Abstract
Microglia are present throughout the central nervous system and are vital in neural repair, nutrition, phagocytosis, immunological regulation, and maintaining neuronal function. In a healthy spinal cord, microglia are accountable for immune surveillance, however, when a spinal cord injury occurs, the microenvironment drastically changes, leading to glial scars and failed axonal regeneration. In this context, microglia vary their gene and protein expression during activation, and proliferation in reaction to the injury, influencing injury responses both favorably and unfavorably. A dynamic and multifaceted injury response is mediated by microglia, which interact directly with neurons, astrocytes, oligodendrocytes, and neural stem/progenitor cells. Despite a clear understanding of their essential nature and origin, the mechanisms of action and new functions of microglia in spinal cord injury require extensive research. This review summarizes current studies on microglial genesis, physiological function, and pathological state, highlights their crucial roles in spinal cord injury, and proposes microglia as a therapeutic target.
Keywords: astrocytes, cytokines, functional recovery, immune regulation, M1/M2 activation, macrophages, microglia, neuroinflammation, spinal cord injury, therapy
Introduction
Spinal cord injury (SCI) is a type of severe neurological dysfunction typically brought on by trauma and results in a total or partial loss of neurological function. While traumas might include car accidents, sports injuries, or simple slips and falls, nontraumatic causes such as discopathies, infections, or tumors can also cause SCI. In addition to the physical and emotional trauma caused by SCI, independent from its cause, families, and society are also burdened economically by its aftermath. As per estimates, over two million people worldwide are affected by SCI (Quadri et al., 2020). To restore injured spinal cord tissues, modern medicine focuses on finding novel treatment strategies, which makes understanding the pathological process and biological mechanisms underlying SCI a current necessity.
The pathological stages of SCI can be roughly categorized into two phases: Mechanical damage results in a primary injury, characterized by laceration, compression, transection, shearing, distraction, or stretching, which cause immediate hemorrhage, vasospasm, and rapid cell death. In the second phase, several biological responses occur, including ischemic injury, an immune-inflammatory response, necrosis, scar formation, and excitotoxicity, developing over weeks and months (Jiang et al., 2023). Notably, inflammation significantly influences the progression of SCI. Microglia and neutrophils respond first during neuroinflammatory responses, followed by monocyte infiltration. As central nervous system (CNS) -resident immune cells, microglia play an essential role in the inflammatory response following SCI, with new functions and modes of action being discovered regularly. This narrative review intends to provide an exhaustive summary of the latest findings on microglia’s roles and therapeutic potential in SCI.
Literature Search Strategy and Data Extraction and Synthesis
A thorough literature search was conducted using the PubMed database, targeting publications from 2010 to 2023. The search terms included “microglia, macrophages, M1/M2 activation, neuroinflammation, spinal cord injury, immune regulation, therapy, functional recovery,” along with specific terms related to the genesis and pathological states of microglia. These terms were chosen to cover the extensive scope of microglial functions, their dynamic responses to SCI, and the evolving strategies in therapeutic interventions.
Data extracted from the selected articles encompassed study design, sample sizes, and key findings relevant to microglial roles in SCI. A detailed narrative synthesis approach was adopted to integrate findings from various study designs, including experimental research and previous review articles. This approach enabled a comprehensive overview of microglial functions, their responses to SCI, and potential therapeutic avenues. Special attention was given to synthesizing information on the physiological and pathological states of microglia, highlighting their complex roles in SCI pathology and recovery.
Overview of Microglia
Genesis and physiological role of microglia
Unlike other glial cells, microglia are the only cells in nerve tissue originating from the mesoderm. c-kit+ myeloid progenitor cells, which arise from the yolk sac, have been revealed to be the actual progenitors of microglia (Lopez‐Atalaya et al., 2018). Studies in the past claimed that only primitive hematopoiesis was the origin of microglia, which was supplemented and corrected by Xu et al.’s finding in 2015 that adult zebrafish microglia arise from secondary hematopoiesis (Xu et al., 2015). With the development of tracing technologies, the origin of microglia may be further identified. Despite their similarities, macrophages and microglia have different gene expression patterns and origins, demonstrating their inter-cellular independence (Table 1). In mice, microglia become immune cells that dwell in the CNS in the early embryonic stage and renew themselves throughout life. In contrast, monocyte-derived macrophages stem from blood-borne leukocytes, and only participate in this population under disease conditions, such as SCI, when the blood–brain barrier is broken (Tay et al., 2017).
Table 1.
Distinction between hematogenous macrophages and microglia
| Microglia | Macrophages | |
|---|---|---|
| Origin | Yolk sac Ventral wall of dorsal aorta region | Myeloid progenitor cells |
| Location | Central nervous system | Peripheral blood and tissue |
| Size | Small | Big |
| Marker | CD11b–/CD45–/TMEM119/CX3CR1+/P2Y12/Hexb | CD11b+/CD45+/CCR2+/CX3CR1– |
Estimates of microglial abundance in the adult brain range from 5%–20% (Yang et al., 2010). Microglia vary in density, shape, and size in different regions of the CNS (Yang et al., 2010). Although the full range of microglial functions is not yet understood, two key roles have been identified: Immunological defense and maintenance of the homeostasis of the neurological system, including myelin phagocytosis, neuronal activity regulation, pruning of neuronal/synapse collaterals, supporting neuronal development and axon regeneration. Microglia have a dynamic surveillance role in the CNS by making direct or indirect contact with neurons, astrocytes, and oligodendrocytes via their multi-level branches and screening for damage and pathogenic breaches by extending and retracting their processes (Nemes-Baran et al., 2020). Conversely, the absence or dysfunction of microglia can cause immune disorders, inflammatory storms, and CNS neuronal death (Xu et al., 2016).
Microglia enter a disease-related state once activated or when exposed to the microenvironment of disorders, after which they phagocytose invaders (Hakim et al., 2021). According to their activation state, microglia can manifest distinct morphologies, for example, amoeboid microglia with efficient phagocytosis contain fewer or even no cell processes and are more rounded (Vidal-Itriago et al., 2022). Molecular signals, such as different cytokines and chemokines generated by damaged cells, like neurons, regulate the migration of microglia (Gülke et al., 2018). Triggering receptors expressed on myeloid cells 2 (TREM-2) and Toll-like receptors (TLRs), two additional important phagocytic receptors that detect invading pathogens and apoptotic cell debris, are expressed by microglia (Vidal-Itriago et al., 2022). Amoeboid microglia expand their processes around extracellular material by reorganizing the actin cytoskeleton, producing a phagosome, and eventually phagocytosing debris and foreign materials (Vidal-Itriago et al., 2022). Microglial phagocytosis protects against various CNS diseases, such as Alzheimer’s disease, by removing pathological accumulations of amyloid-beta and alpha-synuclein proteins (Bartels et al., 2020).
Microglial heterogeneity in the spinal cord
Despite the fact that microglia exist in all regions of the CNS, they appear to play different roles in different regions and physiological conditions and form distinctive characteristics in response to tissue-specific molecular signals. As a response to SCI, microglia modify their gene expression, function, and morphological structure, corresponding to their activation already mentioned. Various activating and function-regulating surface receptors have been identified, including TLRs, purinergic receptors, C-C motif chemokine receptor 2, and receptor of advanced glycation end products (Wang et al., 2022). However, there is still debate over the definition of microglia subtypes.
