Abstract
While cycloaddition reactions of bicyclobutanes (BCBs) have emerged as a potent method for synthesizing (hetero-)bicyclo[n.1.1]alkanes (usually n ≤ 3), their utilization in the synthesis of bicyclo[4.1.1]octane derivatives (BCOs) is still underdeveloped. Here, a palladium-catalyzed formal (4 + 3) reaction of BCBs with 1,4-O/C dipole precursors for the synthesis of oxa-BCOs is described. Unlike previous catalytic polar (3 + X) cycloadditions of BCBs, which are typically achieved through the activation of BCB substrates, the current reaction represents a novel strategy for realizing the cycloaddition of BCBs through the activation of the “X” cycloaddition partner. Moreover, the obtained functionalized oxa-BCOs products can be readily modified through various synthetic transformations.
A novel palladium-catalyzed strategy was employed to achieve higher-order (4 + 3) cycloadditions of bicyclobutanes with various 2-alkylidenetrimethylene carbonates to synthesize 2-oxabicyclo[4.1.1]octanes.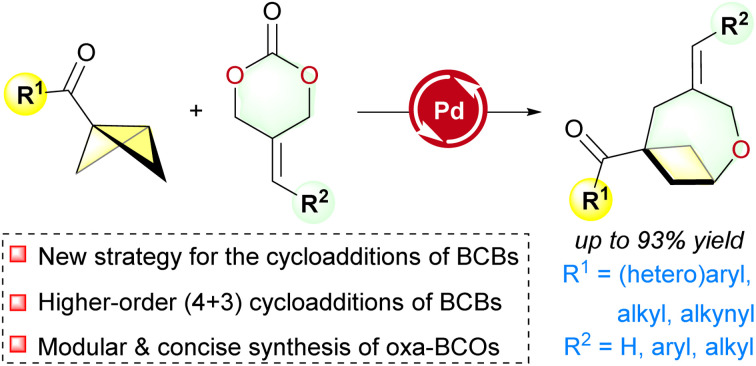
Introduction
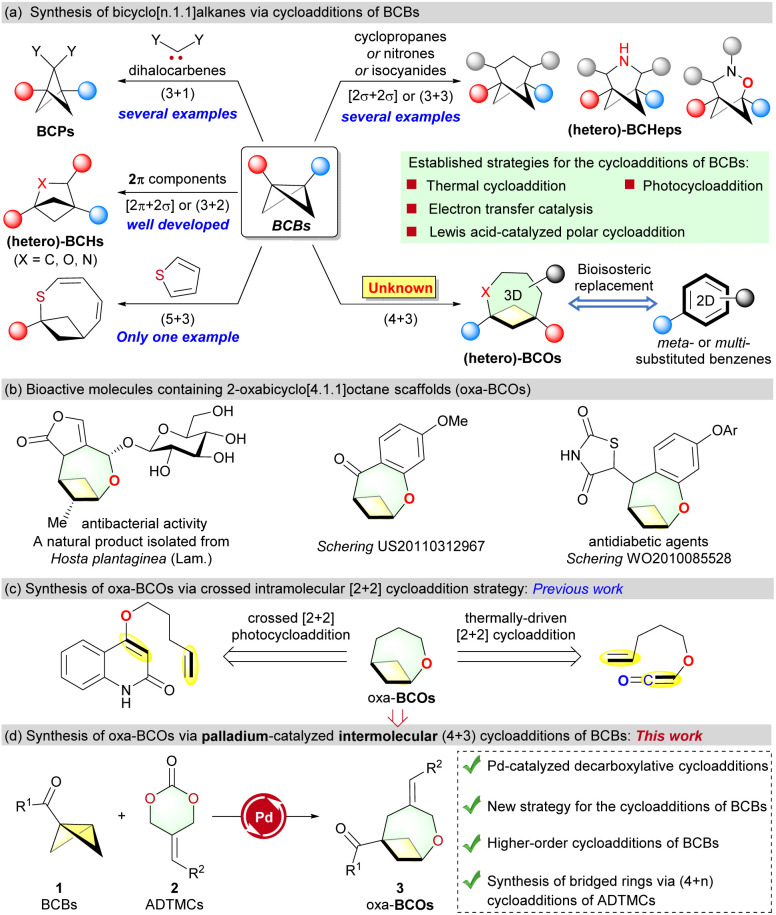
Bridged ring systems have long been coveted by organic and medicinal chemists due to their versatility as the basic structures of natural products and pharmaceuticals.1 Given the success of the “escape from flatland” concept in medicinal chemistry,2 there is a growing interest in bicyclo[n.1.1]alkanes as synthetic targets due to their rigid conformation and metabolic stability, which show great potential in replacing benzene rings.3 For instance, bicyclo[1.1.1]pentanes (BCPs),4 bicyclo[2.1.1]hexanes (BCHs),5 and bicyclo[3.1.1]heptanes (BCHeps)6 can mimic para-, ortho-, meta- and multi-substituted benzenoids in drug design, contingent on their substitution patterns. Additionally, Mykhailiuk's survey showed that incorporating heteroatoms (O- or N-) into BCH and BCHep analogues generally resulted in positive changes in water solubility, metabolic stability, and lipophilicity.5a–c,6a Therefore, developing new strategies to synthesize bicyclo[n.1.1]alkane derivatives is highly desirable and will expedite their application in new drug discovery.
