora balanitis Crous, Jol. Roux & Yuan Yuan Chen, sp. nov. MycoBank MB 852266. Figs 11, 12.
Fig. 11.
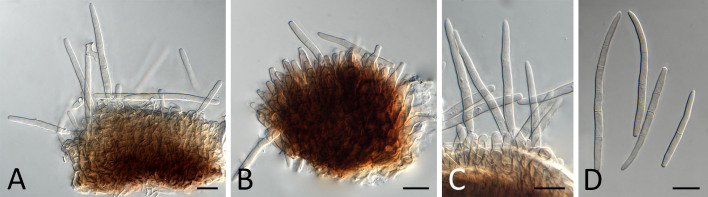
Pseudocercospora balanitis (CPC 25271, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C, D. Fascicles of conidiophores. E–G. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. H. Conidia. Scale bars = 10 μm.
Fig. 12.
Pseudocercospora balanitis (CPC 25271, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Balanites.
Leaf spots amphigenous, sub-circular, 4–8 mm diam, grey brown with dark brown margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, epiphyllous, brown on leaves, up to 120 μm diam and 160 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 100 μm diam and 50 μm high; conidiophores medium brown, smooth, 0–2-septate, subcylindrical, straight to slightly curved, unbranched, 15–30 × 4–5 μm. Conidiogenous cells terminal, pale to medium brown, smooth, tapering to flat-tipped apical loci, proliferating inconspicuously percurrently near apex, 7–15 × 4–5 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight to curved, 1–3-septate, (50–)60–75(–80) × (3–)3.5(–4) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 25271): Mycelium subhyaline to pale brown, smooth to verruculose, 1.5–4 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 16.5–50.5 × 5–7 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, apex conically truncate, 4.5–25 × 4–6.5 μm; scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, obclavate to filiform, apex obtuse to subobtuse, base long obconically truncate to truncate, straight to slightly curved, 35–95 × 3–4.5 μm, 4–10-septate; hila neither thickened nor darkened-refractive, 1–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, rounded margins. Surface smoke grey with patches of greyish sepia; reverse olivaceous. Colonies reaching 22 mm diam.
Typus: South Africa, Limpopo Province, Louis Trichardt, from leaf spots on Balanites sp. (Zygophyllaceae), 2 Oct. 2014, J. Roux (holotype CBS H-25043, culture ex-type CPC 25271 = CBS 149363).
Notes: Pseudocercospora balanitis (Fig. 1, block 15) is introduced here as a new species, and represents the only species of Pseudocercospora known from the genus Balanites, and family Zygophyllaceae. It is phylogenetically closely related to Ps. bonjeaneae-rectae on Lotus rectus and Ps. dovyalidis on Dovyalis zeyheri.
Pseudocercospora bonjeaneae-rectae (Caball.) U. Braun, Schlechtendalia 40: 278. 2023. Fig. 13.
Fig. 13.
Pseudocercospora bonjeaneae-rectae (CPC 31698, ex-epitype culture). A–C. Fascicles with conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Basionym: Cercospora bonjeaneae-rectae Cabal., Fac. Sci. Univ. Barcelona Publ. Secc. Ci. Nat. 12: 104. 1920.
Synonyms: Cercospora bonjeaneae Maire [as ‘bonjeaniae’], Bull. Soc. Hist. Nat. Afrique N. 8: 193. 1917. nom. inval. Art. 36.1(a). Pseudocercospora bonjeaneae (Maire) U. Braun & Crous, in Crous & Braun, CBS Diversity Ser. (Utrecht) 1: 85. 2003, nom. inval. Art. 40.1 (Shenzhen).
Leaf spots amphigenous, irregular to sub-circular, 7–12 mm diam, brown on adaxial and abaxial surface, with light brown borders. Mycelium internal to rarely external, pale brown, consisting of septate, branched, verruculose hyphae, 2–5 μm diam. Caespituli fasciculate, amphigenous, grey brown to olivaceous brown on leaves, up to 155 μm diam and 115 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 75 μm diam and 50 μm high; conidiophores medium brown, smooth, 0–1-septate, clavate, straight to slightly curved, unbranched, usually reduce to conidiogenous cell, 14–32.5 × 5–6.5 μm. Conidiogenous cells terminal, unbranched, medium brown, smooth, tapering to obconically truncate apical loci, proliferating percurrently near apex, 15–25 × 4.5–6.5 μm; scars inconspicuous, 1–2.5 μm diam. Conidia solitary, pale to medium brown, smooth, guttulate, narrowly obclavate, apex obtuse, base long obconically truncate, straight to curved, slightly sinuous, 3–9-septate, 30–125 × 4.5–6.5 μm; hila neither thickened nor darkened-refractive, 1.5–3 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse aerial mycelium, surface irregularly folded, with a prominent network of ridges, grey white becoming pale mouse grey at margin; reverse dark mouse grey. Colonies reaching 28 mm diam.
Typus: Spain, Barcelona, La Planas, on Lotus rectus (≡ Bonjeanea recta), Oct. 1919, A. Caballero 4315 (MA-FunHist 6514). Greece, Rhodos, ca. 3.3 km W Archangelos, creek at the road to Malona, 36°13’06’’N, 28°04’49’’E, ca. 85 m alt, from leaf spots on Lotus rectus (≡ Dorycnium rectum), 19 Sep. 2016, V. Kummer, HPC 1369, U. Braun: Fungi selecti exsiccati 233 (epitype designated here CBS H-25057, MBT 10018174, culture ex-epitype CPC 31698 = CBS 149393).
Notes: Pseudocercospora bonjeaneae-rectae (Fig. 1, block 15) was distributed as Fungi selecti exsiccati ex Herbario Universitatis Halensis No. 233 (duplicated in BPI, BRIP, GZU, HMAS, K, KR, KUS, LE, M, PDD) ( Braun et al. 2017), which was also used to derive this ex-epitype culture. Because the name C. bonjeaniae was invalidly published, the synonym, C. bonjeaneae-rectae, was chosen (see Braun 2023 for explanation). Pseudocercospora bonjeaneae-rectae is phylogenetically closely related to Ps. balanitis on Balanites sp. and Ps. dovyalidis on Dovyalis zeyheri.
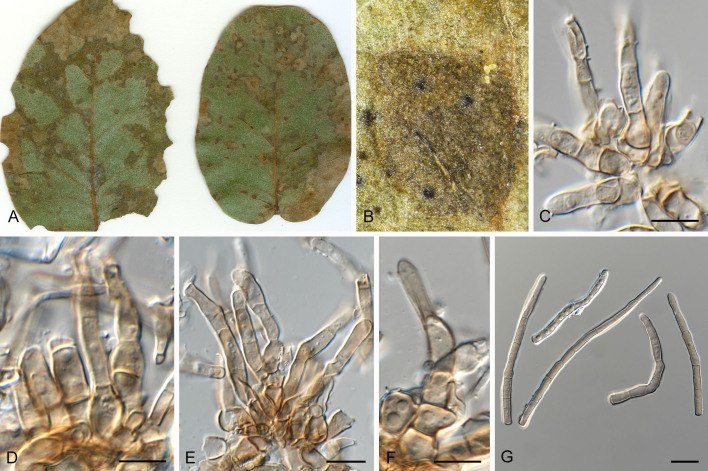
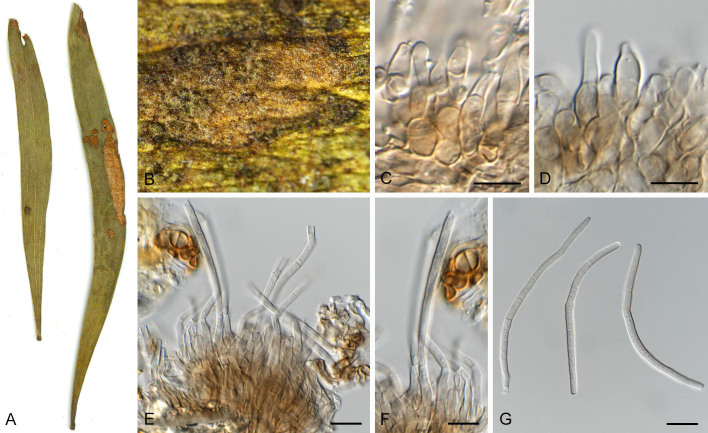
Pseudocercospora dovyalidicola Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852267. Figs 14, 15.
Fig. 14.
Pseudocercospora dovyalidicola (CPC 25273, ex-type culture). A. Leaf spots on upper and lower leaf surface. B, C. Close-up of lesions. D–I. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. J. Conidia. Scale bars = 10 μm.
Fig. 15.
Pseudocercospora dovyalidicola (CPC 25275). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Dovyalis.
Leaf spots amphigenous, sub-circular to circular, 2–7 mm diam, pale brown in centre, darker brown toward raised border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, amphigenous, brown on leaves, up to 120 μm diam and 100 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 80 μm diam and 40 μm high; conidiophores medium brown, finely verruculose to verrucose, 1–3-septate, subcylindrical, straight to geniculate-sinuous, unbranched, 30–60 × 5–7 μm. Conidiogenous cells terminal, unbranched, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating percurrently near apex, with rough, cercostigmina-like annellations, 10–22 × 5–7 μm; scars inconspicuous, 3.5–4.5 μm diam. Conidia solitary, medium brown, finely verruculose, guttulate, obclavate to subcylindrical, apex subobtuse, base obconically truncate, straight to slightly curved, (1–)5–8(–10)-septate, (35–)55–75(–95) × (5–)6–7 μm; hila neither thickened nor darkenedrefractive, (2.5–)3–5 μm diam.
Description in vitro (SNA; CPC 25275): Mycelium pale brown, smooth to slightly verruculose, 1.8–6 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary or fasciculate, smooth or slightly roughened, unbranched, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, multiseptate, 10–213 × 4.5–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 8–37 × 3.8–6 μm, scars inconspicuous, 1.3–2.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, 33.5–62 × 4–5.5 μm, 3–5-septate; hila neither thickened nor darkened-refractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and rough, rounded margins. Surface white to mouse grey; reverse olivaceous to mouse grey. Colonies reaching 12 mm diam.
Typus: South Africa, KwaZulu-Natal Province, Drakensberg, The Hedges, from leaf spots on Dovyalis zeyheri (Salicaceae), 2014, J. Roux (holotype CBS H-25044, culture ex-type CPC 25273 = CBS 149364).
Additional material examined: South Africa, KwaZulu-Natal Province, Drakensberg, The Hedges, from leaf spots on D. zeyheri, 2014, J. Roux (CBS H-25045, culture CPC 25275 = CBS 149365).
Notes: Pseudocercospora dovyalidicola (Fig. 1, block 23) needs to be compared to Ps. dovyalidis (ex-epitype CBS 126002; Fig. 1, block 15) a foliar pathogen of D. zeyheri occurring in Gauteng Province of South Africa (conidiophores 12–34 × 3–6 μm, conidia smooth, pale brown or subhyaline, 1–10-septate, subcylindrical, (20–)30–70(–84) × (3–)3.5–5(–6) μm; Crous et al. 2013a). Although the two species are phylogenetically distinct, they show considerable morphological overlap, with Ps. dovyalidicola distinct in having longer conidiophores, wider conidia, and rougher percurrent proliferations on its conidiogenous cells.
Pseudocercospora encephalarticola Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852268. Fig. 16.
Fig. 16.
Pseudocercospora encephalarticola (CPC 15278, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Encephalartos.
Description in vitro (SNA; CPC 15278): Mycelium pale brown, smooth, 1.5–4 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary, smooth to slightly verruculose, unbranched to branched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, multiseptate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 20–155 × 2–4.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to brown, mono- or polyblastic, proliferating sympodially or percurrently, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex truncate, 3–51.5 × 1.5–4.5 μm, scars inconspicuous, 1–2.5 μm diam. Conidia solitary, pale brown to medium brown, guttulate, cylindrical, apex rounded, base truncate, straight to slightly curved, 33–97 × 2.5–3 μm, 3–10-septate; hila neither thickened nor darkened-refractive, 1–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface folded with a prominent network of ridges, erumpent, spreading, with sparse aerial mycelium, and smooth, lobate margins. Surface smoke grey to white, with patches of dark mouse grey; reverse olivaceous to cinnamon. Colonies reaching 22 mm diam.
Typus: South Africa, Western Cape, from leaf spots on Encephalartos sp. (Zamiaceae), 22 May 2008, A.R. Wood (holotype CBS H-25029, culture ex-type CPC 15278 = CBS 149351).
Notes: Pseudocercospora encephalarticola (Fig. 1, block 7) needs to be compared to Ps. encephalarti (on leaves of Encephalartos barteri, from Benin), which is distinct in that it has obclavate to subacicular conidia, that are also longer and wider, (55–)90–167.5(–190) × 3.5–4.5(–5) μm, 3–8-septate ( Meswaet et al. 2019).
Pseudocercospora erythrophleicola Crous, R.G. Shivas & Yuan Yuan Chen, sp. nov. MycoBank MB 852269; Fig. 17.
Fig. 17.
Pseudocercospora erythrophleicola (CPC 17241, ex-type culture). A–C. Fascicles with conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus from which it was collected, Erythrophleum.
Leaf spots amphigenous, sub-circular, 2–20 mm diam, light brown to dark brown on adaxial and abaxial surface, with dark brown margin. Mycelium internal and rarely external, pale brown, consisting of septate, branched, smooth hyphae, 2–4 μm diam. Caespituli fasciculate, amphigenous, grey brown on leaves, up to 88 μm diam and 140 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 65 μm diam and 50 μm high; conidiophores medium brown, smooth, 0–3-septate, clavate to subcylindrical, straight to variously curved, unbranched, 13–21.5 × 4.5–6 μm. Conidiogenous cells terminal, unbranched, medium brown, smooth, tapering to flat-tipped apical loci, proliferating percurrently near apex, 7–25 × 3.5–6 μm; scars inconspicuous, 3–4 μm diam. Conidia solitary, pale brown, smooth, guttulate, cylindric to obclavate, apex obtuse to subobtuse, base long obconically truncate to truncate, straight to slightly curved, contract at septate, 3–8-septate, 17.5–60(–65) × 4–5.5 μm; hila neither thickened nor darkened-refractive, 2.5–3.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, pale mouse grey becoming lavender grey at margin; reverse dark mouse grey becoming mouse grey at margin. Colonies reaching 25 mm diam.
Typus: Australia, Queensland, from leaf spots on Erythrophleum chlorostachys (Fabaceae), 10 Aug. 2009, P.W. Crous (holotype CBS H-25034, culture ex-type CPC 17241 = CBS 149391).
Notes: Pseudocercospora erythrophleicola (Fig. 1, block 19) is distinguished from Ps. erythrophlei (on Erythrophleum chlorostachys, across northern Australia) by having large stromata and smaller conidia (stromata absent to small, conidia 44–100 × 3–4.5 μm in Ps. erythrophlei; Yuan 1996).
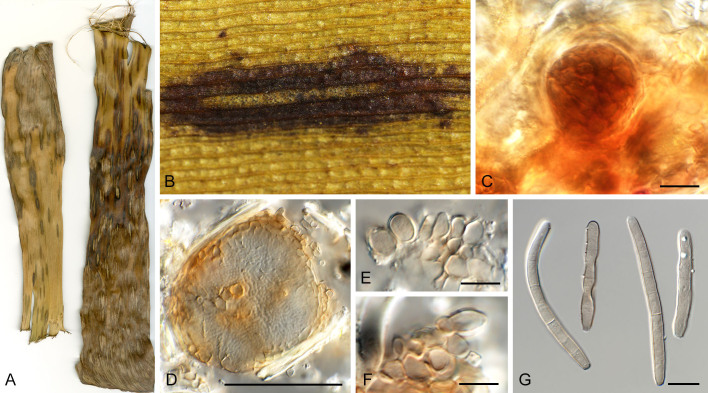
Pseudocercospora eucleae Crous & B. Sutton, J. S. African Bot. 63: 283. 1997. Fig. 18.
Fig. 18.
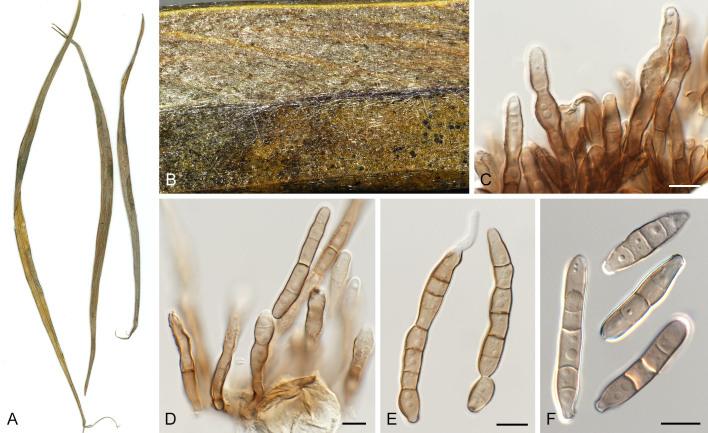
Pseudocercospora eucleae (CPC 32792, ex-epitype culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesions. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Leaf spots amphigenous, irregular to angular, 2–8 mm diam, dark brown with raised dark brown border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, hypophyllous, brown on leaves, up to 60 μm diam and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 30 μm diam and 20 μm high; conidiophores medium brown, smooth, 1–4-septate, subcylindrical, straight to variously curved, unbranched or branched below, 20–40 × 5–7 μm. Conidiogenous cells terminal, pale to medium brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently, 7–16 × 3–4 μm; scars inconspicuous, 1.5–2 μm diam. Conidia solitary, pale brown, smooth, guttulate, narrowly obclavate, apex obtuse, base long obconically truncate, straight to slightly curved, (1–)5–7(–9)-septate, (50–)60–85(–120) × 3.5(–4) μm; hila neither thickened nor darkened-refractive, 1.5–2 μm diam. Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with moderate/sparse to absent aerial mycelium, and smooth, lobate margins. Surface pale olivaceous grey; reverse olivaceous grey. Colonies reaching 13 mm diam.
Typus: South Africa, Gauteng Province, Warmbaths Rd. beyond Pienaars River, Euclea undulata (Ebenaceae), 20 Mar. 1950, P.H.B. Talbot (holotype PREM 39020); KwaZulu-Natal Province, Champagne resort, from leaf spots on Euclea sp., 16 Jan. 2017, P.W. Crous, HPC 1512 (epitype designated here CBS H-25064,MBT 10018178, culture ex-epitype CPC 32792 = CBS 149431).
Notes: Pseudocercospora eucleae (Fig. 1, block 17) was described as a foliar pathogen of Euclea undulata from Gauteng Province in South Africa, characterised by irregular, grey to brown leaf spots up to 7 mm diam, amphigenous caespituli, 1–3-septate conidiophores, 15–45 × 3–6 μm, with inconspicuous percurrent proliferation, and subcylindrical to narrowly obclavate conidia, 1–6-septate, 35–90 × 3–4 μm ( Crous & Sutton 1997). The present collection matches well with the morphology of the type specimen, and is herewith designated as epitype in order to obtain a culture and ex-type reference sequence data which are essential for taxonomic-phylogenetic purposes.