Recent developments in single-cell sequencing technology have made it possible to identify more complex microglial phenotypes in healthy and pathological states. In SCI, within seconds to minutes, microglia respond by extending their processes in the direction of the lesion, which reveals dynamic temporal and spatial regulation across the grey and white matter of the spinal cord (Cătălin et al., 2013). Hakim et al. (2021) identified five subtypes of microglia that changed dynamically over time via a single-cell investigation of injured mice spinal cord tissue (0.5 hours–90 days). Thirty minutes after injury, microglia transformed from a resting state into an inflammatory phenotype with expression of the markers interleukin (IL)-1a and CD83, which further converted into a proliferation-related phenotype with expression of the markers HBEGF and EDN1 after 2 hours. Within 6 and 36 hours, monocyte-activated microglia with high MT2 and MSR1 expression were observed, reaching the state of disease-associated microglia after 3 days, which stabilized and persisted over 21 days up to the chronic phase, enhancing functional recovery. These disease-associated microglia are extremely similar to those found in previous investigations of development, demyelination, and neurodegenerative diseases (Hakim et al., 2021). Milich et al. (2021) also identified several new microglia subtypes with their single-cell mouse SCI model study. In the resting microglia, they found highly expressed genes such as P2RY12, Siglech, and TMEM119, which is consistent with previous findings (Schafer et al., 2023). Based on the differential expression of P2RY12, insulin-like growth factor 1 (IGF-1), MSR1, and CDK1, the non-steady-state microglia were classified as dividing, migrating, and inflammation-associated.
There have been several additional significant developments about noteworthy microglia subtypes in the context of SCI: CD11c+ microglia were associated with neuropathic pain, microglia with high expression of NXPE3, PIRB, and NR4A1, were found to traverse the fibrous scar, neonatal microglia in cluster 3 (MG3) microglia showed specific molecular characteristics that support scar-free wound repair, and microglia expressing the markers CD11b, galectin-3, and CD68 were found at the center of the spinal cord lesion with currently unknown functions (Lund et al., 2022; Gong et al., 2023; Zhou et al., 2023). These single-cell analyses offer specific insights into the dynamic alterations of microglia after SCI by demonstrating the substantial association between microglial heterogeneity and post-injury time points, giving us a better understanding of the diversity of microglia.
Role of Microglia in Spinal Cord Injury
Inflammatory regulation: a double-edged sword
When the CNS shows indications of damage, microglia change into an inflammation-related state in a matter of minutes after SCI, which starts a series of critical inflammatory processes. After activation, microglia exhibit two distinct subtypes: classically activated (M1) and alternatively activated (M2) microglia, each with their own distinct physiological characteristics and functions, playing a relevant role in the survival of neurons and functional recovery (Figure 1). Thus, microglia have a dual role in regulating inflammation after SCI.
Figure 1.
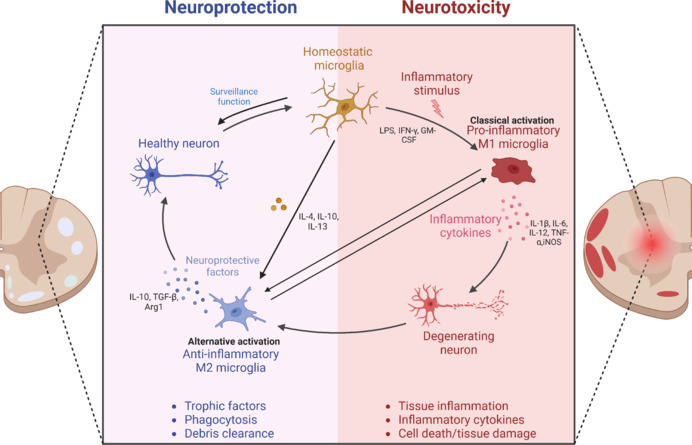
Effects of resting microglia and M1/M2 microglia on neurons.
Homeostatic microglia have a dynamic surveillance role in the CNS. Following spinal cord injury, microglia are activated into pro-inflammatory (M1) and anti-inflammatory (M2) microglia. The pro-inflammatory cytokines produced by M1 microglia, such as IL-1β, TNF-α, and iNOS, further aggravate damage, which could eventually cause the death of neurons, while the anti-inflammatory cytokines produced by M2 microglia, such as IL-10, can restore the damage and play a neuroprotective role. Created with BioRender.com. Arg1: Arginase-1; CNS: central nervous system; GM-CSF: granulocyte-macrophage colony-stimulating factor; IL-1β: interleukin-1β; IL-4: interleukin-4; IL-6: interleukin-6; IL-10: interleukin-10; IL-12: interleukin-12; IL-13: interleukin-13; IFN-γ: Interferon-γ; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; TNF-α: tumor necrosis factor-α; TGF-β: transforming growth factor-β.
Harmful effects of microglia
In reaction to inflammatory agents including lipopolysaccharide (LPS) and Interferon-γ (IFN-γ), microglia in the resting state can transform into the M1 sub- or phenotype. The primary signaling pathways that contribute to this activation towards the M1 phenotype include (1) the regulation of Janus kinase (JAK)1/JAK2 and signal transducers and activator of transcription (STAT) through Th1-cells-secreted IFN-γ, and (2) the stimulation of TLR4 by LPS or damage-associated molecular patterns, initiating the formation of an “activation complex” composed of myeloid differentiation primary response gene 88, nuclear factor kappa B, p65, p38, and interferon regulatory factor (IRF) 3 (Fu et al., 2022). M1 microglia have been shown to release harmful cytokines like IL-6, IL-12, IFN-γ, and tumor necrosis factor (TNF) after SCI, which trigger inflammatory responses from nearby cells, resulting in significant neural cell death, axonal degeneration, and demyelination. The overproduction of reactive oxygen species at the site of injury, which disrupts the redox equilibrium and severely damages deoxyribonucleic acid (DNA), proteins, and lipids, is one of the most critical pro-inflammatory mechanisms, causing accompanying edema, as well as uncontrolled peroxidation (Nukolova et al., 2018). After SCI, animal’s motor function recovery was shown to be markedly enhanced by gene editing that eliminated the IL-1a, TNF, or C1q-encoding genes from their microglia (Deng et al., 2022). Additionally, it has been shown that chronic neuroinflammation is brought on by persistently activated microglia, which results in additional tissue damage and cell death (Zhang et al., 2021b). By inducing astrocyte necroptosis, M1 microglia have been observed to be associated with spinal cord cavity generation and enlargement after SCI (Brockie et al., 2021). Activated microglia also secrete large amounts of chemokines and cell adhesion molecules, possibly helping to recruit immune cells in circulation, including circulating leukocytes, B cells, T cells, dendritic cells, and macrophages to the injury site, further complicating the immune response (David and Kroner, 2011). Although active microglia may improve SCI recovery, M1 microglia also phagocytose necrotic cells and debris to promote CNS recovery, especially M1 microglia are usually overactivated, which can facilitate the accumulation and invasion of inflammatory cells and intensify the immune system’s response, intensifying secondary injury cascades.
Protective role of microglia
Alternatively, activated M2 microglia carry out various tasks, including removing cellular debris, the secretion of neurotrophic factors and anti-inflammatory cytokines, and tissue healing. M2a, M2b, and M2c are three further subtypes of M2 microglia that differ in terms of how they are activated and what their functions are.