The first cycloaddition reaction of bicyclo[1.1.0]butanes (BCBs) was reported in 1966 by Blanchard.7 Only in recent years has it emerged as a crucial synthetic platform for constructing (hetero-)bicyclo[n.1.1]alkane skeletons, owing to the pioneering scientific contributions of Wipf,8 Glorius,9 Brown,10 Leitch,11 Procter,12 Molander,13 Li,14 Wang,15 Waser,16 Studer,17 L. Deng,18 Feng,19 W.-P. Deng,20 Bach,21 Jiang22 and others.23 Generally, there are five state-of-the-art strategies for the cycloaddition of BCBs to obtain (hetero-)bicyclo[n.1.1]alkanes: (a) the formal (3 + 1) cycloaddition of BCBs with dihalocarbenes for synthesizing BCPs pioneered by Applequist,24a Mykhailiuk,24b,c Ma24d,e and Anderson;24f (b) thermally-driven (3 + 2) cycloaddition of BCBs for the synthesis of (hetero-)BCHs;7,8,23a–c (c) energy transfer [2π + 2σ]9a–d,10,21 and [2σ + 2σ]16 photocycloadditions; (d) electron transfer catalysis to construct BCHs and BCHeps via formal (3 + 2)12,14a,15,23d,e and (3 + 3)13,14b cycloaddition of BCBs. (e) Polar cycloadditions of BCBs.9f,g,11,17-20 Despite substantial advancements, the cycloadditions of BCBs continue to encounter numerous obstacles. For example, (i) in contrast to the well-established formal (3 + 1), (3 + 2), and (3 + 3) cycloadditions of BCBs, the higher-order (3 + n) (n > 3) cycloaddition of BCBs to saturated bicyclo[n.1.1]alkanes is still uncommon in the literature (Scheme 1a).25 Currently, there is only one documented formal (5 + 3) example of BCBs reacting with thiophenes to form unsaturated bicyclic rings through a photoredox-induced radical pathway (Scheme 1a, left).9h This rarity may be attributed to the high degree of transannular strain and unfavorable entropic factors during the formation of medium-sized rings. Although Grygorenko's elegant research has shown that bicyclo[4.1.1]octane frameworks (BCOs) are potential bioisosteres of substituted benzene,3c there is no report on the (4 + 3) cycloaddition of BCBs for preparing these valuable C(sp3)-rich bridged ring systems. (ii) The seminal contributions of Leitch's group11 in the realm of polar cycloadditions of BCBs led to the expeditious advancement of formal (3 + n) (n = 2 or 3) cycloadditions between BCBs and imines,11 aldehydes,9f ketenes,17 indoles18,19 and nitrones20 over the past two years, providing a wide range of valuable (hetero-)BCHs and BCHeps. However, all these Lewis acid catalytic systems were used to activate BCB substrates to generate the cycloadducts. The strategy for synthesizing bicyclo[n.1.1]alkanes by catalytically activating the “X” cycloaddition partner in the polar (3 + X) cycloadditions of BCBs remains unknown (Scheme 1a, right). Therefore, it is highly desirable to develop a new cycloaddition strategy to achieve the challenging (4 + 3) cycloaddition of BCBs.
Scheme 1. Synthesis of 2-oxabicyclo[4.1.1]octanes and its scientific context.
2-Oxabicyclo[4.1.1]octane scaffolds (oxa-BCOs) exist in both natural and pharmacologically relevant compounds (Scheme 1b).26 However, only a limited number of preparative methods have been established so far, and they are limited in scope and lack convergence. Current syntheses of oxa-BCOs primarily rely on the intramolecular crossed [2 + 2] cycloaddition of 4-(pent-4-en-1-yloxy)quinolin-2(1H)-ones27a,b or alkenyloxyketenes (Scheme 1c).27c,d
In the field of O-heterocyclic synthesis, Pd-catalyzed (4 + n) dipolar cycloadditions of 2-alkylidenetrimethylene carbonates (ADTMCs)28 have emerged as a powerful method for synthesizing monocyclic rings,29a,b fused rings,29c–e and spirocyclic scaffolds.29f–j However, their application in constructing bridged ring systems is extremely rare. As part of our ongoing research program on designing new reactions involving strained rings,19,30 this paper presents the first hetero-(4 + 3) cycloadditions of BCBs with 1,4-O/C dipole species generated catalytically from ADTMCs. The innovative palladium catalytic strategy in BCB chemistry was used to facilitate the cycloaddition reactions,31 resulting in the production of functionalized oxa-BCOs that were unattainable by other methods.
Results and discussion
Unlike relatively stable 1,3-dipoles, 1,4-dipoles, which are not fully conjugated chemical species, are frequently highly reactive and short-lived, resulting in numerous unexpected reaction pathways.28 Initially, we studied the cycloaddition of ADTMC 2a with 1,3-disubstituted BCB ketone using a palladium catalysis strategy. Regrettably, we failed to achieve the desired cycloadduct as 2a rapidly decomposed.32 Therefore, we opted for the monosubstituted BCB ketone 1a with reduced steric hindrance as the model substrate (Table 1; see the ESI† for the complete set of optimization data). Ligands L1–L3, L6–L8, and L10 were tested as indicated in Table 1. However, none of these ligands resulted in the formation of the desired product 3aa, and 2a underwent rapid decomposition. Phosphinooxazoline (PHOX)-type ligands L4 and L5 produced the (4 + 3) cycloadduct 3aa, albeit with a low yield (<15%). The current Pd-catalyzed cycloaddition of BCB was highly ligand-dependent (entries 1–10). Although Segphos L8 did not produce oxa-BCO 3aa, the sterically-hindered and electron-rich DTBM-Segphos L9 successfully yielded the desired product with a 49% NMR yield (entry 8 versus 9). Further improvement of the yield was achieved with EtOAc, THF or 1,4-dioxane instead of toluene as the solvent (entries 11–15). Besides ligands, the solvent also had substantial effect on the yield but no improvement over 1,4-dioxane was seen. Therefore, the final reaction conditions are described as follows: 1 (1.0 equiv.), 2 (1.2 equiv.), Pd2(dba)3.CHCl3 (5 mol%), commercial available ligand L9 (10 mol%), 1,4-dioxane, 25 °C, and 12 h (conditions A). Moreover, we conducted condition-based sensitivity screening,33 revealing that the O2 level had no significant impact on the reaction. However, this reaction exhibited moderate sensitivity to concentration, moisture, temperature, and scale.
Selected examples of the optimization of reaction conditionsa.
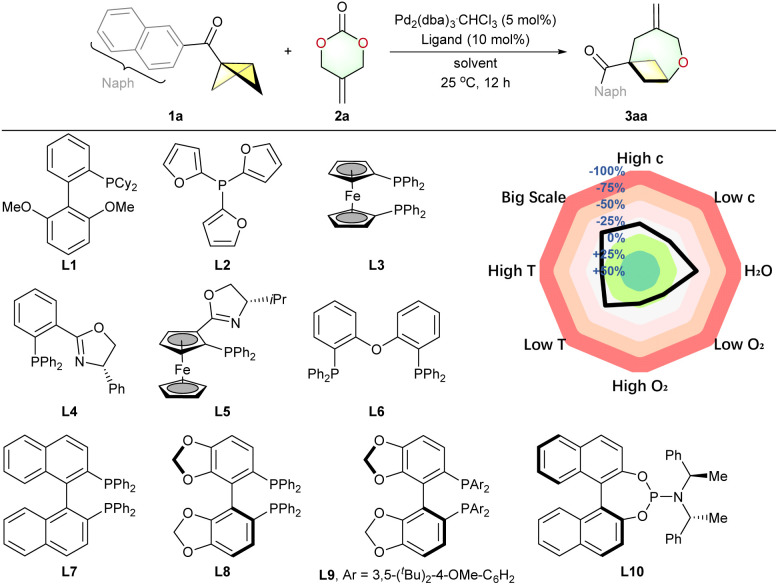
| |||||
|---|---|---|---|---|---|
| Entry | Ligand | Solvent | Conversion of 1ab (%) | Conversion of 2ab (%) | Yieldb (%) |
| 1 | L1 | Toluene | 36 | 100 | 0 |
| 2 | L2 | Toluene | <5 | 100 | 0 |
| 3 | L3 | Toluene | 34 | 100 | 0 |
| 4 | L4 | Toluene | 42 | 100 | 11 |
| 5 | L5 | Toluene | 58 | 100 | 14 |
| 6 | L6 | Toluene | 44 | 100 | 0 |
| 7 | L7 | Toluene | 50 | 100 | 0 |
| 8 | L8 | Toluene | 10 | 100 | 0 |
| 9 | L9 | Toluene | 66 | 100 | 49 |
| 10 | L10 | Toluene | 8 | 73 | 0 |
| 11 | L9 | PhCl | 75 | 100 | 40 |
| 12 | L9 | CH2Cl2 | 30 | 100 | 10 |
| 13 | L9 | EtOAc | 64 | 100 | 53 |
| 14 | L9 | THF | 85 | 100 | 71 |
| 15 | L9 | 1,4-Dioxane | >99 | 100 | 96 |
Reaction conditions: 1a (0.10 mmol), 2a (0.12 mmol), Pd2(dba)3·CHCl3 (5 mol%) and ligand (10 mol%), solvent (2 mL), 25 °C, under N2 for 12 h.
