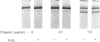Abstract
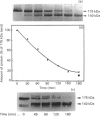
Macrophages express a receptor on the cell surface that functions to clear glycoproteins from the extracellular milieu. The activity of this receptor is sensitive to treatment with trypsin. In inflammatory situations, macrophages are activated and exposed to increased levels of extracellular proteases. Under these conditions, mannose receptor activity on the macrophages is diminished. We therefore decided to study the effects of trypsin treatment on the structure and activity of cell-associated and purified receptor that might contribute to the activation-associated receptor down-regulation. Trypsin treatment (1 microgram/ml for 3 h) resulted in the production of a 140 kDa, trypsin-resistant fragment from both intact cells and isolated receptor. This fragment was no longer able to bind ligand. The remaining 35 kDa fragment apparently is further degraded into smaller fragments, since no evidence of this domain was found on Coomassie Blue-stained gels. The 140 kDa fragment retained immunoreactivity and contained at least a portion of the iodinated tyrosine residues following surface labelling with Na125I. Neither calcium nor ligand protected the receptor from proteolysis. In addition, prior treatment with oxidants did not increase the susceptibility of the receptor to trypsin digestion. We conclude from these results that the macrophage mannose receptor is clipped by the serine protease trypsin at the cell surface, resulting in the release and further degradation of the binding domain, and the production of a membrane-associated 140 kDa fragment. This trypsin-mediated down-regulation of receptor activity might be important in controlling glycoprotein clearance during inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Bozeman P. M., Hoidal J. R., Shepherd V. L. Oxidant-mediated inhibition of ligand uptake by the macrophage mannose receptor. J Biol Chem. 1988 Jan 25;263(3):1240–1247. [PubMed] [Google Scholar]
- Brower M. S., Levin R. I., Garry K. Human neutrophil elastase modulates platelet function by limited proteolysis of membrane glycoproteins. J Clin Invest. 1985 Feb;75(2):657–666. doi: 10.1172/JCI111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley N. J., Clark V. M., Hall M. O. Surface modification of retinal pigment epithelial cells: effects on phagocytosis and glycoprotein composition. Exp Eye Res. 1987 Mar;44(3):377–392. doi: 10.1016/s0014-4835(87)80172-0. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Boches F. S. Oxidized proteins in erythrocytes are rapidly degraded by the adenosine triphosphate-dependent proteolytic system. Science. 1982 Feb 26;215(4536):1107–1109. doi: 10.1126/science.7038874. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Haltiwanger R. S., Hill R. L. The isolation of a rat alveolar macrophage lectin. J Biol Chem. 1986 Jun 5;261(16):7440–7444. [PubMed] [Google Scholar]
- Hatzfeld J., Miskin R., Reich E. Acetylcholine receptor: effects of proteolysis on receptor metabolism. J Cell Biol. 1982 Jan;92(1):176–182. doi: 10.1083/jcb.92.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Kao R. C., Wehner N. G., Skubitz K. M., Gray B. H., Hoidal J. R. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988 Dec;82(6):1963–1973. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz M. R., Cole F. S., Shepherd V. L., Wileman T. E., Stahl P. D. Isolation and characterization of a mannose-specific endocytosis receptor from human placenta. J Biol Chem. 1987 Jul 25;262(21):9942–9944. [PubMed] [Google Scholar]
- Lipson K. E., Kolhatkar A. A., Donner D. B. Cell surface proteolysis and down-regulation of the hepatic insulin receptor. Evidence for selective sorting of intact and degraded receptors after internalization. J Biol Chem. 1988 Jul 25;263(21):10495–10501. [PubMed] [Google Scholar]
- Loeb J. A., Drickamer K. The chicken receptor for endocytosis of glycoproteins contains a cluster of N-acetylglucosamine-binding sites. J Biol Chem. 1987 Mar 5;262(7):3022–3029. [PubMed] [Google Scholar]
- Rabinovitch M., Topper G., Cristello P., Rich A. Receptor-mediated entry of peroxidases into the parasitophorous vacuoles of macrophages infected with Leishmania Mexicana amazonensis. J Leukoc Biol. 1985 Mar;37(3):247–261. doi: 10.1002/jlb.37.3.247. [DOI] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Lewandrowski K. Decrease of the major surface glycoprotein gp 160 in activated macrophages. Cell Immunol. 1982 Jun;70(1):85–94. doi: 10.1016/0008-8749(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Campbell E. J., Senior R. M., Stahl P. D. Characterization of the mannose/fucose receptor on human mononuclear phagocytes. J Reticuloendothel Soc. 1982 Dec;32(6):423–431. [PubMed] [Google Scholar]
- Shepherd V. L., Konish M. G., Stahl P. Dexamethasone increases expression of mannose receptors and decreases extracellular lysosomal enzyme accumulation in macrophages. J Biol Chem. 1985 Jan 10;260(1):160–164. [PubMed] [Google Scholar]
- Shirayoshi Y., Hatta K., Hosoda M., Tsunasawa S., Sakiyama F., Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986 Oct;5(10):2485–2488. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. D., Shepherd V. L. Purification of the human alveolar macrophage mannose receptor. Biochem Biophys Res Commun. 1987 Oct 29;148(2):883–889. doi: 10.1016/0006-291x(87)90958-2. [DOI] [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz A. P., Amatruda J. F., Borhani D., Harrison S. C., Schwartz A. L. A high yield purification of the human transferrin receptor and properties of its major extracellular fragment. J Biol Chem. 1988 Jun 15;263(17):8318–8325. [PubMed] [Google Scholar]
- Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by products of activated lymphoid cells. J Exp Med. 1977 Feb 1;145(2):429–437. doi: 10.1084/jem.145.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott K. R., Searles R. P., Rome L. H. Evidence for ligand- and pH-dependent conformational changes in liposome-associated mannose 6-phosphate receptor. J Biol Chem. 1987 May 5;262(13):6101–6107. [PubMed] [Google Scholar]
- Wileman T. E., Lennartz M. R., Stahl P. D. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2501–2505. doi: 10.1073/pnas.83.8.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]