Abstract
Porcine circovirus 3 (PCV3) was first reported in the United States in 2016; this virus is considered to be involved in diverse pathologies, such as multisystem inflammation, porcine dermatitis and nephropathy syndrome, and reproductive disorders. However, successful isolation of PCV3 using cultured cells has been rare. In this study, we aimed to isolate PCV3 using primary porcine bone marrow-derived cells. Mononuclear cells were isolated from the femur bones of clinically healthy pigs. These primary cells were cultured for 6–10 days post-seeding and infected with PCV3-containing tissue homogenates. The cells were cultured for up to 37 days, and the culture medium was changed every 3–4 days. The growth curve of PCV3 in porcine bone marrow cells revealed a decline in growth during the first 10 days post-infection, followed by an increase leading to > 1010 genomic copies/mL of the cell culture supernatant; moreover, the virus was capable of passaging. The indirect fluorescent antibody assay for PCV3 infection revealed the presence of PCV3 capsid protein in the cytoplasm and nuclei of infected cells. Bone marrow cells were passaged for more than 20 generations (over 5 months), and PCV3 persistently infected the cells. PCV3-infected bone marrow cells expressed mesenchymal markers. These results reflect that primary porcine bone marrow-derived mesenchymal cells are permissive to PCV3 and continuously replicate a high copy number of the PCV3 genome. These findings regarding the high replication rate of PCV3 in bone marrow-derived mesenchymal cells could enhance our understanding of PCV3 pathogenicity.
Keywords: Bone marrow cells, Porcine circovirus 3 (PCV3), Virus isolation
Introduction
Circoviruses, members of the genus Circovirus belonging to the family Circoviridae, are characterized by single-stranded circular DNA genomes [1]. Four species of circovirus infect pigs: porcine circovirus 1 (PCV1), 2 (PCV2), 3 (PCV3), and 4 (PCV4) [2]. While PCV1 is nonpathogenic [3], PCV2 causes pathological conditions referred to as porcine circovirus-associated diseases (PCVAD), which includes various syndromes, such as PCV-2-systemic disease, PCV-2-reproductive disease, PCV-2-subclinical infection, and porcine dermatitis and nephropathy syndrome (PDNS), causing severe economic losses to the global swine industry [4]. PCV3 has been detected in pigs with diverse pathologies, which suggests its involvement in PDNS, systemic inflammation, reproductive disorders, and neurological symptoms in newborn pigs. However, it was also detected in several pigs without syndromes; hence, the pathogenicity of PCV3 remains unclear [5]. Successful isolation of PCV3 using cultured cells has rarely been reported previously. The porcine kidney cell line (PK-15) has been widely used to isolate and produce PCV1 and PCV2; however, previous attempts to isolate PCV3 using PK-15 cells and primary swine testicular cells (ST) failed [6, 7]. In one study, PCV3 was isolated using primary porcine kidney cells. This isolation involved infecting these cells with the supernatant from freeze-thawed PCV3-infected cells, allowing the virus to be passaged up to the 8th generation [8]. Furthermore, the full-length PCV3 genome has been introduced into cells to create infectious clones [9].
The mesenchymal stromal cells (MSCs) were first identified as plastic adherent fibroblast-like cells in the bone marrow. They exhibit multilineage differentiation, immunoregulatory properties, and tissue repair properties [10]. Given their wide range of biological properties, MSCs are a potential tool in the fields of inflammatory diseases, autoimmune diseases, and regenerative medicine. For instance, they are useful for clinical applications in hepatic and cardiovascular diseases as well as in bone regeneration. They have also been shown to suppress immunological and inflammatory responses and facilitate the regeneration of damaged tissues [11].
Given the limited knowledge about isolating PCV3 in cell cultures, we attempted to isolate PCV3 using primary porcine bone marrow-derived cells and conducted comprehensive analyses to explore the growth of this virus in these cells. Here, we, for the first time, demonstrate that porcine bone marrow-derived mesenchymal cells are permissive to PCV3 and can highly replicate the PCV3 genome.