Determined by 1H NMR spectroscopy using CH2Br2 as the internal standard.
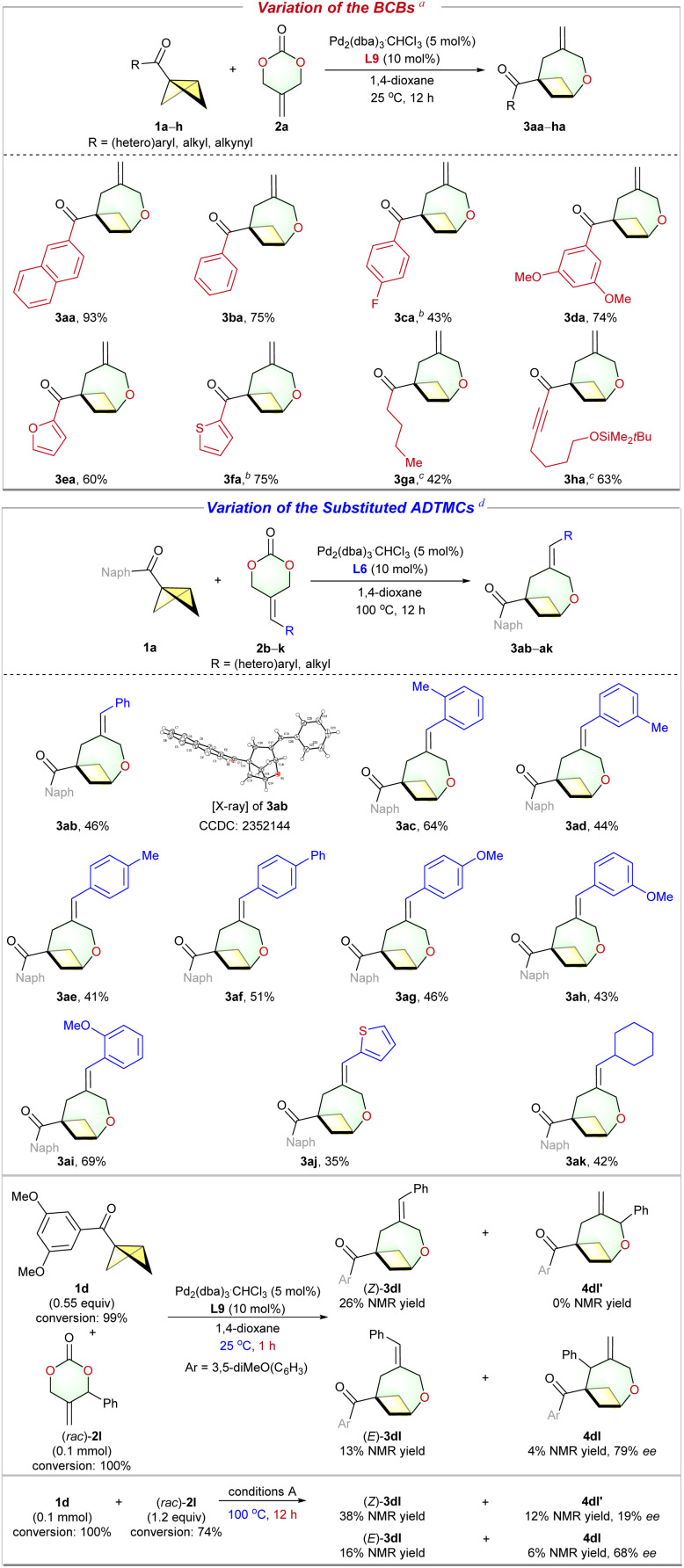
With optimized reaction conditions established, we first examined the substrate scope with respect to the BCBs 1. As shown in Scheme 2, in addition to naphthyl BCB ketone 1a, phenyl BCB ketones (3ba–da) with substituents in the para- and meta-positions were compatible with our catalyst system and afforded the corresponding oxa-BCOs in moderate to good yield. The cycloadditions of furan-2-yl- and thiophen-2-yl-containing BCB ketones were also successful (3ea–fa). Aliphatic BCB ketones, such as 1p, are also tolerated, yet with eroded yield. Notably, the functionalized alkynyl-substituted Malins's BCB,34 not previously reported in cycloaddition reactions, selectively forms the desired cycloadduct 3ha with a 63% yield.
Scheme 2. Substrate scope of the Pd-catalyzed (4 + 3) cycloaddition. a Standard conditions A: 1 (0.20 mmol), 2 (0.24 mmol), Pd2(dba)3·CHCl3 (5 mol%), and L9 (10 mol%) in 1,4-dioxane (4 mL) at 25 °C under a N2 atmosphere for 12 h. Isolated yields of 3 were reported. b Reaction was run at 0.15 mmol scale. c Run at 100 °C. d Standard conditions B: 1 (0.10 mmol), 2 (0.20 mmol), Pd2(dba)3·CHCl3 (5 mol%), and L6 (10 mol%) in 1,4-dioxane (2 mL) at 100 °C under a N2 atmosphere for 12 h.
