Abstract
OBJECTIVES:
There is uncertainty regarding which hospitalized patients with acute gastroenteritis (AGE) benefit from gastrointestinal panel (GIP) testing. Unnecessary testing may lead to increased costs, overdiagnosis, and overtreatment. In general, AGE management and outcomes are most impacted if an actionable (bacterial or parasitic) result is obtained. We aimed to assess which clinical reasons for ordering GIP testing (“order indications”) and patient factors were associated with actionable results.
METHODS:
This is a cross-sectional study of pediatric patients hospitalized between 2015 and 2018 at a large pediatric health care system with diarrhea and a GIP performed. Multivariable regression analysis was used to determine associations between actionable GIP results and order indication, stool frequency, and demographics. Findings were evaluated in patients with complex chronic conditions (CCC) and non-CCC patients.
RESULTS:
There were 1124 GIPs performed in 967 encounters. Non-CCC patients had more actionable results than CCC patients, and reasons for testing differed. Across both cohorts, age ≥1 year old was positively associated with actionable results. For non-CCC patients, actionable results were associated with “diarrhea with blood or pus” order indication and nonwinter season; international travel was associated with non-Clostridioides difficile bacteria and parasites. No order indications were associated with actionable results for CCC patients.
CONCLUSIONS:
Patient factors and order indications that may help identify children hospitalized for AGE with actionable GIP results include older age (regardless of CCC status), as well as bloody stools and international travel in previously healthy children. Prospective validation of these findings could help improve diagnostic stewardship and decrease unnecessary testing.
Acute gastroenteritis (AGE) is a leading cause of morbidity and mortality in children worldwide,1 and accounts for up to 200 000 pediatric hospitalizations and $350 million in health care spending a year in the United States.2,3 Given their convenience, fast turnaround time, and simultaneous detection of multiple pathogens with high sensitivity and specificity, gastrointestinal multiplex polymerase chain reaction (PCR) panels (GIPs) are now a popular testing modality for identifying the etiology of AGE.4,5 At our institution, introduction of the GIP increased stool testing volume by 20%, but only a small subset (3%) of patients tested had improved clinical outcomes, primarily those treated for a detected bacterial or parasitic pathogen.6 This discordance emphasizes the need for diagnostic stewardship interventions to ensure appropriate use, as studies have demonstrated that unnecessary testing can lead to increased health care utilization, increased costs, and overdiagnosis with possible subsequent overtreatment.6–9
There is uncertainty regarding which patients and situations truly benefit from GIP testing as most AGE episodes are self-limited and due to a viral infection for which treatment is supportive.10 Patient management and outcomes are most often affected if an “actionable result” is obtained, meaning the detection of a bacterial or parasitic pathogen, which may facilitate faster initiation or targeting of antimicrobial treatment or closer monitoring of possible associated complications.6,11 Differentiating between viral and bacterial/parasitic AGE is difficult; risk factors for nonviral AGE cited in the literature include high fevers, bloody diarrhea, summertime, frequent stooling, and international travel, but none of these factors are highly discriminatory.10–13 Current AGE guidelines are inconsistent when it comes to testing recommendations (though routine stool testing is universally not recommended), largely on the basis of low-quality evidence or expert consensus opinion, and more robust data are needed to guide testing practices.2,10,11,14,15 To help fill this gap and inform future GIP diagnostic stewardship, the aim of this study was to evaluate which provider-reported reasons for testing (“order indications”) and patient factors, including demographics and stool frequency, were associated with actionable GIP results for hospitalized children with acute diarrhea.
METHODS
Study Design and Population
We performed a cross-sectional study of hospitalized children aged ≤18 years with AGE, diarrhea, or dehydration who had at least 1 GIP performed during their admission, including any GIPs collected in the emergency department before admission. Using similar methods to previous related studies,16–18 we identified patients with diarrheal illnesses using discharge International Classification of Diseases, 10th Revision, diagnosis codes for AGE or diarrhea of determined/presumed infectious etiology (A02.0, A02.9, A03.0, A03.9, A04.0–0.9, A07.1–0.2, A08.0–0.8, A09, B82.9, B96.20–0.23), diarrhea of undetermined/presumed noninfectious etiology (K52.29, K52.89, K52.9, R19.7), and dehydration (E86.0). We included hospitalizations between November 1, 2015, and November 1, 2018, across 5 pediatric hospitals in the Mountain West region of the United States, including a large, 434-bed academic quaternary care pediatric hospital and 4 affiliated sites. Patient demographics, clinical characteristics, discharge diagnoses, and GIP testing information were extracted from the electronic medical record (EMR). This study was approved by the university’s institutional review board.
Gastrointestinal Multiplex PCR Panel
Our hospital system uses the BioFire FilmArray Gastrointestinal Panel (bioMérieux, Marcy-l’Étoile, France), which detects 22 enteric bacterial, viral, and parasitic pathogens (Supplemental Table 4). GIP tests are run by our in-house microbiology laboratory, with an average of 130 GIP tests performed per month on inpatient samples during the study period.