Pseudocercospora gracilis Crous & Alfenas, Mycologia 87: 123. 1995. Fig. 19.
Fig. 19.
Pseudocercospora gracilis (CPC 20850). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion with ascomata. C–E. Asci and ascospores. Scale bars = 10 μm.
Synonym: Mycosphaerella gracilis Crous & Alfenas, Mycologia 87: 123. 1995.
Leaf spots absent, pseudothecial ascomata occurring on leaf litter. Pseudothecia amphigenous, predominantly hypophyllous, black, subepidermal, erumpent to superficial, globose, 40–70 μm diam; apical ostiole 10 μm diam; wall consisting of 2–3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, 8-spored, (30–)35–45(–50) × (8–)9(–10) μm. Ascospores multiseriate, overlapping, hyaline, guttulate, thin-walled, straight to slightly curved, narrowly fusoid-ellipsoidal with subobtuse ends, widest just above septum, medianly 1-septate, slightly constricted at the septum, tapering towards both ends, (14–)15–17(–20) × (2–)2.5(–3) μm.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, glaucous grey to mouse grey with patches of brown; reverse dark mouse grey to fawn; folds appearing cinnamon. Colonies reaching 20 mm diam.
Typus: Indonesia, N. Sumatra, on Eucalyptus urophylla, 22 Nov. 1993, A.C. Alfenas (holotype of Mycosphaerella gracilis PREM 51718, holotype of Pseudocercospora gracilis PREM 51719, culture ex-type CBS 243.94 = CMW 14455 = CPC 730 = STE-U 730).
Additional materials examined: Indonesia, on Eucalyptus urophylla, 12 Mar. 1996, M.J. Wingfield, culture CPC 1314 = CBS 111372; on Eucalyptus urophylla, 12 Mar. 1996, M.J. Wingfield, culture CPC 1315 = CBS 111189; on Eucalyptus sp., Jan. 2011, M.J. Wingfield, culture CPC 19804. Malaysia, from leaf litter of Eucalyptus sp. (Myrtaceae), 22 Jun. 2012, M.J. Wingfield (holotype CBS H-25039, culture ex-type CPC 20850 = CBS 149390; CPC 20852)
Notes: The isolates studied here are sterile in culture. Phylogenetically, they fall within the variation accepted for Ps. gracilis, a foliar pathogen of E. urophylla in Indonesia (Fig. 1, block 7). It is characterised by narrowly fusoid-ellipsoidal ascospores, (10–)15–18(–20) × (2–)2.5–3 μm, and uniformly cylindrical, pale olivaceous conidia ( Crous et al. 2019c).
Pseudocercospora grevilleae Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852270. Figs 20, 21.
Fig. 20.
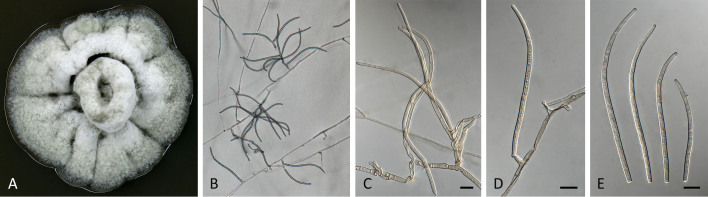
Pseudocercospora grevilleae (CPC 17585, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C, D. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. E, F. Conidia. Scale bars = 10 μm.
Fig. 21.
Pseudocercospora grevilleae (CPC 17585, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C–E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Grevillea.
Leaf spots amphigenous, irregular to elongated, 2–3 mm diam, medium brown. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–3 μm diam. Caespituli fasciculate, hypophyllous, dark brown on leaves, up to 110 μm diam and 160 μm high. Conidiophores aggregated in loose fascicles arising from the upper cells of a brown stroma up to 90 μm diam and 50 μm high; conidiophores medium brown, finely verruculose, 2–12-septate, subcylindrical, straight to variously curved, thick-walled, unbranched, 45–110 × 4–7 μm. Conidiogenous cells terminal, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating percurrently near apex with rough annellations, 10–35 × 4–7 μm; scars inconspicuous, 2.5–3 μm diam. Conidia solitary, medium brown, finely verruculose, guttulate, obclavate to subcylindrical, apex obtuse, base obconically truncate, straight to curved, (3–)5–7(–8)-septate, (30–)45–70(–80) × (5–)6(–7) μm; hila neither thickened nor darkened-refractive, 2.5–3 μm diam.
Description in vitro (SNA; CPC 17585): Mycelium subhyaline to pale brown, smooth, delicate, 1.5–5 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary, smooth, unbranched, cylindrical, straight to sinuous, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 8–35 × 4–6.3 μm. Conidiogenous cells integrated, terminal, pale brown, mono- or polyblastic, proliferating sympodially or percurrently, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 7.5–20 × 4–6 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex rounded, base obconically truncate to long obconically truncate, straight to strongly curved, 57–108 × 3.5–5 μm, 4–9-septate; hila neither thickened nor darkened-refractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, grey green, with patches of rosy buff; reverse vinaceous buff. Colonies reaching 25 mm diam.
Typus: Australia, New South Wales, S17°21’01.8” E144°54’45.1”, from leaf spots on Grevillea sp. (Proteaceae), 10 Aug. 2009, P.W. Crous (holotype CBS H-25035, culture ex-type CPC 17585 = CBS 149356).
Notes: Pseudocercospora grevilleae (Fig. 1, block 24) is phylogenetically and morphologically distinct from Ps. hakeae (see elsewhere in this publication; Fig. 1, block 24). It also needs to be compared to Ps. agharkarii (from Grevillea robusta, India, conidiophores 0–1-septate, 10–45 × 3–4 μm, conidia obclavate to subcylindrical, 3–11-septate, (35–)45–70(–95) × (3–)3.5–4.5(–5) μm; Crous & Palm 1999), from which it is distinguished based on its longer conidiophores and wider conidia.
Pseudocercospora grewiana Crous, H.D. Shin & Yuan Yuan Chen, sp. nov. MycoBank MB 852271. Figs 22, 23.
Fig. 22.
Pseudocercospora grewiana (CPC 25564, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C–H. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 23.
Pseudocercospora grewiana (CPC 25564, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Epithet composed of the name of the host genus on which it occurs, Grewia, + -ana (relating to/belonging to).
Leaf spots amphigenous, irregular to sub-circular, 1–7 mm diam, brown with indistinct border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, amphigenous, brown on leaves, up to 90 μm diam and 80 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 70 μm diam and 30 μm high; conidiophores brown, finely verruculose, 0–2-septate, subcylindrical, straight to slightly curved, unbranched, 17–35 × 5–7 μm. Conidiogenous cells terminal, unbranched, brown, finely verruculose, tapering to flat-tipped apical loci, proliferating percurrently near apex (annellations rough), 7–20 × 4–6 μm; scars inconspicuous, 2.5–3 μm diam. Conidia solitary, brown, finely verruculose, guttulate, obclavate to subcylindrical, apex subobtuse, base obconically truncate, straight to slightly curved, (2–)5–7(–8)-septate, (22–)38–55(–70) × (4–)5(–6) μm; hila neither thickened nor darkened-refractive, 2–3 μm diam.
Description in vitro (SNA; CPC 25564): Mycelium subhyaline to pale brown, smooth to slightly verruculose, 2.5–5.5 μm diam. Conidiophores macronematous, emerging from hyphae or conidia, pale brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–5-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 7–31 × 3–5.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding, apex conically truncate, 7–16 × 3–5.5 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, obclavate, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, 33–69 × 3–5 μm, 4–8-septate; hila neither thickened nor darkenedrefractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and rough, lobate margins. Surface olivaceous to pale mouse grey, with patches of white and cinnamon; reverse mouse grey. Colonies reaching 19 mm diam.
Typus: South Korea, Seoul, from leaf spots on Grewia biloba (Malvaceae), 28 Oct. 2014, P.W. Crous, HPC14 (holotype CBS H-25047, culture ex-type CPC 25564 = CBS 149368).
Notes: Pseudocercospora grewiana clustered with Ps. dovyalicola and Ps. dodonaeae (Fig. 1, block 23). Pseudocercospora grewiicola ( Guo et al. 1998) on Grewia in China has a very similar morphology, but causes different lesions, has shorter, 0–2-septate conidiophores (10–40 μm), and broader conidia, 3–6.5 μm wide. Pseudocercospora grewiigena ( Guo 1994) on Grewia biloba in China has a similar morphology, but the leaf spots are dark brown with a yellowish halo on the upper surface and brown on the lower surface, secondary external mycelium is present, and the conidia are larger, 15–100(–147.5) × 3–6.5(–7.6) μm, and 3–13-septate.
Pseudocercospora hakeae (U. Braun & Crous) U. Braun & Crous, Stud. Mycol. 75: 88. 2012. Figs 24–27.
Fig. 24.
A–E. Verrucisporota proteacearum. A. Leaf spots on lower leaf surface. B. Close-up of lesion and sporulation. C, D. Conidiophores and conidiogenous cells giving rise to conidia. E. Conidia. F–M. Pseudocercospora hakeae (CPC 32190). F. Leaf spots on lower and upper leaf surface. G–L. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. M. Conidia. Scale bars = 10 μm.
Fig. 27.
Pseudocercospora hakeae (CPC 32100). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Basionym: Cercostigmina protearum var. hakeae U. Braun & Crous, Sydowia 46: 206. 1994.
Synonym: Pseudocercospora protearum var. hakeae (U. Braun & Crous) U. Braun & Crous, Mycol. Prog. 1: 22. 2002.
(CBS H-25058): Leaf spots amphigenous, sub-circular to circular, 4–8 mm diam, pale brown with indistinct border. Mycelium internal and external, pale brown, consisting of septate, branched, smooth hyphae, 3–4 μm diam. Caespituli fasciculate, amphigenous, pale brown on leaves, up to 100 μm diam and 130 μm high. Conidiophores arising singly from superficial mycelium, or aggregated in loose fascicles arising from the upper cells of a brown stroma up to 80 μm diam and 60 μm high; conidiophores pale to medium brown, smooth, 3–8-septate, subcylindrical, straight to geniculate-sinuous, branched below and above, 30–60 × 4–5 μm. Conidiogenous cells terminal and intercalary, pale brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially, 7–20 × 4–6 μm; scars inconspicuous, 2.5–3.5 μm diam. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, apex subobtuse, base truncate, straight to slightly curved, (5–)8–16-septate, (50–)80–100(–120) × (3–)4–5(–6) μm; hila neither thickened nor darkened-refractive, 2.5–3 μm diam.
Description in vitro (SNA; CPC 32190): Mycelium subhyaline to pale brown, smooth, 1.54 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown, solitary, smooth, unbranched, cylindrical, straight, geniculate-sinuous or truncate at the apex, 1–6-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 12–83.5 × 2.7–3.8 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially or percurrently; sometimes reduced to hyphal loci, aseptate, unbranched, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate to truncate, 6–28 × 2.5–4.5 μm, scars inconspicuous, 1–2.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate to cylindrical, apex rounded, base obconically truncate to truncate, straight to slightly curved, 46.5–123.5 × 2.5–4.5 μm, 5–11-septate; hila neither thickened nor darkened-refractive, 1–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse aerial mycelium, and smooth, rounded margins. Surface pale to dark mouse grey with patches of cinnamon; reverse fawn, folds iron grey. Colonies reaching 14 mm diam.
Materials examined: Australia, Victoria, Royal Botanic Gardens Melbourne, from leaf spots on Hakea sp. (Proteaceae), 2015, P.W. Crous, CBS H-25058, culture CPC 32190 = CBS 149425; Blue Mountains Botanic Garden, Mount Tomah, on leaves of Grevillea sp. (Proteaceae), Aug. 1999, P.W. Crous, JT 873, culture CPC 3145 = CBS 112226; ibid., Sep. 1999, P.W. Crous, cultures JT 873, CPC 3146 = CBS 112225, CPC 3147 = CBS 114184, and CPC 3148 = CBS 114185; Victoria, Royal Botanic Garden Melbourne, from leaf spots on Hakea sp., 2015, P.W. Crous, CBS H-23798, culture CPC 32100 = CBS 144520; Victoria, Royal Botanic Garden Melbourne, from leaf spots on Acacia sp. (Fabaceae), 2015, P.W. Crous, CBS H-25059 = HPC 1748, culture CPC 32366 = CBS 149426.
Notes: Pseudocercospora hakeae (as Cercostigmina protearum var. hakeae; Fig. 1, block 24) was described from leaves of Hakea saligna collected in the Northern Province of South Africa ( Crous & Braun 1994), but lacked viable cultures. Conidia were described as medium brown, cylindrical, 2–9-septate, 40–90 × 4–5 μm. Recently, Crous et al. (2019a) reported a culture of this fungus from leaves of Hakea sp. collected in Australia (HPC 1756 = CBS H-23798, culture CPC 32100 = CBS 144520), with conidia being (1–)3–6(–7)-septate, (15–)30–50(–65) × 4(–5) μm), thus closely matching the holotype. The present collection (CBS H-25058) is morphologically distinct from Ps. hakeae in that it has much longer, multiseptate pale brown conidia. It was isolated from leaf spots where it was found co-occurring with Verrucisporota proteacearum, a common foliar pathogen on various species of Proteaceae ( Crous et al. 2009). Phylogenetically, however, there is no clear distinction between Ps. hakeae and CBS H-25058, and hence we accept it within the morphological variation of Ps. hakeae.
Pseudocercospora halleriae (Chupp & Doidge) Deighton, Mycol. Pap. 140: 145. 1976. Figs 28, 29.
Fig. 28.
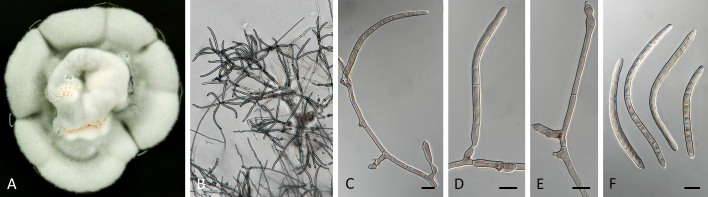
Pseudocercospora halleriae (CPC 21952, ex-epitype culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C. Close-up of fascicles. D–G. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. H. Conidia. Scale bars = 10 μm.
Fig. 29.
Pseudocercospora halleriae (CPC 21952, ex-epitype culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Basionym: Cercospora halleriae Chupp & Doidge, Bothalia 4: 886. 1948.
Leaf spots amphigenous, angular, vein delimited, 3–8 mm diam, medium brown. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, hypophyllous, medium brown on leaves, up to 80 μm diam and 60 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 50 μm diam and 25 μm high; conidiophores medium brown, smooth, 1–2-septate, subcylindrical, straight to slightly curved, unbranched, 15–30 × 4–5 μm. Conidiogenous cells terminal, unbranched, pale to medium brown, smooth, tapering to flat-tipped apical loci, proliferating percurrently near apex, 10–17 × 4–5 μm; scars inconspicuous, 2.5–3 μm diam. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight, 1–3-septate, (17–)20–25 × 3–3.5(–4) μm; hila neither thickened nor darkened-refractive, 2–3 μm diam.
Description in vitro (SNA; CPC 21952): Mycelium subhyaline to pale brown, smooth to verruculose, delicate, uniform in width, 1.5–2.5 μm. Conidiophores macronematous, emerging from hyphae, pale brown, solitary, smooth or finely roughened, unbranched, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 5.5–57 × 3–5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding, apex conically truncate, 5–29 × 3–5 μm, scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, obclavate, apex obtuse to subobtuse, base obconically truncate to truncate, straight to slightly curved, 29–59 × 2–3.5 μm, 2–6-septate; hila neither thickened nor darkened-refractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, lobate margins. Surface dirty white with patches of pale mouse grey; reverse olivaceous to mouse grey. Colonies reaching 19 mm diam.
Typus: South Africa, Mpumalanga Province, Barberton, on leaves of Halleria lucida (Stilbaceae), 16 Oct. 1913, P.A. van der Byl (holotype PREM 7377); Western Cape Province, Cape Town, Kirstenbosch National Botanical Garden, from leaf spots on H. lucida, 29 Dec. 2012, P.W. Crous (epitype designated here CBS H-25042, MBT 10018182, culture ex-epitype CPC 21952 = CBS 149362).
Notes: Pseudocercospora halleriae (Fig. 1, block 13) was originally described from leaves of Halleria lucida collected in Mpumalanga Province, South Africa ( Chupp & Doidge 1948). The present collection correlates well with Ps. halleriae, which causes angular leaf spots and has hypophyllous caespituli, short conidiophores, 10–35 × 2–4 μm, and 1–5-septate, obclavate conidia, 15–70 × 1.5–3 μm. The present collection is herein designated as epitype.
Pseudocercospora lonicerae-japonicae Crous, Y. Zhang ter. & Yuan Yuan Chen, sp. nov. MycoBank MB 852272. Fig. 30.
Fig. 30.
Pseudocercospora lonicerae-japonicae (CPC 30538, ex-type culture). A–D. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus from which it was collected, Lonicera.
Leaf spots amphigenous, irregular to sub-circular, delimited by leaf veins, 1–8 mm diam, sometimes confluent up to 20 mm, purple brown to brown on adaxial surface, yellowish or pale to medium brown on abaxial surface, without definite margin. Mycelium internal and external, pale brown, consisting of septate, branched, smooth hyphae, 1.5–3 μm diam. Caespituli fasciculate, inconspicuous, pale brown on abaxial surface, up to 45 μm diam and 100 μm high. Conidiophores arranged in small loose fascicles, arising from internal hyphae, emerging from very small stromata composed of a few brown hyphal cells, or arising from superficial hyphae; conidiophores pale to medium brown, smooth, 0–7-septate, subcylindrical, straight to variously curved, branched below, 10–80 × 3–5.5 μm. Conidiogenous cells terminal, unbranched, pale brown, smooth, tapering to flat-tipped apical loci, proliferating percurrently near apex, 10–27 × 3–5 μm; scars inconspicuous, 1.5–3 μm diam. Conidia solitary, pale brown, smooth, guttulate, narrowly obclavate, apex obtuse to subobtuse, base long obconically truncate, straight to slightly curved, 3–9-septate, (30–)40–85(–90) × 2.5–4 μm; hila neither thickened nor darkened-refractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, mouse grey becoming white at margin; reverse dark mouse grey becoming smoke grey at margin. Colonies reaching 25 mm diam.
Typus: China, Guizhou Province, from leaf spots on Lonicera japonica (Caprifoliaceae), 2015, J.J. Gan, HPC 1112 = GJJ 160212-31 (holotype CBS H-25054, culture ex-type CPC 30538 = CBS 149389).
Additional material examined: South Korea, from leaf spots on Lonicera japonica (Caprifoliaceae), 7 Nov. 2007, H.D Shin, culture CPC 14801.
Notes: Pseudocercospora lonicerae-japonicae (Fig. 1, block 9) differs from Ps. lonicerae and Ps. lonicerigena in having smaller to absent stromata, and smaller conidia (40–120 × 3.2–6 μm in Ps. lonicerae; 20–100 × 2–4 μm in Ps. lonicerigena; Guo 1994, Braun & Crous 2007). Phylogenetically, these taxa are distinct from Ps. lonicericola, conidia 2–6-septate, 25–64 × 2.5–5 μm (on Lonicera gracilipes var. glabra, Japan) (Fig. 1, block 35) (see Nakashima et al. 2016).