Materials and methods
Isolation and culture of primary bone marrow cells
Primary cells were isolated from euthanized pigs aged 7 days to 16 weeks in laboratory animal experiments. For each of the five PCV3 passages, primary cells were isolated from pigs: Nippon Institute for Biological Sciences (NIBS) miniature pigs [12] for Generation 1 and F1 crossbred (Landrace × Yorkshire) pigs for Generations 2, 3, 4, and 5. These pigs were clinically healthy and had previously exhibited negative PCR results for PCV1, PCV2, PCV3, and PCV4. Femur bones were obtained from pigs, and primary bone marrow cells were isolated following a previously described method with some modifications [13]. To isolate cells, bone marrow was harvested by flushing the femur bones with RPMI 1640 medium (Gibco, NY, USA). The bone marrow cells were filtered through a 70 μm nylon mesh filter (BD Falcon, NJ, USA), and red blood cells were removed using hemolytic buffer (155 mM NH4Cl, 10 mM NaHCO3, 0.1 mM EDTA) to acquire mononuclear cells. Subsequently, cells were washed with RPMI 1640 medium through centrifugation at 400×g for 5 min at 4 °C and plated 1.2 × 106 cells/well onto cell culture 6-well plates (Iwaki, Japan) containing Dulbecco's modified Eagle's medium (DMEM; Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, NY, USA), 2 mM L-glutamine (Gibco, NY, USA), 50 mM 2-mercaptoethanol (Sigma-Aldrich, MO, USA), and 1% antibiotic solution (Gibco, NY, USA). Cell cultures were incubated at 37 °C or 39 °C in a humidified atmosphere containing 95% air and 5% CO2. The culture medium was changed, and non-adherent cells were removed after 3–4 days of culture. Primary cultures became nearly confluent 6–10 days after seeding. At that point, the cells were inoculated with PCV3 or detached with 0.0125% trypsin (Gibco, NY, USA) and stored at − 80 °C in a cell preservation solution containing DMSO (Nacalai Tesque, Japan).
Isolation of PCV3 using bone marrow cells
PCV3, passaged for two generations in miniature pigs, was isolated from the tissue homogenates of tracheobronchial lymph nodes derived from those pigs [14]. The prepared lymph node homogenates were analyzed using PCR, multiplex PCR and multiplex RT-PCR and confirmed to be positive for PCV3 and negative for PCV1, PCV2, PCV4, suid herpesvirus 1, porcine parvovirus, porcine reproductive and respiratory syndrome virus, Japanese encephalitis virus, porcine rotavirus type A, porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and Getah virus [2, 15, 16]. The amount of PCV3 genome in the DNA extracted from the homogenate and supernatants was quantified via real-time PCR using GoTaq® qPCR Master Mix (Promega, WI, USA) following the instructions provided by the manufacturer. The forward and reverse primers were designed as previously described [7]. The resulting lymph node homogenates contained 1.55 × 108 genomic copies per mL.
The 50 μL of 10% homogenate was added to the cell culture medium when the bone marrow cells reached confluence in 6-well plates (Iwaki, Japan); next, the cells were cultured for 9 days, and the culture medium was changed every 3–4 days. No CPE was observed during the culture, and the culture was stopped to stock the culture supernatant. The culture supernatant was collected while changing the medium and stored at − 80 °C for further real-time PCR analysis. The culture supernatant collected on the last day was used to infect newly seeded bone marrow cells and passaged five times. Generations 1, 2, 3, 4, and 5 were cultured for 9, 22, 31, 30, and 37 days, respectively.