Given suspected high rates of asymptomatic colonization, our study excluded the detection of Clostridioides difficile (C. difficile) in patients aged <1 year19–21 and detection of enteroaggregative, enteropathogenic, and enterotoxigenic Escherichia coli in all ages.22,23 These bacteria are not routinely reported by our laboratory and are not visible to providers in the EMR.
Computerized Physician Order Entry Decision Support With Order Indications and Stool Frequency
Between 2015 and 2018, medical providers were required to answer 2 questions in the EMR when ordering a GIP: (1) order indication (select 1 of a prepopulated list) and (2) number of stools in the last 24 hours. The order indication options were “concern for C. difficile,” “diarrhea with blood or pus,” “hemolytic uremic syndrome,” “international travel-associated diarrhea,” “prolonged diarrhea (>7 days),” “severe water-loss diarrhea and dehydration,” “surveillance testing,” “outbreak/epidemiologic testing,” “retesting for return to day care or infection control reason,” and “other.” The “other” indication asked providers to elaborate in an optional free text box. “Surveillance testing”, “outbreak/epidemiologic testing”, and “retesting for return to day care or infection control reason” were grouped into “epidemiologic/surveillance testing” given similarities in testing intent. Providers entered a numerical value for the number of daily stools on the basis of parental report or number of stools charted in the EMR. There were no guidelines or restrictions on GIP usage during the study period outside of completing the computerized physician order entry questions.
Outcomes and Statistical Analysis
Patients were analyzed in 2 cohorts on the basis of underlying medical history: Complex chronic condition (CCC) (presence of at least 1 CCC as defined by Feudtner et al’s pediatric CCC classification system version 2)24 versus non-CCC (“previously healthy”).
GIP results were categorized as “actionable” (defined as any bacteria or parasite detected), “nonactionable” (virus only detected), or “no organism detected.” The primary outcome was proportion of actionable results, and the prevalence of each result type was calculated for each order indication, by CCC status. For each result type (at the encounter level), the median and interquartile range was calculated for the number of stools reported in the previous 24 hours, by CCC status.
Bivariable analyses were used to compare clinical and demographic characteristics between CCC and non-CCC patients. Wilcoxon rank sum tests were used to compare median values of continuous variables, and a Pearson’s χ2 (or Fisher’s exact) test was used to compare proportions for categorical variables.
Separate multivariable analyses were performed for CCC and non-CCC cohorts using Poisson regression with robust error variance to calculate adjusted relative risks (RR) comparing covariates to an actionable result indicator (yes or no). A full data model (with CCC and non-CCC patients) was considered, but CCC status had significant interaction with multiple covariates, and therefore analyses were stratified by CCC status to make results more manageable/interpretable. Several covariates were determined a priori: Age, seasonality, ICU admission any time during hospitalization, and number of daily stools. Age was analyzed in 4 age buckets: <1 year, 1 to 2 years, 2 to 5 years, and ≥5 years. Individual order indications were included in the model if they were associated with actionable results in bivariate analyses with a P value <.2. Poisson regression with robust error variance was chosen to allow us to calculate RR, rather than odds ratios, with improved accuracy of the SE with increasing outcome incidence. To address the possibility of some C. difficile detections representing asymptomatic colonization in age groups >1 year, we conducted sensitivity analyses in which multivariable regression analyses were performed by sequentially excluding C. difficile as an actionable result in patients <2 years old, <3 years old, and in cases when a virus was codetected with C. difficile. We also performed a regression analysis to evaluate risk factors for non-C. difficile bacteria and parasites. The number of daily stools was missing for a small subset (n = 14 of 1124) of GIPs, and these tests were excluded from regression analysis; there were no other missing data.
Data were analyzed using SAS version 9.4 software (Cary, NC). All statistical tests were performed with a level of significance of α = .05.
RESULTS
There were 1124 GIP tests performed for 967 hospital encounters in our study (Table 1). Almost half of encounters were from patients who had at least 1 underlying medical condition (CCC), and 55% of GIP tests performed were from the CCC cohort. More than 1 GIP was performed in a higher proportion of CCC encounters (13.7% vs 5.6%).
TABLE 1.