Pseudocercospora lophostemonigena Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852274. Fig. 31.
Fig. 31.
Pseudocercospora lophostemonigena (CPC 16409, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Etymology: Epithet composed of the name of the host genus on which it occurs, Lophostemon, + -genus (produced in a certain place).
Leaf spots amphigenous, irregular to sub-circular, 1–8 mm diam, grey brown with raised red-purple border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate to sporodochial, amphigenous, grey brown on leaves, up to 110 μm diam and 120 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 85 μm diam and 50 μm high; conidiophores medium brown, smooth, 1–9-septate, subcylindrical, straight to variously curved, unbranched, 25–90 × 4–5 μm. Conidiogenous cells terminal, unbranched, medium brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 10–16 × 3–4 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate, apex subobtuse, base truncate, straight to slightly curved, 3–6-septate, (35–)45–55(–65) × 3(–3.5) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, olivaceous grey with patches of dirty white; reverse grey sepia becoming smoke grey at margin; folds appearing dark slate blue, Colonies reaching 28 mm diam.
Typus: Australia, New South Wales, Washpool National Park, S29°11’201 E152°25’70”, 789 m, from leaf spots on Lophostemon confertus (Myrtaceae), Mar. 2009, B.A. Summerell (holotype CBS H-25030, culture ex-type CPC 16409 = CBS 149352).
Notes: Pseudocercospora lophostemonigena (Fig. 1, block 9) needs to be compared to Ps. sawadae (on Psidium guajava, Taiwan; Fig. 1, block 26a) and Ps. lophostemonicola (on Lophostemon, New Zealand). It can be easily distinguished from both species by having 3–6-septate, narrowly obclavate conidia that are shorter than 65 μm (see Braun et al. 2013b). See also the species notes of Ps. lophostemonis.
Pseudocercospora lophostemonis Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852275. Figs 32, 33.
Fig. 32.
Pseudocercospora lophostemonis (CPC 14517, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C. Closeup of fascicles. D–I. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. J. Conidia. Scale bars = 10 μm
Fig. 33.
Pseudocercospora lophostemonis (CPC 14517, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus on which it occurs, Lophostemon.
Leaf spots amphigenous, irregular to sub-circular, 3–8 mm diam, pale to medium brown with diffuse red-brown border. Mycelium internal pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, amphigenous, grey brown on leaves, up to 130 μm diam and 60 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 110 μm diam and 50 μm high; conidiophores medium brown, finely verruculose, 1–3-septate, subcylindrical, straight to geniculate-sinuous, unbranched or branched below or above, 20–45 × 4–6 μm. Conidiogenous cells terminal or intercalary, medium brown, finely verruculose, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 15–25 × 3.5–4 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, medium brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex obtuse, base obconically truncate, straight, (1–)3-septate, (30–)35–40(–45) × (3.5–)4 μm; hila neither thickened nor darkened-refractive, 2 μm diam.
Description in vitro (SNA; CPC 14517): Mycelium hyaline to pale brown, smooth, delicate, 1.5–2.5 μm. Conidiophores micro- to macronematous, emerging from hyphae, pale brown, solitary, smooth, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, holoblastic, 7–47 × 2.5–5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, aseptate, unbranched, conidiogenous loci at the apex and shoulders, apex conically truncate, 6–30 × 2.5–5 μm, scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, guttulate, narrowly obclavate to filiform, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, 45–90(–95) × 2.5–3.5 μm, 5–10-septate; hila neither thickened nor darkened-refractive, 1 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface folded with a prominent network of ridges, with smooth, lobate margins. Surface olivaceous grey to pale olivaceous grey; reverse olivaceous to mouse grey. Colonies reaching 26 mm diam.
Typus: Australia, Northern Territory, Robin Falls, from leaf spots on Lophostemon lactifluus (Myrtaceae), 23 Sep. 2007, B.A. Summerell (holotype CBS H-25026, culture ex-type CPC 14517 = CBS 149350).
Notes: Pseudocercospora lophostemonis (Fig. 1, block 22) needs to be compared to Ps. lophostemonicola (on Lophostemon confertus, New Zealand, conidiophores solitary on superficial mycelium, conidia 3–10-septate, 30–105 × 3–5 μm), from which it is distinguished based on its dense fasciculate conidiophores, and smaller, (1–)3-septate conidia. It is also distinct from Ps. lophostemonigena (Lophostemon confertus, Australia; Fig. 1, block 9) which has longer conidia with more septa, 3–6-septate, (35–)45–55(–65) × 3(–3.5) μm. For notes on Ps. sawadae (Fig. 1, block 26a), see Braun et al. (2013b).
Pseudocercospora macadamiae Beilharz et al., Australas. Pl. Path. 32: 280. 2003.
Typus: Australia, Queensland, Glasshouse Mountains, on living husks of Macadamia integrifolia var. Hinde H2, 5 Feb. 1999, P. Mayers (holotype VPRI 21900, specimen confirmed lost); lectotype here designated, Australas. Pl. Path. 32: 281. 2003, fig. 1, MBT 10018454. Australia, Queensland, Glasshouse Mountains, on living husks of Macadamia integrifolia var. Hinde H2, 12 Nov. 2011, O.A. Akinsanmi (designated as epitype of Ps. macadamiae Beilharz et al. here: BRIP 55526, MBT 10018192, preserved as metabolically inactive culture, and as specimen, culture ex-epitype BRIP 55526 = CBS 133432).
Notes: Pseudocercospora macadamiae (Fig. 1, block 9) causes husk spot of Macadamia in Australia, resulting in premature fruit abscission, and giving rise to nuts with low oil content ( Beilharz et al. 2003). To help understand the mechanism of disease development, its spread, and subsequent role in fruit abscission, Akinsanmi & Carvalhais (2020) published a draft genome sequence of a culture they accepted as ex-epitype of Ps. macadamiae (BRIP 55526), but this typification was never formally introduced, and thus it is done here. The holotype of Ps. macadamiae has the same collection details as the proposed epitype specimen BRIP 55526 collected from the same farm 12 years later.
Pseudocercospora musarum Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852276. Figs 34, 35.
Fig. 34.
Pseudocercospora musarum (CPC 32815, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesions. C. Close-up of stroma. D–G. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. H. Conidia. Scale bars = 10 μm.
Fig. 35.
Pseudocercospora musarum (CPC 32815, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Musa, in genitive plural.
Leaf spots amphigenous, lens-shaped to irregular, 1–4 mm diam, grey brown with raised border and dark brown margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 1.5–2 μm diam. Caespituli fasciculate, amphigenous, pale brown on leaves, up to 50 μm diam and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 60 μm diam and 20 μm high; conidiophores pale brown, smooth, reduced to conidiogenous cells, ampulliform to subcylindrical, proliferating percurrently near apex, 10–15 × 5–8 μm; scars inconspicuous, 2 μm diam. Conidia solitary, pale olivaceous, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight to variously curved, 3–6-septate, (55–)60–80(–90) × (2.5–)3(–3.5) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 32815): Mycelium hyaline to pale brown, smooth, delicate, 1.5–2.3 μm diam. Conidiophores macronematous, emerging from hyphae or conidia, pale brown, solitary, smooth, unbranched, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, 1-septate, sometimes reduced to conidiogenous cells, straight to curved in segments, unbranched, holoblastic, 7.5–11 × 2.7–3.5 μm. Conidiogenous cells integrated, terminal, pale brown, mono- or polyblastic, proliferating sympodially, unbranched, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 4.5–8 × 2.5–3.5 μm, scars inconspicuous, 1 μm diam. Conidia solitary, hyaline to pale, guttulate, filiform, apex obtuse, base truncate to long obconically truncate, straight to slightly curved, 50–100 × 1.52.5 μm, 3–5-septate; hila neither thickened nor darkened-refractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with moderate aerial mycelium, and smooth, lobate margins. Surface pale olivaceous grey; reverse saffron to smoke grey. Colonies reaching 35 mm diam.
Typus: Malaysia, from leaf spots on Musa sp. (Musaceae), 2010, P.W. Crous, HPC 1610 (holotype CBS H-25065, culture ex-type CPC 32815 = CBS 149516).
Notes: Pseudocercospora musarum (Fig. 1, block 28) is phylogenetically distinct from all Pseudocercospora spp. presently recognised on Musa ( Crous et al. 2021). Using the key provided by Braun et al. (2014), it is similar to Ps. assamensis, though phylogenetically distinct (Fig. 1, block 18).
Pseudocercospora musigena Crous, Alfenas & Yuan Yuan Chen, sp. nov. MycoBank MB 852277; Figs 36, 37.
Fig. 36.
Pseudocercospora musigena (CPC 18460, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C. Close-up of stroma. D. Spermatogonium. E, F. Fascicles with conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Fig. 37.
Pseudocercospora musigena (CPC 18460, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Epithet composed of the name of the host genus on which it occurs, Musa, + -genus (produced in a certain place).
Leaf spots amphigenous, elongated angular, 1–2 mm diam, 4–20 mm long, pale brown with dark brown raised margin.
Spermatogonia amphigenous, chiefly epiphyllous, dark brown, substomatal, erumpent, globose, 50–90 μm diam, with central ostiole; wall consisting of 2–3 layers of brown textura angularis. Mycelium internal pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Sporulation sparse. Conidiophores arising from the upper cells of spermatogonia, pale to medium brown, smooth, reduced to conidiogenous cells, straight, unbranched, holoblastic, 10–30 × 3–4 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, medium brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex obtuse, base narrowly obclavate, straight to slightly curved, (1–)3(–5)-septate, (25–)35–50(–60) × (4–)5(–6.5) μm; hila neither thickened nor darkened-refractive, 3–3.5 μm diam.
Description in vitro (SNA; CPC 18460): Mycelium subhyaline, smooth, delicate, 1–2.5 μm diam. Conidiophores macronematous, pale brown, emerging from hyphae or conidia, solitary, cylindrical, smooth, unbranched, 1–2-septate, straight to curved in segments, geniculate-sinuous at the apex, sometimes reduced to conidiogenous cells, straight, holoblastic, 5–21 × 2–3.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, aseptate, unbranched, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 4–15 × 2–3.5 μm; scars inconspicuous, 1–1.5 μm diam. Conidia solitary, subhyaline to pale brown, guttulate, obclavate to filiform, apex obtuse to subobtuse, base obconically truncate to truncate, straight to slightly curved, 38–110(–135) × 2–3 μm, 3–9(–11)-septate; hila neither thickened nor darkenedrefractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, aerial mycelium absent, with smooth, lobate margins. Surface irregularly folded, pale mouse grey with patches of rosy buff; reverse olivaceous to mouse grey. Colonies reaching 27 mm diam.
Typus: Brazil, Minas Gerais, Viçosa, from leaf spots on Musa sp. (Musaceae), 1990, P.W. Crous (holotype CBS H-25036, culture ex-type CPC 18460 = CBS 149357).
Notes: Pseudocercospora musigena (Fig. 1, block 4) is phylogenetically distinct from all Pseudocercospora spp. presently recognised on Musa ( Crous et al. 2021; Fig. 1, blocks 4, 9, 18, 28, 30, 31, 33, and 46), and is closely related to Ps. norchiensis obtained from Rubus from New Zealand.
Pseudocercospora paracydoniae Crous, H.D. Shin & Yuan Yuan Chen, sp. nov. MycoBank MB 852278. Figs 38, 39.
Fig. 38.
Pseudocercospora paracydoniae (CPC 25478, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C, D. Fascicles with conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Fig. 39.
Pseudocercospora paracydoniae (CPC 25478, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to its morphological similarity to Ps. cydoniae.
Leaf spots amphigenous, irregular to sub-circular, 2–8 mm diam, medium brown with raised border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, epiphyllous, iron-grey on leaves, up to 60 μm diam and 70 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 50 μm diam and 50 μm high; conidiophores medium brown, smooth, 1–3-septate, subcylindrical, straight to variously curved, unbranched, 25–35 × 4–5 μm. Conidiogenous cells terminal, medium brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 12–20 × 4–5 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate, apex subobtuse, base obconically truncate, straight to curved, (1–)3(–6)-septate, (35–)45–55(–65) × (3–)4 μm; hila neither thickened nor darkened-refractive, 2.5–3 μm diam.
Description in vitro (SNA; CPC 25478): Mycelium subhyaline to pale brown, smooth to verruculose, delicate, 1.3–3.5 μm diam. Conidiophores micronematous, emerging from hyphae, pale brown, solitary, smooth or roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 13–37 × 3–5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 6–15.5 × 3–4 μm; scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate to filiform, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, 40–99 × 2.5–3.5 μm, 3–10-septate; hila neither thickened nor darkenedrefractive, 1.5–2 μm diam.
Culture characteristics: Colonies after 2 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, smoke grey to mouse grey with patches of dirty white; reverse olivaceous. Colonies reaching 28 mm diam.
Typus: South Korea, Seoul, from leaf spots on Chaenomeles speciosa (Rosaceae), 28 Oct. 2014, P.W. Crous, HPC 8 (holotype CBS H-25046, culture ex-type CPC 25478 = CBS 149367).
Notes: Pseudocercospora paracydoniae (Fig. 1, block 20) needs to be compared to Ps. cydoniae (on Cydonia japonica, USA; conidia 1–3-septate, 30–45 × 2.5 μm; Fig. 1, block 20). The latter fungus has been reported from Asia [China, Japan (MUCC 1184) and Korea] ( Hsieh & Goh 1990, Shin & Kim 2001), and placed into phylogenetic context by Crous et al. (2013a) based on a Korean isolate (CPC 10678). Shin & Kim (2001) also discuss morphological variation within the Asian collections, but still accepted it as one species. As we have shown here, however, there are several distinct phylogenetic species in Asia, with overlapping morphology. Fresh collections are required from the USA, to determine if the fungus on C. japonica is conspecific with material studied from Asia on C. speciosa.
Pseudocercospora parakaki Crous, H.D. Shin & Yuan Yuan Chen, sp. nov. MycoBank MB 852280. Fig. 40.
Fig. 40.
Pseudocercospora parakaki (CPC 14686, ex-type culture). A–D. Fascicles with conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to its morphological similarity to Pseudocercospora kaki.
Leaf spots amphigenous, irregular to sub-circular, delimited by leaf veins, 1–10 mm diam, red brown on adaxial surface, medium brown on abaxial surface, with raised dark brown borders. Mycelium internal to rarely external, pale brown, consisting of septate, branched, smooth hyphae, 1.5–3.5 μm diam. Caespituli fasciculate, amphigenous, grey brown on leaves, up to 130 μm diam and 145 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 70 μm diam and 60 μm high; conidiophores medium brown, smooth, 1–6-septate, subcylindrical, straight to variously curved, unbranched, 30–62 × 3–4.5. Conidiogenous cells terminal, unbranched, medium brown, smooth, tapering to flat-tipped apical loci, proliferating percurrently near apex, 13.5–25.5 × 3–4.5 μm; scars inconspicuous, 1.5–2.5 μm diam. Conidia solitary, pale brown, smooth, guttulate, narrowly obclavate, apex obtuse to subobtuse, base long obconically truncate, straight to slightly curved, 3–9-septate, (30–)40–75(–85) × 3–4 μm; hila neither thickened nor darkened-refractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, pale mouse grey; reverse dark mouse grey becoming vinaceous buff at margin. Colonies reaching 38 mm diam.
Typus: South Korea, from leaf spots on Diospyros kaki (Ebenaceae), 30 Oct. 2007, H.D. Shin (holotype CBS H-25028, culture ex-type CPC 14686 = CBS 149388).
Additional material examined: South Korea, Gongju, on D. lotus, 28 Oct. 2003, H.D. Shin, CBS H-20871, cultures CBS 132019 = CPC 10837, CPC 10838, and CPC 10839.
Notes: The Pseudocercospora spp. occurring on Diospyros were treated by Braun et al. (2020). Crous et al. (2013a) reported a collection from D. lotus from South Korea (CPC 10837–10839) as Ps. kaki. Braun et al. (2020) subsequently treated the Korean material from D. lotus as a new species, Ps. diospyriphila (Fig. 1, block 25). In the present study we add an additional collection, namely from D. kaki, and show this taxon treated as ‘Ps. kaki’ by Crous et al. (2013a) to represent a new species, Ps. parakaki (Fig. 1, block 7) distinct from Ps. kaki (Fig. 1, block 37) and Ps. diospyriphila (Fig. 1, block 25). Conidia of Ps. diospyriphila are shorter, 15–55 × 2.5–3.5 μm, 0–3-septate, while those of Ps. kaki are similar, (15–)25–80(–100) × 2–4 μm, 1–9-septate, although these species are phylogenetically distinct ( Braun et al. 2020).
Pseudocercospora paramacadamiae Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852281. Fig. 41.
Fig. 41.
Pseudocercospora paramacadamiae (CPC 19150, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to its similarity to Pseudocercospora macadamiae.
Description in vitro (SNA; CPC 19150): Mycelium hyaline to pale brown, smooth to slightly sinuous, delicate, 1.5–3 μm diam. Conidiophores micro- to macronematous, emerging from hyphae or conidia, pale brown, solitary, smooth, unbranched to branched, cylindrical, straight to curved, geniculate-sinuous at the apex, 1–3-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 12–47 × 3–4.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to medium brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, apex conically truncate, 7.5–29 × 3–3.5 μm, scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse to subobtuse, base obconically truncate, straight to slightly curved, 34–61.5 × 2.5–3.5 μm, 3–5-septate; hila neither thickened nor darkened-refractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, lobate margins. Surface dirty white with patches of pale mouse grey; reverse vinaceous grey. Colonies reaching 29 mm diam.
Typus: Australia, New South Wales, Valla, Sullivans Road, Gunder plantation, from leaf spots on Macadamia integrifolia (Proteaceae), 14 Oct. 1997, B. Maier (holotype CBS H-25179, culture ex-type CPC 19150 = CBS 129067).
Additional material examined: Australia, New South Wales, Valla, Sullivans Road, Gunder plantation, from leaf spots on Macadamia integrifolia (Proteaceae), 14 Oct. 1997, B. Maier, culture CPC 19149 = CBS 129066.
Notes: Pseudocercospora paramacadamiae represents a sister clade (Fig. 1, block 9) to Ps. macadamiae (conidia subcylindrical, (0–)5–9-septate, (17–)45–69 × 2–2.5 μm; Beilharz et al. 2003), having wider conidia that on average have fewer septa. Isolates of Ps. paramacadamiae were also isolated from nuts with husk spot disease symptoms, suggesting that two species are associated with the disease in Australia.
Pseudocercospora persooniae Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852282. Figs 42, 43.
Fig. 42.
Pseudocercospora persooniae (CPC 32456, ex-type culture). A. Leaf spot on lower leaf surface. B. Close-up of lesion. C. Close-up of fascicles. D–H. Fascicles with conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 43.
Pseudocercospora persooniae (CPC 32456, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology. Name refers to host genus on which it occurs, Persoonia.
