Abstract
Early viral replication and profound CD4+ T-cell depletion occur preferentially in intestinal tissues of macaques infected with simian immunodeficiency virus (SIV). Here we show that a much higher percentage of CD4+ T cells in the intestine express CCR5 compared with those found in the peripheral blood, spleen, or lymph nodes. In addition, the selectivity and extent of the CD4+ T-cell loss in SIV infection may depend upon these cells coexpressing CCR5 and having a “memory” phenotype (CD45RA−). Following intravenous infection with SIVmac251, memory CD4+ CCR5+ T cells were selectively eliminated within 14 days in all major lymphoid tissues (intestine, spleen, and lymph nodes). However, the effect on CD4+ T-cell numbers was most profound in the intestine, where cells of this phenotype predominate. The CD4+ T cells that remain after 14 days of infection lacked CCR5 and/or were naive (CD45RA+). Furthermore, when animals in the terminal stages of SIV infection (with AIDS) were examined, virtually no CCR5-expressing CD4+ T cells were found in lymphoid tissues, and all of the remaining CD4+ T cells were naive and coexpressed CXCR4. These findings suggest that chemokine receptor usage determines which cells are targeted for SIV infection and elimination in vivo.
Recent studies with the simian immunodeficiency virus (SIV) macaque model of AIDS have demonstrated that most of the viral replication and CD4+ T-cell loss initially occurs within the gastrointestinal tract, regardless of the route of exposure (10, 24, 26, 28). Since SIV and human immunodeficiency virus (HIV) optimally replicate within activated memory CD4+ T cells in vitro, and most lymphocytes in the intestinal lamina propria are of this phenotype (2–5, 13, 15, 16, 22, 23, 25, 27, 31, 32), this partially explains why the intestine is a preferred target for SIV replication and early CD4+ T-cell loss. Moreover, it has recently been demonstrated that, in addition to intestinal CD4+ T-cell depletion, a selective loss of activated memory (CD45RA−) CD4+ T cells (effector CD4+ T cells) can be detected in peripheral blood and lymph nodes of macaques within a few weeks of viral infection (30). However, since peripheral lymphoid tissues consist primarily of resting, naive (CD45RA+) cells, this selective loss does not result in a significant reduction in overall CD4+ T-cell numbers.
Although HIV and SIV require cellular activation for optimal viral replication, it is still not known how these viruses distinguish effector CD4+ T cells from resting, naive CD4+ T cells. Although integrated provirus can be detected within naive CD4+ T cells (33), multiple studies have confirmed that the majority of cell-associated virus is found within memory CD4+ T cells (17, 19, 23). It is apparent that the virus is primarily targeting activated memory CD4+ T cells for viral attachment and entry, as well as for optimal replication. How then, does the virus recognize and distinguish effector from naive CD4+ T cells?
A crucial piece of the puzzle was provided by a series of discoveries involving chemokine receptors. First, the discovery that chemokine receptors were the coreceptors for HIV and SIV infection explained why only certain cells could be infected with HIV. It is now well-established that, in addition to the CD4 molecule, HIV and SIV require one or more chemokine receptors as coreceptors for attachment and entry into host cells. Moreover, like most primary isolates of HIV, SIVmac utilizes both CD4 and the chemokine receptor CCR5 for attachment, fusion, and entry into host cells (1, 14). Later studies demonstrated that a correlation exists between naive and memory lymphocyte subsets and the expression of particular chemokine receptors. In normal human blood, CXCR4 is expressed primarily on naive lymphocytes (CD45RA+), whereas CCR5 expression is primarily limited to memory lymphocytes (CD45RO+) (20). Finally, experiments using SIV/HIV chimera viruses (SHIVs) suggest that chemokine receptor usage may determine which CD4+ T cells are targeted. SHIVs that strictly utilize CXCR4 rapidly deplete CD4+ T cells in peripheral blood, but spare intestinal CD4+ T cells. In contrast, a SHIV that exclusively utilizes CCR5 depletes intestinal CD4+ T cells, but spares peripheral blood CD4+ T cells (8). This study suggested that chemokine receptor usage was involved in determining which CD4+ T cells (mucosal versus peripheral) were depleted in SIV infection. However, chemokine receptor expression on subsets of mucosal CD4+ T cells during acute infection has not been examined.