Characteristics of Study Population
| All Encounters, N = 967 | Non-CCC, N = 478 | CCC, N = 489 | P | |
|---|---|---|---|---|
| Age (y) | 3.7 (1.2–10.7) | 3.0 (1.0–11.2) | 4.5 (1.4–10.4) | .077 |
| Female sex | 446 (46.1%) | 221 (46.2%) | 225 (46.0%) | .95 |
| ICU stay | 238 (24.6%) | 60 (12.6%) | 178 (36.4%) | <.001 |
| Length of stay (h) | 90.8 (47.4–196.6) | 65.0 (41.6–109.1) | 140.5 (69.3–382.6) | <.001 |
| >1 GIP performed | 94 (9.7%) | 27 (5.6%) | 67 (13.7%) | <.001 |
| Time to first GIP (h) | 17.5 (5.5–48.6) | 13.4 (4.7–27.2) | 24.2 (7.0–92.4) | <.001 |
| <72 h to first GIP | 777 (80.4%) | 430 (90.0%) | 347 (71.0%) | <.001 |
Values are displayed as N (%) or median (interquartile range), where N represents the number of hospital encounters. P values from Pearson’s χ2 test (or Fisher’s exact) or the Wilcoxon rank sum test.
GIP Results
One-fourth of tests had an actionable result (versus 19.4% when excluding C. difficile as an actionable result for <3 years old and 19.0% when excluding C. difficile in cases with viral coinfection), and 8.5% had >1 type of pathogen detected. Overall, viruses were the most common pathogen type detected (positive on 38.6% of tests), followed by bacteria (24.2%) and parasites (2.0%) (Supplemental Table 4). C. difficile was the most common bacteria detected (14.5%) and was more prevalent in the CCC cohort (16.3% CCC versus 12.3% non-CCC) and in younger age groups (26.2% 1–2 years, 17.4% 2–5 years, 15.9% >5 years). The prevalence of non-C. difficile bacteria was lower in CCC patients than non-CCC (3.6% CCC versus 17.0% non-CCC). Almost half of C. difficile-positive tests showed a coinfection with another pathogen type, with viruses as the predominant codetected pathogen type (Fig 1).
FIGURE 1.
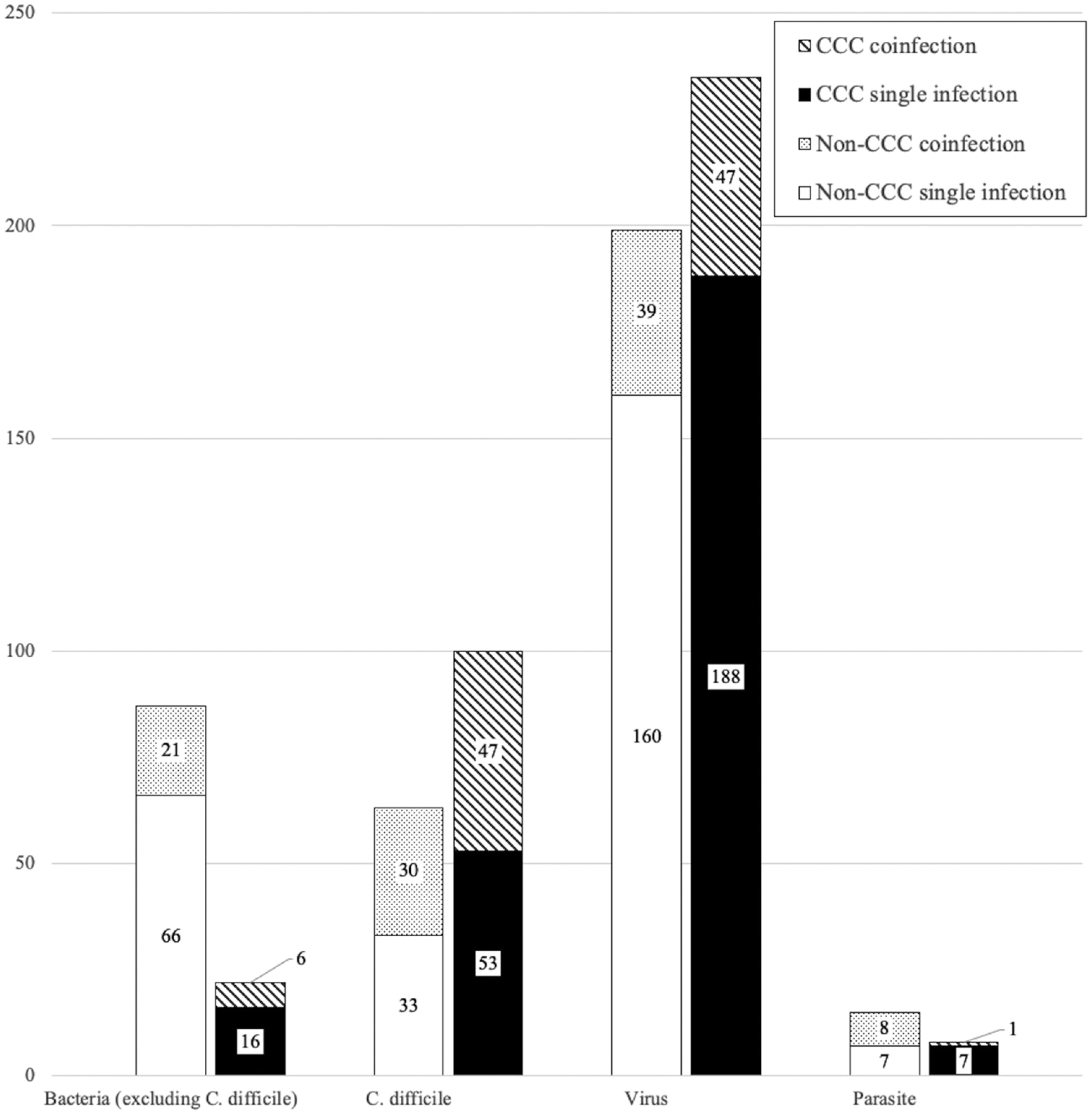
Pathogen types detected on GIP, by CCC status. Number of GIP tests with each pathogen type (C. difficile, non-C. difficile bacteria, virus, and parasite) detected. Figure delineates single infection (only 1 pathogen type detected) versus coinfection (>1 pathogen type detected). Regarding C. difficile coinfections: For CCC cohort, coinfections consisted of 91% virus, 4% bacteria, 2% parasite, and 2% >1 organism type; for non-CCC cohort, coinfections consisted of 73% virus, 13% bacteria, 13% >1 organism type, and 0% parasite. Values are displayed as N where N represents the number of GIP tests.
