ABSTRACT
Most meningiomas are slow–growing tumors that do not rapidly recur after subtotal removal. After subtotal resection of a meningioma a 47–year–old woman developed a large extracranial recurrence 1 year later. The recurrence was resected successfully. On histological examination the typical characteristics of a meningioma were absent. Based on immunohistological and ultrastructural studies, the tumor was classified as a grade III meningioma of the newly recognized rhabdoid subtype.
These tumors behave aggressively and should be treated accordingly.
Keywords: Meningioma, rhabdoid tumor, skull base, case report
Meningiomas have a wide range of histological appearances. Some subtypes recognized in the World Health Organization (WHO) classification1 display aggressive behavior, including atypical, clear cell, and chordoid meningiomas (WHO grade II) and papillary and anaplastic meningiomas (WHO grade III). Recently rhabdoid meningiomas have been added as another grade III subtype.
ILLUSTRATIVE CASE
A 47–year–old woman was referred to the senior author with a tumor in the infratemporal fossa. A year before her referral, she experienced altered sensation in the distribution of the first and second divisions of the trigeminal nerve, caused by a tumor of the middle cranial fossa that extended along the course of the trigeminal nerve. The tumor was resected subtotally, and the histology was reported as a schwannoma. Follow–up imaging 14 months later showed that the tumor had recurred and now extended into the infratemporal fossa, without causing additional symptoms (Fig. 1). Given the patient's relatively young age and the tumor's rapid progression, an infratemporal fossa approach (Fisch type C) was performed. Gross total resection was achieved. The tumor was dissected from the horizontal portion of the carotid artery, well into the cavernous sinus. An extension along the trigeminal nerve was removed from the posterior fossa. The patient recovered well from surgery. An abducens nerve palsy is resolving.
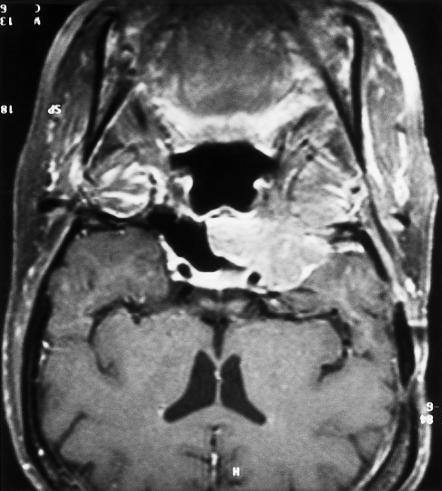
Figure 1.
Coronal MRI shows a tumor in the right infratemporal fossa, extending medially to the carotid artery and into the sphenoid sinus.
Histological Analysis
Histologically, the tumor infiltrated the resected bone, skeletal muscle, and the trigeminal nerve that it encased. Nests of tumor cells were separated by a small amount of stroma rich in capillary blood vessels and containing lymphocytes. The capillaries were often straight and parallel, creating a packeted appearance (Fig. 2). Focally, there was a perivascular pseudopapillary appearance due to discohesion of cells in the center of the nests. The cells had eccentric nuclei, which were only mildly atypical, and abundant glossy eosinophilic cytoplasm (Fig. 3). There were 5 to 10 mitoses per 10 high–power fields and a proliferation rate (Ki67) of about 5 %. There was no necrosis. Whorls and intranuclear cytoplasmic pseudoinclusions were not seen. On immunoperoxidase staining the cells were positive for cytokeratins AE1/AE3 and 19, epithelial membrane antigen, and vimentin, and focally positive for progesterone receptor. Stains for S100 protein, desmin, smooth muscle actin, glial fibrillary acidic protein, chromogranin, synaptophysin, thyroglobulin, calcitonin, estrogen receptors, and a range of anterior pituitary hormones were negative.
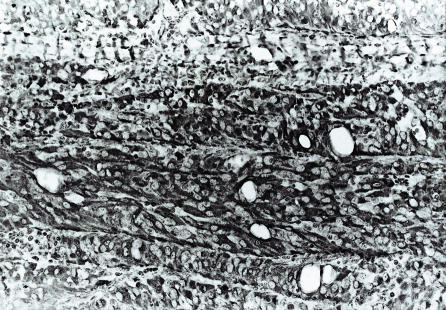
Figure 2.
Packets of tumor cells are separated by stroma rich in capillary blood vessels. Hematoxylin and eosin, original magnification × 20.
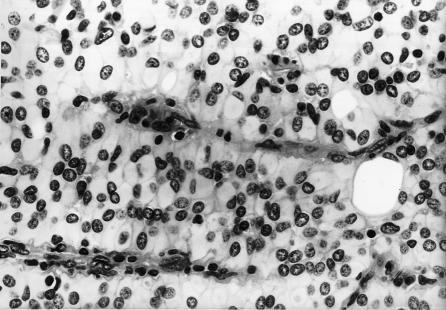
Figure 3.
Tumor cells have eccentric mildly atypical nuclei with glossy eosinophilic “rhabdoid” cytoplasm. Hematoxylin and eosin, original magnification × 40.