Detection of PCV3 by indirect fluorescent antibody assay (IFA)
Rabbit antiserum against PCV3 capsid protein (Cap) was used to detect PCV3 via IFA. The full-length PCV3 Cap (PCV3 rCap) was expressed in silkworm pupae and purified. The construction of a baculovirus transfer vector encoding the full length of Cap and the expression of PCV3 rCap by infection of silkworm pupae with the baculovirus was carried out using the ProCube service (Sysmex Corporation, Japan). The silkworm pupae infected with recombinant baculovirus were homogenized with PBS and sonicated by 30 s × 6 times at 4 °C. After centrifugation at 11,200×g for 15 min at 4 °C, the supernatant was layered on 40% sucrose and then centrifuged at 128,000×g for 16 h at 4 °C. Pellets were resuspended in PBS and sonicated by 15 s × 6 times at 4 °C. Samples were layered in a discontinuous Optiprep density gradient (Serumwerk Bernburg AG, Germany) and then centrifuged at 128,000×g for 20 h 4 °C. Fractions containing PCV3 rCap were collected and dialyzed with saline and used for antiserum preparation. Two 11-week-old Std:JW/CSK female rabbits (Japan SLC, Japan) were immunized with purified PCV3 rCap to generate polyclonal antibodies. Rabbits were immunized subcutaneously with 70 μg purified PCV3 rCap with complete Freund’s adjuvant (BD Falcon, NJ, USA) and boosted with 70 μg purified PCV3 rCap with incomplete Freund’s adjuvant (BD Falcon, NJ, USA) at 3 weeks interval. At 2 weeks post-boosted immunization, rabbits were 3rd-immunized intravenously with 10 μg purified PCV3 rCap. At a week post-3rd immunization, serum was collected from each immunized rabbit and used for IFA. The IFA was conducted as follows: PCV3 was cultured for 42 days in bone marrow cells. PCV3-infected cells were fixed and permeabilized using Cytofix/Cytoperm (BD Falcon, NJ, USA) following the instructions provided by the manufacturer. The samples were blocked with Blockace (Megmilk Snow Brand, Japan) for 1 h at 20–25 °C, followed by sequential incubation with anti-PCV3 antibody (primary antibody) and Alexa Fluor 488 goat anti-rabbit IgG (secondary antibody) (Invitrogen, CA, USA) for 2 h at 20–25 °C and 1.5 h at 20–25 °C, respectively. Primary and secondary antibodies were diluted at 1:500 and 1:200, respectively, in PBST (0.1% Tween 20). Images were captured using a fluorescence microscope.
Passage of PCV3-infected bone marrow cells
To validate the persistence of the infection, PCV3-infected bone marrow cells were repeatedly passaged. Fourth-passage PCV3-infected cells were used to infect newly seeded bone marrow cells. Nineteen days post PCV3 infection, the cells were detached using 0.125% trypsin (Gibco, NY, USA) and passaged (1:2 split). The cells were similarly passaged once a week and subcultured up to passage 20.
Phenotypic marker analysis of bone marrow cells
Phenotypic analysis of bone marrow cells was performed using flow cytometry. For this analysis, bone marrow cells were detached by treating them with 0.125% trypsin–EDTA. The formalin-fixed single-cell suspension was stained using the following primary antibodies for 1 h at room temperature: mouse anti-human/porcine/equine CD29 (Bio-techne, MN, USA), mouse anti-CD44 (Invitrogen, CA, USA), mouse anti-CD90 (Proteintech, IL, USA), and mouse anti-pig CD45 (Bio-Rad, CA, USA). After washing through centrifugation at 400×g for 5 min at 4 °C, the cells were incubated with an Alexa Fluor 488 goat anti-mouse IgG antibody (Invitrogen) in the dark at room temperature for 1 h. Relevant isotypes and secondary antibodies were used as controls for non-specific binding. The cells were acquired using a FACSCanto II flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and analyzed using FlowJo ver. 10 Software (Becton Dickinson).
