Abstract
Provided herein are novel cyanopyridine compounds as KHK inhibitors, pharmaceutical compositions, use of such compounds in treating NAFLD, NASH, and type II diabetes, and processes for preparing such compounds.
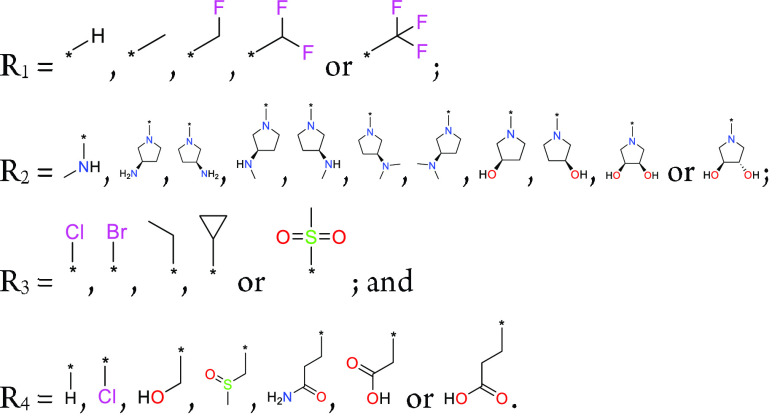
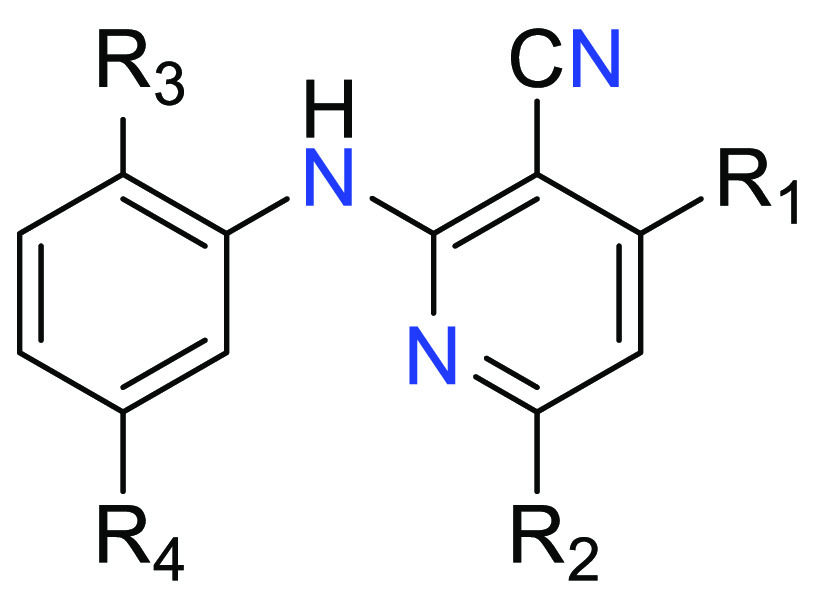
Important Compound Classes
Title
New Cyanopyridine KHK Inhibitor Compounds
Patent Publication Number
WO 2024/121183 A1
Publication Date
June 13, 2024
Priority Application
EP 22212311.9
Priority Date
December 8, 2022
Inventors
Kley, J.; Gottschling, D.; Heine, N.; Nosse, B.; Pautsch, A.; Weber, A.
Assignee Company
Boehringer Ingelheim International GmbH, Germany
Disease Area
NAFLD, NASH, and type II diabetes
Biological Target
KHK
Summary
Ketohexokinase (KHK) catalyzes the phosphorylation of fructose to fructose-1-phosphate (F-1-P). Enzymatic activity of human KHK-C in the liver in combination with elevated fructose consumption leads to increased synthesis of fatty acids and triglycerides. Fructose metabolism by KHK is thought to contribute to a number of diseases, e.g., nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and type 2 diabetes (T2DM).
The present application describes a series of novel cyanopyridine compounds as KHK inhibitors for the treatment of NAFLD, NASH and type II diabetes. Further, the application discloses compounds, their preparation, use, and pharmaceutical composition, and treatment.
Key Structures
Definitions
Biological Assay
The human KHK-C inhibition assay was performed. The compounds described in this application were tested for their ability to inhibit KHK. The KHK IC50 values (nM) are shown in the following table.
Biological Data
The table below shows representative
compounds that were tested for KHK inhibition and the biological data
obtained from testing representative examples.
Claims
Total claims: 16
Compound claims: 9
Pharmaceutical composition claims: 3
Salt claims: 1
Method of preparation claims: 2
Medicament claims: 1
Recent Review Articles
The author declares no competing financial interest.
References
- Esler W. P.; Cohen D. E. Pharmacologic inhibition of lipogenesis for the treatment of NAFLD. J. Hepatol. 2024, 80, 362–377. 10.1016/j.jhep.2023.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Yu W.; Li Y.; Wang A.; Cao H.; Fu Y. Drug development advances in human genetics-based targets. MedComm 2024, 5, e481. 10.1002/mco2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y.; Zhang Q.; Wang H.; Yang X.; Mu H. Alternative splicing and related RNA binding proteins in human health and disease. Sig. Transduct. Target. Ther. 2024, 9, 26. 10.1038/s41392-024-01734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.; Patial V. Insights into the molecular mechanisms of malnutrition-associated steatohepatitis: A review. Liver Int. 2024, 15932. 10.1111/liv.15932. [DOI] [PubMed] [Google Scholar]
- Inci M. K.; Park S.-H.; Helsley R. N.; Attia S. L.; Softic S. Fructose impairs fat oxidation: Implications for the mechanism of western diet-induced NAFLD. J. Nutr. Biochem. 2023, 114, 109224. 10.1016/j.jnutbio.2022.109224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.; Chen S.; Han L.; Liu J. Pharmacotherapies of NAFLD: updated opportunities based on metabolic intervention. Nutr. Metab. 2023, 20, 30. 10.1186/s12986-023-00748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]