Electron microscopy showed basal lamina surrounding packets of cells. Adjacent cells showed focal complex interdigitation of cytoplasmic membranes, with some simple intercellular junctions and a few well–formed desmosomes. The cytoplasm was packed with randomly oriented intermediate filaments. Organelles were sparse, with no tonofilament bundles or Z bands. These features were incompatible with the original diagnosis of schwannoma.
Because of the unusual histology and epithelial phenotype, clinical investigations, including computed tomography of the abdomen, were performed to exclude a possible primary site elsewhere. None was found, and a rhabdoid meningioma (WHO grade III) was diagnosed. Review of sections obtained from the original operation showed a similar rhabdoid appearance focally. Other cellular areas with a syncytial sheeted appearance were consistent with atypical meningioma of the conventional type. Given the previous behavior of the tumor and the histological appearances, the patient underwent radiotherapy.
DISCUSSION
Rhabdoid morphology in tumors refers to resemblance of the cells to rhabdomyoblasts, without true skeletal muscle differentiation. The cytologic features include abundant eosinophilic cytoplasm, eccentric nuclei, and intracytoplasmic hyaline inclusions. Ultrastructurally, the latter represent whorls of intermediate filaments expressing vimentin and sometimes cytokeratin. The term malignant rhabdoid tumor (MRT) was first used to describe a distinctive pediatric renal tumor.2 The term was then extended to similar extrarenal pediatric tumors (extrarenal MRT), including atypical teratoid/rhabdoid tumor (AT/RT) of the central nervous system.3 These tumors commonly show mutation of the INI1 gene on 22q11.2. A rhabdoid phenotype has also been described in a variety of tumors with a different histogenesis, including carcinomas, sarcomas, gliomas, and melanomas (composite extrarenal rhabdoid tumors, or CERT).4 These tumors do not commonly show 22q11.2 deletions.5 Rhabdoid morphology is related to aggressive behavior and poor outcomes, irrespective of the tumor's histogenesis.
In 1998, Kepes et al6 and Perry et al7 described the first two series of meningiomas with rhabdoid transformation. These meningiomas often recur, and the rhabdoid features become more apparent in subsequent biopsies. Brain invasion, anaplasia, and extracranial metastasis have been reported. Besides the rhabdoid morphology, cytoarchitectural features of atypical meningioma (four or more mitoses per 10 high-power fields, high cellularity, sheeting architecture, nuclear atypia, and necrosis) are seen in most cases. Expression of cytokeratin is common. A histologic overlap with the papillary subtype of meningioma, which is also aggressive, has been noted 6, 7, 8 and was seen in the present case.
The wide histological differential diagnosis includes metastatic carcinoma, metastatic melanoma, glioma, and sarcoma, as well as AT/RT in children. Diagnosis depends on finding evidence of meningothelial differentiation either by light microscopy (whorls, intranuclear pseudoinclusions), immunohistochemistry (expression of vimentin, epithelial membrane antigen, and progesterone–receptor positivity), or electron microscopy (interdigitating cytoplasmic membranes, intercellular junctions). The case described here was initially thought to be a trigeminal nerve schwannoma, and its clinical and radiological appearance supported this diagnosis. However, the histology of the recurrent lesion was that of a rhabdoid meningioma, and review of the pathology from the first operation showed areas of conventional, though atypical, meningioma.
CONCLUSION
It is important to recognize rhabdoid morphology in meningiomas because this subtype is associated with recurrences, sometimes frank malignancy, and poor outcomes. Close follow up and aggressive treatment of these tumors are warranted.
REFERENCES
- Kleihues P, Cavenee WK. World Health Organization Classification. Pathology and Genetics of Tumors of the Nervous System. Lyon, France: IARC Press. 2000:176–184. [Google Scholar]
- Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney: a distinctive renal tumor of children. Hum Pathol. 1981;12:646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- Wick MR, Ritter JH, Dehner LP. Malignant rhabdoid tumors: a clinicopathological review and conceptual discussion. Semin Diagn Pathol. 1995;12:233–248. [PubMed] [Google Scholar]
- Fuller CE, Pfeifer J, Humphrey P, Bruch LA, Dehner LP, Perry A. Chromosome 22q dosage in composite extrarenal rhabdoid tumors: clonal evolution or a phenotypic mimic? Hum Pathol. 2001;32:1102–1108. doi: 10.1053/hupa.2001.28252. [DOI] [PubMed] [Google Scholar]
- Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas: a histological indication of increased proliferative activity. Report of four cases. Am J Surg Pathol. 1998;22:231–238. doi: 10.1097/00000478-199802000-00012. [DOI] [PubMed] [Google Scholar]
- Perry A, Scheithauer BW, Stafford SL, Abell–Aleff PC, Meyer FB. “Rhabdoid” meningioma. An aggressive variant. Am J Surg Pathol. 1998;22:1482–1490. doi: 10.1097/00000478-199812000-00005. [DOI] [PubMed] [Google Scholar]
- Hojo H, Abe M. Rhabdoid papillary meningioma. Am J Surg Pathol. 2001;25:964–969. doi: 10.1097/00000478-200107000-00018. [DOI] [PubMed] [Google Scholar]