Results
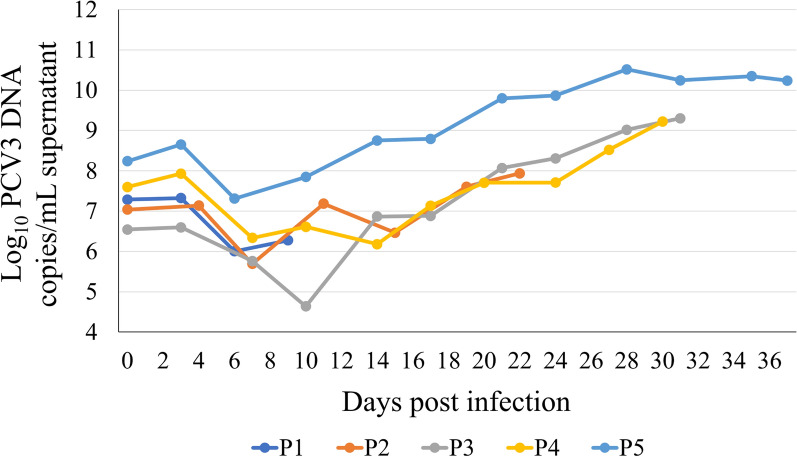
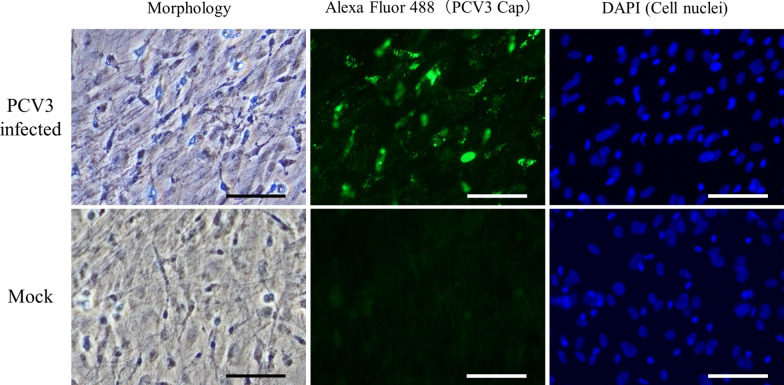
A fibroblast-like spindle-shaped morphology of cells isolated from porcine bone marrow was detected (Fig. 1A); these cells became nearly confluent 6–10 days after seeding (Fig. 1B). Bone marrow cells were infected with PCV3-containing tissue homogenates, and the subcultures were passaged five times. The growth curve of PCV3 in bone marrow cells is shown in Fig. 2. In all passages, the copy number of the PCV3 genome detected in the supernatant decreased for approximately 10 days post-PCV3 infection and subsequently increased. The highest copy number was 3.3 × 1010 copies/mL (28 days post-infection, fifth generation). No cytopathic effects were observed in infected bone marrow cells. Therefore, IFA with rabbit antiserum against PCV3 Cap was performed to confirm PCV3 infection in primary bone marrow cells, which revealed PCV3-positive signals in PCV3-infected cells (Fig. 3), which was predominant in the cytoplasm; moreover, some signals were found in the nuclei.
Fig. 1.