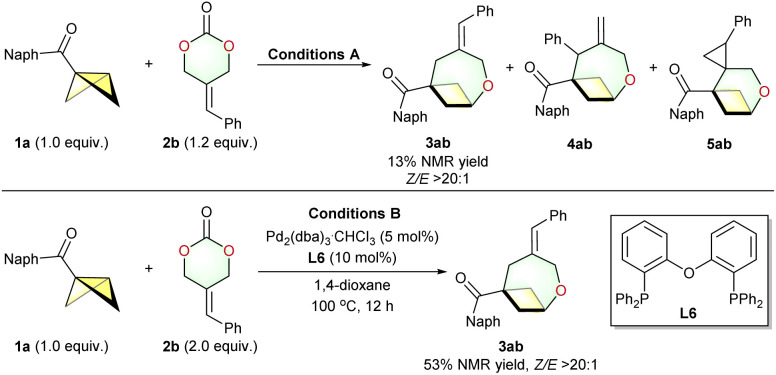
Although (4 + 2) cycloadditions of ADTMCs are well established,28 the corresponding (4 + 3) reactions are relatively rare. Currently, there have been only two successful examples.29g,h However, the (4 + 3) reactions reported independently by Chen29g and Li29h are not compatible with substituted ADTMCs. This result can be attributed to the additional challenges in controlling regioselectivity (such as the by-products 4ab and 5ab in Scheme 3) and Z/E selectivity when using the substituted ADTMCs as oxa-1,4-dipole synthons. Under conditions A, 1a and phenyl-2-alkylidenetrimethylene carbonate 2b were converted into product 3ab, but the yield was low (13% NMR yield). The structure and E/Z geometry of the C C bond in 3ab were determined using X-ray single crystal diffraction.35 After numerous attempts, the optimized reaction conditions B successfully facilitated the (4 + 3) cycloaddition of 1a and 2b, resulting in a 53% NMR yield of the desired 3ab together with some unidentified by-products (<10% NMR yield) (Scheme 3 bottom, see the ESI for details). Next, we investigated the reaction of the substituted ADTMCs with substituents at the ortho-, meta- or para-position of the phenyl ring (Scheme 2, middle, 3ab–ai). In general, substrates (3ac and 3ai) with a substituent group at the ortho-position of the phenyl ring showed higher yields compared to substrates with meta- or para-substituted phenyl rings. Gratifyingly, the reaction was not limited to ADTMCs with substituted phenyl group; the heteroaryl substrate 2j, and the cycloalkyl substrate 2k also underwent the reaction smoothly. Moreover, the reaction between BCB 1d (0.55 equiv.) and racemic 5-methylene-4-phenyl-1,3-dioxan-2-one 2l yielded a mixture of (Z)-3dl and (E)-3dl, along with chiral (4 + 3) cycloadduct 4dl, with a 4% NMR yield and 79% ee after 1 hour at room temperature. When the reaction was conducted at 100 °C with 1.2 equivalents of (rac)-2l, it produced four isomers, namely (Z)-3dl, (E)-3dl, 4dl′ (19% ee), and 4dl (68% ee), in a ratio of about 52 : 24 : 16 : 8. The unreacted ADTMC 2l was obtained with an enantiomeric excess of 1% (Scheme 2 bottom).
Scheme 3. Optimized reaction conditions B for the (4 + 3) cycloadditions of BCBs with the substituted ADTMCs.
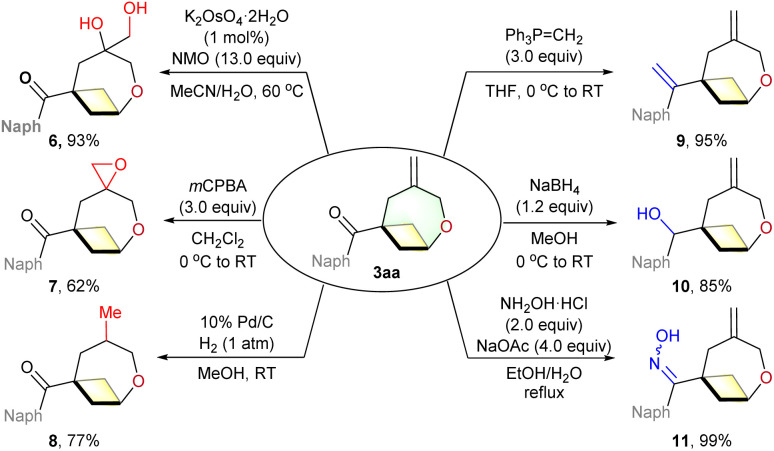
To demonstrate the synthetic utility of these (4 + 3) cycloadducts, several transformations of 3aa were investigated (Scheme 4). Firstly, we found that the dihydroxylation of the exo-methylene moiety of 3aa using K2OsO4–2H2O/NMO afforded diol 6 in 93% yield. Moreover, the epoxidation of the double bond was accomplished with m-CPBA (3aa → 7). Alternatively, the double bond could be hydrogenated to produce the corresponding 8 in good yield. In addition, adduct 3aa could be converted into a variety of oxa-bicyclo[4.1.1]octane derivatives by Wittig olefination (9), reduction (10) and condensation reaction (11).
Scheme 4. Synthetic transformations.
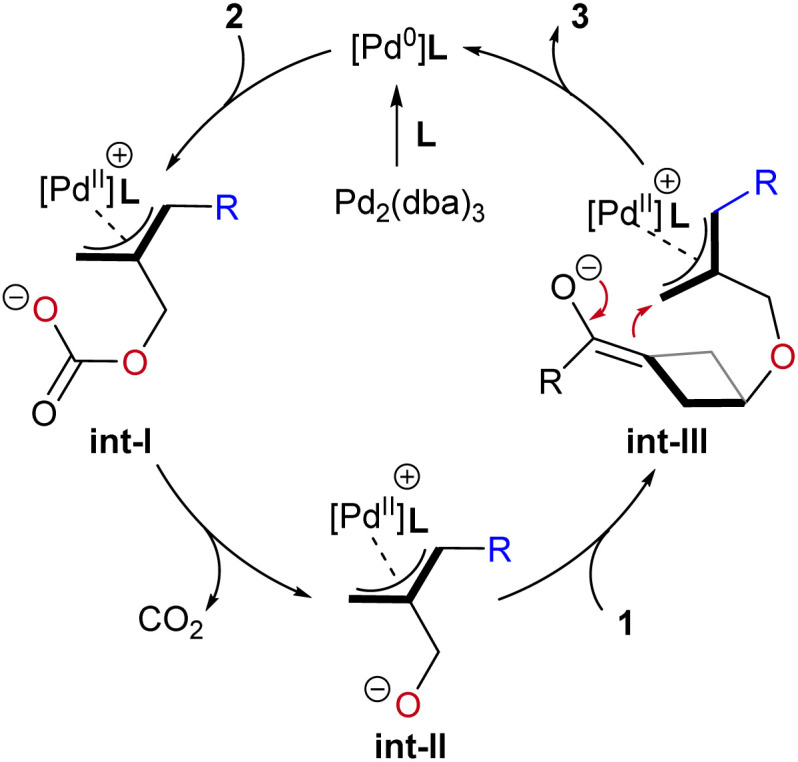
Based on our experimental observations and relevant literature studies, we proposed a plausible mechanism to illustrate the catalytic cycle (Scheme 5). First, Pd2(dba)3.CHCl3 and the P-ligand formed a [Pd0]/L complex. Then, oxidative addition of the ADTMCs 1 to palladium(0) gives π-allylpalladium carbonate int-I. Successive decarboxylation forms π-allyl palladium 1,4-O/C dipole species int-II. The oxygen anion in the zwitterionic allylpalladium intermediates int-II facilitates nucleophilic ring-opening of BCBs, generating intermediate int-III. Finally, intramolecular allyation of int-III affords the target (4 + 3) product 3 and regenerates the palladium catalyst. Therefore, in the O-homoconjugate addition process (int-II → int-III), the monosubstituted BCB ketones could serve as more effective electrophiles compared to the 1,3-disubstituted BCB ketones for producing the desired cycloadducts.