M2a microglia are the major M2 subtype, typically induced by IL-4 or IL-13 and interacting with the IL-4 receptor (IL-4R) and IL-13 receptor (IL13Ra), respectively. This M2 subtype triggers transcription factors such as STAT6, peroxisome-proliferator activated receptor γ, and IRF4, which in turn lead to the release of growth factors including IGF-1, transforming growth factor beta (TGF-β) and IL-10, causing the further transformation of M1 to M2 microglia and improving tissue repair (Shan et al., 2020). M2a microglia express neuroprotective markers such as arginase-1 or CD206 and promote axon regeneration and functional rehabilitation after SCI, while being involved in tissue fibrosis.
M2b microglia are activated by immune complexes or viral infections and have pro- and anti-inflammatory effects at the same time. This “dual effect” indicates their complex role in regulating various aspects of the inflammatory response.
M2c microglia are activated by IL-10 and TGF-β and in the context of SCI have been found to express the marker suppressor of cytokine signaling (SOCS) 3 and the IL4R-a (Kisucká et al., 2021). This M2 subtype participates in phagocytosis, synaptic remodeling, and wound repair (Kisucká et al., 2021). Furthermore, M2c and M2a microglia were shown to reduce secondary tissue injury caused by inflammation and to support neurological recovery following SCI, particularly during the initial post-injury phases (Kisucká et al., 2021). Finally, enhancing M2c but also M2b microglia promoted SCI repair by coordinating the proliferative stage of wound healing (Gensel and Zhang, 2015).
With the advancement of microglia investigation, the classification of microglia into the M1 and M2 phenotypes is not deemed reasonable anymore. The polarization of microglia into the M1/M2 phenotype is instead recognized as a dynamic process. Moreover, there are some mixed phenotypes of microglia with complex functions, such as those exposed to LPS, IL-4, and IL-13, and harbor great repair potential (Mishra et al., 2021). Following SCI, microglia become briefly activated into the M2 phenotype and play an anti-inflammatory role. However, due to the continued stimulation of the disease microenvironment over the following few hours to several days, microglia quickly multiply and gradually transform into the M1 phenotype, which becomes predominate in the subacute and chronic phases after SCI, constantly aggravating tissue injury and impeding tissue healing. Findings in the literature indicate that the depletion of M1-like macrophages in some tissues impedes the healing process and that the absence of reparative M2 microglia led to a failure of nerve tissue repair (Martin and Garcia, 2021). Such reports suggest that each macrophage/microglia phenotype plays a vital role in tissue repair. To effectively treat SCI, it will be essential to limit M1 microglia activity while preserve M2 microglia activation at the optimal inflammatory window. Furthermore, due to the complexity of microglia, each microglial cell cannot be defined entirely in terms of neurotoxic and neuroprotective effects.
Microglia and excitotoxicity
Excitotoxicity is a type of neurotoxicity that occurs when glutamate overstimulates neuronal glutamate receptors, promoting ischemia and neuronal death. Mammals express roughly three pannexins and 20 connexins, which are the primary structure of gap junctions. Each gap channel is made up of a combination of two connexin hemichannels (Pohl, 2020). After SCI, glutamate is excessively released through gap junction hemichannels by activated microglia and astrocytes, leading to glutamate excitotoxicity (Brockie et al., 2021). In various studies, connexin inhibitors have been shown to suppress neuroinflammation and improve electrophysiological and functional recovery (Guo et al., 2022). Research on pannexins’ function in the pathophysiology of SCI, however, remains limited. Activated microglia produce pro-inflammatory cytokines, including IFN-γ, and TNF, which inhibit and reverse glutamate reuptake mediated by the excitatory amino acid transporter of astrocytes and directly promote glutamate production and reduce absorption in microglia via protein kinase C, cAMP-responsive element-binding protein, and CAAT-enhancer-binding protein-beta signals (Rao et al., 2013). The production of quinolinic acid by activated microglia increases glutamate release from synaptosomes and astroglia (Lindhout et al., 2021). Excess accumulation of glutamate from disrupted astrocytes, microglia, and neurons causes ionic disorder in postsynaptic cells, which increases glutamatergic signaling and glial cell depolarization, causing calcium influx and overproduction of reactive oxygen species (Brockie et al., 2021). Excitotoxicity is recognized to play a substantial role in secondary cell death and since microglia and glutamate hyperfunction have a complicated and intimate relationship, follow-up research should focus on more mechanisms and treatment strategies behind it.
Microglia and vascular injury
Dysfunction in the blood-spinal cord barrier (BSCB) and vascular disruption significantly affect SCI pathogenesis. The vascular leakage caused by hypoxia and ischemia is related to the accumulation and activation of perivascular microglia. In the aftermath of SCI, cytokine signaling-induced vasoconstriction places more strain on the blood vessels, affecting their structural integrity and resulting in protracted vascular leakage (Allison and Ditor, 2015). Reactive oxygen species generated by activated microglia cause the BSCB to become more permeable, damage endothelial cells, and make peripheric leukocytes penetrate the CNS parenchyma (Jaffer et al., 2023). Additionally, by generating matrix metalloproteinase and pro-inflammatory cytokines, microglia can break down extracellular matrix proteins and damage the BSCB. Interestingly, as a double-edged sword, microglia also play a role in vascular protection (Choi et al., 2023). According to Mastorakos et al. (2021), after a vascular injury to the CNS, local microglia stabilize injured vasculature in a purinergic receptor-dependent way and form tube-like structures around wounded or occluded vessels to act as the first line of protection, decreasing the degree of infiltrating into the parenchyma. In normoxic environments, pharmacologically depleting microglia with the colony-stimulating factor (CSF)-1 receptor inhibitor PLX5622 showed little impact, but caused significant vascular leakage because endothelial tight junction proteins were lost. In addition, the inhibition of the interaction between fibrinogen and its Mac-1 integrin receptor diminished the microglia-mediated vascular repair and maintenance (Halder and Milner, 2019). In a different study, it has been demonstrated that STAT3/SOCS3 activation and NK-κB inhibition by TMP (tetramethyl pyrazine)-treated M2 microglia improve BSCB integrity in an experimental autoimmune encephalomyelitis model (Zhang et al., 2021a). To sum up, pro-inflammatory factors secreted by microglia increase the permeability of blood vessels, while anti-inflammatory factors facilitate angiogenesis, highlighting the role of microglia in BSCB maintenance and repair. Thus, further research on the barrier-sealing properties of microglia and their eventual transformation into proangiogenic repair-associated microglia should be promoted.