Leaf spots amphigenous, associated with tip blight, medium brown with raised red-purple border. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate to sporodochial, hypophyllous, grey brown on leaves, up to 160 μm diam. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 100 μm diam and 40 μm high; conidiophores medium brown, smooth to finely verruculose, 1–4-septate, subcylindrical, straight to geniculate-sinuous, unbranched or branched above, 25–50 × 4–6 μm. Conidiogenous cells terminal or intercalary, unbranched, pale brown, smooth to finely roughened, tapering to flat-tipped apical loci, proliferating sympodially to rarely percurrently near apex, 12–20 × 3–5 μm; scars inconspicuous, 2–3 μm diam. Conidia solitary, pale brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex obtuse, base truncate, straight to curved to once geniculate, (3–)5–7-septate, (35–)45–65(–70) × (3–)3.5–4 μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 32456): Mycelium subhyaline to pale brown, smooth to slightly verruculose, delicate, 1.5–3.5 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, multiseptate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 7–110 × 3–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, unbranched to branched above, conidiogenous loci at the apex and shoulders, protruding truncate, apex conically truncate or truncate, 6.5–23 × 3–6 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse, base obconically truncate, straight to slightly curved, (38–)45–75 × (3–)3.5–4.5 μm, 4–10-septate; hila neither thickened nor darkened-refractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, aerial mycelium absent, with smooth, lobate margins. Surface irregularly folded, grey olivaceous becoming pale lavender grey to dirty white at margin; reverse vinaceous grey to olivaceous. Colonies reaching 22 mm diam.
Typus: Australia, Victoria, Royal Botanical Gardens Melbourne, from leaf spots on Persoonia sp. (Proteaceae), 2015, P.W. Crous, HPC 1920 (holotype CBS H-25063, culture ex-type CPC 32456 = CBS 149430).
Additional material examined: Australia, Victoria, Sardine Creek, from leaves of unidentified tree host, 7 Nov. 2006, P.W. Crous, CBS H-25052, culture CPC 28349 = CBS 149421.
Notes: Pseudocercospora persooniae (Fig. 1, block 9) represents the second Pseudocercospora species described from this host. It is phylogenetically distinct from the first species, Ps. wuchienshiungiae (Fig. 1, block 14). Several species have to date been recorded from Proteaceae ( Crous et al. 2013b), which all differ from Ps. persooniae phylogenetically. It is closely related to Ps. christellae (from Christella parasitica (Thelypteridaceae), Thailand; Fig. 1, block 9), but morphologically distinct in that the latter has longer conidia (3–9-septate, 53–105 × 2–4 μm), and shorter conidiophores (0–1-septate, 9–14 × 2–4 μm; Phengsintham et al. 2013a). Pseudocercospora christellae is currently only known from an ITS sequence and its position in the phylogenetic tree is not stable between different analyses; in the IQ-TREE analysis (Fig. 1) it clusters with Pseudocercospora sp. 014 in block 9 and in the RAxML analysis it clusters among strains of Ps. badjensis (see Figshare; equivalent of clustering in Fig. 1, block 9) (Suppl. Table S3). The ITS sequence of Ps. christellae is 99 % (identity 339/340, including one gap) similar to the ITS sequences of Pseudocercospora sp. 014 and Ps. badjensis.
Pseudocercospora pseudocydoniae Crous, H.D. Shin & Yuan Yuan Chen, sp. nov. MycoBank MB 852283. Fig. 44.
Fig. 44.
Pseudocercospora pseudocydoniae (CPC 14665, ex-type culture). A–C. Fascicles with conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the epithet of Pseudocercospora cydoniae, with which it was confused in the past, expressed by adding the prefix pseudo-.
Leaf spots amphigenous, irregular to angular, delimited by leaf veins, 1–10 mm diam, sometimes confluent up to 20 mm, dark brown with grey center on adaxial surface, dark brown on abaxial surface, without definite margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–4.5 μm diam. Caespituli fasciculate to sporodochial, amphigenous, grey on leaves, up to 115 μm diam and 150 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 100 μm diam and 100 μm high; conidiophores pale brown, smooth, 0–3-septate, subcylindrical to obclavate, straight to variously curved, unbranched, 11–32 × 3–4.5 μm. Conidiogenous cells terminal, unbranched, pale brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially to percurrently, 5–21.5 × 3–5 μm; scars inconspicuous, 1.5–3 μm diam. Conidia solitary, subhyaline, smooth, narrowly obclavate, apex subobtuse, base long obconically truncate, straight to slightly curved, 3–5-septate, (30–)35–65 × 2.5–3.5 μm; hila neither thickened nor darkened-refractive, 1.5–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, rounded margins. Surface irregularly folded, pale mouse grey to mouse grey; reverse olivaceous to smoke grey. Colonies reaching 40 mm diam.
Typus: South Korea, from leaf spots on Chaenomeles lagenaria (Rosaceae), 22 Oct. 2007, H.D. Shin (holotype CBS H-25027, culture ex-type CPC 14665 = CBS 149392).
Notes: Asian specimens of this species were originally identified as Ps. cydoniae (on Cydonia japonica, USA; Fig. 1, block 20) ( Guo et al. 1998). However, Ps. pseudocydoniae (Fig. 1, block 51) has much larger stromata and conidia (20–60 μm diam and 20–65 × 2–3.5 μm, respectively in Ps. cydoniae).
Pseudocercospora pultenaeae Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852284. Fig. 45.
Fig. 45.
Pseudocercospora pultenaeae (CPC 28318, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus it was collected from, Pultenaea.
Description in vitro (SNA; CPC 28318): Mycelium subhyaline to pale brown, smooth to verruculose, 1.5–4.5 μm diam.
Conidiophores micro- to macronematous, emerging from hyphae, pale brown, solitary, smooth, unbranched, cylindrical to clavate, straight to slightly sinuous, geniculate-sinuous at the apex, 1–3-septate, 11.5–78.5 × 4.5–7 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and obconically truncate, apex truncate, 11–25 × 4.5–7 μm; scars inconspicuous, 2–3 μm diam. Conidia solitary, pale brown, guttulate, obclavate to cylindrical, apex obtuse to subobtuse, base obconically truncate to truncate, straight to slightly curved, 50–85(–95) × 3.5–4.5 μm, 3–5-septate; hila neither thickened nor darkened-refractive, 2–3 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, spreading, with sparse to absent aerial mycelium, and smooth, rounded margins. Surface irregularly folded, white to pale mouse grey; reverse olivaceous to mouse grey. Colonies reaching 21 mm diam.
Typus: Australia, Victoria, Genoa, from leaf spots on Pultenaea daphnoides (Fabaceae), 10 Dec. 2000, unknown collector (holotype CBS H-25051, culture ex-type CPC 28318 = CBS 149419).
Notes: Pseudocercospora pultenaeae (Fig. 1, block 9) is introduced here as a new species, and represents the only species of Pseudocercospora known from the genus Pultenaea. It is phylogenetically related to Ps. paramacadamiae (see elsewhere in this publication), which causes husk spot on Macadamia in Australia, and to Ps. ackamae on Ackama rosifolia and Ps. aristoteliae on Aristotelia serrata in New Zealand.
Pseudocercospora rhododendri Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852285. Figs 46, 47.
Fig. 46.
Pseudocercospora rhododendri (CPC 21544, ex-type culture). A. Leaf spots on lower and upper leaf surface. B, C. Close-up of lesions. D, E. Close-up of fascicles. F–H. Fascicles with conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 47.
Pseudocercospora rhododendri (CPC 21544, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Rhododendron.
Leaf spots amphigenous, irregular to sub-circular, 3–12 mm diam, brown with raised dark brown border and at times with diffuse brown margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 1.5–2 μm diam. Caespituli fasciculate, amphigenous, pale brown on leaves, up to 180 μm diam and 40 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a pale brown stroma up to 170 μm diam and 30 μm high; conidiophores pale brown, smooth, 3–6-septate, subcylindrical, straight to variously curved, branched below or above, 35–55 × 4–5 μm. Conidiogenous cells terminal and intercalary, pale brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 10–25 × 3–3.5 μm; scars inconspicuous, 2–2.5 μm. Conidia solitary, pale brown, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight to variously curved, 6–12-septate, (60–)75–95(–100) × 2.5(–3) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 21544): Mycelium pale brown, smooth, delicate, 1.5–4 μm diam. Conidiophores micronematous, emerging from hyphae, pale brown, solitary, smooth or slightly roughened, unbranched to branched below, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, 1–6-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 5.5–58.5 × 2.5–4 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially or percurrently, conidiogenous loci at the apex and shoulders, apex conically truncate to truncate, 5.5–33 × 2.5–3.5 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, cylindrical to filiform, apex obtuse to subobtuse, base obconically truncate to truncate, straight to strongly curved, 63–118 × 2.5–3 μm, 5–8-septate; hila neither thickened nor darkened-refractive, 1.5–2 μm diam.
Culture characteristics: Colonies after 2 wk at 25 °C in the dark on MEA; surface folded with a prominent network of ridges, white with patches of greyish yellow green; reverse olivaceous. Colonies reaching 25 mm diam.
Typus: Netherlands, Utrecht Province, Utrecht, Utrecht Botanical Garden, from leaf spots on Rhododendron sp. (Ericaceae), 3 Oct. 2012, P.W. Crous (holotype CBS H-25040, culture ex-type CPC 21544 = CBS 149360).
Notes: Several species of Pseudocercospora are known from Rhododendron, including Ps. rhododendricola (conidiophores 1–2-septate, conidia 3–6-septate, 54–96 × 2–2.5 μm; Yen 1966) (Fig. 1, block 35), Ps. handelii (now Chuppomyces handelii) and Ps. rhododendri-indici (conidiophores 0–2-septate, conidia 1–4-septate, (35–)40–55(–65) × (2–)3 μm) (Fig. 1, block 9), and Ps. rhododendrigena (conidiophores 1–3-septate, conidia 4–12-septate, 28–90 × 2–4 μm) ( Nakashima et al. 2010, Crous et al. 2013a) (Fig. 1, block 51). Morphologically, Ps. rhododendri (Fig. 1, block 7) is most similar to Ps. rhododendrigena (NBRC105400), but the two species are phylogenetically distinct (Fig. 1, blocks 7 and 51). The ITS sequences of Ps. rhododendri and Ps. rhododendrigena are 97 % (identity 456/472, including five gaps) similar. Pseudocercospora rhododendrigena is only represented by an ITS sequence in the current dataset and its position depends on the phylogenetic analysis: sister to Ps. stranvaesiae in block 51 (Fig. 1, IQ-TREE analysis) or sister to Pseudocercospora sp. 033 (see Figshare; equivalent of clustering in Fig. 1, block 41) in the RAxML analysis (Suppl. Table S3). The ITS sequence of Ps. rhododendrigena is 100 % (identity 468/468 and 472/472) identical to the ITS sequences of Ps. stranvaesiae and Pseudocercospora sp. 033.
Pseudocercospora rubigena Crous, Y. Zhang ter. & Yuan Yuan Chen, sp. nov. MycoBank MB 852286. Figs 48, 49.
Fig. 48.
Pseudocercospora rubigena (CPC 30628, ex-type culture). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C–E. Fascicles with conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Fig. 49.
Pseudocercospora rubigena (CPC 30628, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Epithet composed of the name of the host genus on which it occurs, Rubus, + -genus (produced in a certain place).
Leaf spots amphigenous, irregular to angular, vein delimited, 3–5 mm diam, brown with raised border and wide purple margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2–2.5 μm diam. Caespituli fasciculate, hypophyllous, grey brown on leaves, up to 80 μm diam and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 50 μm diam and 30 μm high; conidiophores medium brown, smooth, 1–3-septate, subcylindrical, straight to slightly curved, unbranched, 30–50 × 4–6 μm. Conidiogenous cells terminal, unbranched, pale to medium brown, smooth, tapering to flat-tipped apical loci, proliferating percurrently near apex, 14–25 × 3.5–4 μm; scars inconspicuous, 2.5–3 μm diam. Conidia solitary, medium brown, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight to slightly curved, 1–4-septate, (30–)45–60(–70) × (3.5–) 4 μm; hila neither thickened nor darkened-refractive, 2–3 μm diam.
Description in vitro (SNA; CPC 30628): Mycelium hyaline to pale brown, smooth to slightly verruculose, 1.5–5 μm diam. Conidiophores macronematous, emerging from hyphae, pale brown to medium brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous or truncate at the apex, 1–6-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 13.5–56.5 × 3–4.5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, unbranched, conidiogenous loci at the apex and shoulders, apex conically truncate to truncate, 11.5–39.5 × 3–4.5 μm; scars inconspicuous, 1.5–2 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse to subobtuse, base long obconically truncate, straight to slightly curved, 43–123.5 × 3–4 μm, (3–)5–10-septate; hila neither thickened nor darkenedrefractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, pale mouse grey to olivaceous grey with patches of dirty white; reverse dark mouse grey to olivaceous; folds appearing dark slate blue. Colonies reaching 27 mm diam.
Typus: China, Guizhou, from leaf spots on Rubus sp. (Rosaceae), 2015, J.J. Gan, HPC 1144 = GJJ160212-01 (holotype CBS H-25056, culture ex-type CPC 30628 = CBS 149424).
Notes: Using the key provided by Guo & Hsieh (1995), Ps. rubigena (Fig. 1, block 9) is distinct from other species on Rosaceae in having medium brown, subcylindrical, 1–4-septate conidia that are narrower than 4 μm, being closely related to Ps. paederiae (on Paederia scandens, Taiwan; conidia 2–7-septate, 25–80 × 3.5–5.5 μm, cylindrical-obclavate, subhyaline to pale olivaceous brown), from which it is morphologically distinct and to Ps. hibbertiae-asperae (on Hibbertia aspera, Australia). Only an ITS sequence is available for the authentic culture of Ps. hibbertiae-asperae included in the present study and sequences from additional protein-coding genes are needed for a proper comparison between the species. Due to the availability of only ITS, the position of Ps. hibbertiae-asperae in the phylogenetic tree is not stable between different analyses; in the IQ-TREE analysis (Fig. 1) it clusters sister to Ps. rubigena in block 9 and in the RAxML analysis it clusters sister to Ps. encephalarticola (see Figshare; equivalent of clustering in Fig. 1, block 7) (Suppl. Table S3). The ITS sequence of Ps. hibbertiae-asperae is 100 % (identity 464/464) identical to the ITS sequences of Ps. rubigena and Ps. encephalarticola.
Pseudocercospora tectonigena Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852287. Figs 50, 51.
Fig. 50.
Pseudocercospora tectonigena (CPC 21663, ex-type culture). A. Leaf spots on upper and lower leaf surface. B, C. Close-up of lesions with fascicles. D, E. Fascicles with conidiogenous cells giving rise to conidia. F. Conidia. Scale bars = 10 μm.
Fig. 51.
Pseudocercospora tectonigena (CPC 21663, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Epithet composed of the name of the host genus on which it occurs, Tectona, + -genus (produced in a certain place).
Occurring on leaf litter. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, amphigenous, pale brown on leaves, up to 60 μm diam and 50 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a brown stroma up to 25 μm diam and 20 μm high; conidiophores medium brown, smooth, 1–3-septate, subcylindrical, straight to variously curved, unbranched, 25–50 × 4–5.5 μm. Conidiogenous cells terminal, medium brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 15–20 × 4–5 μm; scars inconspicuous, 1.5–2 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate, apex subobtuse, base long obconically truncate, straight to curved, 3–8-septate, (45–)60–75(–80) × (3–)3.5 μm; hila neither thickened nor darkened-refractive, 2 μm diam.
Description in vitro (SNA; CPC 21663): Mycelium pale brown, smooth to slightly verruculose, 1.5–4 μm diam. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary, smooth or finely roughened, unbranched to branched below and above, cylindrical, straight to curved in segments, geniculate-sinuous at the apex, multiseptate, 24.5–295 × 4–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown to brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding, apex conically truncate to truncate, 8.5–32 × 4–7 μm, scars inconspicuous, 1–2 μm diam. Conidia solitary, pale brown, guttulate, narrowly obclavate, apex obtuse to subobtuse, base obconically truncate to long obconically truncate, straight to slightly curved, 35–80 × 3–4 μm, 5–10-septate; hila neither thickened nor darkened-refractive, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface folded with a prominent network of ridges, mouse grey to pale mouse grey, with patches of dirty white; reverse dark mouse grey. Colonies reaching 27 mm diam.
Typus: Thailand, Chiang Mai, Chiang Mai Botanical Garden, from leaf spots on Tectona grandis (Lamiaceae), Nov. 2012, P.W. Crous (holotype CBS H-25041, culture ex-type CPC 21663 = CBS 149361).
Additional material examined: Thailand, Chiang Mai Botanical Garden, on leaves of Curcuma sp. (Zingiberaceae), 5 Nov. 2012, P.W. Crous, culture CPC 21861.
Notes: Two species of Pseudocercospora are known from Tectona, namely Ps. tectonae (forms distinct leaf spots, and has obclavate to obclavate-cylindrical conidia that are 2–5-septate, 35–63 × 3–4 μm), and Ps. tectonicola (lacks distinct leaf spots, stromata lacking or up to 20 μm diam, and has much broader conidia, 30–100 × 6.5–8 μm, that can be up to 9-septate; Phengsintham et al. 2013b). Pseudocercospora tectonigena (Fig. 1, block 11) more closely resembles Ps. tectonicola, but is distinct in that it has narrower conidia, and larger stromata. The apparent occurrence on Curcuma sp. (?), needs further clarification.
Pseudocercospora tristaniopsidis Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852288. Fig. 52.
Fig. 52.
Pseudocercospora tristaniopsidis (CPC 28315, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to host genus on which it occurs, Tristaniopsis.
Description in vitro (SNA; CPC 28315): Mycelium smooth to verruculose, hyaline and 1–2 μm diam when young, pale brown and 1.5–5 μm diam when mature. Conidiophores micro- to macronematous, emerging from hyphae, pale brown to brown, solitary or slightly fasciculate, smooth, unbranched to branched, cylindrical, straight to slightly sinuous, geniculate-sinuous at the apex, multiseptate, 15–217(–230) × 3.5–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and truncate, apex truncate, 7.5–25 × 2–5 μm, scars inconspicuous, 1–2.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse, base obconically truncate to long obconically truncate, straight to slightly curved, (30–)47–85 × 3.5–5 μm, (3–)5–12-septate; hila neither thickened nor darkened-refractive, 1.5–2.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, olivaceous becoming mouse grey at margin; reverse olivaceous becoming dark mouse grey at margin. Colonies reaching 24 mm diam.
Typus: Australia, New South Wales, Morton National Park, from leaf spots on Tristaniopsis collina (Myrtaceae), 25 Nov. 2000, B.A. Summerell (holotype CBS H-25050, culture ex-type CPC 28315 = CBS 149418).
Additional material examined: Australia, New South Wales, Northern Tablelands, on Eucalyptus approximans (Myrtaceae), 27 Mar. 2009, B.A. Summerell, culture CPC 16373.