GIP Order Indications and Proportion of Actionable Results
The most common GIP order indication (besides “other”) for both cohorts was “severe water-loss diarrhea and dehydration” (Table 2). “Diarrhea with blood or pus” and “international travel-associated diarrhea” were more commonly selected for non-CCC patients, whereas “concern for C. difficile” and “epidemiologic/surveillance testing” were more common indications for CCC patients.
TABLE 2.
Prevalence of Provider-Selected GIP Order Indications
| All Encounters, N = 1124 | Non-CCC, N = 512 | CCC, N = 612 | P | |
|---|---|---|---|---|
| Diarrhea with blood or pus | 185 (16.5%) | 106 (20.7%) | 79 (12.9%) | <.001 |
| Concern for C. difficile | 169 (15.0%) | 50 (9.8%) | 119 (19.4%) | <.001 |
| Prolonged diarrhea (>7 d) | 137 (12.2%) | 79 (15.4%) | 58 (9.5%) | .002 |
| Severe water-loss diarrhea/dehydration | 229 (20.4%) | 109 (21.3%) | 120 (19.6%) | .49 |
| Hemolytic uremic syndrome | 8 (0.7%) | 6 (1.2%) | 2 (0.3%) | .15 |
| International travel-associated diarrhea | 19 (1.7%) | 18 (3.5%) | 1 (0.2%) | <.001 |
| Epidemiologic/surveillance testing | 67 (6.0%) | 19 (3.7%) | 48 (7.8%) | .004 |
| Other | 310 (27.6%) | 125 (24.4%) | 185 (30.2%) | .030 |
Values are displayed as N (%) where N represents the number of GIP tests. P values from Pearson’s χ2 test (or Fisher’s exact).
Overall, GIPs from non-CCC patients had a higher percentage of actionable results (29.7% non-CCC versus 20.6% CCC) but a similar percentage of nonactionable results (Fig 2). In both cohorts, “diarrhea with blood or pus” had the highest yield for actionable results. “Hemolytic uremic syndrome” and “international travel-associated diarrhea” had high percentages of actionable results but very small sample sizes. Conversely, “severe water-loss diarrhea and dehydration” and “epidemiologic/surveillance testing” had higher proportion of viruses.
FIGURE 2.