Microglia and the glial scar
A thick border formation around the post-traumatic spinal cord lesion known as the glial scar comprises microglia, astrocytes, and oligodendrocyte precursor cells. For the formation of protective glial scarring, astrocytes and microglia need to collaborate. Removing microglia hinders the clustering of astrocytes at the lesion site, amplifies the infiltration of immune cells into the parenchyma, diminishes neuronal survival, intensifies axonal death, and obstructs the recovery of locomotion (Jakovčevski et al., 2021). Activated microglia position themselves where invading leukocytes and astrocytes intersect, facilitating the creation of astrocytic scars. This positioning is influenced by substances produced by the microglia, particularly IGF-1. While these scars somewhat inhibit axonal regeneration, they play a crucial role in confining inflammation to the lesion site and isolating invading macrophages, thereby limiting further damage (Zheng et al., 2023). The “microglial scar” concept was thus proposed, highlighting the close temporal and spatial connections between astrocytes and microglia in glial scars after CNS injury (Bellver-Landete et al., 2019). Microglia and macrophages dynamically modulate the way they react to injury via various mechanisms, including their role in wound containment and compression via Plexin-B2 signaling (Zhou et al., 2020). Plexin-B2 is expressed more abundantly in activated macrophages and microglia early in the course of an injury, restricting their ability to diffuse away from the injury site, concentrating their impact, and reducing extensive tissue damage (Zhou et al., 2020). These findings highlight the crucial role of microglia in forming scars SCI, acting as a protective barrier for the CNS tissue. Additionally, components of extracellular matrix in glial scars, such as chondroitin sulfate proteoglycans, interact with microglia. For instance, chondroitin sulfate proteoglycans can trigger the conversion of anti-inflammatory microglia into pro-inflammatory cells. Conversely, the removal of chondroitin sulfate proteoglycans can aid in reducing inflammation and promoting tissue recovery (Francos-Quijorna et al., 2022). Different developmental stages of microglia appear to affect scar formation differently. In the process of SCI repair in neonatal mice, the microglia-mediated healing without scarring and subsequent inflammatory response enables the regeneration of a large number of axons, which is different from that in adult mice (Li et al., 2020b). In neonatal mice, microglia are the main organizers of this reparative damage response, having to do with the momentary activation and spontaneous return to equilibrium of microglia during the acute phase of spinal cord damage.
Overall, microglia are essential for the development of scars following SCI. Investigating strategies to reduce the harmful effects of the scarring process and encouraging recovery without scars is essential. Additionally, further investigation is required on microglia’s gene expression patterns associated with scars and their interactions with other cells.
Microglia and myelin regeneration
Axon demyelination and death of oligodendrocytes are crucial events in the cascades of secondary injuries following SCI and result in persistent neurodegeneration. Oligodendrocyte precursor cells (OPCs) migrate to the demyelinating lesion site after being exposed to a demyelinating insult, then proliferate and mature into myelinating oligodendrocytes that produce the myelin sheath surrounding neuronal axons and ensuring impulse transmission. TNF, IL-1β, NO, and nerve growth factor precursor (proNGF) are secreted by activated microglia to promote the death of oligodendrocytes and OPCs (Kalafatakis and Karagogeos, 2021). In contrast, the anti-inflammatory phenotype microglia mediate myelin regeneration by secreting activin-A (Miron et al., 2013). Following SCI, microglia’s phagocytosis eliminates debris and facilitates myelin regeneration via the TREM2 signaling pathway (Gervois and Lambrichts, 2019). After CNS injury, inadequate myelin clearance by microglia inhibits the recruitment of OPCs, resulting in disorganized and defective axonal remyelination. A study by Bellver-Landete et al. (2019) showed that a lower number of oligodendrocytes were observed at the lesion site in the CSF-1 receptor inhibitor PLX5622 group than in the vehicle group 35 days after SCI, indicating that microglia are essential for the survival of oligodendrocytes. With the results of RNA sequencing technology, another study found that by selectively reducing CD11c+ microglia, oligodendrocyte development, and functioning recovery were impeded (Jia et al., 2023). Since oligodendrocytes need iron to produce myelin cholesterol and fatty acids, it has been postulated that microglia provide oligodendrocytes with extracellular iron by secreting ferritin. By inhibiting the oxygen-glucose deprivation/reperfusion–induced bone morphogenetic proteins/hepcidin pathway, bone morphogenetic protein antagonists may induce reactive microglia to transition from a phenotype that stores iron to one that releases iron, resulting in microglial iron release and oligodendrocyte myelin synthesis (Shi et al., 2021). Based on the above findings, it appears that depending on the specific environment and their phenotype, microglia may have protective or deleterious effects on oligodendrocytes and OPCs. However, due to the heterogeneity of microglia, clarifying the specific processes will require more investigation.
Microglia in coordinating cellular interactions and the crosstalk with other cells
After SCI, microglia release IL-1α, TNF, and C1q to stimulate the proliferation and activation of astrocytes, converting them from a neuroprotective into a neurotoxic state (Kwon and Koh, 2020). Depletion of microglia in mice leads to lower responsiveness of astrocytes compared to wildtype controls in an inflammatory paradigm caused by LPS (Liddelow et al., 2020). In turn, astrocytes can facilitate microglial activation and TNF-α production by secreting pro-inflammatory substances such as granulocyte-macrophage colony-stimulating factor (Kano et al., 2019). Interestingly, in a CNS inflammation study, astrocytes inhibited the activation of microglia by up-regulating TGF-β and Galectin-1, while TGF-β and fibroblast growth factor were shown to inhibit the activation of neurotoxic astrocytes (Kabba et al., 2018). As a seemingly contradictory result, other authors reported that microglia can stimulate both, astrocyte proliferation and necroptosis, illustrating the complexity of the crosstalk between those cell types (Fan et al., 2016). To enhance our understanding of recovery following SCI, future research, employing cell-specific techniques, should focus on exploring the interactions between astrocytes and microglia and deciphering how these collectively influence recovery processes and their mechanisms.
A major factor in determining functional recovery after SCI is the preservation of neurons. Following SCI, microglia engage in phagocytosis of axon fragments and secrete pro/anti-inflammatory cytokines, which can impact neuronal regeneration. After CNS injury, it has been observed that microglial activation persists, leading to chronic neuroinflammation, which can exacerbate neurological deficits (Bellver-Landete et al., 2019). What is more, a recent study showed that certain medications, like bumetanide, can profoundly influence neurons by acting through microglia. The research revealed that bumetanide helps modify the characteristics of microglia, improving their production of brain-derived neurotrophic factor and interaction with neurons. This enhancement leads to increased neurogenesis and recovery (Tessier et al., 2023). It also indicated that the microglia-neuron interface exists in physical interactions, which can lead to nerve regeneration and phagocytosis. In the core spinal cord lesions occurring after SCI, microglial scars can protect axons from cytotoxic immune cells, and it has been demonstrated that the elimination of microglia is detrimental to neuronal regrowth (Bellver-Landete et al., 2019). Sustained reduction of microglia has a negligible impact on neurogenesis in the setting of CNS damage, however, it has been shown that functional neurogenesis is stimulated following traumatic brain injury when microglia switch to a neuroprotective phenotype (Willis et al., 2020). The proper time frame during the acute phase of damage is crucial for these repopulating microglia to have a neuroprotective impact.
Neural stem/progenitor cells (NSPCs) are important for nervous system regeneration after injury because of their ability to proliferate and differentiate into other nervous system cells, such as neurons, oligodendrocytes, and astrocytes. However, it has been reported that LPS or IFN-γ activated microglia produce mediators that prevent the proliferation of NSPCs (Osman et al., 2019). Surprisingly, studies have shown that anti-inflammatory microglia display a preference for oligodendrogenesis and migration of NPSCs, whereas pro-inflammatory microglia promote neurogenesis (Matsui and Mori, 2018). Further research indicated that M2c microglia promote NSPCs to differentiate into oligodendrocytes by secreting Wnt7a (Mecha et al., 2020). Nevertheless, some investigations contradict such findings, more specifically that M2 microglia support neurogenesis (Vay et al., 2018). This may be due to different experimental conditions, such as cell origin, primary cells, and cell lines, in vivo and in vitro experiments. It appears that the microglia phenotype has a significant impact on cell renewal and the direction of differentiation of NSPCs. Additionally, NSPCs primarily regulate microglial function by releasing proteins such as chemokines and cytokines, as well as extracellular vesicles and metabolites (de Almeida et al., 2023). Even though a few studies have investigated how different types of microglia affect NSPCs, the exact mechanisms are still unclear.