Notes: Pseudocercospora tristaniopsidis (Fig. 1, block 9) is introduced here as a new species, and represents the only species of Pseudocercospora known from the genus Tristaniopsis. Phylogenetically it is closely related to Ps. persooniae (conidia subcylindrical, (3–)5–7-septate, (35–)45–65(–70) × (3–)3.5–4 μm), thus being morphologically clearly distinct. It is also phylogenetically distinct from species known from Eucalyptus.
Pseudocercospora victoriae Crous, Summerell & Yuan Yuan Chen, sp. nov. MycoBank MB 852289. Figs 53–55.
Fig. 53.
Pseudocercospora victoriae (CPC 32428). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesions. C–F. Fascicles with conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Fig. 55.
Pseudocercospora victoriae (CPC 32442, ex-type culture). A, B. Leaf spots on upper leaf surface. C–G. Fascicles with conidiogenous cells giving rise to conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the state in Australia where it was collected, Victoria.
Leaf spots amphigenous, irregular to angular, 2–8 mm diam, medium brown with raised border and red purple margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, amphigenous, pale grey brown on leaves, up to 80 μm diam and 70 μm high. Conidiophores aggregated in loose fascicles arising from the upper cells of a brown stroma up to 25 μm diam and 20 μm high; conidiophores medium brown, smooth, 1–3-septate, subcylindrical, straight to slightly curved, unbranched, 35–70 × 4–5 μm. Conidiogenous cells terminal, unbranched, pale brown, smooth, tapering to flat-tipped apical loci, proliferating sympodially and percurrently near apex, 18–30 × 3.5–4 μm; scars inconspicuous, 2–2.5 μm diam. Conidia solitary, medium brown, smooth, guttulate, narrowly obclavate, apex subobtuse, base long obconically truncate, straight to slightly curved, (3–)5–7-septate, (45–)60–75(–80) × (3–)4(–5) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 32428): Mycelium subhyaline to pale brown, smooth to slightly verruculose, 1.5–3.5 μm diam. Conidiophores micro- to macronematous, emerging from hyphae or germinated conidia, pale brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, multiseptate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 19–193 × 4.5–6 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, unbranched, conidiogenous loci at the apex and shoulders, protruding and truncate, apex conically truncate, 8.5–19 × 4–7 μm, scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, guttulate, obclavate, apex obtuse, base obconically truncate, straight to slightly curved, 33.5–67 × 3.5–5.5 μm, 5–10-septate; hila neither thickened nor darkenedrefractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; erumpent, with moderate to absent aerial mycelium, and roughed, lobate margin. Surface pale mouse grey to white with patches of cinnamon; reverse smoke grey to hazel. Colonies reaching 23 mm diam.
Typus: Australia, Victoria, Mount Best, Tin Mine road, from leaf spots on Eucalyptus globoidea (Myrtaceae), 2015, P.W. Crous (holotype CBS H-25062, culture ex-type CPC 32442 = CBS 149429).
Additional materials examined: Australia, Victoria, from leaf spots on Eucalyptus radiata (Myrtaceae), 2015, P.W. Crous, HPC 1828 = CBS H-25061, culture CPC 32428 = CBS 149428; New South Wales, Wingecarribee Shire, Paddy’s River, on leaves of E. dives, Mar. 2009, B.A. Summerell, culture CPC 16385; Queensland, on leaves of Eucalyptus sp. on cultivated forest tree, Aug. 2005, A. Carnegie, culture VPRI 40611 = CPC 28347.
Notes: Pseudocercospora victoriae (Fig. 1, block 9) is described as a new foliar pathogen of Eucalyptus dives, E. globoidea and E. radiata, being closely related to Ps. subulata (percurrent annellations conspicuous, conidia broadly acicular to obclavate-cylindrical, 3–10-septate, 10–100 × 4–7 μm; Braun & Dick 2002; Crous et al. 2019c).
Pseudocercospora wingfieldii Crous & Yuan Yuan Chen, sp. nov. MycoBank MB 852290. Fig. 56, 57.
Fig. 56.
Pseudocercospora wingfieldii (CPC 26287, ex-type culture). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Fig. 57.
Pseudocercospora wingfieldii (CPC 26287, ex-type culture). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Named in honour of Michael J. Wingfield, who collected this fungus, and many others, with PWC during a collection trip to Réunion.
Leaf spots amphigenous, irregular to sub-circular, 2–3 mm diam, medium brown with raised dark brown border. Mycelium internal, subhyaline, consisting of septate, branched, smooth hyphae, 1.5–2 μm diam. Caespituli fasciculate, hypophyllous, pale olivaceous on leaves, up to 60 μm diam and 70 μm high. Conidiophores aggregated in dense fascicles arising from the upper cells of a pale brown stroma up to 45 μm diam and 20 μm high; conidiophores pale olivaceous, smooth, 1–2-septate, subcylindrical, straight to slightly curved, unbranched, 15–35 × 3–5 μm. Conidiogenous cells terminal, unbranched, pale olivaceous, smooth, tapering to flat-tipped apical loci, proliferating sympodially and inconspicuously percurrently near apex, 7–15 × 2.5–4 μm; scars inconspicuous, 1.5–2 μm diam. Conidia solitary, pale olivaceous, smooth, guttulate, subcylindrical, apex obtuse, base truncate, straight to curved, 5–10(–22)-septate, (65–)75–100(–160) × (2.5–)3(–3.5) μm; hila neither thickened nor darkened-refractive, 2–2.5 μm diam.
Description in vitro (SNA; CPC 26287): Mycelium subhyaline to pale brown, smooth to slightly verruculose, delicate, uniform in width, 1–3 μm. Conidiophores micronematous, emerging from hyphae or conidia, pale brown, solitary, smooth, unbranched, cylindrical, straight, geniculate-sinuous at the apex, 1-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 5–15 × 2.5–4.5 μm. Conidiogenous cells integrated, terminal, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding and conically truncate, apex conically truncate, 5–10.5 × 3–4 μm; scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, cylindrical to filiform, apex obtuse to subobtuse, base obconically truncate to truncate, slightly curved to curved, 84.5–118(–130) × 2.5–3 μm, 8–11-septate; hila neither thickened nor darkened-refractive, 1–2 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface irregularly folded, with a prominent network of ridges, greyish green and becoming dirty white at margin; reverse fawn to olivaceous. Colonies reaching 25 mm diam.
Typus: France, Réunion, S21°5’45.7” E55°33’3.6 from leaf spots on Acacia heterophylla (Fabaceae), 7 Mar. 2015, P.W. Crous & M.J. Wingfield, HPC 257 (holotype CBS H-25048, culture ex-type CPC 26287 = CBS 149369).
Notes: See discussion under Ps. acaciicola above (Fig. 1, block 25). Pseudocercospora wingfieldii (Fig. 1, block 26a) is distinct from other species of Pseudocercospora known from this host genus based on its long, narrow, multiseptate, pale olivaceous, subcylindrical conidia.
Unresolved species complexes and synonymies
Pseudocercospora diplusodonis Meir. Silva et al. [as ‘diplusodonii’], Persoonia 37: 153. 2016.
Hosts and distribution: Brazil, on Diplusodon sp. (Lythraceae), culture ex-type COAD 1476. South Korea, on Diospyros lotus (Ebenaceae), cultures CPC 14778 and CPC 14804; on Ilex verticillata (Aquifoliaceae), culture CPC 25429; on Rosa multiflora (Rosaceae), culture CPC 25419.
New synonym: Pseudocercospora ershadii M. Bakhshi et al., Fungal Syst. Evol. 6: 105. 2020.
Hosts and distribution: Iran, on Diospyros lotus (Ebenaceae), IRAN 16456F – holotype, CCTU 1206 = CBS 136114 – ex-type cultures, additional cultures IRAN 3431C, IRAN 3429C, IRAN 3428C, IRAN 3430C.
Note: Although Ps. diplusodonis (Fig. 1, block 43) was described from Diplusodon sp. in Brazil, it is here recorded from several unrelated hosts in South Korea and Iran.
Pseudocercospora nogalesii (Urries) U. Braun & M.A. Dick, Australas. Pl. Path. 32: 91. 2003.
Hosts and distribution: New Zealand, Chamaecytisis proliferus (Fabaceae), culture CBS 115022; Virgilia divaricara (Fabaceae), culture CBS 122501. Spain (Canary Islands), Cytisus proliferus (original description).
Note: Originally described from leaves of Cytisus proliferus (Fabaceae), Canary Islands, both strains in the present study were also isolated from Fabaceae hosts (Fig. 1, block 4).
Pseudocercospora norchiensis Crous, Fungal Diversity 26: 172. 2007.
Hosts and distribution: Italy, Eucalyptus sp. (Myrtaceae; original description). New Zealand, Eucalyptus sp. (Myrtaceae), culture CBS 120738 = CPC 13049, Rubus sp. (Rosaceae), cultures CBS 114641 and CBS 118413.
Note: Originally described from Eucalyptus leaves in Italy, and here reported on an unrelated host family, Rosaceae, in New Zealand (Fig. 1, block 4).
Pseudocercospora trichogena Guatim. et al., Persoonia 37: 135. 2016.
Hosts and distribution: Brazil, on leaves of Deparia petersenii (Athyriaceae), culture COAD 1088; on leaves of Macrothelypteris torresiana (Thelypteridaceae), culture ex-type COAD 1087.
Note: The two involved host families of the Polypodiopsida (ferns) are allied and pertain to the Polypodiales, Aspleniineae, so that it is not surprising that the two isolates appear phylogenetically identical (similarities 504/504 nt ITS, 198/198 nt actA, 471/471 nt tef1) (Fig. 1, block 11).
Pseudocercospora wuchienshiungiae Y.P. Tan et al. [as ‘wuchienshiung’], Index of Australian Fungi 6: 11. 2023.
New synonym: Pseudocercospora blackwoodiae Y.P. Tan et al., Persoonia 50: 283. 2023.
Notes: Pseudocercospora blackwoodiae was published as a Fungal Planet description sheet in Persoonia with an effective online publication date of 29 June 2023 ( Crous et al. 2023), while Ps. wuchienshiungiae (as ‘wuchienshiung’) (Fig. 1, block 14) was published as an Index of Australian Fungi entry with an effective online publication date of 9 May 2023 ( Tan & Shivas 2023). The two species were described from Queensland on Persoonia falcata. The metabolically inactive culture as holotype and GenBank numbers of derived sequences are identical between the two publications and therefore the later published Ps. blackwoodiae is reduced to synonymy under the first published name.
Pseudocercospora wusaulaniae Y.P. Tan et al. [as ‘wusaulan’], Index of Australian Fungi 6: 12. 2023.
New synonym: Pseudocercospora dalyelliae Y.P. Tan et al., Persoonia 50: 285. 2023.
Notes: Pseudocercospora dalyelliae was published as a Fungal Planet description sheet in Persoonia with an effective online publication date of 29 June 2023 ( Crous et al. 2023), while Ps. wusaulaniae (as ‘wusaulan’) (Fig. 1, block 19) was published as an Index of Australian Fungi entry with an effective online publication date of 9 May 2023 ( Tan & Shivas 2023). The two species were described from Queensland on Senna alata. The metabolically inactive culture as holotype and GenBank numbers of derived sequences are identical between the two publications and therefore the later published Ps. dalyelliae is reduced to synonymy under the first published name.
Block 4
Pseudocercospora luzardii Furlan. & Dianese, Mycol. Res. 103: 1207. 1999.
Host and distribution: Brazil, on leaves of Hancornia speciosa (Apocynaceae), culture ex-epitype COAD 1505 = CPC 25196 and culture CPC 2556 = STE-U 2556.
Pseudocercospora convoluta (Crous & Den Breeÿen) U. Braun et al., Stud. Mycol. 87: 312. 2017.
Basionym: Passalora convoluta Crous & Den Breeÿen, Fungal Diversity 23: 96. 2006.
Host and distribution: Costa Rica, on leaves of Chromolaena odorata (Asteraceae), ex-type CBS 113377.
Note on Block 4 : Although phylogenetically similar (ITS: 485/487 nt; additional genes needed) (Fig. 1, block 4), they occur on different host families, and are morphologically distinct (Den Breeÿen et al. 2006, Silva et al. 2016).
Block 10
Pseudocercospora balsaminae (Syd.) Deighton, Mycol. Pap. 140: 139. 1976.
Basionym: Cercoseptoria balsaminae Syd., Ann. Mycol. 33: 69. 1935.
Hosts and distribution: India, Uttar Pradesh, on leaves of Impatiens balsamina (Balsaminaceae) (type). South Korea, on leaves of Impatiens textorii, cultures CBS 131882 = CPC 10044 and CBS 132020 = CPC 10699.
Pseudocercospora boehmeriigena U. Braun, in Braun & Mel’nik, Trudy Botanicheskogo Instituta im. V.L. Komarova 20: 42. 1997. Replaced synonym: Cercospora boehmeriae Peck, Ann. Rep. N.Y. St. Mus. nat. Hist. 34: 48. 1883. [1881].
Host and distribution: Brazil, on leaves of Boehmeria nivea (Urticaceae), culture ex-epitype COAD 1562 = CPC 25243.
New synonym: Cercospora fukuii W. Yamam. [as ‘fukui’], Jour. Soc. Trop. Agric. Formosa 6: 601. 1934.
Pseudocercospora fukuii (W. Yamam.) W.H. Hsieh & Goh, Trans. Mycol. Soc. R.O.C. 2: 115. 1987.
Host and distribution: Japan, Tokyo, Akiruno, Itsukaichi, on leaves of Boehmeria nivea var. concolor f. nipononivea (Urticaceae), culture ex-epitype MAFF 238121 = MUCC 1297.
Pseudocercospora crocea Crous et al., Stud. Mycol. 75: 83. 2012. [2013].
Host and distribution: South Korea, on leaves of Pilea hamaoi (Urticaceae), culture ex-type CBS 126004 = CPC 11668.
Pseudocercospora cyatheicola Crous & R.G. Shivas [as ‘cyathicola’], Crous et al., Persoonia 26: 121. 2011.
Host and distribution: Australia, on fronds of Cyathea australis (Cyatheaceae), culture ex-type CBS 129520 = CPC 17047.
Pseudocercospora dianellae U. Braun & C.F. Hill, Australas. Pl. Path. 32: 89. 2003.
Hosts and distribution: New Zealand, on leaves of Dianella nigra (Asphodelaceae) (type); on leaves of Dianella caerulea, culture CBS 117746.
Pseudocercospora farfugii C. Nakash., I. Araki & Ai Ito, in Chen et al., Stud. Mycol. 101: 540. 2022.
Host and distribution: Japan, on leaves of Farfugium japonicum (Asteraceae), culture ex-type MUCC 978.
Notes: The present species formed a clade with Ps. textariae on Tectaria harlandii (Tectariaceae). However, the sequencing data of the latter species is only known as an rDNA ITS, and its accurate phylogenetic position is unclear. The morphological characteristics are also different from each other in the size and shape of conidia and conidiogenesis. See Chen et al. (2022) and Kirschner & Wang (2015).
Pseudocercospora fatouae Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and Similar Fungi from Taiwan (Taipei): 236. 1990.
Hosts and distribution: Taiwan, on leaves of Fatoua villosa (Moraceae) (type). South Korea, on leaves of Fatoua villosa, culture CPC 11648.
Pseudocercospora hachijokibushi C. Nakash., H. Horie & Tak. Kobay., Mycoscience 45(1): 50. 2004.
Host and distribution: Japan, on leaves of Stachyurus praecox var. matsuzakii (Stachyuraceae), culture ex-type MAFF 238479 = MUCC 1337.
Pseudocercospora humulicola Crous et al., Stud. Mycol. 75: 89. 2012. [2013].
Host and distribution: South Korea, on leaves of Humulus scandens (Cannabaceae), culture ex-type CBS 131585 = CPC 11358.
Pseudocercospora lygodiicola Y.L. Guo & U. Braun, in Braun, Nakashima & Crous, IMA Fungus 4(2): 317. 2013.
Hosts and distribution: China, on leaves of Lygodium japonicum (Lygodiaceae) (type). Brazil, on fronds of Lygodium volubile, culture COAD 1745 = CPC 25755.
Pseudocercospora macarangae (Syd. & P. Syd.) Deighton, Mycol. Pap. 140: 47. 1976.
Basionym: Cercospora macarangae Syd. & P. Syd., Annls mycol. 12(6): 575. 1914.
Hosts and distribution: Philippines, on leaves of Macaranga tanarius (Euphorbiaceae) (type). Laos, on leaves of Macaranga denticulata, culture P564.
Pseudocercospora plectranthi G.C. Hunter et al., Stud. Mycol. 75: 96. 2012. [2013]
Host and distribution: South Korea, from leaves of Plectranthus sp. (Lamiaceae), culture ex-type CBS 131586 = CPC 11462.
Pseudocercospora profusa (Syd. & P. Syd.) Deighton, Trans. Brit. Mycol. Soc. 88: 388. 1987.
Basionym: Cercospora profusa Syd. & P. Syd., Ann. Mycol. 7: 175. 1909.
Host and distribution: South Korea, on leaves of Acalypha australis (Euphorbiaceae), culture ex-epitype CPC 10713 and culture CBS 132306 = CPC 10055.
Pseudocercospora pteridicola U. Braun & Y.L. Guo, in Braun & Mel’nik, Trudy Botanicheskogo Instituta im. V.L. Komarova 20: 84. 1997.
Hosts and distribution: China, Sichuan, on leaves of Pteris vittata (Pteridaceae) (type). Taiwan, on leaves of Pteris semipinnata, specimen R. Kirschner 4166.
Pseudocercospora pteridophytophila Goh & W.H. Hsieh, Trans. Mycol. Soc. Rep. China 4(2–3): 25–38. 1989.
Host and distribution: Taiwan, on leaves of Cyclosorus acuminatus (Thelypteridaceae) (type); on leaves of Cyclosorus parasiticus, voucher TNM 3602.
Pseudocercospora rhabdothamni U. Braun & C.F. Hill, Australas. Pl. Path. 33: 489. 2004.
Host and distribution: New Zealand, on leaves of Rhabdothamnus solandri (Gesneriaceae), culture ex-type CBS 114872 = ICMP 15289.
Pseudocercospora rumohrae Goh & W.H. Hsieh, Trans. Mycol. Soc. Rep. China 4: 29. 1989.
Hosts and distribution: Taiwan, on leaves of Rumohra adiantiformis (Dryopteridaceae) (type). New Zealand, on leaves of Ptisana (=Marattia) salicina (Marattiaceae), culture CBS 117747.
Pseudocercospora sp. 016
Host and distribution: New Zealand, on Rubus × barkeri (Rosaceae), culture CPC 14813.
Pseudocercospora tectariae R. Kirschner, in Kirschner & Wang, Mycol. Progr. 14(65): 7. 2015.
Host and distribution: Taiwan, on living sterile fronds of Tectaria harlandii (Tectariaceae), from type and from authentic culture BCRC FU30377.
Notes on Block 10 : Although similar in DNA phylogeny (Fig. 1, block 10), these taxa are morphologically different, and mostly occur on unrelated host families, some of which are restricted in their distribution, suggesting that the genes used here lack the resolution to properly distinguish these species. See also Ps. farfugii. Although Ps. fatouae, Ps. macarangae, Ps. pteridicola, Ps. pteridophytophila and Ps. tectariae are represented by only an ITS sequence each in the current dataset, their phylogenetic association stays the same between the IQ-TREE and RAxML analyses (Suppl. Table S3).