Scheme 5. Proposed mechanism.
Conclusions
In conclusion, a new strategy based on the palladium catalysis toward the atom-economic synthesis of uncommon yet significant 2-oxabicyclo[4.1.1]octane frameworks (oxa-BCOs) has been presented. This represents the first hetero-(4 + 3) cycloaddition of BCBs.36 The utilization of easily accessible starting materials, gentle reaction conditions, broad functional group tolerance, and versatile functionalizations of the functionalized oxa-BCOs products renders this approach highly practical and appealing. The increasing significance of bicyclo[4.1.1]octanes in the quest for new bridged bicyclic molecules with bioisosteric properties leads us to believe that our protocol would not only enrich the toolkit of synthetic chemists but also greatly enlarge the (hetero-)bicyclo[n.1.1]alkanes library accessible for drug discovery.
Data availability
The data supporting this article have been included as part of the ESI.† All detailed procedures, characterization data and NMR spectra are available in the ESI.†
Author contributions
X.-Y. G. and L. T. performed all of the experiments and analysed their results. X.-Y. G., X. Z. and J.-J. F. wrote the manuscript. J.-J. F. conceived the catalytic system and directed the project.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We are grateful to the Fundamental Research Funds for the Central Universities. The 1H, 13C NMR spectra, HRMS (ESI) and single crystal X-ray diffraction were performed at Analytical Instrumentation Center of Hunan University.
Electronic supplementary information (ESI) available. CCDC 2352144. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02998d
Notes and references
- (a) Xue Y. Dong G. Acc. Chem. Res. 2022;55:2341–2354. doi: 10.1021/acs.accounts.2c00400. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Min L. Liu X. Li C.-C. Acc. Chem. Res. 2020;53:703–718. doi: 10.1021/acs.accounts.9b00640. [DOI] [PubMed] [Google Scholar]; (c) Liu J. Liu X. Wu J. Li C.-C. Chem. 2020;6:579–615. doi: 10.1016/j.chempr.2019.12.027. [DOI] [Google Scholar]; (d) Liu C.-H. Yu Z.-X. Angew. Chem., Int. Ed. 2017;56:8667–8671. doi: 10.1002/anie.201702288. [DOI] [PubMed] [Google Scholar]; (e) Stockdale T. P. Williams C. M. Chem. Soc. Rev. 2015;44:7737–7763. doi: 10.1039/C4CS00477A. [DOI] [PubMed] [Google Scholar]; (f) Ruiz M. López-Alvarado P. Giorgi G. Menéndez J. C. Chem. Soc. Rev. 2011;40:3445–3454. doi: 10.1039/C1CS15018A. [DOI] [PubMed] [Google Scholar]
- (a) Lovering F. Bikker J. Humblet C. J. Med. Chem. 2009;52:6752–6756. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]; (b) Lovering F. MedChemComm. 2013;4:515–519. doi: 10.1039/C2MD20347B. [DOI] [Google Scholar]
- (a) Subbaiah M. A. M. Meanwell N. A. J. Med. Chem. 2021;64:14046–14128. doi: 10.1021/acs.jmedchem.1c01215. [DOI] [PubMed] [Google Scholar]; (b) Mykhailiuk P. K. Org. Biomol. Chem. 2019;17:2839–2849. doi: 10.1039/C8OB02812E. [DOI] [PubMed] [Google Scholar]; (c) Semeno V. V. Vasylchenko V. O. Fesun I. M. Ruzhylo L. Yu. Kipriianov M. O. Melnykov K. P. Skreminskyi A. Iminov R. Mykhailiuk P. Vashchenko B. V. Grygorenko O. O. Chem.–Eur. J. 2023:e202303859. doi: 10.1002/chem.202303859. [DOI] [PubMed] [Google Scholar]
- For representative reviews, see:; (a) Shire B. R. Anderson E. A. JACS Au. 2023;3:1539–1553. doi: 10.1021/jacsau.3c00014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson J. M. Measom N. D. Murphy J. A. Poole D. L. Angew. Chem., Int. Ed. 2021;60:24754–24769. doi: 10.1002/anie.202106352. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pramanik M. M. D. Qian H. Xiao W.-J. Chen J.-R. Org. Chem. Front. 2020;7:2531–2537. doi: 10.1039/D0QO00460J. [DOI] [Google Scholar]; (d) He F.-S. Xie S. Yao Y. Wu J. Chin. Chem. Lett. 2020;31:3065–3072. doi: 10.1016/j.cclet.2020.04.023. [DOI] [Google Scholar]; (e1) Kanazawa J. Uchiyama M. Synlett. 2019;30:1–11. doi: 10.1055/s-0037-1610314. [DOI] [Google Scholar]; ; For selected examples, see:; (f) Zhao J.-X. Chang Y.-X. He C. Burke B. J. Collins M. R. Bel M. D. Elleraas J. Gallego G. M. Montgomery T. P. Mousseau J. J. Nair S. K. Perry M. A. Spangler J. E. Vantourout J. C. Baran P. S. Proc. Natl. Acad. Sci. U. S. A. 2021;28:e2108881118. doi: 10.1073/pnas.2108881118. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhang X. Smith R. T. Le C. McCarver S. J. Shireman B. T. Carruthers N. I. MacMillan D. W. C. Nature. 