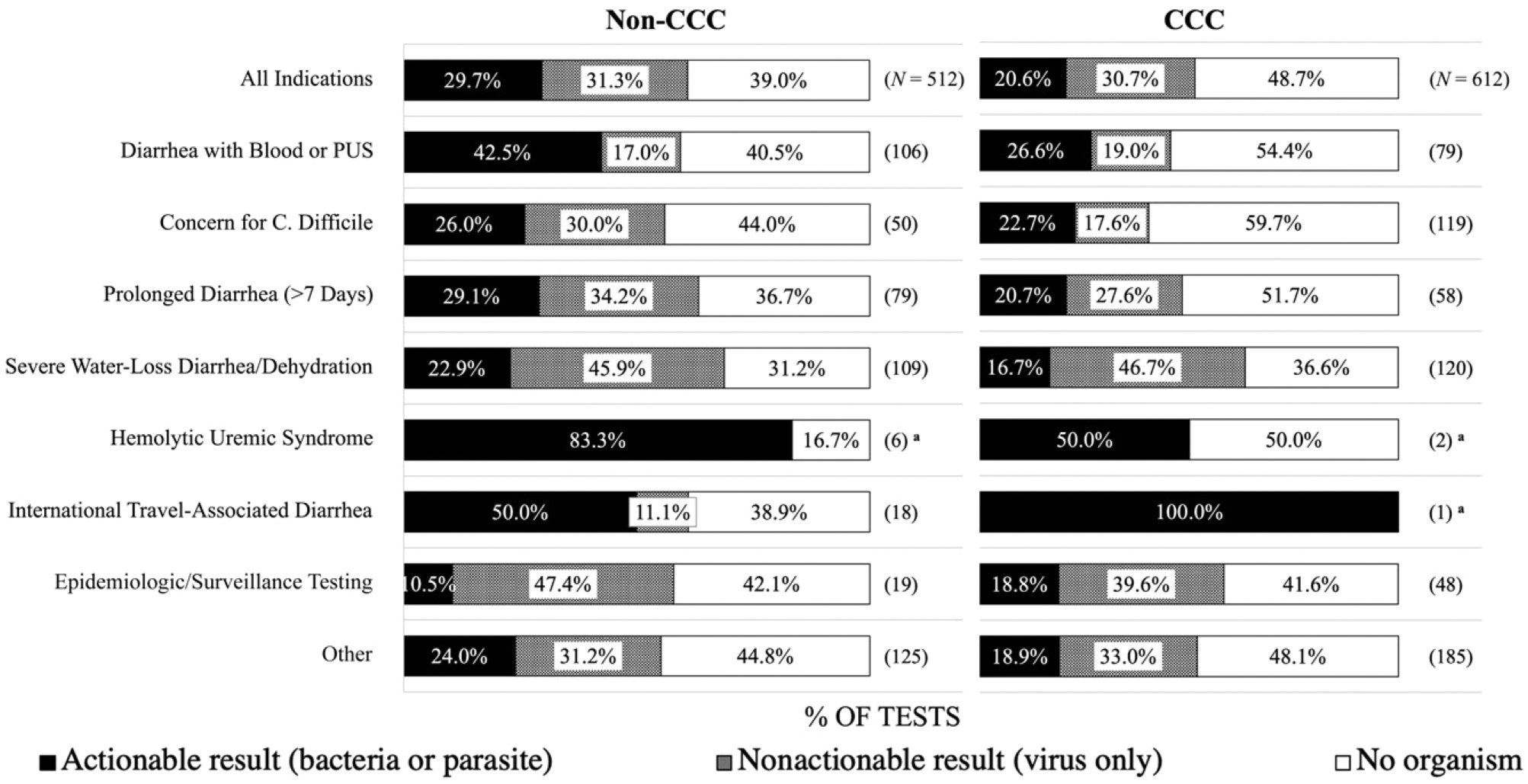
Prevalence of GIP result type by order indication. Proportion (%) of GIP result types for each order indication, by CCC status (left: Non-CCC, right: CCC). Result types include actionable (bacteria or parasite) result, nonactionable (virus only) result, and no organism detected. Bar graphs add up to 100%. Values in parentheses are displayed as N where N represents the number of GIP tests. a N < 10.
Multivariable Logistic Regression Analysis of Actionable Results
For non-CCC patients, we found positive associations between actionable results and all age buckets ≥1 year old versus <1 (RR range 3.33–4.49, P < .001 for all), order indication “diarrhea with blood or pus” (RR 1.52, P = .015), and summer/spring versus winter (RR range 1.56–1.92, P value range .002–.039) (Table 3). In sensitivity analyses, regression results did not materially change when sequentially excluding C. difficile as an actionable result in patients aged <2 years, <3 years, and in viral coinfection cases (Supplemental Table 5). When evaluating detection of non-C. difficile bacteria and parasites, all age buckets ≥1 years remained positively associated with actionable results for the non-CCC cohort, though with mild decreases in RR. Conversely, RR for “diarrhea with blood or pus” and seasonality were larger when excluding C. difficile. Additionally, the order indication “international travel-associated diarrhea” (RR 3.06, P < .001), as well as ≥4 stools per day (RR 1.61, P = .03) were positively associated with non-C. difficile bacteria and parasites (Supplemental Table 5).
TABLE 3.