Block 20c - Pseudocercospora pruni-persicicola complex
Pseudocercospora sp. 069 on Buxus megistophylla in China. Figs 58, 59.
Fig. 58.
Pseudocercospora sp. 069 (CPC 30540). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesion. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Fig. 59.
Pseudocercospora sp. 069 (CPC 30540). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
(CBS H-25055): Leaf spots amphigenous, sub-circular to circular, 4–10 mm diam, medium brown with raised border and red-brown margin. Mycelium internal, pale brown, consisting of septate, branched, smooth hyphae, 2.5–3 μm diam. Caespituli fasciculate, chiefly hypophyllous, brown on leaves, up to 100 μm diam and 120 μm high. Conidiophores in dense fascicles arising from the upper cells of a brown stroma up to 65 μm diam and 40 μm high; conidiophores brown, verrucose, 1–2-septate, subcylindrical, straight to variously curved, unbranched, 20–35 × 4–5 μm. Conidiogenous cells terminal, pale to medium brown, verruculose, proliferating percurrently near apex, with rough annellations, 10–15 × 4–5 μm; scars inconspicuous, 2.5–3 μm diam. Conidia solitary, pale brown, verruculose with longitudinal striations, guttulate, narrowly obclavate, apex subobtuse, base obclavate, straight to slightly curved, (2–)3–5(–8)-septate, (27–) 45–60(–80) × (2.5–)3 μm; hila neither thickened nor darkened, 2 μm diam.
Description in vitro (SNA; CPC 30540): Mycelium subhyaline to pale brown, smooth to slightly verruculose, delicate, 1.5–3.5 μm diam. Conidiophores micro- to macronematous, emerging from hyphae or conidia, pale brown, solitary, smooth or slightly roughened, unbranched, cylindrical, straight to slightly curved in segments, geniculate-sinuous at the apex, 1–2-septate, sometimes reduced to conidiogenous cells, straight, unbranched, holoblastic, 12–26.5 × 3.5–5 μm. Conidiogenous cells integrated, terminal or intercalary, pale brown, mono- or polyblastic, proliferating sympodially, conidiogenous loci at the apex and shoulders, protruding, apex conically truncate, 5–16.5 ×3–4 μm; scars inconspicuous, 1–1.5 μm diam. Conidia solitary, pale brown, obclavate, apex obtuse to subobtuse, base long obconically truncate, straight to slightly curved, 25–75 × 2.5–3 μm, (1–)3–8-septate; hila neither thickened not darkened, 1–1.5 μm diam.
Culture characteristics: Colonies after 3 wk at 25 °C in the dark on MEA; surface folded with a prominent network of ridges, surface smoke grey with patches of lavender blue; reverse mouse grey becoming olivaceous at the margin. Colonies reaching 28 mm diam.
Material examined: China, Guizhou, from leaf spots on Buxus megistophylla (Buxaceae), 2015, J.J. Gan, HPC 1113 = GJJ 160210-01 = CBS H-25055, culture CPC 30540 = CBS 149423.
Species involved in this complex based on Fig. 1:
Pseudocercospora eupatorii-formosani U. Braun & Bagyan., Sydowia 51: 8. 1999.
Synonyms: Cercospora eupatorii-formosani Sawada, Rep. Dept. Agric. Gov. Res. Inst. Formosa 86: 169. 1943, nom. inval. (Art. 39.1).
Pseudocercospora eupatorii-formosani J.M. Yen [as ‘(Sawada) J.M. Yen’], Gard. Bull. Singapore 33: 175. 1980, nom. inval. (Art. 39.1).
Pseudocercospora eupatorii-formosanae J.M. Yen ex Y.L. Guo & W.H. Hsieh [as ‘(Sawada ex Y.L. Guo & W.H. Hsieh) J.M. Yen’], Mycosystema Monographicum Series 2: The genus Pseudocercospora in China: 67. 1995, nom. inval. (Art. 40.1, Art. 40.4).
Host and distribution: Laos, on Chromolaena odorata (Asteraceae), cultures CPC 17319 = NOUL p.9 and CPC 19195 = LC 0395. South Africa, on leaves of Solanum mauritianum (Solanaceae), culture CPC 25040. Taiwan, on leaves of Eupatorium sp. (Asteraceae), culture ex-epitype NCHUPP_ L1606, and from unknown host, culture CPC 20054. Unknown, no collection information, cultures CBS 112825 = C 439 = CPC 4087 = STE-U 4087 and CBS 115494 = C 444 = CPC 4088 = STE-U 4088. USA, unknown host, culture CBS 115493 = C 448 (a) = CPC 4073 = STE-U 4073.
Pseudocercospora ginkgoana R. Kirschner, Pl. Pathol. Quarant. 8: 12. 2018.
Host and distribution: Taiwan, on dead margin of living leaves of Ginkgo biloba (Ginkgoaceae), culture R. Kirschner 3563.
Pseudocercospora pruni-persicicola (J.M. Yen) J.M. Yen, Bull. Soc. Mycol. Fr. 97: 94. 1981.
Synonym: Cercospora pruni-persicicola J.M. Yen, Rev. Mycol. (Paris): 63. 1978.
Host and distribution: South Korea, on leaves of Prunus avium (Rosaceae), culture KACC 47019.
Pseudocercospora sp. 069
Host and distribution: China, on leaves of Buxus megistophylla (Buxaceae), culture CPC 30540 = CBS 149423.
Notes on Block 20c: Because there are presently no records of Pseudocercospora spp. known from Buxus, the Chinese material was initially assumed to represent a new species (Fig. 1, block 20c). However, based on the phylogenetic data, the Pseudocercospora on Chinese Buxus pertains to a complex (clade; Block 20c) within Block 20 comprised of sequences retrieved from several species, including Ps. pruni-persicicola, Ps. eupatorii-formosani, and Ps. ginkgoana, with insufficient resolution based on the currently applied markers. The involvement of a single plurivorous species cannot be excluded with absolute certainty, but it is highly unlikely that we are dealing with a single species. The hosts involved in this complex are completely unrelated, ranging from Ginkgo (gymnosperms) to Chromolaena odorata (Compositae, i.e., advanced dicots). Furthermore, the morphology of the taxa involved is strongly different from each other. The Pseudocercospora on Buxus is, in contrast to the other involved species, characterised by having internal mycelium in vivo, distinctly verruculose conidiophores and conidia, and percurrently proliferating conidiogenous cells with conspicuous rough annellations. This type of proliferation and annellation is not present in all other species of this complex, and there are additional significant morphological differences in the formation of stromata, external mycelium, and in the dimension and shape of the conidiophores and conidia.
Pseudocercospora pruni-persicicola is currently represented by a single isolate with only an ITS sequence in the dataset and its phylogenetic position varies depending on the phylogenetic analysis used, namely clustering among isolates of Ps. eupatoriiformosani in block 20 (Fig. 1, IQ-TREE analysis) or sister to Ps. sphendamnophila (see Figshare; equivalent of clustering in Fig. 1, block 25) in the RAxML analysis (Suppl. Table S3). The ITS sequence of Ps. pruni-persicicola is 99 % (identities 466/469 and 473/474, both including one gap) similar to the ITS sequences of Ps. eupatorii-formosani and Ps. sphendamnophila. Pseudocercospora ginkgoana is also represented only by an ITS sequence, but it clusters with the same association between the IQ-TREE and RAxML analyses (Suppl. Table S3). The Pseudocercospora pruni-persicicola complex requires the application of additional makers for a better resolution at species level, and further pathogenicity studies are needed to confirm the phylogenetic-taxonomic all species involved in this clade (see Choi et al. 2014).
Block 26a
Pseudocercospora chionanthi-retusi Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and Similar Fungi from Taiwan (Taipei): 249. 1990.
Host and distribution: Taiwan, on leaves of Chionanthus retusus var. serrulatus (Oleaceae), culture ex-epitype NCHUPP L1605.
Pseudocercospora marginalis G.C. Hunter et al., Stud. Mycol. 75: 92. 2012. [2013].
Host and distribution: South Korea, on leaves of Fraxinus rhynchophylla (Oleaceae), ex-type CBS 131582 = CPC 12497.
Note on Block 26a: Although phylogenetically similar (ITS: 475/477 nt; actA: 220/220 nt; tef1: 311/313 nt) (Fig. 1, block 26), the host genera and fungal morphology are distinct.
Block 28 – Pseudocercospora atromarginalis complex
Pseudocercospora atromarginalis (G.F. Atk.) Deighton, Mycol. Pap. 140: 139. 1976.
Basionym: Cercospora atromarginalis G.F. Atk., J. Elisha Mitchell scient. Soc. 8: 59. 1892.
Synonyms: See Braun (2017).
Hosts and distribution: Brazil, on leaves of Solanum americanum (Solanaceae), culture COAD 1975 = CPC 25230. Iran, on leaves of Solanum nigrum (Solanaceae), cultures CBS 136112 = CCTU 1056, CCTU 1052, CCTU 1091 and CCTU 1193. New Zealand, on Solanum sp. (Solanaceae), culture CBS 114640. South Korea, on leaves of Solanum nigrum (Solanaceae), cultures CPC 14649 and CBS 132010 = CPC 11372. USA, on Solanum nigrum (Solanaceae) (type).
Pseudocercospora chengtuensis (F.L. Tai) Deighton, Mycol. Pap. 140: 141. 1976.
Basionym: Cercospora chengtuensis F.L. Tai, Lloydia 11: 40. 1948.
Hosts and distribution: China, on Lycium chinense (Solanaceae) (type). South Korea, on Lycium chinense (Solanaceae), culture CBS 131924 = CPC 10696.
Pseudocercospora cruenta (Sacc.) Deighton, Mycol. Pap. 140: 142. 1976.
Basionym: Cercospora cruenta Sacc., Michelia 2(no. 6): 149. 1880.
Hosts and distribution: USA, on leaves of Phaseolus (Fabaceae) (type). Trinidad and Tobago, on Vigna sp. (Leguminosae), culture CBS 132021 = CPC 10846 (block 28).
Pseudocercospora dingleyae U. Braun & C.F. Hill [as ‘dingleyi’], Mycol. Progr. 1(1): 23. 2002.
Competing homonym: non Pseudocercospora haloragis (Hansf.) U. Braun [as ‘haloragidis’], Monogr. Cercosporella, Ramularia Allied Genera (Phytopath. Hyphom.) 1: 109. 1995.
Replaced synonym: Cercospora haloragis Dingley [as ‘haloragi’], N.Z. Jl agric. Res. 8(4): 913. 1965.
Host and distribution: New Zealand, on Haloragis erecta (Haloragaceae), type as well as culture CBS 114645.
Pseudocercospora fuligena (Roldan) Deighton, Mycol. Pap. 140: 144. 1976.
Basionym: Cercospora fuligena Roldan, Philipp. J. Sci. 66: 8. 1938.
Hosts and distribution: Philippines, on leaves of Solanum lycopersicum (Solanaceae) (type). Thailand, on Solanum lycopersicum, culture CBS 132017 = CPC 12296. Taiwan, on Solanum lycopersicum, culture CBS 109729. USA, on Solanum lycopersicum, culture PF001.
Pseudocercospora mangiferae Tamakaew & Cheew., Chiang Mai J. Sci. 49(3): 687. 2022.
Host and distribution: Thailand, on leaves of Mangifera indica (Anacardiaceae), culture SDBR-CMU401 and culture ex-type SDBR-CMU403.
Pseudocercospora musarum Crous & Yuan Yuan Chen, sp. nov. Present study.
Host and distribution: Malaysia, on living leaves of Musa sp. (Musaceae), culture ex-type CBS 149516 = CPC 32815.
Pseudocercospora rauvolfiicola A. Singh, P.N. Singh & N.K. Dubey, Phytotaxa 545(2): 131. 2022.
Host and distribution: India, on living leaves of Rauvolfia serpentina (Apocynaceae), culture ex-type NFCCI 4586.
Pseudocercospora solanicola Arch. Singh, et al., Turk. J. Bot. 46: 508. 2022.
Host and distribution: India, on leaves of Solanum nigrum (Solanaceae), culture ex-type NFCCI 5015.
Pseudocercospora sp. 059
Hosts and distribution: Australia, on Santalum acuminatum (Santalaceae), culture CBS 149420 = CPC 28327; on Santalum lanceolatum (Santalaceae), culture CBS 149417 = CPC 27618.
Pseudocercospora sp. 060
Hosts and distribution: Australia, on Solanum sp. (Solanaceae), culture CPC 28350. New Zealand, on Gunnera tinctoria (Gunneraceae), culture CBS 115122. Unknown, unknown host, culture CBS 114642.
Pseudocercospora tabernaemontanae (Syd. & P. Syd.) Deighton, Mycol. Pap. 140: 154. 1976.
Basionyn: Cercospora tabernaemontanae Syd. & P. Syd., Philipp. J. Sci., C, Bot. 8(5): 507. 1913.
Hosts and distribution: Laos, on leaves of Tabernaemontana coronaria (Apocynaceae), culture CPC 19198 (ex-epitype). Philippines, on leaves of Tabernaemontana pandacaqui (Apocynaceae) (type).
Notes on Block 28 : Wang et al. (1995) and Braun (2017) proposed a synonymy between Ps. atromarginalis and Ps. fuligena based on overlapping host ranges, lack of distinguishing morphological features and lack of sufficient numbers of nucleotide differences to support the two species as distinct. However, Braun (2017) did state that the designation of epitypes for both species is required to finally clarify the taxonomic status of these species. Therefore the present study treats them as separate pending the collection and sequencing of material that can be designated as epitypes for both species. Currently there are only an ITS and LSU sequence available for Ps. solanicola (GenBank MZ474952 and MZ474953, respectively), of which the ITS sequence was used in the phylogenetic analyses. Interestingly, this sequence clustered with Ps. hakeae in the IQ-TREE analysis (Fig. 1, block 24), but intermingled with strains in the Ps. atromarginalis complex in the RAxML analysis (see Figshare; equivalent of clustering in Fig. 1, block 28). The ITS sequence differs by four gaps and one substitution (484/489 (99 %) identity; the region containing the three gaps was at the very end of the ITS sequence and excluded from the analyses as they appear to be consistent with base-calling problems) from that of Ps. hakeae culture CBS 112226 (GenBank GU269784) and with a single gap (477/478 (99 %) identity) from that of Ps. atromarginalis culture CBS 136112 (GenBank KM452851).
Block 38
Pseudocercospora zelkovae X.J. Liu & Y.L. Guo [as ‘zelkowae’], Acta Mycol. Sin. 12: 33. 1993.
Hosts and distribution: Japan, on leaves of Zelkova serrata (Ulmaceae), culture ex-neotype MAFF 410008 = MUCC 1398 and culture MAFF 238237 = MUCC 872. South Korea, on leaves of Zelkova serrata, cultures CBS 132106 = CPC 14484 and CBS 132118 = CPC 14717.
Pseudocercospora riachueli var. horiana (Togashi & Katsuki) U. Braun & Crous, in Crous & Braun, CBS Diversity Ser. (Utrecht) 1: 354. 2003.
Host and distribution: Japan, on leaves of Parthenocissus tricuspidate (Vitaceae), culture ex-epitype MUCC 2141.
Notes on Block 38: Although occurring on unrelated hosts, the two species appear phylogenetically similar (ITS: 478/478 nt; actA: 219/219 nt; tef1: 307/308 nt; rpb2: 673/673 nt) (Fig. 1, block 38). The ex-type culture was located in the clade of Ps. zelkovae (Fig. 1, block 38). The epitypified specimen of Ps. riachueli var. horiana was collected from the fringe of woods near a populated area, which was planted with Zelkova trees. The morphological characteristics of both specimens are similar. Pseudocercospora zelkovae might temporarily colonise Parthenocissus. More detailed examinations with fresh samples are required.
Block 51
Pseudocercospora amelanchieris C. Nakash. et al., Mycol. Progr. 15: 1097. 2016.
Host and distribution: Japan, on leaves of Amelanchier canadensis (Rosaceae), culture ex-type MAFF 237782 = MUCC 885.
Pseudocercospora corylopsidis (Togashi & Katsuki) C. Nakash. & Tak. Kobay., Mycoscience 40: 270. 1999.
Basionym: Cercospora corylopsidis Togashi & Katsuki, Bot. Mag., Tokyo 65: 20. 1952.
Host and distribution: Japan, on leaves of Corylopsis spicata (Hamamelidaceae), culture ex-epitype MAFF 237795 = MUCC 908.
Pseudocercospora chibaensis Tak. Kobay. & Nagash., Trans. Mycol. Soc. Japan 32: 328. 1991.
Host and distribution: Japan, on leaves of Nyssa sylvatica (Cornaceae), culture ex-epitype MUCC 1670.
Pseudocercospora cotoneastri (Katsuki & Tak. Kobay.) Deighton [as ‘cotoneasteris’], Trans. Brit. Mycol. Soc. 88: 389. 1987. Basionym: Cercospora cotoneastri Katsuki & Tak. Kobay. [as ‘cotoneasteris’], Trans. Mycol. Soc. Japan 17: 276. 1976.
Host and distribution: Japan, on leaves of Cotoneaster dammeri (Rosaceae), culture ex-type MAFF 410089 = MUCC 1416.
Pseudocercospora daphniphylli (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88(3): 389. 1987.
Basionym: Cercospora daphniphylli Katsuki & Tak. Kobay., Trans. Mycol. Soc. Japan 23: 44. 1982.
Host and distribution: Japan, on leaves of Daphniphyllum macropodum (Daphniphyllaceae), culture ex-type MAFF 410009 = MUCC 1399.
Pseudocercospora emmoticola Meir. Silva, R.W. Barreto & Crous [as ‘emmotunicola’], in Silva, Barreto, Pereira, Freitas, Groenewald & Crous, Persoonia 37: 153. 2016.
Host and distribution: Brazil, on leaves of Emmotum nitens (Icacinaceae), culture ex-type COAD 1491 = CPC 25187.
Pseudocercospora eriodendri (Racib.) U. Braun, Nova Hedwigia 55(1–2): 217. 1992.
Basionym: Ramularia eriodendri Racib., Parasit. Alg. Pilze Java’s (Jakarta) 1: 35. 1900.
Host and distribution: Java, on Eriodendron anfractuosum (Bombacaceae) (lectotype). Somalia, on Ceiba pentandra (Bombacaceae), authentic culture CBS 130.32.
Pseudocercospora formosana (W. Yamam.) Deighton, Mycol. Pap. 140: 144. 1976.
Basionym: Cercospora formosana W. Yamam., Journal of the Society of Tropical Agriculture, Formosa 6: 600. 1934.
Host and distribution: Japan, on Lantana camara (Verbenaceae), culture ex-epitype MAFF 238239 = MUCC 879 and culture MUCC 855. Malaysia, on Lantana sp., culture MUCC 2612.
Pseudocercospora forsythiae (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora forsythiae Katsuki & Tak. Kobay., Trans. Mycol. Soc. Japan 17: 273. 1976.