2020;580:220–226. doi: 10.1038/s41586-020-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yu S. Jing C. Noble A. Aggarwal V. K. Angew. Chem., Int. Ed. 2020;59:3917–3921. doi: 10.1002/anie.201914875. [DOI] [PubMed] [Google Scholar]; (i) Yang Y. Tsien J. Hughes J. M. E. Peters B. K. Merchant R. R. Qin T. Nat. Chem. 2021;13:950–955. doi: 10.1038/s41557-021-00786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Denisenko A. Garbuz P. Voloshchuk N. M. Holota Y. Al-Maali G. Borysko P. Mykhailiuk P. K. Nat. Chem. 2023;15:1155–1163. doi: 10.1038/s41557-023-01222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Levterov V. V. Panasyuk Y. Pivnytska V. O. Mykhailiuk P. K. Angew. Chem., Int. Ed. 2020;59:7161–7167. doi: 10.1002/anie.202000548. [DOI] [PubMed] [Google Scholar]; (c) Levterov V. V. Panasiuk Y. Shablykin O. Stashkevych O. Sahun K. Rassokhin A. Sadkova I. Lesyk D. Anisiforova A. Holota Y. Borysko P. Bodenchuk I. Voloshchuk N. M. Mykhailiuk P. K. Angew. Chem., Int. Ed. 2024:e202319831. doi: 10.1002/anie.202319831. [DOI] [PubMed] [Google Scholar]; (d) Denisenko A. Garbuz P. Shishkina S. V. Voloshchuk N. M. Mykhailiuk P. K. Angew. Chem., Int. Ed. 2020;59:20515–20521. doi: 10.1002/anie.202004183. [DOI] [PubMed] [Google Scholar]; (e) Denisenko A. Garbuz P. Makovetska Y. Shablykin O. Lesyk D. Al-Maali G. Korzh R. Sadkova I. V. Mykhailiuk P. K. Chem. Sci. 2023;14:14092–14099. doi: 10.1039/D3SC05121H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Jeyaseelan R. Utikal M. Daniliuc C. G. Næsborg L. Chem. Sci. 2023;14:11040–11044. doi: 10.1039/D3SC03242F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Reinhold M. Steinebach J. Golz C. Walker J. C. L. Chem. Sci. 2023;14:9885–9891. doi: 10.1039/D3SC03083K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Posz J. M. Sharma N. Royalty P. A. Liu Y. Salome C. Fessard T. C. Brown M. K. J. Am. Chem. Soc. 2024;146:10142–10149. doi: 10.1021/jacs.4c01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Dibchak D. Snisarenko M. Mishuk A. Shablykin O. Bortnichuk L. Klymenko-Ulianov O. Kheylik Y. Sadkova I. V. Rzepa H. S. Mykhailiuk P. K. Angew. Chem., Int. Ed. 2023:e202304246. doi: 10.1002/anie.202304246. [DOI] [PubMed] [Google Scholar]; (b) Harmata A. S. Spiller T. E. Sowden M. J. Stephenson C. R. J. J. Am. Chem. Soc. 2021;143:21223–21228. doi: 10.1021/jacs.1c10541. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Iida T. Kanazawa J. Matsunaga T. Miyamoto K. Hirano K. Uchiyama M. J. Am. Chem. Soc. 2022;144:21848–21852. doi: 10.1021/jacs.2c09733. [DOI] [PubMed] [Google Scholar]; (d) Frank N. Nugent J. Shire B. R. Pickford H. D. Rabe P. Sterling A. J. Zarganes-Tzitzikas T. Grimes T. Thompson A. L. Smith R. C. Schofield C. J. Brennan P. E. Duarte F. Anderson E. A. Nature. 2022;611:721–726. doi: 10.1038/s41586-022-05290-z. [DOI] [PubMed] [Google Scholar]
- Cairncross A. Blanchard Jr E. P. J. Am. Chem. Soc. 1966;88:496–504. doi: 10.1021/ja00955a021. [DOI] [Google Scholar]
- (a) Wipf P. Walczak M. A. A. Angew. Chem., Int. Ed. 2006;45:4172–4175. doi: 10.1002/anie.200600723. [DOI] [PubMed] [Google Scholar]; (b) Walczak M. A. A. Krainz T. Wipf P. Acc. Chem. Res. 2015;48:1149–1158. doi: 10.1021/ar500437h. [DOI] [PubMed] [Google Scholar]
- (a) Kleinmans R. Pinkert T. Dutta S. Paulisch T. O. Keum H. Daniliuc C. G. Glorius F. Nature. 2022;605:477–482. doi: 10.1038/s41586-022-04636-x. [DOI] [PubMed] [Google Scholar]; (b) Liang Y. Kleinmans R. Daniliuc C. G. Glorius F. J. Am. Chem. Soc. 2022;144:20207–20213. doi: 10.1021/jacs.2c09248. [DOI] [PubMed] [Google Scholar]; (c) Kleinmans R. Dutta S. Ozols K. Shao H. Schäfer F. Thielemann R. E. Chan H. T. Daniliuc C. G. Houk K. N. Glorius F. J. Am. Chem. Soc. 2023;145:12324–12332. doi: 10.1021/jacs.3c02961. [DOI] [PubMed] [Google Scholar]; (d) Dutta S. Lu Y.-L. Erchinger J. E. Shao H. Studer E. Schäfer F. Wang H. Rana D. Daniliuc C. G. Houk K. N. Glorius F. J. Am. Chem. Soc. 2024;146:5232–5241. doi: 10.1021/jacs.3c11563. [DOI] [PubMed] [Google Scholar]; (e) Dutta S. Lee D. Ozols K. Daniliuc C. G. Shintani R. Glorius F. J. Am. Chem. Soc. 2024;146:2789–2797. doi: 10.1021/jacs.3c12894. [DOI] [PubMed] [Google Scholar]; (f) Liang Y. Paulus F. Daniliuc C. G. Glorius F. Angew. Chem., Int. Ed. 2023:e202305043. doi: 10.1002/anie.202305043. [DOI] [PubMed] [Google Scholar]; (g) Liang Y. Nematswerani R. Daniliuc C. G. Glorius F. Angew. Chem., Int. Ed. 2024:e202402730. doi: 10.1002/anie.202402730. [DOI] [PubMed] [Google Scholar]; (h) Wang H. Shao H. Das A. Dutta S. Chan H. T. Daniliuc C. Houk K. N. Glorius F. Science. 2023;381:75–81. doi: 10.1126/science.adh9737. [DOI] [PubMed] [Google Scholar]
- Guo R. Chang Y.-C. Herter L. Salome C. Braley S. E. Fessard T. C. Brown M. K. J. Am. Chem. Soc. 2022;144:7988–7994. doi: 10.1021/jacs.2c02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Dhake K. Woelk K. J. Becica J. Un A. Jenny S. E. Leitch D. C. Angew. Chem., Int. Ed. 2022;61:e202204719. doi: 10.1002/anie.202204719. [DOI] [PubMed] [Google Scholar]; (b) Woelk K. J. Dhake K. Schley N. D. Leitch D. C. Chem. Commun. 2023;59:13847–13850. doi: 10.1039/D3CC04234K. [DOI] [PubMed] [Google Scholar]