In the current study, we have addressed the role that CCR5 plays in the selective elimination of effector CD4+ T cells in early and late stages of SIV infection. The following questions were specifically addressed. How does CCR5 expression correlate with activation and memory markers on macaque CD4+ T cells in various lymphoid tissues?. Are there larger percentages of CCR5-expressing CD4+ T lymphocytes in the intestine than there are CCR5-expressing peripheral blood or lymph node CD4+ T lymphocytes? Finally, are CCR5-expressing CD4+ T lymphocytes selectively depleted in peripheral and mucosal lymphoid tissues in early SIV infection? We hypothesized that if the depletion of effector CD4+ T cells was dependent on coreceptor usage, animals sacrificed in all stages of infection should be markedly depleted of effector CD4+ T cells coexpressing CCR5.
MATERIALS AND METHODS
Animals and virus.
A total of 15 rhesus macaques (Macaca mulatta) were used to examine lymphocyte subsets in peripheral blood, systemic lymphoid tissues, and intestine. In prospective studies, sequential changes in peripheral blood and intestinal lymphocyte subsets were examined in six 2- to 3-year-old rhesus macaques. Three of these animals (mm158-99, mm169-99, and mm170-99) were killed at 14 days postinfection (p.i.), and changes in lymphocyte subsets in the spleen and axillary and mesenteric lymph nodes were also examined. Sampling occurred before infection (day 0) and at 7, 14, 21, 35, and 63 days after intravenous infection with 50 ng of SIVmac251. Controls consisted of blood and intestinal biopsies collected from three uninfected macaques and blood, spleen, axillary and mesenteric lymph nodes collected from two additional uninfected macaques killed for other studies. The same tissues were collected at necropsy from an additional four macaques that developed AIDS within 4 to 6 months of intravenous infection with SIVmac251. All animals were maintained in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and the guidelines of the Committee on Animals of Harvard Medical School.
Cell isolation and flow cytometry.
Peripheral blood and intestinal lymphocytes were stained and analyzed by flow cytometry as previously described (28–30). Briefly, jejunal pinch biopsies were incubated with 1 mM EDTA in Hanks balanced salt solution for 30 min, followed by 1 h in RPMI containing 20 U of collagenase per ml while rapidly shaking at 37°C. No attempts were made to separate intestinal epithelial lymphocytes from lamina propria lymphocytes in biopsy samples. Biopsies were further disrupted, and single-cell suspensions were prepared by pipetting 5 to 10 times with a 16-g feeding needle. Lymphocytes were then enriched by Percoll density gradient centrifugation. To ensure that CCR5 expression was not being up-regulated on intestinal cells due to these isolation procedures, whole blood from one macaque was similarly treated, and levels of CCR5 expression on lymphocytes were compared before and after treatment. A slightly lower percentage of CCR5 receptors were detected on lymphocytes after treatment (2.9% CCR5+) when compared to whole blood staining (3.1% CCR5+). Since Ficoll purification of cells has been reported to transiently down-regulate chemokine receptor expression (11, 12), the Percoll density gradient centrifugation step was most likely responsible for this slight decrease.
Peripheral blood samples were stained by a whole-blood staining procedure, with the exception of samples obtained from macaques mm352, mm353, and mm356-96 in which peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll density gradient separation. However, since we were examining changes in chemokine receptor expression over time, and since PBMCs were always used in these three animals (before and after infection), this did not significantly affect the results of this study.
Cells from all tissues were stained for four-color flow cytometry with monoclonal antibodies to CD4 allophycocyamin (APC), CD8PerCP, CD45RA-fluorescein isothiocyanate (FITC) conjugate (Becton Dickinson), and either CCR5-phycoerythrin (PE) conjugate (clone 3A9) or CXCR4-PE conjugate (Pharmingen). Since CD45RO does not reliably cross-react with macaque lymphocytes, CD4+ lymphocytes are defined in this report as either naive (CD45RA+) or memory (CD45RA−) by a previously described rationale and methodology (28). Samples were acquired on a FACS Calibur flow cytometer and analyzed with Cell Quest software (Becton Dickinson).