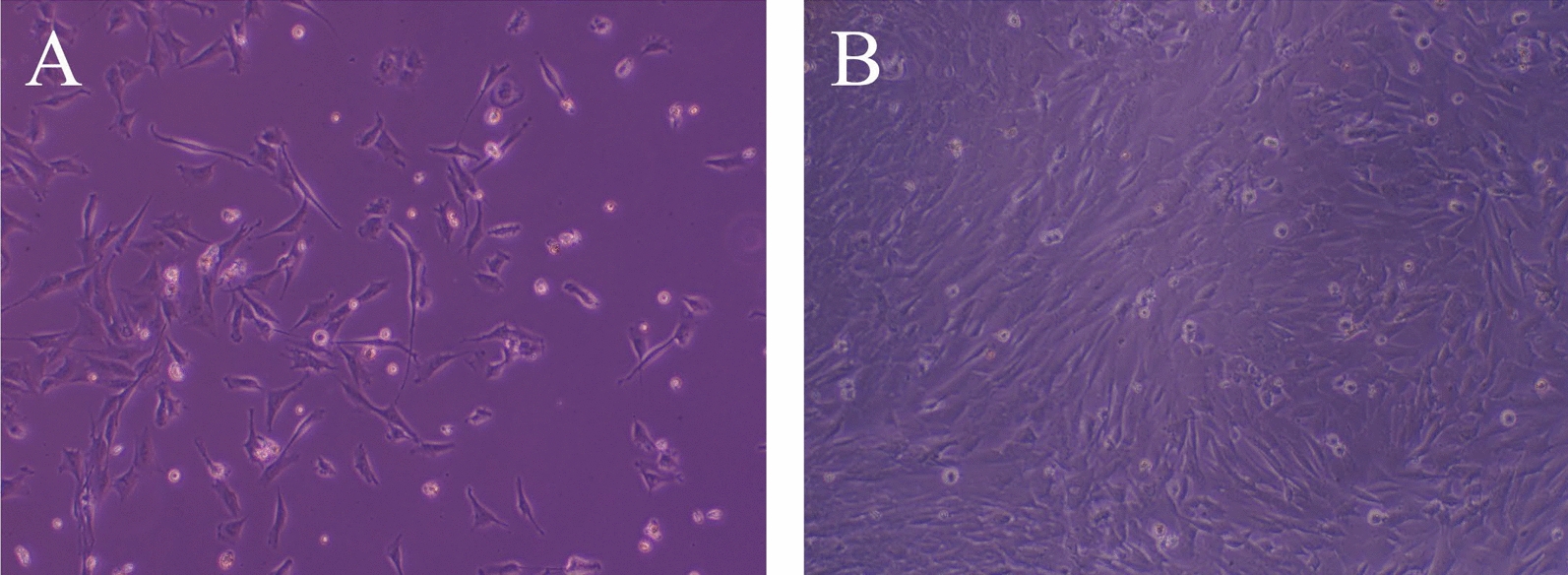
Morphology of the primary porcine bone marrow-derived cells. Bone marrow cells were isolated from the femur bones of pigs. A Morphology of cells recorded 5 days after isolation; the cells exhibited a fibroblast-like, spindle-shaped morphology. B Morphology of cells 7 days post-isolation, when the cells became nearly confluent
Fig. 2.
Viral load of PCV3 in culture supernatants quantified via real-time PCR. Serial passaging of PCV3 in primary porcine bone marrow cells; the generations 1–5 were cultured for 9, 22, 31, 30, and 37 days, respectively. Viral replication in infected culture supernatants was measured using real-time PCR. Real-time PCR data are expressed as log10 genomic copies per 1 mL supernatant
Fig. 3.
IFA of porcine bone marrow-derived cells. IFA of PCV3-infected porcine bone marrow cell using rabbit antiserum against the PCV3 Cap. Nuclei were stained with DAPI. In PCV3-infected cells, PCV3-positive signals were detected, which were predominant in the cytoplasm of the infected cells; moreover, some signals were identified in the nuclei. Mock cells exhibited no PCV3-positive signal. Scale bar = 80 µm
To confirm the persistence of infection, PCV3-infected bone marrow cells were passaged at 19 days post infection and were passaged for over 20 generations (over 5 months). The PCV3 genome copy number in the supernatant initially decreased for approximately 10 days post-PCV3 infection and then increased during subsequent cell passages. The PCV3 genome in the culture supernatant reached 5.10 × 1011/copies/mL at the 12th passage (95 days post-infection), indicating that PCV3 persistently infects bone marrow cells.
