Abstract
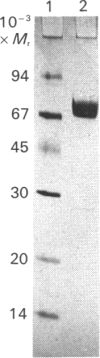
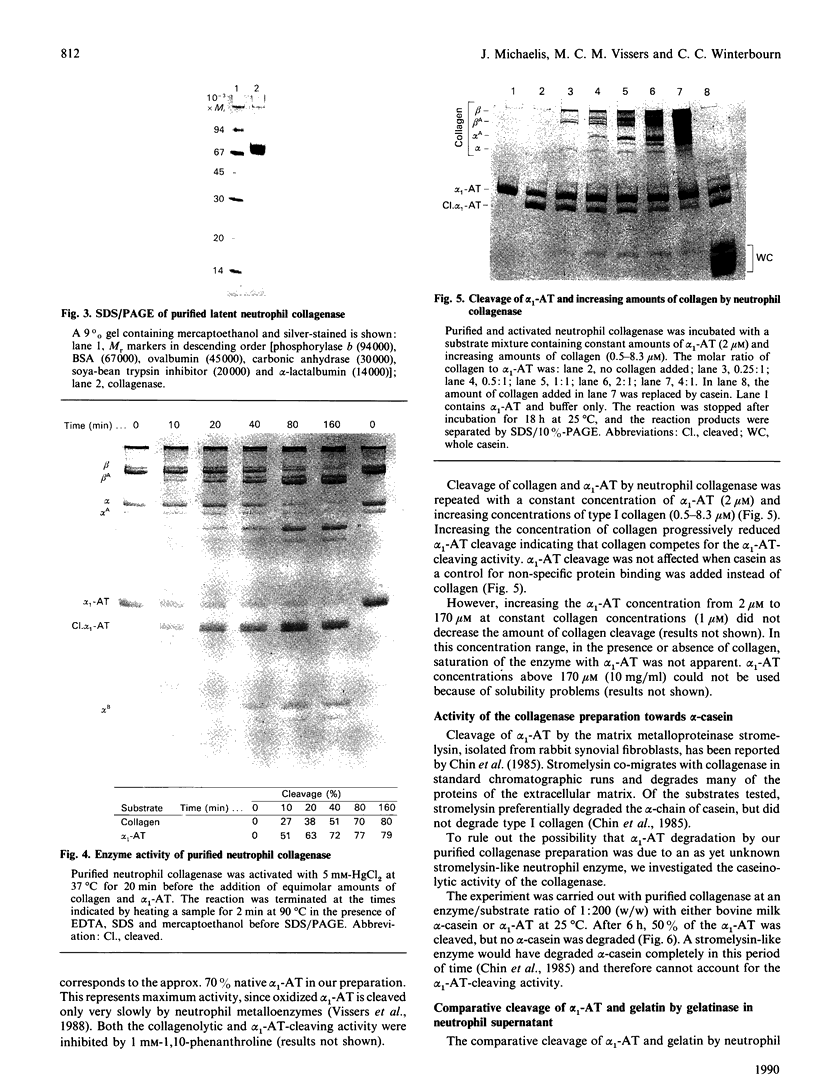
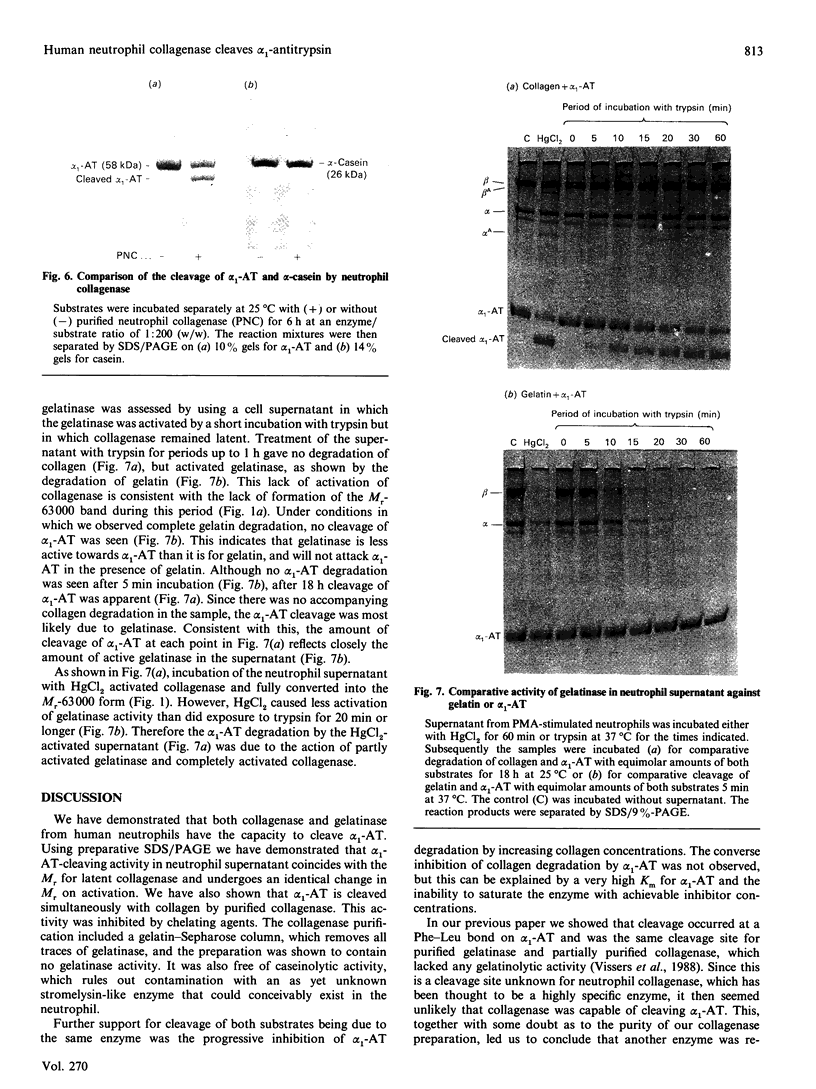
Inactivation of the plasma serine-proteinase inhibitor alpha 1-antitrypsin (alpha 1-AT) by neutrophil metalloproteinases has been reported [Vissers, George, Bathurst, Brennan & Winterbourn (1987) Fed. Proc. Fed. Am. Soc. Exp. Biol. 46, 1390a; (1988) J. Clin. Invest. 82, 706-711; Desrochers & Weiss (1988) J. Clin. Invest. 81, 1646-1650]. To identify the enzyme responsible, supernatant from neutrophils stimulated with phorbol 12-myristate 13-acetate was subjected to preparative SDS/PAGE, both with and without activation of latent metalloproteinases with HgCl2. The lanes were subsequently sliced into pieces, the slices incubated with equimolar amounts of type I collagen and alpha 1-AT in the presence of HgCl2, and the reaction products separated by SDS/PAGE. With the latent supernatant, the characteristic collagen-cleavage products and cleaved alpha 1-AT were present in the same slices, corresponding to an Mr of 80,000-85,000. On treatment with HgCl2 both degradative activities underwent the same molecular-mass shift to a position corresponding to Mr 60,000-65,000. Western blots of neutrophil supernatants, using a polyclonal antibody to purified collagenase, showed Mr values of 83,000 for the latent enzyme and 63,000 for the HgCl2-activated enzyme. Neutrophil collagenase was purified to homogeneity and shown also to exist in a second latent form with Mr 70,000. When activated to the Mr-63,000 form by HgCl2 and incubated with equimolar amounts of collagen and alpha 1-AT, collagenase cleaved alpha 1-AT at almost twice the rate at which collagen was cleaved. alpha 1-AT cleavage was inhibited by 1,10-phenanthroline and by high concentrations of collagen. That the purified collagenase did not contain a contaminant proteinase such as stromelysin was indicated by inability of the preparation to cleave casein. Taken together these results lead us to conclude that neutrophil collagenase is capable of degrading alpha 1-AT. Neutrophil gelatinase also cleaved alpha 1-AT, but cleavage was slow when compared with its activity against gelatin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods Enzymol. 1987;144:140–171. doi: 10.1016/0076-6879(87)44177-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Desrochers P. E., Weiss S. J. Proteolytic inactivation of alpha-1-proteinase inhibitor by a neutrophil metalloproteinase. J Clin Invest. 