Statistics.
Statistical analyses were performed with a paired Student's t test and commercial statistical software (Sigma Plot, SPSS). P values <0.05 were considered significant.
RESULTS
Lymphocytes from the intestine have much higher CCR5 expression than lymphocytes from blood.
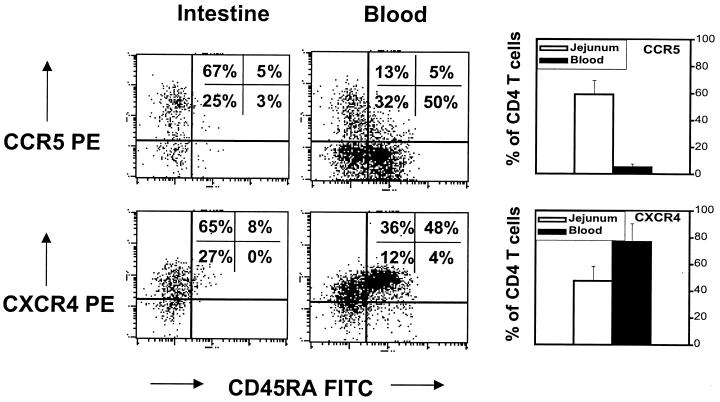
First, we established that chemokine receptor expression patterns were similar between macaque lymphocytes and those reported for human lymphocytes (20). We examined the expression of CCR5 and CXCR4 in combination with CD45RA (a naive cell marker) on both CD4+ and CD8+ T cells by four-color flow cytometry. As in humans (20), the majority of CD4+ and CD8+ T cells from macaque blood that expressed CCR5 were memory (CD45RALO), whereas most of the T cells in the blood that were naive (CD45RAHI) coexpressed CXCR4 (Fig. 1).
FIG. 1.
Comparison of CCR5 and CXCR4 expression on normal (uninfected) CD4+ T lymphocytes from the jejunum to those of peripheral blood. Note that very few (∼5%) peripheral blood CD4+ lymphocytes express CCR5, whereas most (∼60%) intestinal CD4+ lymphocytes express CCR5. Also note that CCR5 expression and CD45RA expression are relatively mutually exclusive; most naive CD4 cells (CD45RAHI) do not express CCR5, whereas CCR5HI cells are CD45RALO. In contrast, most peripheral blood CD4 cells express CXCR4. CD45RAHI cells are usually CXCR4HI. The dot plots shown were generated by first gating through CD4+ T lymphocytes and are representative of uninfected animals (except that the macaque with the highest CCR5 expression in the peripheral blood was selected for illustration of CD45RA coexpression). Graphs represent the mean of six animals examined ± standard deviation.
We then determined and compared baseline expression of chemokine receptors on naive and memory CD4+ T cells from lymphocytes isolated from the intestine, lymph nodes, and peripheral blood of normal macaques. Significant (P < 0.05) differences in the percentages of lymphocytes expressing chemokine receptors were observed between CD4+ T cells obtained from peripheral lymphoid tissues and those from the intestine (Fig. 1). Only a small percentage (5%) of CD4+ lymphocytes from peripheral blood expressed CCR5, whereas the majority (60%) of intestinal CD4+ T cells were CCR5 bright (Fig. 1). Conversely, most CD4+ T lymphocytes in peripheral blood expressed CXCR4 (77%), whereas less than half of the intestinal CD4+ T cells expressed CXCR4 (Fig. 1). Dual expression of CXCR4 and CCR5 receptors was evident on a significant proportion of intestinal CD4+ T cells (data not shown). In blood, CD4+ T cells expressing CXCR4 were predominantly CD45RAHI, but since the vast majority of intestinal CD4+ T cells lack CD45RA, this was not always true for normal intestinal CD4+ T cells. In other words, intestinal CD4+ T cells that expressed CXCR4 usually lacked CD45RA, but CD4+ T cells from both blood and intestine that expressed CCR5+ usually lacked CD45RA expression (Fig. 1).
CD4+ T cells expressing CCR5 are selectively eliminated in acute SIV infection.