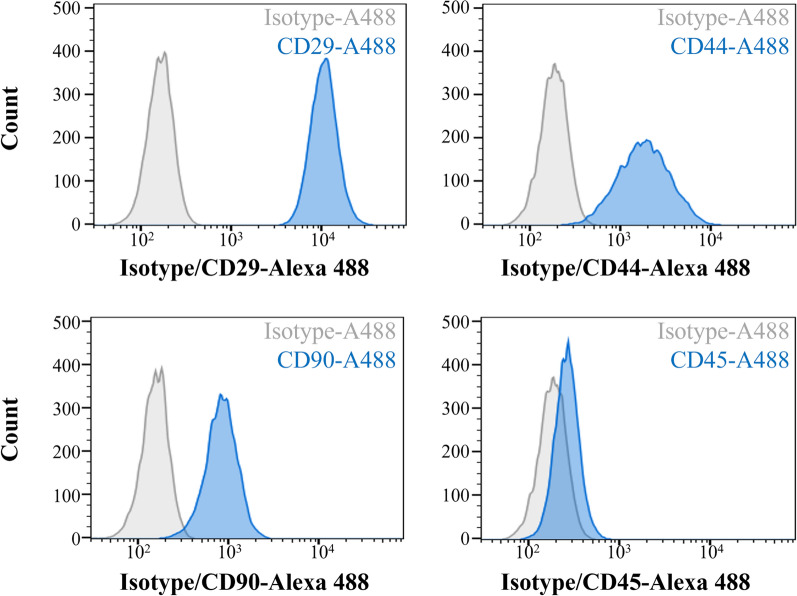
Phenotypically, PCV3-infected cells acquired from the 20th passage expressed the mesenchymal markers CD29, CD44, and CD90 and did not express the hematopoietic marker CD45, which confirms the mesenchymal lineage (Fig. 4). Analysis of non-infected bone marrow cells revealed the same mesenchymal characteristics; however, a lower percentage of CD90-positive cells was recorded compared to that of PCV3-infected cells.
Fig. 4.
Phenotype of PCV3 infected bone marrow cells in 20th passage. Flow cytometric analysis of the cell phenotypes. Bone marrow cells were positive for the mesenchymal markers CD29, CD44, and CD90 and negative for the hematopoietic marker CD45, indicating that these cells belong to the mesenchymal lineage. Gray line: isotype control; blue line: specific antibody
Discussion
In this study, we, for the first time, isolated PCV3 using primary porcine bone marrow-derived mesenchymal cells, which effectively supported PCV3 replication. In porcine bone marrow-derived mesenchymal cells, PCV3 exhibited a decline in the growth curve for 10 days post-infection, followed by increased growth. Similar growth curves detected in successive passages of infected cells indicate that PCV3 persistently infected the bone marrow cells for an extended period. Although PCV3 growth in porcine bone marrow cells is slow, these cells continuously produce high copy numbers of PCV3. PCV3 Cap was detected in the cytoplasm and nuclei of the infected cells through IFA.These outcomes reflect that primary porcine bone marrow-derived mesenchymal cells are useful tools for isolating and replicating PCV3.
Bone marrow cells include hematopoietic stem cells and MSCs [17]. MSCs become morphologically homogeneous after several passages and lose some of their potential for multilineage differentiation [18]. Using long-term in vitro cultivation of bone marrow-derived porcine MSCs for 20 passages, Zimmermann et al. demonstrated that the expression of surface markers varied with the passage number [11]. Similarly, in our cells, CD45-positive cells were detected using IFA at the 5th passage (data not shown), indicating the mixture of haematopoietic cells at low passages. We detected cell surface marker characteristics in PCV3-infected cells at passage 20 comparable to those of MSCs at passage 20. These data suggest that the cells we used have mesenchymal characteristics and that PCV3 is likely to replicate in bone marrow-derived mesenchymal cells.