Microglia also receive signals from neurons and astrocytes via direct membrane-bound contact and secretion, creating a dynamic feedback loop between these cell groups (Liddelow et al., 2020). Due to the separation in space between neurons and astrocytes, microglia serve as crucial functional network coordinators by maintaining contact between glial cells and nerve cells. The CNS-resident glia and infiltrating leukocyte damage responses are coordinated by microglia, which is essential for SCI healing. Additionally, bioinformatics studies point to the possibility that successful SCI recovery depends on monocyte-derived macrophages, astrocytes, and microglia cooperating in important ligand-receptor interactions (Brennan et al., 2022). Targeting these networks and their interconnections, therefore, provides a promising treatment strategy for SCI (Figure 2).
Figure 2.
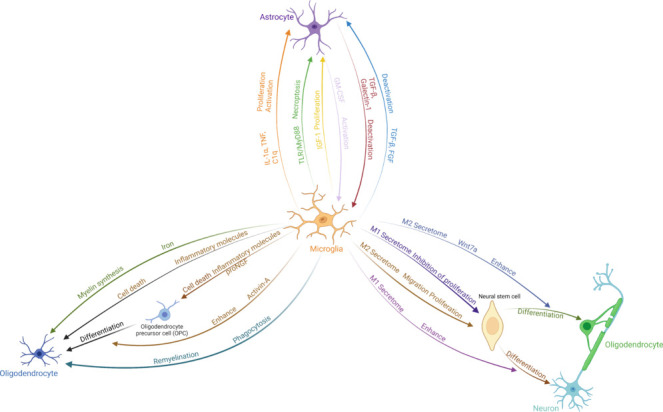
Interplay between microglia and other cells in the central nervous system.
Microglia change their morphology and function and release neurotransmitters and immunomodulators such as cytokines, chemokines, and complement factors, which modulate the activity and fate of other cells in the environment. Created with BioRender.com. FGF: Fibroblast growth factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; IGF-1: insulin-like growth factor 1; IL-1α: interleukin-1α; Myd88: myeloid differentiation primary response gene 88; proNGF: nerve growth factor precursor; TGF-β: transforming growth factor-β; TLR: Toll-like receptor; TNF: tumor necrosis factor.
Therapeutic Potential of Microglia
Microglia M1/M2 shifts
Given the high plasticity of microglia, current treatment strategies focus on inhibiting M1 microglia, which amplify neuroinflammation, and fostering their transition to the M2 phenotype.
Modifying the microglia phenotype via drugs and cytokines
Some drugs and cytokines can encourage microglia to polarize to the M2 anti-inflammatory and pro-regenerative phenotype, which reduces neuroinflammation brought on by spinal cord damage. To enhance M2 polarization of macrophages/microglia following SCI, for example, IL-4 has been given systemically, which leads to a drastic decrease of pro-inflammatory cytokines and markers (Lima et al., 2017). Similarly, Resveratrol can suppress M1 polarization while simultaneously promote M2 polarization of microglia via peroxisome proliferator-activated receptor coactivator-1α (Yang et al., 2017). Furthermore, lowering IL-6 signaling prevented M1 and encouraged M2 macrophages/microglia activation after SCI, promoting functional recovery (Guerrero et al., 2012). Additionally, cytokines like IL-10, TGF-β, and vascular endothelial growth factor can regulate the inflammatory conditions and prevent microglia from becoming pro-inflammatory.
Regulating the polarization of microglia via miRNAs
MiRNAs, a group of short non-coding RNAs, which regulate transcription, have been found to be essential for the growth, genesis, and regeneration of new neurons in the CNS. Due to their regulatory role in pathophysiological processes, these molecules have also been identified as promising treatments for SCI. For instance, it has been demonstrated that blocking miRNA-27b-3p prevents microglial apoptosis following SCI and decreases microglial production of cytokines associated with inflammation, including TNF-α and IL-1β (Li et al., 2021). Additionally, miRNA-423-5p specifically targets NOD-like receptor pyrin domain containing 3 to prevent microglia from polarizing into the M1 phenotype (Cheng et al., 2021). Another study found that miRNAs influence macrophage and microglia activity and differentiation by modifying the signaling of key transcription factors, including STATs, CCAAT-enhancer-binding proteins, C-MYC, IRFs, and peroxisome-proliferator activated receptor (Li et al., 2018). Given such findings and their high specificity, target diversity, target reversibility, and low immunogenicity, miRNAs are a promising therapeutic strategy for regulating neuroinflammation after SCI.
Affecting the microglia phenotype via mesenchymal stem cells and exosomes
The ability of mesenchymal stem cells (MSCs) to secrete a variety of anti-inflammatory factors allows them to polarize macrophages and microglia towards a healing phenotype after transplantation. The resulting reduction of inflammation and promotion of tissue remodeling may be part of the explanation for their therapeutic efficacy shown after SCI by some authors. According to recent research, in addition to the anti-inflammatory cytokines secreted by MSCs, exosomes also significantly contribute to their therapeutic effects (Sun et al., 2018). Those small extracellular vesicles are a vital paracrine component and, after being released from MSCs, prevent apoptosis, maintain the BSCB’s integrity, modulate the immunological response, and promote axonal regeneration and angiogenesis after SCI (Liu et al., 2019). For instance, exosomes containing miR-124-3p derived from MSCs diminished spinal cord and nerve injuries by reducing apoptosis and polarizing macrophages/microglia towards the M2 phenotype (Li et al., 2020a). Since many medications cannot cross the blood-brain barrier or BSCB, treating diseases of the CNS can be challenging. Exosomes can not only cross the BSCB, but also have other advantages such as low immunogenicity, high stability, and biocompatibility which render them a promising therapeutic strategy for SCI.
Targeting microglia polarization via bioactive materials
In recent years, achievements have also been made in bioactive material treatment targeting microglia, such as hydrogels and nanomaterials. Liu et al. (2020) have developed a novel class of bionic nanoparticles with a range of enzyme activities that efficiently remove reactive oxygen species and promote microglia towards the M2 phenotype. In a different study, PLX3397 hydrogel greatly alleviated the persistent inflammatory response after SCI, thereby reducing the generation of pro-inflammatory cytokines (Ma et al., 2020). For effective tissue repair, a balance between M1-like and M2-like features is essential, which includes a synergistic transformation of phenotypes, a sustained inflammatory response, and finely tuned proliferation and reconstruction processes. The M1-like response is vital in the initial stage of the healing process. Thus, the transition from the M1 to the M2 phenotype should be carefully managed. Inappropriate metabolic activities during this transition could worsen fibrosis and dysfunction in the scarred regions. To avoid the harmful effects of long-term immunosuppression on tissue regeneration, timing is crucial when considering the polarization of microglia as a therapeutic option.