Changes in chemokine receptor expression on CD4+ T cells were then examined with intestinal and peripheral blood lymphocytes in very early SIV infection. Six adult macaques were intravenously inoculated with SIVmac251, three of which were killed at 14 days p.i. The other three were monitored for 9 weeks p.i. Immunophenotyping of blood and intestinal lymphocytes was performed before viral inoculation (day 0) and on days 14, 21, 35, and 63 p.i. As expected from previous studies, intestinal CD4+ T cells were markedly (and selectively) depleted within 14 days p.i. in all animals. Although CD4+ T cells comprised 50 to 60% of the total intestinal T-cell population before infection, only 5 to 10% of the remaining T cells expressed CD4 by 14 days of infection (Fig. 2).
FIG. 2.
Dot plots showing CD4 T-cell depletion due to SIV infection in the intestinal mucosa of three macaques. Intestinal lymphocytes were obtained from endoscopic pinch biopsies from the jejunum of the same animals at different time points (prospective studies). Plots were generated by gating through CD3+ lymphocytes. From left to right, note the rapid loss of CD4+ T cells within 14 days after infection. Also note that CD4+ CD8+ DP cells (upper right quadrants) are more profoundly eliminated than the CD4+ SP population. DP cells were essentially eliminated by 14 days p.i. and did not return in the time points examined, whereas some SP CD4+ T cells return by 35 days p.i. (lower right quadrants).
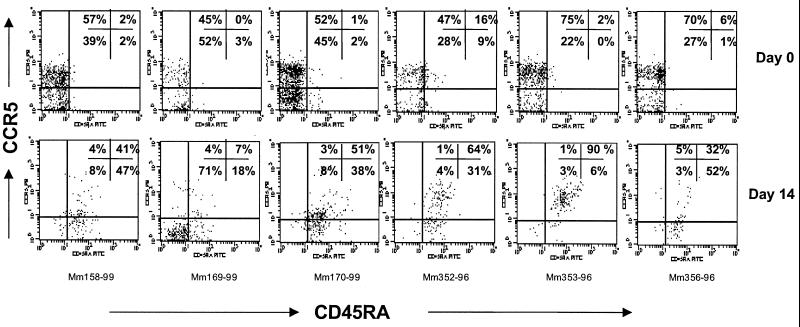
Gating through the remaining CD4+ T cells revealed that virtually all of the memory (CD45RALO) CCR5+ helper cells had been eliminated by 14 days p.i. in all six animals (Fig. 3). Since 45 to 75% of the CD4+ T cells were CD45RALO and CCR5HI in the same animals just 14 days prior (day 0), this indicated that a profound elimination of memory CD4+ T cells coexpressing CCR5 had occurred within this short time frame. It is important to emphasize, however, that not all CCR5-expressing CD4+ T cells were eliminated by this time point. In three animals (mm170-99, mm352-96, and mm353-96), over half of the remaining CD4+ T cells coexpressed CCR5 (Fig. 3). However, these residual CCR5-expressing cells were virtually all naive (CD45RAHI), suggesting that they were either recently recruited to or newly formed within the intestinal mucosa (Fig. 3).
FIG. 3.
Dot plots demonstrating selective depletion of the CD45RALO CCR5HI subset of CD4+ T cells in the intestine within 14 days p.i. All plots were generated by first gating through CD4+ T lymphocytes; top panels are from intestinal biopsies taken before infection (day 0), and bottom panels correspond to the same animals and region 14 days after SIV infection (prospective analysis). Before infection, 45 to 75% of the CD4+ T cells are both memory (CD45RALO) and CCR5HI (upper left quadrants). After infection, virtually all of this memory CCR5HI subset of CD4+ T cells has been selectively depleted in all six animals examined. The remaining CD4+ T cells are either CD45RAHI CCR5HI (upper right) or lack CCR5 expression (lower left and right).