MSCs were reported to possess immunoregulatory properties against innate and adaptive immune cells; viral infection of MSCs may result in immune dysregulation and altered differentiation properties [19, 20]. Khatri et al. [19] suggested that porcine bone marrow MSCs infected with influenza virus potentially cause hyperactivation and proliferation of inflammatory cells and suppress humoral immune responses. Additionally, viral infection of MSCs may affect regenerative medicine and viral pathogenesis. Previous reports have indicated that in immunocompromised individuals, such as transplant recipients, transplantation of virus-infected MSCs results in the transmittance of infection and leads to reactivation of the virus in the host [20].
Previously, the PCV3 genome was detected in the bone marrow of experimental miniature pigs inoculated with a PCV3-positive tissue homogenate [14]. Moreover, in colostrum-deprived pigs, PCV3 was reported to cause viremia and an IgM antibody response; no significant IgG seroconversion was detected [21]. Long-term surveillance of wild boars revealed PCV3 viremia in specimens collected from the same individual 5 months apart [22]. Like influenza virus infection, further studies are needed to determine whether PCV3 infection of bone marrow mesenchymal cells involves systemic immunoregulation and suppressing humoral immune responses in vivo.
In conclusion, for the first time, we demonstrated that PCV3 persistently infects and replicates in porcine bone marrow-derived mesenchymal cells. The high replication of PCV3 in bone marrow cells, which exceeded 1010 genomic copies/mL, can potentially facilitate our understanding of the pathogenicity of isolated PCV3. Since primary culture cells were used in this study, differences in cell quality and virus susceptibility may arise depending on the level of the operator's skill as well as the breed and age of the source pigs. Establishing a cell line from porcine bone marrow-derived mesenchymal cells could considerably advance future PCV3 research. Further studies on the effects of PCV3 infection on MSC functions should elucidate the in vivo infection dynamics of PCV3 and the role of bone marrow mesenchymal cells.
Acknowledgements
The authors thank Dr. Chihiro Sasakawa for his helpful advice. We thank Mr. Kazuki Oroku, Mr. Yoshiaki Furuya, and Mr. Yoshiyuki Ohshima for animal and laboratory research support.
Abbreviations
- Cap
Capsid protein
- IFA
Indirect fluorescent antibody assay
- MSCs
Mesenchymal stromal cells
- PCV1
Porcine circovirus 1
- PCV2
Porcine circovirus 2
- PCV3
Porcine circovirus 3
- PCV4
Porcine circovirus 4
- PCVAD
Porcine circovirus-associated diseases
- PDNS
Porcine dermatitis and nephropathy syndrome
Author contributions
SH contributed to the conception and study design, acquisition, analysis, interpretation of data, and manuscript drafting. TS contributed to the study design, administrated and supervised the collection and analysis of data, interpreted the data, and drafted the manuscript. FK and TM contributed to the acquisition and interpretation of data and revised the manuscript. NT and KS supervised the study and revised the manuscript.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The femur bones used for cell isolation were obtained from euthanized pigs; the NIBS Experimental Animal Care and Use Committee approved these experiments. The production of rabbit antiserum was approved by the NIBS Experimental Animal Care and Use Committee, and it was conducted following the NIBS Animal Experimentation Regulations (Approval Number: 19kenkyu-023B).