Microglia transplantation
Cell transplantation employing a variety of cells with therapeutic potential, such as NSPCs, MSCs, embryonic stem cells, or Schwann cells, has been successfully used to treat SCI in multiple studies. However, also microglial cell transplantation has attracted a growing amount of attention. According to a recent report, microglial cells polarized to the M2 phenotype in vitro that were then transplanted to the injury site in an animal SCI model in vivo, enhanced the recovery of limb function (Kobashi et al., 2020). Additionally, a human-mouse chimera model based on single-cell RNA sequencing data has been created by inducing human pluripotent stem cells into microglial precursors and transplanting them into mice brains, which provides an excellent in vivo platform for studying human microglia (Svoboda et al., 2019; Schafer et al., 2023). In another study, transplanting neonatal or adult microglia that had been incubated with the peptidase inhibitors E64 and serpinA3N significantly enhanced recovery after SCI and led to considerable axon regeneration in adult mice (Li et al., 2020b). However, ethical controversies concerning the source of cells, logistics and timing of the transplantation, and long-term safety are persisting issues that may limit the clinical application of microglia transplantation. Efforts to address these concerns will be crucial for making microglia transplantation a feasible clinical option in the treatment of SCI.
Microglia regeneration and replacement
In the treatment of CNS disorders, it is becoming more and more important to utilize microglia replacement and regeneration since it has demonstrated remarkable therapeutic potential. In 2014, a CSF1-receptor inhibitor was used by Green et al. to eradicate brain microglia nearly entirely. After halting inhibition and a brief period of recovery, they discovered that microglia, originating from a group of cells that briefly expressed Nestin rapidly proliferated, dispersed throughout the whole brain, and reverted to their original morphology within a week. This was the first report of microglial repopulation, a widespread process of CNS regeneration (Elmore et al., 2014). Further investigation by Willis et al. (2020) led to the discovery that repopulated microglia support hippocampus neuron regeneration and enhance spatial learning and memory in an IL-6-dependent manner (Willis et al., 2020). After complete SCI, Ma et al. observed that a 2-week period of microglia eradication in rats improved the local inflammatory microenvironment, electrophysiological activity, and neurological function (Ma et al., 2020). Intriguingly, a study by Poulen et al. (2021) revealed that sustained depletion of microglia had no positive effects on tissue regeneration following spinal cord hemisection, but temporary depletion greatly improved functional recovery and the reduction of tissue damage in rats and non-human primates. Overall, the impact of microglia depletion on SCI remains a subject of debate. Various factors, such as the techniques used for microglia depletion, the duration of depletion, and the timing relative to SCI, significantly influence the outcomes, which can be either positive or negative. However, emerging data suggest that a selective and strategic approach to microglia removal, as opposed to continuous depletion, may enhance tissue healing following SCI.
The Gut–CNS axis
The gut–CNS axis, a connection between the gastrointestinal tract and the central nervous system, can be helpful for comprehending the nature of microglia and CNS disorders. The use of antibiotics to eradicate gut bacteria has been found to significantly alter microglia characteristics and decrease bacterial diversity, leading to microglia dysfunction. Moreover, dysbiosis, or an imbalance in gut bacteria, has been closely linked to microglia function following SCI. According to a recently published study, the lack of gastrointestinal microbiota can lead to straightforward mutations in the gene expression of CNS cells, particularly the microglia (Huang et al., 2023). In addition, the gastrointestinal microbiome has been demonstrated to modulate the transformation of microglial subtypes (Huang et al., 2023). Thus, using Lactobacillus and Bifidobacterium preparations to treat SCI by managing the gut flora has been highlighted as a viable treatment strategy (Huang et al., 2023). The role of intestinal flora metabolites in promoting and inhibiting inflammation is comparable to that of microglia, indicating that they may be related and work together to regulate the development of inflammation after SCI. These findings suggest that future SCI treatment strategies may target the gastrointestinal microbiome to a greater extent.
Conclusion and Perspectives
SCI is a complex disorder triggered by various traumatic events, leading to immediate physical damage and necrotic cell death in the spinal cord. This initial phase is followed by a secondary stage of injury, marked by inflammation, further cellular death, and scar formation, involving both resident and infiltrating immune cells in the inflammatory process.
This review delineates the origins, physiology, heterogeneity, and therapeutic roles of microglia, highlighting their multifaceted regulatory functions in SCI. Ordinarily, microglia maintain the central nervous system in a state of homeostasis. However, disruptions in this balanced state compel microglia to engage in numerous complex pathological processes. Microglia possess the capacity to exert both beneficial and detrimental influences on the disease course, playing a pivotal role in orchestrating and modulating the entire trajectory of SCI. Thus, further exploration is necessary to discern strategies to amplify the positive impacts of microglia while mitigating their adverse effects in SCI recovery.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
Open peer reviewers: Giovane Galdino, Federal University of Alfenas, Brazil; Elena Giusto, University of Padova, Italy.
P-Reviewers: Galdino G, Giusto E; C-Editors: Zhao M, Zhao LJ, Qiu Y; T-Editor: Jia Y
References
- Allison D, Ditor D. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–18. doi: 10.1038/sc.2014.184. [DOI] [PubMed] [Google Scholar]
- Bartels T, De Schepper S, Hong S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science. 2020;370:66–69. doi: 10.1126/science.abb8587. [DOI] [PubMed] [Google Scholar]
- Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle M-E, Vernoux N, Tremblay M-È, Fuehrmann T, Shoichet MS. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan FH, Li Y, Wang C, Ma A, Guo Q, Li Y, Pukos N, Campbell WA, Witcher KG, Guan Z. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat Commun. 2022;13:4096. doi: 10.1038/s41467-022-31797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie S, Hong J, Fehlings MG. The role of microglia in modulating neuroinflammation after spinal cord injury. Int J Mol Sci. 2021;22:9706. doi: 10.3390/ijms22189706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cătălin B, Mitran S, Albu C, Iancău M. Comparative aspects of microglia reaction in white and gray matter. Curr Health Sci J. 2013;39:151. [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Hao J, Jiang X, Ji J, Wu T, Chen X, Zhang F. Ameliorative effects of miR-423-5p against polarization of microglial cells of the M1 phenotype by targeting a NLRP3 inflammasome signaling pathway. Int Immunopharmacol. 2021;99:108006. doi: 10.1016/j.intimp.2021.108006. [DOI] [PubMed] [Google Scholar]