Host and distribution: Japan, on leaves of Forsythia suspensa (Oleaceae), culture ex-type MAFF 410087 = MUCC 1414.
Pseudocercospora hiratsukana (Togashi & Katsuki) Deighton, Mycol. Pap. 140: 34. 1976.
Basionym: Cercospora hiratsukana Togashi & Katsuki, J. Jap. Bot. 28: 286. 1953.
Host and distribution: Japan, on leaves of Dioscorea tokoro (Dioscoreaceae), culture ex-epitype MAFF 238300 = MUCC 1105.
Pseudocercospora izuohshimensis C. Nakash. et al., Mycoscience 45: 49. 2004.
Host and distribution: Japan, on leaves of Helwingia japonica (Helwingiaceae), culture ex-type MAFF 238478 = MUCC 1336.
Pseudocercospora mangifericola R.G. Shivas, A.J. Young & Grice, Persoonia 23: 197. 2009.
Host and distribution: Australia, on leaves of Mangifera indica (Anacardiaceae), culture ex-type BRIP 52776b.
Pseudocercospora mombin (Petr. & Cif.) Deighton, Mycol. Pap. 140: 148. 1976.
Basionym: Cercospora mombin Petr. & Cif., Annls mycol. 30(3/4): 322. 1932.
Hosts and distribution: Dominican Republic, on living leaves of Spondias mombin (Anacardiaceae) (lectotype). Thailand, on Spondias pinnata, culture P625.
Pseudocercospora pallida (Ellis & Everh.) H.D. Shin & U. Braun, Mycotaxon 74(1): 114. 2000.
Basionym: Cercospora pallida Ellis & Everh., J. Mycol. 3(2): 21. 1887.
Hosts and distribution: USA, on living leaves of Tecoma radicans (Bignoniaceae) (type). South Korea, on Campsis grandiflora (Bignoniaceae), culture CBS 131889.
Pseudocercospora perae Meir. Silva, R.W. Barreto & Crous, in Silva, Barreto, Pereira, Freitas, Groenewald & Crous, Persoonia 37: 158. 2016.
Host and distribution: Brazil, on leaves of Pera glabrata (Euphorbiaceae), culture ex-type COAD 1465 = CPC 25171.
Pseudocercospora prunicola (Ellis & Everh.) U. Braun, in Braun & Mel’nik, Trudy Botanicheskogo Instituta im. V.L. Komarova 20: 82 (1997)
Basionym: Cercospora prunicola Ellis & Everh., J. Mycol. 3: 17. 1887.
Hosts and distribution: USA, on leaves of Prunus americana (Rosaceae) (type). South Korea, on leaves of Prunus yedoensis, cultures CBS 132107 = CPC 14511, CPC 14792, and on leaves of Prunus sp., culture CPC 25431.
Pseudocercospora pruni-yedoensis Sawada ex Goh & W.H. Hsieh, in Hsieh & Goh, Cercospora and Similar Fungi from Taiwan (Taipei): 282. 1990.
Hosts and distribution: Taiwan, on leaves of Prunus yedoensis (Rosaceae) (type); on Prunus campanulate, voucher CEO13.
Pseudocercospora pseudocydoniae sp. nov. (see elsewhere in this publication)
Host and distribution: South Korea, on leaf spots on Chaenomeles lagenaria (Rosaceae), culture ex-type CBS 149392 = CPC 14665.
Pseudocercospora puerariicola (W. Yamam.) Deighton, Mycol. Pap. 140: 151. 1976.
Basionym: Cercospora puerariicola W. Yamam. [as ‘pueraricola’], Trans. Sapporo nat. Hist. Soc. 13(2–3): 142. 1934.
Hosts and distribution: Japan, on living leaves of Pueraria thunbergiana (Leguminosae) (original description). Taiwan, on Pueraria montana, culture BCRC FU30030 (block 51).
Pseudocercospora pyracanthae (Katsuki) C. Nakash. & Tak. Kobay., Ann. phytopath. Soc. Japan 63(4): 313. 1997.
Basionym: Cercospora pyracanthae Katsuki [as ‘pyrecanthae’], Bull. agr. impr. Sect. Econ. Dept. Fukuoka Prefecture Japan 1: 19. 1949.
Host and distribution: Japan, on Pyracantha angustifolia (Rosaceae), culture ex-epitype MAFF 237140 = MUCC 1226.
Pseudocercospora rhododendrigena C. Nakash., in Nakashima, Oetari, Kanti, Saraswati, Widyastuti & Ando, Mycosphere 1(6): 318. 2011.
Host and distribution: Indonesia, on leaves of Rhododendron sinense (Ericaceae), culture ex-type NBRC 105400 = BTCC F-62.
Pseudocercospora sp. 039
Host and distribution: Thailand, on Prunus ceraseidos (Rosaceae), culture CPC 21670.
Pseudocercospora sp. 040
Host and distribution: Brazil, unknown, culture CPC 18501.
Pseudocercospora sp. 041
Host and distribution: China, on fruit of Citrus grandis (Rutaceae), culture CPC 24510.
Pseudocercospora stephanandrae (Tak. Kobay. & H. Horie) C. Nakash. & Tak. Kobay., Mycoscience 41(1): 27 (2000)
Basionym: Cercospora stephanandrae Tak. Kobay. & H. Horie, in Kobayashi, Horie & Sasaki, Trans. Mycol. Soc. Japan 20(3): 331. 1979.
Host and distribution: Japan, on leaves of Stephanandra incisa (Rosaceae), culture ex-epitype MAFF 237799 = MUCC 914.
Pseudocercospora stranvaesiae (Katsuki & Tak. Kobay.) Deighton, Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora stranvaesiae Katsuki & Tak. Kobay. [as ‘stranvasiae’], Trans. Mycol. Soc. Japan 17: 278. 1976.
Host and distribution: Japan, on leaves of Photinia davidiana (Rosaceae), culture ex-type MAFF 410090 = MUCC 1417.
Pseudocercospora tamarindi Goonas. & K.D. Hyde, in Liu et al., Fungal Diversity: 10.1007/s13225-015-0324-y, [131]. 2015.
Host and distribution: Thailand, on living leaves of Tamarindus indica (Leguminosae), culture ex-type MFLUCC 14-0805.
Pseudocercospora togashiana (S. Ito & Tak. Kobay.) C. Nakash. & Tak. Kobay., Stud. Mycol. 101: 548. 2022.
Basionym: Mycosphaerella togashiana S. Ito & Tak. Kobay., Bull. Govt Forest Exp. Stn Meguro 59: 23. 1953.
Host and distribution: Japan, on leaves of Populus simonii (Salicaceae), culture ex-type MAFF 410006.
Pseudocercospora vassobiae Meir. Silva, R.W. Barreto & Crous, in Silva, Barreto, Pereira, Freitas, Groenewald & Crous, Persoonia 37: 167. 2016.
Host and distribution: Brazil, on leaves of Vassobia breviflora (Solanaceae), culture ex-type COAD 1572 = CPC 25251.
Pseudocercospora weigelae (Ellis & Everh.) Deighton [as ‘weigeliae’], Trans. Brit. Mycol. Soc. 88: 389. 1987.
Basionym: Cercospora weigelae Ellis & Everh. [as ‘weigeliae’], Proc. Acad. Nat. Sci. Philad. 45: 170. 1893.
Hosts and distribution: USA, on leaves of Weigela sp. (Caprifoliaceae) (type). Japan, on leaves of Weigela coraeensis, culture MAFF 237794 = MUCC 899.
Notes on Block 51: Similar to the other clades containing diverse species, this clade (Fig. 1, block 51) is also unresolved. Two isolates deposited as Ps. formosana do not cluster with the ex-epitype culture and could represent an additional species on Lantana in Asia (ITS: 474–477/477 nt; actA: 189/190 and 192/198 nt; tef1: 302–305/305 nt; rpb2: 636–637/673 nt). Besides unrelated host families and species, there are also significant morphological differences between the species involved. Species belonging this block are share Rosaceae plants as a common ancestral host plant. The Rosaceae hosts, Ameranchier, Chaenomeles, Cotoneaster, Nyssa, Photinia, Prunus, Pyracantha, and Stephanandra, are diffuse on this clade and species from various plants are mixed. The distribution of these Pseudocercospora species is mainly in Asian countries. The phylogeny of these species suggest that the genes used here lack the resolution to properly distinguish these species. They may be showing the host expansion and speciation at the terminal clade on Rosaceae hosts. Pseudocercospora mangifericola is only represented by an ITS sequence in the present analyses and either clusters in block 51 in the IQ-TREE analysis (Fig. 1; sister to Ps. pruni-yedoensis) or sister to Ps. kenyirana (see Figshare; equivalent of clustering in Fig. 1, block 21) in the RAxML analysis (Suppl. Table S3). The ITS sequence of Ps. mangifericola is 99 % (identities 467/470 and 468/470, both including one gap) similar to the ITS sequences of Ps. pruni-yedoensis and Ps. kenyirana. Likewise for Ps. mombin, which is sister on long branches to Ps. pyracanthae (Fig. 1, block 51) or clusters among strains of Ps. flavomarginata (see Figshare; equivalent of clustering in Fig. 1, block 22) in the RAxML analysis (Suppl. Table S3). The ITS sequence of Ps. mombin is 95 % (identities 392/414 and 393/415, including 21 and 22 gaps, respectively) similar to the ITS sequences of Ps. pyracanthae and Ps. flavomarginata. However, Ps. eriodendri and Ps. tamarindi are also represented by only an ITS sequence each but have a similar clustering in both the IQ-TREE and RAxML analyses (Suppl. Table S3). Although Ps. pruni-yedoensis is also represented by only an ITS sequence, in both analyses it clusters with species in block 51: sister to Ps. rhododendrigena in the IQ-TREE analysis and sister to Ps. vassobiae in the RAxML analysis. The ITS sequence of Ps. pruni-yedoensis is 99 % (identity 472/473, including one gap) or 100 % (identity 473/473, including one gap) similar to the ITS sequences of Ps. rhododendrigena and Ps. vassobiae. Also see the notes under Ps. puerariicola and Ps. rhododendrigena.
Problematic application of names
During the present study, numerous isolates were encountered for which it was impossible to unequivocally assign a species identification. This was due to several reasons, e.g., i) multiple species known from the substrate and/or locality but sequence data for the species are not available from (ex-)type or authentic material, ii) the loci commonly used for phylogenetic reconstruction in the genus do not provide sufficient resolution, iii) availability of limited sequence data, e.g. only ITS, for many species, iv) different interpretations of the application of a name by authors, and v) cultures proved to be sterile or the fungus could not be located on the original substrate material. For these reasons, we have chosen not to formally name a large number of the obtained cultures in this study (as Pseudocercospora sp. in Suppl. Table S1). A few problematic species for which a name could be or was applied to a culture or specimen in past studies or for which a culture was supplied under the given name in the present study, but of which the derived sequences show them not to be conspecific in the present study, are discussed below.
Pseudocercospora catappae (Henn.) Y.L. Guo & X.J. Liu, Mycosystema 2: 230. 1989.
Basionym: Cercospora catappae Henn., Bot. Jb. 34: 56. 1904.
Notes: The two cultures included in the present study (Suppl. Table S1) (Fig. 1, blocks 34 and 48) both were isolated from Terminalia catappa in Japan and were published separately by Crous et al. (2013a) and Videira et al. (2017). The study of Videira et al. (2017) used ITS and rpb2 while that of Crous et al. (2013a) used ITS, actA and tef1. The ITS sequences of the two cultures are 99 % similar (478/481 nt). Unfortunately, no other markers are shared between the two cultures and further comparison on more informative loci are therefore not possible. The species was described from Terminalia catappa in Tanzania and needs to be recollected from the type locality to confirm its phylogenetic placement.
Pseudocercospora cornicola (Tracy & Earle) Y.L. Guo & X.J. Liu, Mycosystema 2: 232. 1989.
Basionym: Cercospora cornicola Tracy & Earle, Bull. Torrey bot. Club 23(5): 205. 1896.
Notes: The two cultures included in the present study (Suppl. Table S1) (Fig. 1, blocks 12 and 20) were isolated from different host species and different countries, namely Cornus alba var. sibirica in Japan ( Crous et al. 2013a) and Cornus officinalis in South Korea ( Choi et al. 2022). In their publication, Choi et al. (2022) indicated that the sequences from their material do not match the earlier published sequences of Ps. cornicola (ITS: 478/500 nt including seven gaps; actA: 162/198 nt including 12 gaps; tef1: 236/318 nt including 32 gaps). Pathogenicity of their isolates on the host was confirmed, fulfilling Koch’s postulates. The species was described from Cornus florida in Mississippi, USA, and needs to be recollected from the type locality to confirm its phylogenetic placement.
Pseudocercospora cruenta (Sacc.) Deighton, Mycol. Pap. 140: 142. 1976.
Basionym: Cercospora cruenta Sacc., Michelia 2(no. 6): 149. 1880.
Notes: The two cultures included in the present study (Suppl. Table S1) (Fig. 1, blocks 13 and 28) were isolated from different host genera and different countries, namely Vigna sp. in Trinidad and Tobago (CBS 132021; Crous et al. 2013a) and Phaseolus vulgaris in Taiwan (CBS 117232; Crous et al. 2013a). The authors treated CBS 117232 as “Pseudocercospora cf. cruenta” (ITS: 495/500 nt including one gap; actA: 209/231 nt; tef1: 276/317 nt including nine gaps). The species was described from Phaseolus in South Carolina, USA, and needs to be recollected from the type locality to confirm its phylogenetic placement.
Pseudocercospora jahnii (Syd.) U. Braun & Crous, in Crous & Braun, CBS Diversity Ser. (Utrecht) 1: 230. 2003.
Basionym: Cercospora jahnii Syd., Annls Mycol. 28(1/2): 214. 1930.
Notes: The two cultures included in the present study (Suppl. Table S1) (Fig. 1, blocks 44 and 45) were isolated from different host species and different countries, namely Tabebuia pallida in Philippines (CBS 138757; Acabal Jr et al. 2014) and Tabebuia chrysotricha in Thailand (CPC 19214; present study). The two cultures are genetically distant (ITS: 446/448 nt including one gap; tef1: 438/513 nt including 17 gaps). In their publication, Acabal Jr et al. (2014) indicated that this is a morphologically variable taxon, with conidia from the type specimen (not known from sequence data) are larger than those from Asian collections. The species was described from Tabebuia rosea in Venezuela, and needs to be recollected from the type locality to confirm its phylogenetic placement.
Pseudocercospora platylobii Beilharz & Cunningt., Mycol. Res. 107: 446. 2003.
Notes: Five cultures are included in the present study under this species name (Suppl. Table S1) (Fig. 1, blocks 7 and 9). The two cultures in block 7 include one representing an authentic culture for the name and both are represented only by ITS sequences in the multigene alignment, while the two of the three cultures in block 9 (all three having complete datasets) are from the same country and host [Australia: Platylobium formosum (Leguminosae)] but phylogenetically distinct in the IQ-TREE analysis but not in the RAxML analysis (Suppl. Table S3; see Figshare). The remaining culture was isolated from Grevillea ilicifolia (Proteaceae) in Australia. To establish whether there are more Pseudocercospora species on Platylobium formosum in Australia than described to date would require multigene datasets for the strains in block 7. The ITS sequences of the strains are identical (460/460 or 465/465 nt), with the exception of the ITS sequence of strain CPC 28321 which has one substitution.
Pseudocercospora puerariicola (W. Yamam.) Deighton, Mycol. Pap. 140: 151. 1976.
Basionym: Cercospora puerariicola W. Yamam. [as ‘pueraricola’], Trans. Sapporo nat. Hist. Soc. 13(2–3): 142. 1934.
Notes: The two cultures included in the present study (Suppl. Table S1) (Fig. 1, blocks 22 and 51) were isolated from different host species and different countries, namely Pueraria montana in Taiwan (R. Kirschner 3769; Kirschner & Liu 2014) and Pueraria phaseoloides in Laos (CPC 19193; present study). The two cultures are genetically distant (only ITS available for both: 467/475 nt including two gaps; also see Supp. Table S3). In their publication, Kirschner & Liu (2014) only refer to the collection of this species in a Table and do not treat the species itself in the text. In addition, three isolates from leaves of Pueraria montana var. lobata from South Korea are lodged in GenBank under this species name and can be found in yet another clade (Fig. 1, block 25; Choi et al. in GenBank) and these differ with two (KUS F33308) to six (KUS F33294 and KUS F33343) nucleotides from the strain from Taiwan. The species was described from Pueraria thunbergiana in Japan, and needs to be recollected from the type locality to confirm its phylogenetic placement.
DISCUSSION
Pseudocercospora is a common foliar pathogen, occurring on a wide range of hosts in most subtropical to tropical regions of the world. Despite the more than 1 700 Pseudocercospora names that are presently known, the present study describes a further 29 novel species of Pseudocercospora from 413 host species in 297 host genera occurring in 60 countries. Furthermore, epitypes are designated for a further four existing species of Pseudocercospora to help fix the phylogenetic application of their names, including in addition a lectotype for one of the four species. A major complication in the taxonomy of Pseudocercospora (and cercosporoid fungi in general) is the fact that most existing names are not linked to cultures or DNA data, and that descriptions based on material in vivo, differs from what is observed in vitro. We have also demonstrated this in several cases where cultures sporulated in the present study. However, even if material is studied in vivo, abundant moisture or shade could also influence the morphology of the species, generally making comparisons difficult, and further underlying the importance of DNA-based studies for comparisons and identification.
Some large host genera saw the addition of new Pseudocercospora species in this study, e.g. the genus Eucalyptus, which has up to 700 species, the majority of which are endemic to Australia ( Ladiges et al. 2003). Currently 27 species of Pseudocercospora are known from Eucalyptus ( Crous et al. 2019c), with the present study adding a further three species, namely Ps. australiensis, Ps. badjensis and Ps. victoriae. Musa presently has 11 Pseudocercospora species ( Crous et al. 2021), with Ps. musigena and Ps. musarum adding an additional two species. Three Pseudocercospora species are known from Acacia, with Ps. acaciicola and Ps. wingfieldii adding a further two. Several species are introduced here representing the first Pseudocercospora species from a specific host genus, namely Ps. anopteri from Anopterus, Ps. asplenii from Asplenium, Ps. balanitis from Balanites, Ps. pultenaeae from Pultenaea, and Ps. tristaniopsidis from Tristaniopsis. Although not much is known about the pathology of most of these species, some have been associated with prominent disease symptoms, e.g., Ps. paramacadamiae, which similar to Ps. macadamiae, also causes husk spot of Macadamia in Australia ( Beilharz et al. 2003).