Multivariable Logistic Regression Analysis: Factors Associated With Actionable Results, by CCC Status
| Non-CCC | CCC | |||
|---|---|---|---|---|
| Variablea | RR (95% CI) | P | RR | P |
| Age (y) | ||||
| <1 | 1 | — | 1 | — |
| 1–2 | 4.49 (2.52–7.99) | <.001 | 7.56 (2.75–20.81) | <.001 |
| 2–5 | 3.33 (1.85–5.98) | <.001 | 7.04 (2.56–19.37) | <.001 |
| ≥5 | 3.67 (2.11–6.38) | <.001 | 5.56 (2.05–15.08) | <.001 |
| Season | ||||
| Winter | 1 | — | 1 | — |
| Spring | 1.56 (1.02–2.39) | .039 | 0.85 (0.56–1.29) | .45 |
| Summer | 1.92 (1.27–2.92) | .002 | 0.95 (0.61–1.47) | .8 |
| Fall | 1.51 (0.97–2.33) | .066 | 1.14 (0.76–1.7) | .54 |
| ICU admission | 0.96 (0.65–1.41) | .82 | 0.67 (0.48–0.93) | .016 |
| ≥4 stools per d | 1.22 (0.91–1.65) | .19 | 0.73 (0.54–0.99) | .042 |
| Order indicationb | ||||
| All other indications | 1 | — | 1 | — |
| Diarrhea with blood or pus | 1.52 (1.09–2.13) | .015 | 1.33 (0.88–2.01) | .17 |
| Severe water-loss diarrhea | 0.83 (0.55–1.24) | .36 | — | — |
| International travel | 1.59 (0.94–2.67) | .081 | — | — |
| Epi/surveillance testing | 0.38 (0.1–1.4) | .14 | — | — |
| Other | 0.98 (0.66–1.47) | .94 | — | — |
CI, confidence interval; epi, epidemiologic. —, no value. Boldface indicates P < 0.05.
The variables age, seasonality, ICU admission, and number of daily stools were determined a priori to be included in the multivariable logistic regression models.
Individual GIP order indications were included if they were associated with actionable results in bivariate analyses with a P value < .2.
For CCC patients, all age buckets ≥1 year were positively associated with actionable results (RR range 5.56–7.56, P < .001 for all). ICU admission (RR 0.67, P = .016) was negatively associated with actionable results (Table 3). When excluding C. difficile as an actionable result for patients aged <2 years, <3 years, and in viral coinfection cases, the age bucket 1 to 2 years was no longer positively associated with actionable results, but age buckets 2 to 5 years and ≥5 years remained positively associated. When evaluating non-C. difficile bacteria and parasites, age was no longer associated with actionable results (Supplemental Table 6).
Daily Stool Burden and Result Type
The median number of stools reported in a 24-hour period was 4 to 5, regardless of CCC status or result type (Supplemental Table 7). There was no statistical difference in the median number or interquartile range of daily patient stools on the basis of GIP result.
Time to Testing and Repeat GIP Yield
The yield of actionable results was higher for first-time GIPs collected within 72 hours of admission compared with those collected >72 hours after admission (30.0% vs 15.8%, P < .001). We performed a subsample analysis to evaluate whether repeating a GIP within 1 week of the first test might increase the yield of pathogen detection. Of 41 hospital encounters with a negative GIP test followed by a repeat test performed within 7 days of the previous test, 80% of repeat tests (33 of 41) were again negative. In 5% (2 of 41) of encounters, C. difficile was newly detected on the repeat test. New viruses were detected in 14% (6 of 41). No other bacteria or parasites were newly detected on repeat testing.
DISCUSSION
To our knowledge, this is the first study to incorporate provider-selected GIP order indications and their association with actionable results to inform future diagnostic stewardship practices. For both non-CCC and CCC cohorts, age ≥1 year was positively associated with actionable results. For non-CCC patients, actionable results were also associated with “diarrhea with blood or pus” order indication, nonwinter season, and international travel when excluding C. difficile. For CCC patients, there was a lower percentage of actionable results overall, and actionable results were negatively associated with ICU admission; no order indications were predictive of actionable results. We found repeat GIPs and testing performed >72 hours after hospital admission to be low-yield practices.
The positive association between age ≥1 year and actionable results across both cohorts aligns with previous studies showing higher proportions of viral (compared with bacterial) pathogens in young infants with AGE.13,25 In our study, the yield of actionable results for all patients aged <1 year was only 6.7%, compared with 24.7% overall. Despite this low yield, 20% of GIP tests ordered were on patients aged <1 year, suggesting opportunities for targeted diagnostic stewardship in this age group.