1988 May;81(5):1646–1650. doi: 10.1172/JCI113500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986 Apr 25;261(12):5645–5650. [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Pierce J., Persson U., Thulin E. Purification of alpha1-antitrypsin from plasma through thiol-disulfide interchange. Eur J Biochem. 1975 Sep 1;57(1):107–113. doi: 10.1111/j.1432-1033.1975.tb02281.x. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Ultrastructural aspects of acute inflammation. Pathol Annu. 1970;5:343–353. [PubMed] [Google Scholar]
- Okada Y., Harris E. D., Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts. Purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988 Sep 15;254(3):731–741. doi: 10.1042/bj2540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Sopata I. Further purification and some properties of a gelatin-specific proteinase of human leucocytes. Biochim Biophys Acta. 1982 Jul 16;717(1):26–31. doi: 10.1016/0304-4165(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Sorsa T. A. Activation of latent collagenase purified from human leukocytes. Scand J Rheumatol. 1987;16(3):167–175. doi: 10.3109/03009748709165270. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Birkedal-Hansen H. Human fibroblast collagenase-alpha-macroglobulin interactions. Localization of cleavage sites in the bait regions of five mammalian alpha-macroglobulins. J Biol Chem. 1989 Jan 5;264(1):393–401. [PubMed] [Google Scholar]
- Stephano J. L., Gould M., Rojas-Galicia L. Advantages of picrate fixation for staining polypeptides in polyacrylamide gels. Anal Biochem. 1986 Feb 1;152(2):308–313. doi: 10.1016/0003-2697(86)90414-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sundberg L., Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifuctional oxiranes. J Chromatogr. 1974 Mar 13;90(1):87–98. doi: 10.1016/s0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., George P. M., Bathurst I. C., Brennan S. O., Winterbourn C. C. Cleavage and inactivation of alpha 1-antitrypsin by metalloproteinases released from neutrophils. J Clin Invest. 1988 Aug;82(2):706–711. doi: 10.1172/JCI113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. Human skin fibroblast collagenase. Assessment of activation energy and deuterium isotope effect with collagenous substrates. J Biol Chem. 1981 Sep 25;256(18):9516–9521. [PubMed] [Google Scholar]