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ICTV, Taxonomy [Internet]. 2024 [cited 2024 Aug 1]. Available from: https://ictv.global/taxonomy
- 2.Zhang H, Hu W, Li J, Liu T, Zhou J, Opriessnig T, et al. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4. Hunan province, China. Transbound Emerg Dis. 2020;67:1057–61. 10.1111/tbed.13446 [DOI] [PubMed] [Google Scholar]
- 3.Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271–6. 10.1007/BF01314286 [DOI] [PubMed] [Google Scholar]
- 4.Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169:326–36. 10.1016/j.tvjl.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Klaumann F, Correa-Fiz F, Franzo G, Sibila M, Núñez JI, Segalés J. Current knowledge on porcine circovirus 3 (PCV-3): A novel virus with a yet unknown impact on the swine industry. Front Vet Sci. 2018;5:315. 10.3389/fvets.2018.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bera BC, Choudhary M, Anand T, Virmani N, Sundaram K, Choudhary B, et al. Detection and genetic characterization of porcine circovirus 3 (PCV3) in pigs in India. Transbound Emerg Dis. 2020;67:1062–7. 10.1111/tbed.13463 [DOI] [PubMed] [Google Scholar]
- 7.Palinski R, Piñeyro P, Shang P, Yuan F, Guo R, Fang Y, et al. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol. 2017;91:e01879-16. 10.1128/JVI.01879-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh T, Chae C. First isolation and genetic characterization of porcine circovirus type 3 using primary porcine kidney cells. Vet Microbiol. 2020;241:108576. 10.1016/j.vetmic.2020.108576 [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Wang D, Wang J, Zhu S, She R, Ren X, et al. Induction of porcine dermatitis and nephropathy syndrome in piglets by infection with porcine circovirus type 3. J Virol. 2019;93:e02045-18. 10.1128/JVI.02045-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. 10.1038/nri2395 [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann CE, Mackens-Kiani L, Acil Y, Terheyden H. Characterization of porcine mesenchymal stromal cells and their proliferative and osteogenic potential in long-term culture. J Stem Cells Regen Med. 2021;17(2):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimatsu Y, Yamada K, Horii W, Hirakata A, Sakamoto Y, Waki S, et al. Production of cloned NIBS (Nippon Institute for Biological Science) and α-1, 3-galactosyltransferase knockout MGH miniature pigs by somatic cell nuclear transfer using the NIBS breed as surrogates. Xenotransplantation. 2013;20:157–64. 10.1111/xen.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn P, Bork S, Wagner W. Standardized isolation of human mesenchymal stromal cells with red blood cell lysis. Methods Mol Biol. 2011;698:23–35. 10.1007/978-1-60761-999-4_3 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi S, Sato T, Ono H, Ito S, Takai R, Shibuya K, et al. Experimental inoculation of a tissue homogenate containing porcine circovirus type 3 obtained after two in vivo passages in NIBS miniature pigs. Vet Microbiol. 2023;281:109740. 10.1016/j.vetmic.2023.109740 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa H, Taira O, Hirai T, Takeuchi H, Nagao A, Ishikawa Y, et al. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J Virol Methods. 2009;160:210–4. 10.1016/j.jviromet.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 16.Quintana J, Segalés J, Rosell C, Calsamiglia M, Rodríguez-Arrioja GM, Chianini F, et al. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet Record. 2001;149:357–61. 10.1136/vr.149.12.357 [DOI] [PubMed] [Google Scholar]
- 17.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–5. 10.1073/pnas.141221698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–8. 10.1073/pnas.97.7.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri M, Saif YM. Influenza virus infects bone marrow mesenchymal stromal cells in vitro: implications for bone marrow transplantation. Cell Transpl. 2013;22(3):461–8. 10.3727/096368912X656063 [DOI] [PubMed] [Google Scholar]
- 20.Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal Stromal cells and viral infection. Stem Cells Int. 2015;2015:860950. 10.1155/2015/860950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora-Díaz J, Piñeyro P, Shen H, Schwartz K, Vannucci F, Li G, et al. Isolation of PCV3 from perinatal and reproductive cases of PCV3-associated disease and in vivo characterization of PCV3 replication in CD/Cd growing pigs. Viruses. 2020;12:219. 10.3390/v12020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaumann F, Dias-Alves A, Cabezón O, Mentaberre G, Castillo-Contreras R, López-Béjar M, et al. Porcine circovirus 3 is highly prevalent in serum and tissues and may persistently infect wild boar (Sus scrofa scrofa). Transbound Emerg Dis. 2019;66:91–101. 10.1111/tbed.12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.