ABSTRACT
Sarcomas of the skull base occurring in the field of prior radiotherapy are rare; only a few have been reported in the English literature. We report a fatal osteosarcoma of the skull base arising in a patient with McCune–Albright syndrome and severe fibrous dysplasia of the skull base 22 years after radiation therapy for a pituitary adenoma. This malignancy fulfills Cahan's criteria for a radiation–induced tumor in that it arose in the radiation field of a pituitary adenoma with a latency of 22 years. The literature on radiation–induced sarcomas of the skull base is reviewed, and the predisposition of patients with isolated fibrous dysplasia or McCune–Albright syndrome to develop radiation–induced tumors is discussed. Although the risk of radiation–induced malignancy is low in the general population, special consideration should be given when contemplating the use of radiotherapy for benign disease in patients with McCune–Albright syndrome or isolated fibrous dysplasia.
Keywords: Radiation therapy, sarcoma, fibrous dysplasia, McCune–Albright syndrome
Neoplasms of the skull base that develop after prior radiotherapy are rare events. We report an osteosarcoma that arose in a patient with McCune–Albright syndrome and fibrous dysplasia of the skull base 22 years after he received radiation therapy for a pituitary adenoma. McCune–Albright syndrome is characterized by polyostic fibrous dysplasia, café–au–lait spots, and multiple endocrinological abnormalities. This case fulfills Cahan's criteria for a radiation–induced sarcoma1 and represents the first report of an osteosarcoma of the skull base following radiation therapy for a pituitary adenoma in a patient with McCune–Albright syndrome.
CASE REPORT
A 29–year–old male with McCune–Albright syndrome and severe polyostic fibrous dysplasia that involved most of the skeleton, including the long bones of all extremities, pelvis, thorax, spine, facial bones, and skull base, experienced left facial numbness and rapid deterioration of vision in his left eye for 3 weeks. The patient's history was significant for an eosinophilic macroadenoma of the pituitary gland resected when he was 7 years old and treated with postoperative radiation therapy. This therapy consisted of 5000 rads administered in 25 daily treatments of 200 rads for 31 days, delivered with a Cobalt–60 source 4 MeV linear accelerator with right and left lateral fields measuring 5 × 5 cm.
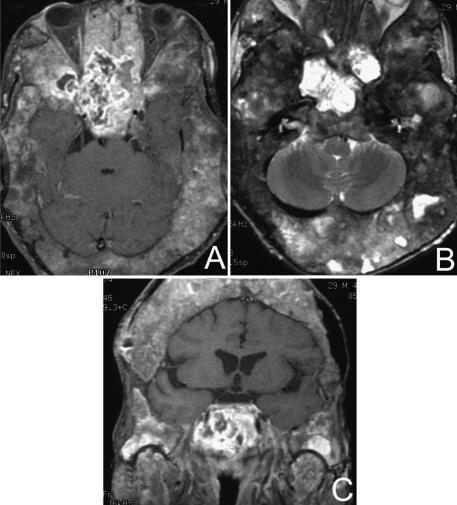
His left visual field was significantly reduced and his right visual field was moderately impaired. Magnetic resonance imaging (MRI) and computer tomography (CT) showed extensive fibrous dysplasia of the skull and skull base with narrowing of the optic foramina bilaterally and of the left internal auditory canal. There was an extensive enhancing 9 × 5 cm irregular mass involving the anterior frontal floor, planum sphenoidale, and sella with extension into the clivus (Fig. 1). The cavernous sinus and carotid arteries were involved bilaterally.
Figure 1.
MRIs of a radiation–induced osteosarcoma in a patient with severe fibrous dysplasia of the skull and skull base. (A) Gadolinium–enhanced, T1–weighted axial image with fat suppression shows a large tumor in the region of the sphenoid and sella. (B) T2–weighted fast spin–echo, axial and (C) gadolinium–enhanced, T1–weighted coronal image with fat suppression of the same lesion.