- Choi BR, Johnson KR, Maric D, McGavern DB. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat Immunol. 2023;24:1110–1123. doi: 10.1038/s41590-023-01521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- de Almeida MM, Goodkey K, Voronova A. Regulation of microglia function by neural stem cells. Front Cell Neurosci. 2023;17:1130205. doi: 10.3389/fncel.2023.1130205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Meng F, Zhang K, Gao J, Liu Z, Li M, Liu X, Li J, Wang Y, Zhang L. Emerging roles of microglia depletion in the treatment of spinal cord Injury. Cells. 2022;11:1871. doi: 10.3390/cells11121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Zhang K, Shan L, Kuang F, Chen K, Zhu K, Ma H, Ju G, Wang YZ. Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol Neurodegener. 2016;11:14. doi: 10.1186/s13024-016-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francos-Quijorna I, Sánchez-Petidier M, Burnside ER, Badea SR, Torres-Espin A, Marshall L, de Winter F, Verhaagen J, Moreno-Manzano V, Bradbury EJ. Chondroitin sulfate proteoglycans prevent immune cell phenotypic conversion and inflammation resolution via TLR4 in rodent models of spinal cord injury. Nat Commun. 2022;13:2933. doi: 10.1038/s41467-022-30467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SP, Chen SY, Pang QM, Zhang M, Wu XC, Wan X, Wan WH, Ao J, Zhang T. Advances in the research of the role of macrophage/microglia polarization-mediated inflammatory response in spinal cord injury. Front Immunol. 2022;13:1014013. doi: 10.3389/fimmu.2022.1014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 20151619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Gervois P, Lambrichts I. The emerging role of triggering receptor expressed on myeloid cells 2 as a target for immunomodulation in ischemic stroke. Front Immunol. 2019;10:1668. doi: 10.3389/fimmu.2019.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Gu Y, Han X, Luan C, Liu C, Wang X, Sun Y, Zheng M, Fang M, Yang S. Spatiotemporal dynamics of the molecular expression pattern and intercellular interactions in the glial scar response to spinal cord injury. Neurosci Bull. 2023;39:213–244. doi: 10.1007/s12264-022-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9:40. doi: 10.1186/1742-2094-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülke E, Gelderblom M, Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 2018;11:1756286418774254. doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Zhang H, Li H, Chiu A, García-Rodríguez C, Lagos CF, Sáez JC, Lau CG. Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2022;119:e2213162119. doi: 10.1073/pnas.2213162119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim R, Zachariadis V, Sankavaram SR, Han J, Harris RA, Brundin L, Enge M, Svensson M. Spinal cord injury induces permanent reprogramming of microglia into a disease-associated state which contributes to functional recovery. J Neurosci. 2021;41:8441–8459. doi: 10.1523/JNEUROSCI.0860-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Milner R. A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc Natl Acad Sci U S A. 2019;116:26029–26037. doi: 10.1073/pnas.1912178116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wu J, Zhang H, Li Y, Wen L, Tan X, Cheng K, Liu Y, Pu J, Liu L. The gut microbiome modulates the transformation of microglial subtypes. Mol Psychiatry. 2023;28:1611–1621. doi: 10.1038/s41380-023-02017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffer H, Andrabi SS, Petro M, Kuang Y, Steinmetz MP, Labhasetwar V. Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model. J Control Release. 2023;364:109–123. doi: 10.1016/j.jconrel.2023.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovčevski I, Förster E, Reiss G, Schachner M. Impact of depletion of microglia/macrophages on regeneration after spinal cord injury. Neuroscience. 2021;459:129–141. doi: 10.1016/j.neuroscience.2021.02.010. [DOI] [PubMed] [Google Scholar]
- Jia J, Zheng L, Ye L, Chen J, Shu S, Xu S, Bao X, Xia S, Liu R, Xu Y. CD11c+ microglia promote white matter repair after ischemic stroke. Cell Death Dis. 2023;14:156. doi: 10.1038/s41419-023-05689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, He F, Ding G, Wu J. Dopamine inhibits pyroptosis and attenuates secondary damage after spinal cord injury in female mice. Neurosci Lett. 2023;792:136935. doi: 10.1016/j.neulet.2022.136935. [DOI] [PubMed] [Google Scholar]
- Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T. Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol. 2018;38:53–71. doi: 10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafatakis I, Karagogeos D. Oligodendrocytes and microglia: key players in myelin development, damage and repair. Biomolecules. 2021;11:1058. doi: 10.3390/biom11071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano SI, Choi EY, Dohi E, Agarwal S, Chang DJ, Wilson AM, Lo BD, Rose IV, Gonzalez S, Imai T. Glutathione S-transferases promote proinflammatory astrocyte-microglia communication during brain inflammation. Sci Signal. 2019;12:eaar2124. doi: 10.1126/scisignal.aar2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisucká A, Bimbová K, Bačová M, Gálik J, Lukáčová N. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells. 2021;10:1943. doi: 10.3390/cells10081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobashi S, Terashima T, Katagi M, Nakae Y, Okano J, Suzuki Y, Urushitani M, Kojima H. Transplantation of M2-deviated microglia promotes recovery of motor function after spinal cord injury in mice. Mol Ther. 2020;28:254–265. doi: 10.1016/j.ymthe.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:1–12. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jiang T, Li MQ, Zheng XL, Zhao GJ. Transcriptional regulation of macrophages polarization by microRNAs. Front Immunol. 2018;9:42. doi: 10.3389/fimmu.2018.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Qi C, Liu Y, Shen Y, Zhao X, Qin H, Zhang Y, Yu T. MicroRNA miR-27b-3p regulate microglial inflammation response and cell apoptosis by inhibiting A20 (TNF-α-induced protein 3) Bioengineered. 2021;12:9902–9913. doi: 10.1080/21655979.2021.1969195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020a;22:75. doi: 10.1186/s13075-020-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He X, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, Yang Z, Chen B, Shi Z, Meng H. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature. 2020b;587:613–618. doi: 10.1038/s41586-020-2795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Marsh SE, Stevens B. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol. 2020;41:820–835. doi: 10.1016/j.it.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Lima R, Monteiro S, Lopes JP, Barradas P, Vasconcelos NL, Gomes ED, Assunção-Silva RC, Teixeira FG, Morais M, Sousa N. Systemic interleukin-4 administration after spinal cord injury modulates inflammation and promotes neuroprotection. Pharmaceuticals. 2017;10:83. doi: 10.3390/ph10040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhout IA, Murray TE, Richards CM, Klegeris A. Potential neurotoxic activity of diverse molecules released by microglia. Neurochem Int. 2021;148:105117. doi: 10.1016/j.neuint.2021.105117. [DOI] [PubMed] [Google Scholar]
- Liu H, Han Y, Wang T, Zhang H, Xu Q, Yuan J, Li Z. Targeting microglia for therapy of Parkinson’s disease by using biomimetic ultrasmall nanoparticles. J Am Chem Soc. 2020;142:21730–21742. doi: 10.1021/jacs.0c09390. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- Lopez‐Atalaya JP, Askew KE, Sierra A, Gomez‐Nicola D. Development and maintenance of the brain’s immune toolkit: Microglia and non‐parenchymal brain macrophages. Dev Neurobiol. 2018;78:561–579. doi: 10.1002/dneu.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MC, Ellman DG, Nissen M, Nielsen PS, Nielsen PV, Jørgensen C, Andersen DC, Gao H, Brambilla R, Degn M. The inflammatory response after moderate contusion spinal cord injury: a time study. Biology. 2022;11:939. doi: 10.3390/biology11060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zhao Y, Huang L, Xiao Z, Chen B, Shi Y, Shen H, Dai J. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020;237:119830. doi: 10.1016/j.biomaterials.2020.119830. [DOI] [PubMed] [Google Scholar]