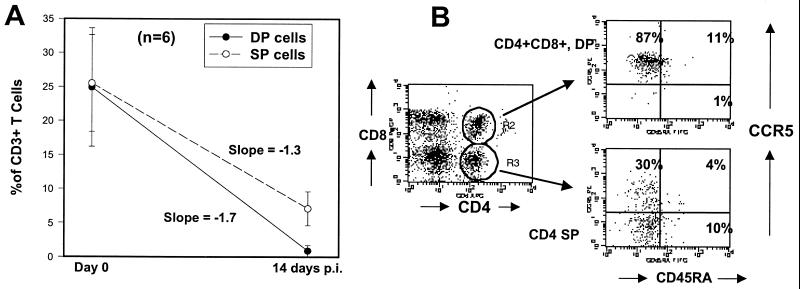
Additional evidence for selective targeting and depletion of memory CCR5+ T cells came from four-color analysis of a unique population of T cells in the proximal intestine. As previously described, macaques have a substantial (yet variable) number of CD4+ CD8+ double-positive (DP) T cells in the lamina propria of the jejunum (28, 29). This particular population of DP cells appears to be unique, since they do not share the same phenotypic markers as DP cells found in the thymus. These DP cells do not express CD34 (a stem cell marker) or CD45RA, but are all CD69HI, suggesting they are activated memory cells rather than immature precursor cells (data not shown). Moreover, these DP cells are consistently eliminated from the intestine in early SIV infection at a rate that exceeds the loss of single-positive (SP) CD4+ T cells (Fig. 2). The rate of cell loss as determined by the mean slope of the line between day 0 and day 14 was −1.7 for DP cells versus −1.3 for SP cells (Fig. 4A). This is most likely an underestimation, since almost all of the DP cells had been eliminated by day 14 p.i. This suggests that intestinal DP T cells are better targets for viral infection and destruction than SP CD4+ T cells.
FIG. 4.
(A) Comparison of the rate of DP CD4+ CD8+ to SP (CD4+) intestinal T-cell depletion after 14 days of SIV infection. Note that approximately equal percentages of DP and SP cells are present in the jejunum of uninfected animals, but the DP cells are virtually eliminated by day 14 p.i. A few (mean, 7%) SP cells remain, indicating that a selective depletion of DP cells is occurring, as indicated by the slope of the lines. Points represent the mean of six animals ± standard deviation. Percentages were determined by gating through CD3+ lymphocytes. (B) Four-color flow cytometry demonstrating that DP intestinal T cells from normal (uninfected) macaques are virtually all CCR5HI, whereas a significant proportion of the SP T cells lack CCR5 expression. Also note that both DP and SP T cells lack significant CD45RA expression. Plots were generated by gating through intestinal lymphocytes.
To account for their increased rate of elimination in early SIV infection, we hypothesized that more DP cells would express CCR5, which in fact, proved to be the case. By four-color flow cytometry, DP cells from uninfected animals were virtually all CCR5+ (Fig. 4B) and also demonstrated significantly lower expression of CXCR4 than their CD4+ SP counterparts (data not shown). Combined, these data suggest that intestinal DP cells are highly activated, terminally differentiated effector lymphocytes, and not newly formed T-cell progenitor cells like the DP cells found in the thymus. We hypothesize that it is the coexpression of both CD4 and CCR5 in conjunction with their high level of activation that leads to optimal (and presumably lytic) viral replication, which would explain why they are eliminated from the intestinal tract of animals at a rate that exceeds the loss of SP cells.
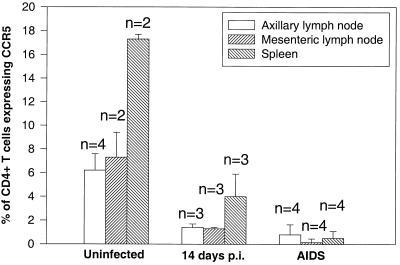
The loss of CCR5+ effector CD4+ T cells was not limited to the intestinal tract. Examination of mesenteric and peripheral lymph nodes and spleen (in animals that were killed) demonstrated that CD4+ T cells expressing CCR5 were markedly decreased in these tissues compared to that in uninfected animals (Fig. 5). Moreover, examination of both peripheral and mucosal tissue sites by flow cytometry provided additional evidence that the loss of these specific T-cell subsets was due to elimination and not redistribution or compartmentalization.