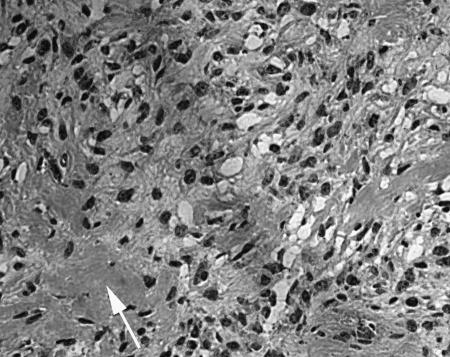
The patient underwent a bifrontal craniotomy with left superolateral orbitotomy and decompression of the optic nerve and biopsy of the skull base mass. Pathologic examination of the biopsy specimen revealed a spindled–cell neoplasm with frequent atypical mitotic figures and an associated osteoid matrix (Fig. 2). Electron microscopy showed osteoid within the matrix of a mesenchymal tumor, confirming the diagnosis of osteosarcoma.
Figure 2.
Photomicrograph of a light microscopy image from a section of the tumor shows a spindle–cell neoplasm with multiple mitotic figures. Arrow indicates area of osteoid formation. Original magnification = 20×.
The tumor was deemed unresectable, and further radiation therapy was not advised. The patient was considered for systemic palliative chemotherapy. However, the disease progressed rapidly with symptoms including blindness, deafness, and lower cranial neuropathies. He died before treatment could be instituted.
DISCUSSION
Cahan proposed four criteria for a sarcoma to be considered radiation induced: (1) the second neoplasm must arise in the irradiated field and be proved histologically; (2) a latent period of at least several years must have elapsed between radiation exposure and development of the second neoplasm; (3) there must be histologic and radiographic evidence of the pre–existing condition and its nonmalignancy in addition to microscopic proof of a tumor; and (4) the second tumor must be histologically different from the first tumor.1
This case report documents the occurrence of a skull base osteosarcoma arising in the field of previous radiation therapy in a patient with McCune–Albright syndrome and severe fibrous dysplasia of the skull base. His initial tumor was an eosinophilic macroadenoma of the pituitary gland. The osteosarcoma developed within the radiation field after a latency of 22 years. Rare cases of radiation–induced malignant transformation of pituitary adenomas into pituitary fibrosarcomas have been reported.2, 3 In this case, both light microscopy and electron microscopy demonstrated osteoid formation within the tumor. These findings confirm that this tumor represented an osteosarcoma arising within fibrous dysplasia and was not a malignant transformation of the pituitary adenoma.
Although difficult to estimate, the incidence of radiation–induced tumors is extremely low.4 Sarcomatous tumors account for most radiation–induced neoplasms involving the head and neck, and most occur in the facial skeleton. Only a very few involve the skull base.5, 6, 7, 8 Other histological types of skull base neoplasms that have been reported to follow radiation therapy include meningiomas, schwannomas, astrocytomas, and squamous cell carcinomas.
Fibrous dysplasia is thought to predispose to the development of sarcomas, and most reported cases have followed previous radiation therapy.9, 10 Rare cases of spontaneous sarcomas developing in fibrous dysplasia without prior radiation therapy have also been described.11, 12, 13, 14 Therefore, it remains possible that this patient's sacrcoma represents a sporadiac neoplasm. Fibrous dysplasia was present throughout most of this patient's skeleton, yet the malignancy arose in the central skull base within the field of radiation therapy. This case fulfills Cahan's criteria for a radiation–induced tumor, and we consider it more likely to be a consequence of radiation therapy than a spontaneous event.
In addition to Cahan's criteria, documentation of new mutations in genes implicated in malignant transformation after radiation therapy, such as the p53 and retinoblastoma tumor suppressor genes, further supports induction of malignancy.15 This requires the availability of a prior tissue specimen or, less ideally, uninvolved tissue adjacent to the new tumor. Lack of adequate pathological specimens prevented such analysis in this case. Many spontaneous sarcomas demonstrate similar genetic alterations, emphasizing that such mutations provide support, not proof, that a given malignancy is a consequence of prior radiation therapy.16, 17, 18, 19 Thus, even in the contemporary era of molecular genetics, it remains difficult to assert absolute proof that a malignancy was induced by radiation therapy and Cahan's criteria remain vital.