- Martin KE, Garcia AJ. Macrophage phenotypes in tissue repair and the foreign body response: implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021;133:4–16. doi: 10.1016/j.actbio.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P, Mihelson N, Luby M, Burks SR, Johnson K, Hsia AW, Witko J, Frank JA, Latour L, McGavern DB. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci. 2021;24:245–258. doi: 10.1038/s41593-020-00773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui TK, Mori E. Microglia support neural stem cell maintenance and growth. Biochem Biophys Res Commun. 2018;503:1880–1884. doi: 10.1016/j.bbrc.2018.07.130. [DOI] [PubMed] [Google Scholar]
- Mecha M, Yanguas-Casás N, Feliú A, Mestre L, Carrillo-Salinas FJ, Riecken K, Gomez-Nicola D, Guaza C. Involvement of Wnt7a in the role of M2c microglia in neural stem cell oligodendrogenesis. J Neuroinflammation. 2020;17:88. doi: 10.1186/s12974-020-01734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich LM, Choi JS, Ryan C, Cerqueira SR, Benavides S, Yahn SL, Tsoulfas P, Lee JK. Single-cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord. J Exp Med. 2021;218:e20210040. doi: 10.1084/jem.20210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, Van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MK, Rawji KS, Keough MB, Kappen J, Dowlatabadi R, Vogel HJ, Chopra S, Distéfano-Gagné F, Dufour A, Gosselin D. Harnessing the benefits of neuroinflammation: generation of macrophages/microglia with prominent remyelinating properties. J Neurosci. 2021;41:3366–3385. doi: 10.1523/JNEUROSCI.1948-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes-Baran AD, White DR, DeSilva TM. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 2020;32:108047. doi: 10.1016/j.celrep.2020.108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukolova N, Aleksashkin A, Abakumova T, Morozova A, Gubskiy I, Kirzhanova Е, Abakumov M, Chekhonin V, Klyachko N, Kabanov A. Multilayer polyion complex nanoformulations of superoxide dismutase 1 for acute spinal cord injury. J Control Release. 2018;270:226–236. doi: 10.1016/j.jconrel.2017.11.044. [DOI] [PubMed] [Google Scholar]
- Osman AM, Rodhe J, Shen X, Dominguez CA, Joseph B, Blomgren K. The secretome of microglia regulate neural stem cell function. Neuroscience. 2019;405:92–102. doi: 10.1016/j.neuroscience.2017.10.034. [DOI] [PubMed] [Google Scholar]
- Pohl U. Connexins: key players in the control of vascular plasticity and function. Physiol Rev. 2020;100:525–572. doi: 10.1152/physrev.00010.2019. [DOI] [PubMed] [Google Scholar]
- Poulen G, Aloy E, Bringuier CM, Mestre-Francés N, Artus EV, Cardoso M, Perez JC, Goze-Bac C, Boukhaddaoui H, Lonjon N. Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics. 2021;11:8640. doi: 10.7150/thno.61833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS, Claus CF, Fiani B, Rahman M, Ramachandran A. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43:425–441. doi: 10.1007/s10143-018-1008-3. [DOI] [PubMed] [Google Scholar]
- Rao KVR, Brahmbhatt M, Norenberg MD. Microglia contribute to ammonia-induced astrocyte swelling in culture. Metab Brain Dis. 2013;28:139–143. doi: 10.1007/s11011-012-9339-1. [DOI] [PubMed] [Google Scholar]
- Schafer ST, Mansour AA, Schlachetzki JC, Pena M, Ghassemzadeh S, Mitchell L, Mar A, Quang D, Stumpf S, Ortiz IS. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 2023;186:2111–2126. doi: 10.1016/j.cell.2023.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, Li J, Chen H, Wang X, Wang R. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med. 2020;24:8045–8056. doi: 10.1111/jcmm.15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity. 2021;54:1527–1542. doi: 10.1016/j.immuni.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Svoboda DS, Barrasa MI, Shu J, Rietjens R, Zhang S, Mitalipova M, Berube P, Fu D, Shultz LD, Bell GW. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. Proc Natl Acad Sci U S A. 2019;116:25293–25303. doi: 10.1073/pnas.1913541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Savage JC, Hui CW, Bisht K, Tremblay MÈ. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595:1929–1945. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier M, Saez Garcia M, Goubert E, Blasco E, Consumi A, Dehapiot B, Tian L, Molinari F, Laurin J, Guillemot F. Bumetanide induces post-traumatic microglia–interneuron contact to promote neurogenesis and recovery. Brain. 2023;146:4247–4261. doi: 10.1093/brain/awad132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vay SU, Flitsch LJ, Rabenstein M, Rogall R, Blaschke S, Kleinhaus J, Reinert N, Bach A, Fink GR, Schroeter M. The plasticity of primary microglia and their multifaceted effects on endogenous neural stem cells in vitro and in vivo. J Neuroinflammation. 2018;15:226. doi: 10.1186/s12974-018-1261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Itriago A, Radford RA, Aramideh JA, Maurel C, Scherer NM, Don EK, Lee A, Chung RS, Graeber MB, Morsch M. Microglia morphophysiological diversity and its implications for the CNS. Front Immunol. 2022;13:997786. doi: 10.3389/fimmu.2022.997786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang L, Shi Q, Yang B, He Q, Wang J, Weng Q. Targeting innate immune responses to attenuate acetaminophen-induced hepatotoxicity. Biochem Pharmacol. 2022;202:115142. doi: 10.1016/j.bcp.2022.115142. [DOI] [PubMed] [Google Scholar]
- Willis EF, MacDonald KP, Nguyen QH, Garrido AL, Gillespie ER, Harley SB, Bartlett PF, Schroder WA, Yates AG, Anthony DC, Rose-John S, Ruitenberg WJ, Vukovic J. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 2020;180:833–846. doi: 10.1016/j.cell.2020.02.013. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, Qu JY, Wen Z. Temporal-spatial resolution fate mapping reveals distinct origins for embryonic and adult microglia in zebrafish. Dev Cell. 2015;34:632–641. doi: 10.1016/j.devcel.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Xu L, He D, Bai Y. Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol. 2016;53:6709–6715. doi: 10.1007/s12035-015-9593-4. [DOI] [PubMed] [Google Scholar]
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xu S, Qian Y, Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu X, Gong L, Cui L, Zhang H, Zhao W, Jiang P, Hou G, Hou Y. Tetramethylpyrazine protects blood-spinal cord barrier integrity by modulating microglia polarization through activation of STAT3/SOCS3 and inhibition of NF-к B signaling pathways in experimental autoimmune encephalomyelitis mice. Cell Mol Neurobiol. 2021a;41:717–731. doi: 10.1007/s10571-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tian T, Gong SX, Huang WQ, Zhou QY, Wang AP, Tian Y. Microglia-associated neuroinflammation is a potential therapeutic target for ischemic stroke. Neural Regen Res. 2021b;16:6. doi: 10.4103/1673-5374.286954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Wu H, Wang X, Zhang G, Xu W, Xu S, Fang Y, Zhang A, Shao A, Chen S. Temporal dynamics of microglia-astrocyte interaction in neuroprotective glial scar formation after intracerebral hemorrhage. J Pharm Anal. 2023;13:862–879. doi: 10.1016/j.jpha.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He X. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci. 2020;23:337–350. doi: 10.1038/s41593-020-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZY, Chang TF, Lin ZB, Jing YT, Wen LS, Niu YL, Bai Q, Guo CM, Sun JX, Wang YS. Microglial Galectin3 enhances endothelial metabolism and promotes pathological angiogenesis via Notch inhibition by competitively binding to Jag1. Cell Death Dis. 2023;14:380. doi: 10.1038/s41419-023-05897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