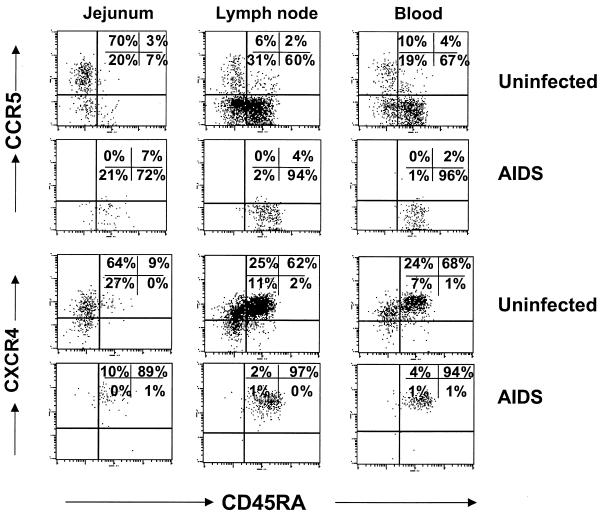
FIG. 5.
Comparison of CCR5 expression on CD4+ T cells from peripheral lymphoid tissues of uninfected macaques to those sacrificed at 14 days after SIV infection and those sacrificed with advanced SIVmac251 infection (AIDS). Note that there are markedly fewer CCR5-expressing CD4+ T cells in tissues from acutely infected macaques, and animals with AIDS show profound depletion of CCR5-expressing CD4+ T cells in all lymphoid tissues. Graphs represent the mean of two to four different animals per group (as indicated) ± standard deviation. Data were generated by gating through CD4+ T lymphocytes.
In the three animals that were monitored for 9 weeks (mm352-96, mm353-96, and mm356-96), effector CD4+ T cells did not return to normal levels. However, two animals (mm352-96 and mm356-96) did have a slight increase in intestinal CD4+ T lymphocytes (up to 20% of CD3+ T cells) by day 35 p.i. (Fig. 2). However, as observed in the 14-day samples, the majority of these repopulating CD4+ T cells were CD45RAHI. This suggests that attempts at intestinal CD4+ T-cell replacement are being made in the early stage of infection, but this supply apparently does not keep pace with the destruction of effector CD4+ T lymphocytes.
CD4+ T cells expressing CCR5 are eliminated in macaques with AIDS.
Marked differences in chemokine receptor expression on CD4+ T cells were also detected in macaques sacrificed in the later stages of SIV infection. In four out of four SIV-infected animals sacrificed at the onset of AIDS (4 to 6 months p.i.), virtually all of the CD4+ T cells remaining in the peripheral blood, lymph nodes, and intestinal tract were CCR5LO and CXCR4HI (Fig. 6). In addition, virtually all of the remaining CD4+ T cells in peripheral blood, lymph nodes, and spleen coexpressed CD45RA, indicating that all of the host's effector CD4+ T cells had been eliminated, leaving only naive CD4+ T cells that lacked CCR5. As in early infection, the loss of CCR5 effector cells was limited to the CD4+ T-cell subset, since significant proportions of CD8+ T cells continued to express CCR5 and were CD45RA+/− (data not shown).
FIG. 6.
Dot plots demonstrating profound loss of CCR5-expressing CD4+ T cells in macaques sacrificed with advanced SIV disease (AIDS). All plots were generated by gating through CD4+ T lymphocytes. Top panels are representative of uninfected macaques demonstrating CCR5HI cells in (from left to right) the jejunum, axillary lymph node, and peripheral blood. Note that in animals with AIDS, essentially all of the CCR5HI as well as the memory (CD45RALO) CD4+ T cells have been eliminated, leaving only CXCR4HI and CD45RAHI cells. Plots are representative of four animals with AIDS examined.
DISCUSSION
These data demonstrate that the selectivity and degree of CD4+ T-cell depletion in early SIV infection depend upon both their level of CCR5 expression and the level of cell activation and memory. Effector CD4+ T cells expressing CCR5 were selectively eliminated within 14 days of SIV infection in all major lymphoid tissues in all animals examined, whereas the CD4+ T cells that remained lacked CCR5 and/or were naive (CD45RA+). Furthermore, the fact that significantly higher percentages of intestinal CD4+ T cells express CCR5 may explain why these cells are rapidly depleted in early SIV infection and also why no profound changes in the percentage or number of CD4+ T cells are detected in the peripheral lymphoid tissues during acute infection.