- Agasti S. Beltran F. Pye E. Kaltsoyannis N. Crisenza G. E. M. Procter D. J. Nat. Chem. 2023;15:535–541. doi: 10.1038/s41557-023-01135-y. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Huang W. Dhungana R. K. Granados A. Keess S. Makvandi M. Molander G. A. J. Am. Chem. Soc. 2022;144:23685–23690. doi: 10.1021/jacs.2c11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Xu M. Wang Z. Sun Z. Ouyang Y. Ding Z. Yu T. Xu L. Li P. Angew. Chem., Int. Ed. 2022;61:e202214507. doi: 10.1002/anie.202214507. [DOI] [PubMed] [Google Scholar]; (b) Yu T. Yang J. Wang Z. Ding Z. Xu M. Wen J. Xu L. Li P. J. Am. Chem. Soc. 2023;145:4304–4310. doi: 10.1021/jacs.2c13740. [DOI] [PubMed] [Google Scholar]
- Liu Y. Lin S. Li Y. Xue J.-H. Li Q. Wang H. ACS Catal. 2023;13:5096–5103. doi: 10.1021/acscatal.3c00305. [DOI] [Google Scholar]
- Nguyen T. V. T. Bossonnet A. Wodrich M. D. Waser J. J. Am. Chem. Soc. 2023;145:25411–25421. doi: 10.1021/jacs.3c09789. [DOI] [PubMed] [Google Scholar]
- Radhoff N. Daniliuc C. G. Studer A. Angew. Chem., Int. Ed. 2023:e202304771. doi: 10.1002/anie.202304771. [DOI] [PubMed] [Google Scholar]
- Ni D. Hu S. Tan X. Yu Y. Li Z. Deng L. Angew. Chem., Int. Ed. 2023:e202308606. doi: 10.1002/anie.202308606. [DOI] [PubMed] [Google Scholar]
- Tang L. Xiao Y. Wu F. Zhou J.-L. Xu T.-T. Feng J.-J. Angew. Chem., Int. Ed. 2023;62:e202310066. doi: 10.1002/anie.202310066. [DOI] [PubMed] [Google Scholar]
- Zhang J. Su J.-Y. Zheng H. Li H. Deng W.-P. Angew. Chem., Int. Ed. 2024:e202318476. doi: 10.1002/anie.202318476. [DOI] [PubMed] [Google Scholar]
- de Robichon M. Kratz T. Beyer F. Zuber J. Merten C. Bach T. J. Am. Chem. Soc. 2023;145:24466–24470. doi: 10.1021/jacs.3c08404. [DOI] [PubMed] [Google Scholar]
- Fu Q. Cao S. Wang J. Lv X. Wang H. Zhao X. Jiang Z. J. Am. Chem. Soc. 2024;146:8372–8380. doi: 10.1021/jacs.3c14077. [DOI] [PubMed] [Google Scholar]
- (a) Meijere A. D. Wenck H. Seyed-Mahdavi F. Tetrahedron. 1986;42:1291–1297. doi: 10.1016/S0040-4020(01)87348-8. [DOI] [Google Scholar]; (b) Schwartz B. D. Smyth A. P. Nashar P. E. Gardiner M. G. Malins L. R. Org. Lett. 2022;24:1268–1273. doi: 10.1021/acs.orglett.1c04071. [DOI] [PubMed] [Google Scholar]; (c) Wang M. Huang Y. Li C. Lu P. Org. Chem. Front. 2022;9:2149–2153. doi: 10.1039/D2QO00167E. [DOI] [Google Scholar]; (d) Yan H. Liu Y. Feng X. Shi L. Org. Lett. 2023;25:8116–8120. doi: 10.1021/acs.orglett.3c03222. [DOI] [PubMed] [Google Scholar]; (e) Ren H. Li T. Xing J. Li Z. Zhang Y. Yu X. Zheng J. Org. Lett. 2024;26:1745–1750. doi: 10.1021/acs.orglett.4c00421. [DOI] [PubMed] [Google Scholar]; (f) Yang L. Wang H. Lang M. Wang J. Peng S. Org. Lett. 2024;26:4104–4110. doi: 10.1021/acs.orglett.4c01219. [DOI] [PubMed] [Google Scholar]
- (a) Applequist D. E. Wheeler J. W. Tetrahedron Lett. 1977;18:3411–3412. doi: 10.1016/S0040-4039(01)83253-6. [DOI] [Google Scholar]; (b) Bychek R. Mykhailiuk P. K. Angew. Chem., Int. Ed. 2022;61:e202205103. doi: 10.1002/anie.202205103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bychek R. M. Hutskalova V. Bas Y. P. Zaporozhets O. A. Zozulya S. Levterov V. V. Mykhailiuk P. K. J. Org. Chem. 2019;84:15106–15117. doi: 10.1021/acs.joc.9b01947. [DOI] [PubMed] [Google Scholar]; (d) Ma X. Sloman D. L. Han Y. Bennett D. J. Org. Lett. 2019;21:7199–7203. doi: 10.1021/acs.orglett.9b02026. [DOI] [PubMed] [Google Scholar]; (e) Ma X. Pinto W. Pham L. N. Sloman D. L. Han Y. Eur. J. Org Chem. 2020:4581–4605. doi: 10.1002/ejoc.202000679. [DOI] [Google Scholar]; (f) McNamee R. E. Thompson A. L. Anderson E. A. J. Am. Chem. Soc. 2021;143:21246–21251. doi: 10.1021/jacs.1c11244. [DOI] [PubMed] [Google Scholar]
- (a) Sujansky S. J. Ma X. Asian J. Org. Chem. 2024:e202400045. doi: 10.1002/ajoc.202400045. [DOI] [Google Scholar]; (b) Bellotti P. Glorius F. J. Am. Chem. Soc. 2023;145:20716–20732. doi: 10.1021/jacs.3c08206. [DOI] [PubMed] [Google Scholar]; (c) Golfmann M. Walker J. C. L. Commun. Chem. 2023;6:9. doi: 10.1038/s42004-022-00811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kelly C. B. Milligan J. A. Tilley L. J. Sodano T. M. Chem. Sci. 2022;13:11721–11737. doi: 10.1039/D2SC03948F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Turkowska J. Durkaab J. Gryko D. Chem. Commun. 2020;56:5718–5734. doi: 10.1039/D0CC01771J. [DOI] [PubMed] [Google Scholar]; (f) Fawcett A. Pure Appl. Chem. 2020;92:751–765. doi: 10.1515/pac-2019-1007. [DOI] [Google Scholar]