We detected toxigenic C. difficile in 14.5% of all tests, with higher detection rates and C. difficile coinfections in children aged <5 years. These results align with findings by Stockmann et al, where 16% of all children and 19% of children aged 2 to 4 years presenting with diarrhea had C. difficile detected on GIP.26 Our institution excludes C. difficile detection in infants aged <12 months because rates of asymptomatic carriage are up to 40% to 70% in this age group.19–21 Given concerns for continued higher rates of asymptomatic carriage in children aged >1 year (up to 13% in children aged 1–2 years and 8% in children aged 2–5 years based on a meta-analysis by Tougas et al),20 we performed sensitivity analyses sequentially recategorizing C. difficile as “nonactionable” for younger age groups and cases with coinfections to account for the possibility of C. difficile colonization. Although this sensitivity analysis did not result in significant changes in multivariable regression analysis, it did decrease proportions of actionable results, most notably in patients 1–2 years of age, where actionable results decreased from 34% to 10% when excluding C. difficile. Our findings underscore the importance of differentiating between true C. difficile infection and colonization, and therefore, whether to initiate treatment. The distinction remains challenging, particularly for younger CCC patients (aged <3 years) who have a high C. difficile detection rate but also more risk factors for true infection (eg, more hospital encounters and antibiotic exposure, underlying gastrointestinal pathology, or immunocompromised state). Because C. difficile detection has become faster and more sensitive with PCR testing, the likelihood of overdiagnosis leading to overtreatment is a growing concern.27–29 Although national guidelines recommend against routine testing for C. difficile in children aged <2 years,21 guidance on how to interpret positive results and decide on treatment is lacking and remains an important focus of future research.20,29
For non-CCC patients, our findings support previously identified clinical and epidemiologic risk factors for bacterial or parasitic AGE, including bloody or mucoid stools, recent international travel, and nonwinter season, and emphasize the importance of considering these factors when deciding on testing for an otherwise healthy child.2,11,13,25,26,30,31
For the CCC cohort, yield of both actionable and positive results was lower than for the non-CCC cohort, similar to findings by Stockmann et al,26 and no order indications or clinical factors other than age were associated with actionable results. The lack of predictive factors for this cohort is not unexpected, because there is more underlying complexity and a broader differential diagnosis for acute diarrhea in this population. Although detection of an actionable pathogen is generally of higher clinical utility, a viral or negative GIP result may aid decision-making and management in certain populations, a sentiment in line with a recent Delphi study addressing testing priorities for pediatric AGE.32 For example, for patients with inflammatory bowel disease, a negative GIP can expedite initiation of immunomodulatory therapy. For immunocompromised patients, detection of a viral infection may warrant antiviral therapy or prevent further invasive diagnostic testing. A potential future area of research is to focus on specific populations within the CCC cohort to better characterize GIP utilization.
Across both cohorts, the most common reason for ordering testing was “severe water-loss diarrhea or dehydration”; however, this order indication had a low yield of actionable results and the highest yield of viral, nonactionable results. This aligns with previous studies demonstrating that AGE caused by rotavirus was associated with more severe dehydration and increased likelihood of hospitalizations compared with other etiologies.10,30 Additionally, ICU admission was negatively associated with actionable results for CCC patients. These findings suggest that certain markers of disease severity, such as ICU admission or severe dehydration, without other compelling risk factors are not good predictors of actionable results and should be lower-priority reasons for testing.
GIPs performed >72 hours after admission and repeat tests were also lower-yield practices, a similar trend reported by previous studies where only 6% to 8% of tests were positive in these scenarios.33,34 Out of the 41 hospital encounters in our study that had repeat GIP testing done after an initial negative test, only 2 new actionable results (both C. difficile) were detected. In these instances, obtaining a C. difficile toxin PCR test would be more cost-effective and targeted. Overall, our findings support restricting repeat testing and testing >72 hours after admission.
Strengths of our study include a large sample size consisting of patients with and without underlying medical conditions. The inclusion of testing order indications adds insight into provider reasons for testing, in addition to consideration of other patient and clinical factors. One study limitation is that 25% to 30% of GIPs had an order indication of “other” without further explanation, limiting our interpretation for these tests. Additionally, this study was conducted within a single hospital system, with a high percentage of CCC patients at all sites, possibly impacting the generalizability of our results.
CONCLUSIONS
For previously healthy children hospitalized with acute diarrhea, providers should consider limiting GIP testing unless there are factors such as bloody stools or international travel, especially in infants aged <1 year. For medically complex patients, C. difficile is the most common actionable pathogen detected, so providers should evaluate for risk factors for C. difficile and consider C. difficile-specific testing rather than a GIP, unless other results (viral, no organism) will change patient management and outcomes.