In addition, selective targeting of CCR5-expressing cells in the intestine was shown by examining DP cells in the intestine before and after SIV infection. DP T cells (which disappear even more rapidly than SP CD4+ T cells) were shown to be virtually all CCR5 positive before infection, and these cells were selectively and essentially eliminated by 14 days p.i. If intestinal CD4+ T cells were randomly eliminated after SIV infection, then an equal proportion of the DP cells would be expected to be eliminated. This demonstrates that selective elimination of CCR5-expressing CD4+ effector T cells occurs in lymphoid tissues in the first few days of SIV infection.
Unfortunately, these data do not fully explain the disappearance of intestinal CD4+ T cells that did not (at least initially) express CCR5, as demonstrated in Fig. 3. However, this is likely a reflection of the dynamic processes that are occurring within the intestinal immune system during acute SIV infection. For example, the CD4+ T cells that were initially CCR5 negative may have upregulated CCR5 expression due to immune activation associated with SIV infection, thus becoming susceptible targets for viral infection and lysis. Alternatively, these cells may have been eliminated through other nonlytic mechanisms, such as “bystander apoptosis” (6). Additional studies are needed to definitively determine the mechanisms and dynamics of the intestinal CD4+ T-cell depletion. However, the loss of CCR5-expressing cells in the peripheral tissues in both early and later stages of SIV infection is clearly evident from this study, indicating that selective loss of CCR5-expressing CD4+ T cells is occurring in SIVmac251 infection.
Combined, these data show that early SIV infection is associated with selective depletion of effector CD4+ T cells expressing CCR5. The speed of the intestinal CD4+ T-cell depletion and the estimated magnitude of viral production in HIV-infected individuals (9) are in marked contrast to the designation of HIV and SIV as “lenti,” or “slow,” viruses. If we consider the possibility that these viruses only lytically replicate within highly specified target cells (such as CD4+ CCR5+ CD45RO+ lymphocytes), then the pathogenesis of SIV and HIV infection begins to appear more similar to that of other viral infections. However, the major difference appears to be the unique ability of SIV and HIV to specifically eliminate effector CD4+ T cells within the first few days or weeks of infection. This almost certainly cripples the initiation of an effective immune response to the virus. Moreover, it appears that as new effector CD4+ T cells are generated, they continue to be eliminated, preventing the development of an effective immune response.
Since this study involved SIVmac251, a virus which utilizes CCR5 (and not CXCR4) for cellular entry, this provided an invaluable model for demonstrating the effects of a virus that utilizes a specific chemokine receptor in an in vivo model of infection. Whether these findings can be reproduced in HIV-infected humans is uncertain, since samples from infected patients are rarely obtained within the first few days of exposure, and they may harbor numerous viral strains, particularly in the latter stages of infection, which may or may not have similar patterns of chemokine receptor utilization. However, studies of human lymphoid tissue have shown that CXCR4 utilization is sufficient to deplete lymph node and peripheral blood CD4+ T cells in vitro (18). Other studies have shown that CCR5-utilizing (R5) strains of HIV deplete CCR5-expressing PBMCs in vitro; however (as shown here in the SIV model), this depletion does not substantially change PBMC numbers, because very few human PBMCs express CCR5 (7). Other studies have suggested that the rapid decline in CD4+ T-cell counts and the progression to AIDS are associated with a switch from CCR5-tropic to CXCR4-tropic variants of HIV (21). Combined, these studies strongly support the hypothesis that chemokine receptor usage determines the speed and course of HIV infection.
The presence of very large numbers of effector CD4+ T cells expressing CCR5 in mucosal surfaces helps explain why SIV and HIV are readily transmitted across mucosal surfaces and why most primary infections are with R5 viruses. Moreover, the abundance of lymphocytes, macrophages, and dendritic cells in the gastrointestinal tract, combined with its sheer size, makes the gut a very large reservoir for viral persistence in both a latent form and (as effector CD4+ T cells attempt to replenish the intestine) a continual source of active viral replication.
Finally, since CCR5-utilizing strains of HIV appear to be the predominant virus transmitted in new infections, these findings may be of major importance in designing vaccines and therapeutic strategies to combat HIV infection. Perhaps vaccines may be designed that selectively block CCR5-mediated attachment and entry of virus into these target cells, which could conceivably allow effector CD4+ T cells to survive long enough to mount an effective immune response against HIV.