- (a) Josien H. B., Clader J. W., Greenlee W. J., Mayer M. J., Davis J. L. and Wan S., WO pat. 2010085528 A1, 2010; (b) Josien H. B., Clader J. W., Greenlee W. J., Mayer M. J., Davis J. L. and Wan S., US pat. 20110312967 A1, 2011; (c) Wang Q. Han J. Bao B. J. Food Biochem. 2017;41:e12320. doi: 10.1111/jfbc.12320. [DOI] [Google Scholar]
- (a) Mülle C. Bauer A. Maturi M. M. Cuquerella M. C. Miranda M. A. Bach T. J. Am. Chem. Soc. 2011;133:16689–16697. doi: 10.1021/ja207480q. [DOI] [PubMed] [Google Scholar]; (b) Austin K. A. B. Herdtweck E. Bach T. Angew. Chem., Int. Ed. 2011;50:8416–8419. doi: 10.1002/anie.201103051. [DOI] [PubMed] [Google Scholar]; (c) Oku A. Sawada Y. Schroeder M. Higashikubo I. Yoshida T. Ohki S. J. Org. Chem. 2004;69:1331–1336. doi: 10.1021/jo0301806. [DOI] [PubMed] [Google Scholar]; (d) Snider B. B. Hui R. A. H. F. J. Org. Chem. 1985;50:5167–5176. doi: 10.1021/jo00225a038. [DOI] [Google Scholar]; (e) Snider B. B. Hui R. A. H. F. Kulkarni Y. S. J. Am. Chem. Soc. 1985;107:2194–2196. doi: 10.1021/ja00293a074. [DOI] [Google Scholar]
- Cho H.-J. Kim J. H. Org. Biomol. Chem. 2023;21:9507–9518. doi: 10.1039/D3OB01619F. [DOI] [PubMed] [Google Scholar]
- (a) Li M.-M. Qu B.-L. Xiao Y.-Q. Xiao W.-J. Lu L.-Q. Sci. Bull. 2021;66:1719–1722. doi: 10.1016/j.scib.2021.04.037. [DOI] [PubMed] [Google Scholar]; (b) Xie H. Chen L. Han Z. Yang Z. Sun J. Huang H. Org. Lett. 2023;25:5011–5016. doi: 10.1021/acs.orglett.3c01646. [DOI] [PubMed] [Google Scholar]; (c) Liu Q. Liu T.-X. Ru Y. Zhu X. Zhang G. Chem. Commun. 2019;55:14498–14501. doi: 10.1039/C9CC07950E. [DOI] [PubMed] [Google Scholar]; (d) Dou P.-H. Yuan S.-P. Chen Y. Zhao J.-Q. Wang Z.-H. You Y. Zhang Y.-P. Zhou M.-Q. Yuan W.-C. J. Org. Chem. 2022;87:6025–6037. doi: 10.1021/acs.joc.2c00276. [DOI] [PubMed] [Google Scholar]; (e) Chen L. Xie H. Xue Y. Han Z. Sun J. Huang H. Chin. J. Chem. 2024;42:829–834. doi: 10.1002/cjoc.202300668. [DOI] [Google Scholar]; (f) Shintani R. Moriya K. Hayashi T. Chem. Commun. 2011;47:3057–3059. doi: 10.1039/C0CC05308B. [DOI] [PubMed] [Google Scholar]; (g) Chen Z. Chen Z.-C. Du W. Chen Y.-C. Org. Lett. 2021;23:8559–8564. doi: 10.1021/acs.orglett.1c03279. [DOI] [PubMed] [Google Scholar]; (h) Meng Y. Song M. Wang Y. Wang Y. Li E.-Q. Org. Chem. Front. 2023;10:2648–2652. doi: 10.1039/D3QO00501A. [DOI] [Google Scholar]; (i) Uno H. Kawai K. Araki T. Shiro M. Shibata N. Angew. Chem., Int. Ed. 2022;61:e202117635. doi: 10.1002/anie.202117635. [DOI] [PubMed] [Google Scholar]; (j) Mao B. Liu H. Yan Z. Xu Y. Xu J. Wang W. Wu Y. Guo H. Angew. Chem., Int. Ed. 2020;59:11316–11320. doi: 10.1002/anie.202002765. [DOI] [PubMed] [Google Scholar]
- (a) Zhu C.-Z. Feng J.-J. Zhang J. Angew. Chem., Int. Ed. 2017;56:1351–1355. doi: 10.1002/anie.201609608. [DOI] [PubMed] [Google Scholar]; (b) Tang L. Huang Q.-N. Wu F. Xiao Y. Zhou J.-L. Xu T.-T. Wu W.-B. Qu S. Feng J.-J. Chem. Sci. 2023;14:9696–9703. doi: 10.1039/D3SC03258B. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xiao Y. Xu T.-T. Zhou J.-L. Wu F. Tang L. Liu R.-Y. Wu W.-B. Feng J.-J. Chem. Sci. 2023;14:13060–13066. doi: 10.1039/D3SC04457B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For Pd-catalyzed ring-opening of BCBs, see:; (a) Fawcett A. Biberger T. Aggarwal V. K. Nat. Chem. 2019;11:117–122. doi: 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]; (b) Zhang Z. Gevorgyan V. J. Am. Chem. Soc. 2022;144:20875–20883. doi: 10.1021/jacs.2c09045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- We presented a compilation of unsuccessful substrate scopes in the ESI. The importance of failed experiments, see: ; Strieth-Kalthoff F. Sandfort F. Kühnemund M. Schäfer F. R. Kuchen H. Glorius F. Angew. Chem., Int. Ed. 2022;61:e202204647. doi: 10.1002/anie.202204647. [DOI] [PubMed] [Google Scholar]
- Pitzer L. Schäfers F. Glorius F. Angew. Chem., Int. Ed. 2019;58:8572–8576. doi: 10.1002/anie.201901935. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D. Zhang M. Y. Attard R. H. Gardiner M. G. Malins L. R. Chem.–Eur. J. 2020;26:2808–2812. doi: 10.1002/chem.201905539. [DOI] [PubMed] [Google Scholar]
- CCDC 2352144 (3ab)
- During the peer review of our manuscript, two studies on the (4 + 3) cycloadditions of BCBs were conducted using a Lewis acid catalysis approach; (a) Wang J.-J. Tang L. Xiao Y. Wu W.-B. Wang G. Feng J.-J. Angew. Chem., Int. Ed. 2024;63:e202405222. doi: 10.1002/anie.202405222. [DOI] [PubMed] [Google Scholar]; (b) Nicolai S. Waser J. Chem. Sci. 2024;15:10823–10829. doi: 10.1039/D4SC02767A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.† All detailed procedures, characterization data and NMR spectra are available in the ESI.†