Supplementary Material
CONFLICT OF INTEREST DISCLOSURES:
Dr Dominguez receives grant support and serves as a consultant for bioMérieux (formerly BioFire Diagnostics). BioMérieux was not involved in this study and was not a study sponsor. The other authors have indicated they have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Troeger C, Blacker BF, Khalil IA, et al. GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and etiologies of diarrhea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1–16 [PubMed] [Google Scholar]
- 3.Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA. 2015; 313(22):2282–2284 [DOI] [PubMed] [Google Scholar]
- 4.Buss SN, Leber A, Chapin K, et al. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol. 2015;53(3):915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malachira A, Beard K, Brendish N, Clark T. 1717. The impact of routine molecular point-of-care testing for gastrointestinal pathogens in adults hospitalized with suspected gastroenteritis: results of a pragmatic randomized controlled trial (GastroPOC). Open Forum Infect Dis. 2018;5(Suppl 1):S51. [DOI] [PubMed] [Google Scholar]
- 6.Cotter JM, Thomas J, Birkholz M, Ambroggio L, Holstein J, Dominguez SR. Clinical impact of a diagnostic gastrointestinal panel in children. Pediatrics. 2021;147(5):e2020036954. [DOI] [PubMed] [Google Scholar]
- 7.Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013–1023 [DOI] [PubMed] [Google Scholar]
- 8.Klatte JM, Selvarangan R, Jackson MA, Myers AL. Reducing over-utilization of testing for Clostridium difficile infection in a pediatric hospital system: a quality improvement initiative. Hosp Pediatr. 2016;6(1):9–14 [DOI] [PubMed] [Google Scholar]
- 9.Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017; 55(3):715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132–152 [DOI] [PubMed] [Google Scholar]
- 11.Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017; 65(12):e45–e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiegering V, Kaiser J, Tappe D, Weissbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15(6):e401–e407 [DOI] [PubMed] [Google Scholar]
- 13.Klein EJ, Boster DR, Stapp JR, et al. Diarrhea etiology in a children’s hospital emergency department: a prospective cohort study. Clin Infect Dis. 2006;43(7):807–813 [DOI] [PubMed] [Google Scholar]
- 14.National Collaborating Centre for Women’s and Children’s Health. Diarrhea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment, and Management in Children Younger Than 5 Years. London, UK: RCOG Press; 2009 [PubMed] [Google Scholar]
- 15.Tarr GAM, Chui L, Lee BE, et al. Performance of stool-testing recommendations for acute gastroenteritis when used to identify children with 9 potential bacterial enteropathogens. Clin Infect Dis. 2019;69(7):1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto JM, Petrova A. Detection of acute gastroenteritis etiology in hospitalized young children: associated factors and outcomes. Hosp Pediatr. 2017;7(9):536–541 [DOI] [PubMed] [Google Scholar]
- 17.Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124(6):e1081–e1087 [DOI] [PubMed] [Google Scholar]
- 18.Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics. 2014;134(1):15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010;51(1):2–7 [DOI] [PubMed] [Google Scholar]
- 20.Tougas SR, Lodha N, Vandermeer B, et al. Prevalence of detection of Clostridioides difficile among asymptomatic children: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(10):e212328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–e48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nycz BT, Pretty K, Gomez-Trujillo A, Sanchez B, Dominguez SR. Description of enteropathic Escherichia coli species in pediatric patients at a quaternary children’s hospital. J Pediatric Infect Dis Soc. 2020;9(5):573–579 [DOI] [PubMed] [Google Scholar]
- 23.Enserink R, Scholts R, Bruijning-Verhagen P, et al. High detection rates of enteropathogens in asymptomatic children attending day care. PLoS One. 2014;9(2):e89496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friesema IHM, de Boer RF, Duizer E, et al. Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. Eur J Clin Microbiol Infect Dis. 2012;31(4):405–415 [DOI] [PubMed] [Google Scholar]
- 26.Stockmann C, Pavia AT, Graham B, et al. Detection of 23 gastrointestinal pathogens among children who present with diarrhea. J Pediatric Infect Dis Soc. 2017;6(3):231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwenk HT, Pollock NR, Vaughan-Malloy AM. Pediatric clostridioides difficile infection: diagnosis and diagnostic stewardship. J Pediatric Infect Dis Soc. 2021;10(Supplement 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 28.Kociolek LK, Patel SJ, Zheng X, Todd KM, Shulman ST, Gerding DN. Clinical and microbiologic assessment of cases of pediatric community-associated Clostridium difficile infection reveals opportunities for improved testing decisions. Pediatr Infect Dis J. 2016;35(2):157–161 [DOI] [PubMed] [Google Scholar]
- 29.Cotter JM, Thomas J, Birkholz M, et al. Impact of multiplex testing on the identification of pediatric Clostridiodes difficile. J Pediatr. 2020;218:157–165.e3 [DOI] [PubMed] [Google Scholar]
- 30.Glass RI, Parashar U, Patel M, Tate J, Jiang B, Gentsch J. The control of rotavirus gastroenteritis in the United States. Trans Am Clin Climatol Assoc. 2012;123:36–52, discussion 53 [PMC free article] [PubMed] [Google Scholar]
- 31.Denno DM, Shaikh N, Stapp JR, et al. Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis. 2012;55(7):897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarr GAM, Persson DJ, Tarr PI, Freedman SB. Enteric pathogen testing importance for children with acute gastroenteritis: a modified Delphi study. Microbiol Spectr. 2022;10(5):e0186422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Hitchcock MM, Gomez CA, Banaei N. Is follow-up testing with the FilmArray gastrointestinal multiplex PCR panel necessary? J Clin Microbiol. 2017;55(4):1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hitchcock MM, Gomez CA, Banaei N. Low yield of FilmArray GI panel in hospitalized patients with diarrhea: an opportunity for diagnostic stewardship intervention. J Clin Microbiol. 2018; 56(3):e01558–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