ACKNOWLEDGMENTS
We thank Michael O'Connell for study coordination, Kristen Toohey for graphical support, and Elaine Roberts and Pam Wooten for technical assistance and animal care.
This work was supported in part by NIH grants DK50550, RR00168, HD36310, and HL59787 and by Elizabeth Glaser Pediatric AIDS Foundation grant PG-50861. A. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award.
REFERENCES
- 1.Berger E A, Doms R W, Fenyo E-M, Korber T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Soedroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 2.Borvak J, Chou C-S, Bell K, Van Dyke G, Zola H, Ramilo O, Vitetta E S. Expression of CD25 defines peripheral blood mononuclear cells with productive versus latent HIV infection. J Immunol. 1995;155:3196–3204. [PubMed] [Google Scholar]
- 3.Chou C-S, Ramilo O, Vitetta E S. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun T-W, Engel D, Mizell S B, Ehler L A, Fauci A S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Maria R, Fais S, Testi R. Persistent in vivo activation and transient anergy to TCR/CD3 stimulation of normal human intestinal lymphocytes. In: Mestecky J, editor. Advances in mucosal immunology. New York, N.Y: Plenum Press; 1995. pp. 43–46. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;2:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 7.Grivel J C, Margolis L B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 8.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 9.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 10.Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup F J, Stallmach A, Zeitz M. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee B, Doranz B J, Rana S, Yi Y, Mellado M, Frade J M R, Martinez-A C, O'Brien S J, Dean M, Collman R G, Doms R W. Influence of the CCR2–V64I polymorphism on human immunodeficiency virus type 1 coreceptor activity and on chemokine receptor function of CCR2b, CCR3, CCR5, and CXCR4. J Virol. 1998;72:7450–7458. doi: 10.1128/jvi.72.9.7450-7458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee B, Sharron M, Montaner L J, Weissman D, Doms R W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahalingham M, Peakman M, Davies E T, Pozniak A, McManus T J, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx P A, Chen Z. The function of simian chemokine receptors in the replication of SIV. Semin Immunol. 1998;10:215. doi: 10.1006/smim.1998.0135. [DOI] [PubMed] [Google Scholar]
- 15.McDougal J S, Mawle A, Cort S P, Nicholson J K A, Cross G D, Schleppler-Campbell J A, Hicks D, Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985;135:3151–3162. [PubMed] [Google Scholar]
- 16.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski M A, Chun T-W, Justement S J, Motola I, Spinelli M A, Adelsberger J, Ehler L A, Mizell S B, Hallahan C W, Fauci A S. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn M L, Grivel J C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg Z F, Fauci A S. Immunopathogenesis of HIV infection. FASEB J. 1991;5:2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarlatti G E, Tresoldi A, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine mediated suppression. Nat Med. 1997;3:1259. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 22.Schieferdecker H L, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 23.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smit-McBride Z, Mattapallil J J, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investig. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl-Hennig C, Steinman R M, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 27.Van Noesel C J, Gruters R A, Terpstra F G, Schellekens P T, van Lier R A, Miedema F. Functional and phenotypic evidence for a selective loss of memory T cells in asymptomatic human immunodeficiency virus-infected men. J Clin Investig. 1990;86:293–299. doi: 10.1172/JCI114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 29.Veazey R S, Rosenzweig M, Shvetz D E, Pauley D R, DeMaria M, Chalifoux L V, Johnson R P, Lackner A A. Characterization of gut-associated lymphoid tissues (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 30.Veazey R S, Tham I C, Mansfield K G, DeMaria M A, Forand A E, Shvetz D E, Chalifoux L V, Sehgal P K, Lackner A A. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willerford D M, Gale M J, Jr, Benveniste R E, Clark E A, Gallatin W M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J Immunol. 1990;144:3779–3783. [PubMed] [Google Scholar]
- 32.Zeitz M, Greene W C, Peffer N J, James S P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z-Q, Schuler T, Zupanic M, Wietgrefe S, Staskus K A, Reimann K A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]