By adding data from secondary barcode loci such as actA, tef1 and rpb2, a major conclusion made by Nakashima et al. (2016) was that the genus Pseudocercospora was monophyletic. Several pseudocercospora-like taxa were subsequently allocated to other genera, such as Chuppomyces, Collarispora, Madagascaromyces, Mycosphaerelloides, Nothopseudocercospora, Pallidocercospora, Paracercospora, Parapallidocercospora, Pteridopassalora, Rhachisphaerella, Scolecostigmina and Trochophora ( Videira et al. 2017, Crous et al. 2022). In addition, some genera were regarded as synonyms of Pseudocercospora, namely Cercostigmina, Ciferriella, Helicomina, Marcosia, Microcyclus, Neopseudocercospora, Pantospora, Phaeoisariopsis, Prathigada, Pseudophaeoramularia, Pseudopuccinia and Stigmina. In the case of other pseudocercospora-like genera, however, their status remains unclear, pending fresh collections and DNA data, namely Denticularia, Elletevera, Eriocercospora, Eriocercosporella, Rhopaloconidium and Semipseudocercospora ( Videira et al. 2017).
The use of ITS has been recommended as the official universal barcode for fungi ( Schoch et al. 2012), but ITS sequences tend to have less resolution in cercosporoid fungi. For example, in Cercospora, the ITS phylogeny recovered only 2.7 % of the accepted phylogenetic clades, whereas phylogenetic trees derived from other loci such as translation elongation factor 1-alpha recovered 45 %, calmodulin recovered 46.6 %, actin recovered 58.9 %, and histone H3 recovered 63 % of the accepted phylogenetic clades ( Groenewald et al. 2010, 2013). Likewise for Pseudocercospora, where it was found that the ITS phylogeny uncovered only 25 of the 146 treated taxa (17 %; Crous et al. 2013a). As shown in the present study (Fig.1, Suppl. Table S3), the phylogenetic inference seems to be sensitive in some cases to the algorithms used for the placement of strains when only ITS is representing that strain in a multigene alignment. This difference in placement appears to be an artifact in the different tree-building strategies employed by RAxML and IQ-TREE when a large amount of missing data is present for a given strain or there are sequence length differences between highly similar sequences. Interestingly, not all strains represented by only an ITS sequence are influenced by this as 18 of the 45 strains had a similar phylogenetic position between the two analyses (Suppl. Table S3); also see the Phylogenetic results section. The phylogenetic position of strains included in an analysis and represented by only an ITS sequence available in the multigene alignment should therefore be subjected to more scrutiny in the interpretation of phylogenetic trees. Although the inclusion of actA, tef1, and in more recent studies, rpb2, does provide increased resolution, none of these genes are perfect for resolving all or most species. In addition, rpb2 is not available for many previously accepted species. We did attempt to obtain sequences for this marker for some of those species, but it is not the easiest of markers to amplify in the cercosporoids ( Videira et al. 2017), with variable success between different laboratories which could be related to the PCR machines or the Taq polymerase used or a combination thereof. A more concerted effort is needed to identify markers with a higher resolution for more or most accepted species, or to supplement those that are currently in use. One approach could be whole genome comparisons to identify better loci; however, at the time of writing there were only 11 genomes listed under the name Pseudocercospora in the NCBI genomes database, namely for Ps. cruenta, Ps. crystallina (now called Pallidocercospora crystallina), Ps. eumusae, Ps. fijiensis (two genomes), Ps. fuligena, Ps. macadamiae, Ps. musae, Ps. pinidensiflorae, Ps. ulei, and Ps. vitis. A higher sampling of genomes from morphologically diverse but phylogenetically closely related species is needed to attempt such a comparison.
As reported earlier ( Braun et al. 2013a, Crous et al. 2013a, Nakashima et al. 2016, Videira et al. 2017), except for a few cases, Pseudocercospora species were observed to be highly host-specific. In most cases, however, host specificity is still based on host association, while cross pathogenicity experiments to confirm true hosts are still largely lacking, which also highlights future questions to be addressed regarding host specificity within families, and genera. In some cases, cercosporoid species have been reported to be able to colonise non-host plants ( Groenewald et al. 2013), a phenomenon coined as the pogostick hyphothesis ( Crous & Groenewald 2005), underlining chance or secondary infections which confuse or cloud the true host range of these fungi. Results obtained in the present study help contribute to a better understanding of Pseudocercospora biodiversity occurring on a range of hosts and countries and could help to prevent epidemics on important host crops. Additional surveys remain essential therefore to help elucidate the host range and distribution of these plant parasitic fungi, many of which represent important pathogens or quarantine organisms in various countries on their respective host plants, and to fix the application of existing names through epi- or neotypification.
Fig. 3.
Pseudocercospora acaciicola (CPC 17121). A. Leaf spots on upper and lower leaf surface. B. Close-up of lesion. C–F. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Fig. 25.
Pseudocercospora hakeae (CPC 32190). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Fig. 26.
Pseudocercospora hakeae (CPC 32100). A. Leaf spots on lower and upper leaf surface. B. Close-up of lesions. C–H. Fascicles with conidiophores and conidiogenous cells giving rise to conidia. I. Conidia. Scale bars = 10 μm.
Fig. 54.
Pseudocercospora victoriae (CPC 32428). A. Colony on MEA. B. Sporulation on SNA. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
ACKNOWLEDGEMENTS
We are grateful to the European Union’s Horizon 2020 research and innovation program (RISE) under the Marie Skłodowska-Curie grant agreement No. 101008129, project acronym ‘Mycobiomics’, and the Dutch NWO Roadmap grant agreement No. 2020/ENW/00901156, project ‘Netherlands Infrastructure for Ecosystem and Biodiversity Analysis – Authoritative and Rapid Identification System for Essential biodiversity information’ (acronym NIEBA-ARISE) for funding. We thank Marjan Vermaas for assistance with the photographic plates. The National Natural Science Foundation of China supported Ying Zhang (31770015) and Yuanyuan Chen (31900017); Yuanyuan Chen also received a grant from the China Scholarship Council (202008410340). We are also thankful to Luuk Veenendaal and Omar Sprockel who generated quite a number of sequences for this publication during their interships at the Westerdijk Institute and to Mieke Starink-Willemse and Arien van Iperen for technical support. We are grateful to all collectors who kindly made their specimens and/or cultures available to us for study.
Conflict of interest:
The authors declare that there is no conflict of interest.
Supplementary Material: https://fuse-journal.org/
Metadata and GenBank accession numbers of Pseudocercospora isolates included in the morphological and/or phylogenetic analyses.
Summary of phylogenetic information for the different analyses in this study.
Comparison of the phylogenetic position of strains represented by only ITS in the sequence dataset.
REFERENCES
- Acabal BD, Jr, Groenewald JZ, Crous PW, et al. (2014). First report of Pseudocercospora jahnii in the Philippines. Australasian Plant Disease Notes 9: 148. [Google Scholar]
- Akinsanmi OA, Carvalhais LC. (2020). Draft genome of the macadamia husk spot pathogen, Pseudocercospora macadamiae . Phytopathology 110: 1503–1506. [DOI] [PubMed] [Google Scholar]
- Bakhshi M, Arzanlou M, Babai-Ahari A.et al. (2014). Multi-gene analysis of Pseudocercospora spp. from Iran. Phytotaxa 184: 245–264. [Google Scholar]
- Beilharz V, Mayers PE, Pascoe IG. (2003). Pseudocercospora macadamiae sp. nov., the cause of husk spot of macadamia. Australasian Plant Pathology 32: 279–282. [Google Scholar]
- Braun U. (1995). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 1. Eching: IHW Verlag. [Google Scholar]
- Braun U. (2017). Pseudocercospora solanacea revisited and a survey of Pseudocercospora spp. on Solanum with a key to the species. Schlechtendalia 32: 51–65. [Google Scholar]
- Braun U. (2023). Reassessment of the name Pseudocercospora bonjeaneae. Schlechtendalia 40: 278–279. [Google Scholar]
- Braun U, Crous PW. (2007). The diversity of cercosporoid hyphomycetes -new species, combinations, names and nomenclatural clarifications. Fungal Diversity 26: 55–72. [Google Scholar]
- Braun U, Dick M. (2002). Leaf spot diseases of eucalypts in New Zealand caused by Pseudocercospora species. New Zealand Journal of Forestry Science 32: 221–234. [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2014). Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae). IMA Fungus 5: 203–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2015a). Cercosporoid fungi (Mycosphaerellaceae) 3. Species on monocots (Poaceae, true grasses). IMA Fungus 6: 25–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2015b). Cercosporoid fungi (Mycosphaerellaceae) 4. Species on dicots (Acanthaceae to Amaranthaceae). IMA Fungus 6: 373–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Crous PW, Nakashima C. (2016). Cercosporoid fungi (Mycosphaerellaceae) 5. Species on dicots (Anacardiaceae to Annonaceae). IMA Fungus 7: 161–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Kummer V, Moreno-Rico O. (2017). Fungi selecti exsiccati ex Herbario Universitatis Halensis – nos. 221–240. Schlechtendalia 32: 67–73. [Google Scholar]
- Braun U, Nakashima C, Bakhshi M.et al. (2020). Taxonomy and phylogeny of cercosporoid ascomycetes on Diospyros spp. with special emphasis on Pseudocercospora spp. Fungal Systematics and Evolution 6: 95–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Nakashima C, Crous PW. (2013a). Cercosporoid fungi (Mycosphaerellaceae) 1. Species on other fungi, Pteridophyta and Gymnospermae. IMA Fungus 4: 265–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Piątek M, Scheuer C. (2013b). New species and new records of several phytopathogenic hyphomycetes. Schlechtendalia 25: 41–48. [Google Scholar]
- Chang TC, Salvucci A, Crous PW.et al. (2016). Comparative genomics of the Sigatoka disease complex on banana suggests a link between parallel evolutionary changes in Pseudocercospora fijiensis and Pseudocercospora eumusae and increased virulence on the banana host. PLoS Genetics 12: e1005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Bakhshi M, Balci Y.et al. (2022) Genera of phytopathogenic fungi: GOPHY 4. Studies in Mycology 101: 417–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I-Y, Braun U, Park JH.et al. (2014). First report of leaf spot caused by Pseudocercospora pruni-persicicola on sweet cherry in Korea. Plant Disease 98: 693. [DOI] [PubMed] [Google Scholar]
- Choi I-Y, Ju H-J, Abasova L.et al. (2022). Identification and characterization of Pseudocercospora cornicola causing leaf spots on Cornus officinalis. The Korean Journal of Mycology 50: 131–136. [Google Scholar]
- Chupp C. (1954). A monograph of the fungus genus Cercospora. Published by the author, Ithaca, New York. [Google Scholar]
- Chupp C, Doidge EM. (1948). Cercospora species recorded from southern Africa. Bothalia 4: 881–893. [Google Scholar]
- Churchill ACL. (2011). Mycosphaerella fijiensis, the black leaf streak pathogen of banana: progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Molecular Plant Pathology 12: 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. [Google Scholar]
- Crous PW, Braun U. (1994). Cercospora species and similar fungi occurring in South Africa. Sydowia 46: 204–224. [Google Scholar]
- Crous PW, Braun U. (2003). Mycosphaerella and its Anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series no. 1, Utrecht, CBS-KNAW Fungal Biodiversity Centre. [Google Scholar]
- Crous PW, Groenewald JZ. (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470. [Google Scholar]
- Crous PW, Palm ME. (1999). Systematics of selected foliicolous fungi associated with leaf spots of Proteaceae. Mycological Research 103: 1299–1304. [Google Scholar]
- Crous PW, Sutton BC. (1997). New cercosporoid fungi from southern Africa. South African Journal of Botany 63: 280–285. [Google Scholar]
- Crous PW, Begoude BAD, Boers J.et al. (2022). New and Interesting Fungi. 5. Fungal Systematics and Evolution 10: 19–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC.et al. (2013a). Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Wingfield MJ.et al. (2009). Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Carlier J, Roussel V. et al. (2021). Pseudocercospora and allied genera associated with leaf spots of banana (Musa spp.). Fungal Systematics and Evolution 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Denman S, Taylor JEet al. (2013b). Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. 2nd edn. CBS Biodiversity Series 13. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich Ket al. (2004b). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469. [Google Scholar]
- Crous PW, Liebenberg MM, Braun Uet al. (2006). Re-evaluating the taxonomic status of Phaeoisariopsis griseola, the causal agent of angular leaf spot of bean. Studies in Mycology 55: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Osieck ER, Shivas RG, et. al. (2023). Fungal Planet description sheets: 1478–1549. Persoonia 50: 158–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov Aet al. (2019a). New and Interesting Fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZet al. (eds). (2019b). Fungal Biodiversity. Westerdijk Laboratory Manual Series 1. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ, Cheewangkoon Ret al. (2019c). Foliar pathogens of eucalypts. Studies in Mycology 94: 125–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa, a synonym of M. molleriana. Mycological Research 95: 628–632. [Google Scholar]
- Deighton FC. (1976). Studies on Cercospora and allied genera. VI. Pseudocercospora Speg., Pantospora Cif. and Cercoseptoria Petr. Mycological Papers 140: 1–168. [Google Scholar]
- Deighton FC. (1979). Studies on Cercospora and allied genera. VII. New species and redispositions. Mycological Papers 144: 1–56. [Google Scholar]
- Deighton FC. (1983). Studies on Cercospora and allied genera. VIII. Further notes on Cercoseptoria and some new species and redispositions. Mycological Papers 151: 1–13. [Google Scholar]
- Deighton FC. (1987). New species of Pseudocercospora and Mycovellosiella, and new combinations into Pseudocercospora and Phaeoramularia. Transactions of the British Mycological Society 88: 365–391. [Google Scholar]
- Deighton FC. (1990). Observations on Phaeoisariopsis. Mycological Research 94: 1096–1102. [Google Scholar]
- Den Breeÿen A, Groenewald JZ, Verkley GJMet al. (2006). Morphological and molecular characterisation of Mycosphaerellaceae associated with the invasive weed, Chromolaena odorata. Fungal Diversity 23: 89–110. [Google Scholar]
- Groenewald JZ, Groenewald M, Braun Uet al. (2010). Cercospora speciation and host range. In: Cercospora leaf spot of sugar beet and related species (Lartey RT, Weiland JJ, Panella L, Crous PW, Windels CE, eds). APS Press, Minnesota USA: 21–37. [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa Jet al. (2013). Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatimosim E, Schwartsburd PB, Barreto RWet al. (2016). Novel fungi from an ancient niche: cercosporoid and related sexual morphs on ferns. Persoonia 37: 106–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL. (1994). Four new species of Pseudocercospora. Mycosystema 7: 119–127 [Google Scholar]
- Guo YL, Hsieh WH. (1995). The genus Pseudocercospora in China. Mycosystema Monographicum Series 2: 1–388. [Google Scholar]
- Guo YL, Liu XJ, Hsieh WH. (1998). Flora Fungorum Sinicorum. Vol. 9, Pseudocercospora. Science Press, Beijing. [Google Scholar]
- Hora BTd, Júnior, de Macedo DM, Barreto RW. et al. (2014). Erasing the past: a new identity for the damoclean pathogen causing South American leaf blight of rubber. PLoS ONE 9: e104750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh WH, Goh TK. (1990). Cercospora and similar fungi from Taiwan. Maw Chang Book Company, Taipei, Taiwan. [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKFet al. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal, Singh RP. (1980). Fungi of Gorakhpur. XIX. Pseudocercospora. Sydowia 33: 157–161. [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson Aet al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R, Liu L-C. (2014). Mycosphaerellaceous fungi and new species of Venustosynnema and Zasmidium on ferns and fern allies in Taiwan. Phytotaxa 176: 309–323. [Google Scholar]
- Kirschner R, Wang H. (2015) New species and records of mycosphaerellaceous fungi from living fern leaves in East Asia. Mycological Progress 14: 65. [Google Scholar]
- Kirschner R, Hou CL, Chen CJ. (2009). Co-occurrence of Pseudocercospora species and rhytismatalean ascomycetes on maple and camellia in Taiwan. Mycological Progress 8: 1–8. [Google Scholar]
- Ladiges PY, Udovicic F, Nelson G. (2003). Australian biogeographic connections and the phylogeny of large genera in the plant family Myrtaceae. Journal of Biogeography 30: 989–998. [Google Scholar]
- Maddison WP, Maddison DR. (2023). Mesquite: a modular system for evolutionary analysis. Version 3.70. http://www.mesquiteproject.org.
- Meswaet Y, Mangelsdorff R, Yorou NSet al. (2019). A new species of Pseudocercospora on Encephalartos barteri from Benin. Asian Journal of Mycology 2: 101–109. [Google Scholar]
- Nakashima C, Motohashi K, Chen C-Yet al. (2016). Species diversity of Pseudocercospora from Far East Asia. Mycological Progress 15: 1093–1117. [Google Scholar]
- Nakashima C, Oetari A, Kanti Aet al. (2010). New species and newly recorded species of Cercospora and allied genera from Indonesia. Mycosphere 1: 315–323. [Google Scholar]
- Nguyen L-T, Schmidt HA, Von Haeseler Aet al. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phengsintham P, Braun U, McKenzie EHCet al. (2013a). Monograph of cercosporoid fungi from Thailand. Plant Pathology & Quarantine 3: 67–138. [Google Scholar]
- Phengsintham P, Chukeatirote E, McKenzie EHCet al. (2013b). Monograph of cercosporoid fungi from Laos. Current Research in Environmental & Applied Mycology 3: 34–158. [Google Scholar]
- Pons N, Sutton BC. (1988). Cercospora and similar fungi on yams (Dioscorea species). Mycological Papers 160: 1–78. [Google Scholar]
- Pretorius MC, Crous PW, Groenewald JZet al. (2003). Phylogeny of some cercosporoid fungi from Citrus. Sydowia 55: 286–305. [Google Scholar]
- Quaedvlieg W, Groenewald JZ, de Jesús Yáñez-Morales M, et al. (2012). DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia 29: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, England. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf SMet al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HD, Kim JD. (2001). Cercospora and allied genera from Korea. Plant Pathogens of Korea 7: 1–302. National Institute of Agricultural Science and Technology. Suwon, Korea. [Google Scholar]
- Shivas RG, Marney TS, Tan YPet al. (2015). Novel species of Cercospora and Pseudocercospora (Capnodiales, Mycosphaerellaceae) from Australia. Fungal Biology 119: 362–369. [DOI] [PubMed] [Google Scholar]
- Silva M, Barreto RW, Pereira OLet al. (2016). Exploring fungal mega-diversity: Pseudocercospora from Brazil. Persoonia 37: 142–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1844–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Shivas RG. (2023). Nomenclatural novelties. Index of Australian Fungi 6: 1–17. [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. (2011). SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27: 171–180. [DOI] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Braun Uet al. (2016). All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima Cet al. (2017). Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Verkley GJMet al. (2015). The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119: 823–843. [DOI] [PubMed] [Google Scholar]
- Yen JM. (1966). Étude sur les champignons parasites du Sud-Est asiatique IV. Troisième note sur quelques nouvelles espèces de Cercospora de Singapour Revue Mycologique 31: 109–149. [Google Scholar]
- Yuan ZQ. (1996). Fungi and associated tree diseases in Melville Island, Northern Territory, Australia. Australian Systematic Botany 9: 337–360. [Google Scholar]
- Zhang Z, Schwartz S, Wagner Let al. (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metadata and GenBank accession numbers of Pseudocercospora isolates included in the morphological and/or phylogenetic analyses.
Summary of phylogenetic information for the different analyses in this study.
Comparison of the phylogenetic position of strains represented by only ITS in the sequence dataset.