McCune–Albright syndrome is caused by a somatic mutation in the alpha subunit of the G protein (Gs alpha) that stimulates adenylate cyclase and cyclic adenosine 3',5'–cyclic monophosphate (AMP) formation.20 This mutation occurs early in embryonic development and results in a mosaic expression pattern in a variety of tissues. The hyperactivity of intracellular signaling resulting from this mutation may predispose to the development of neoplasms, including those induced by radiation therapy.21 The risk of radiation–induced malignancy remains extremely low for the general population. Nonetheless, the use of radiotherapy to treat benign lesions in patients with factors known to predispose to malignant transformation, including fibrous dysplasia and McCune–Albright syndrome, should be considered carefully.
REFERENCES
- Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: report of eleven cases, 1948. Cancer. 1998;82:8–34. doi: 10.1002/(sici)1097-0142(19980101)82:1<8::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ahmad K, Fayos JV. Pituitary fibrosarcoma secondary to radiation therapy. Cancer. 1978;42:107–110. doi: 10.1002/1097-0142(197807)42:1<107::aid-cncr2820420118>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Shi T, Farrell MA, Kaufmann JC. Fibrosarcoma complicating irradiated pituitary adenoma. Surg Neurol. 1984;22:277–284. doi: 10.1016/0090-3019(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Poen J, Tran LM, Fu YS, Selch MT, Parker RG. Postirradiation sarcomas. A single–institution study and review of the literature. Cancer. 1994;73:2653–2662. doi: 10.1002/1097-0142(19940515)73:10<2653::aid-cncr2820731030>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72:887–893. doi: 10.1002/1097-0142(19930801)72:3<887::aid-cncr2820720338>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Patel SG, See AC, Williamson PA, Archer DJ, Evans PH. Radiation–induced sarcoma of the head and neck. Head Neck. 1999;21:346–354. doi: 10.1002/(sici)1097-0347(199907)21:4<346::aid-hed9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lee YY, Van Tassel P, Nauert C, Raymond AK, Edeiken J. Craniofacial osteosarcomas: plain film, CT, and MR findings in 46 cases. Am J Roentgenol. 1988;150:1397–1402. doi: 10.2214/ajr.150.6.1397. [DOI] [PubMed] [Google Scholar]
- Chang SM, Barker FG 2nd, Larson DA, Bollen AW, Prados MD. Sarcomas subsequent to cranial irradiation. Neurosurgery. 1995;36:685–690. doi: 10.1227/00006123-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Mock D, Rosen IB. Osteosarcoma in irradiated fibrous dysplasia. J Oral Pathol. 1986;15:1–4. doi: 10.1111/j.1600-0714.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73:1411–1424. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Taconis WK. Osteosarcoma in fibrous dysplasia. Skeletal Radiol. 1988;17:163–170. doi: 10.1007/BF00351001. [DOI] [PubMed] [Google Scholar]
- Present D, Bertoni F, Enneking WF. Osteosarcoma of the mandible arising in fibrous dysplasia. A case report. Clin Orthop. 1986:238–244. [PubMed] [Google Scholar]
- Kaushik S, Smoker WR, Frable WJ. Malignant transformation of fibrous dysplasia into chondroblastic osteosarcoma. Skeletal Radiol. 2002;31:103–106. doi: 10.1007/s002560100436. [DOI] [PubMed] [Google Scholar]
- Heller AJ, DiNardo LJ, Massey D. Fibrous dysplasia, chondrosarcoma, and McCune–Albright syndrome. Am J Otolaryngol. 2001;22:297–301. doi: 10.1053/ajot.2001.24829. [DOI] [PubMed] [Google Scholar]
- Brachman DG, Hallahan DE, Beckett MA, Yandell DW, Weichselbaum RR. p53 gene mutations and abnormal retinoblastoma protein in radiation–induced human sarcomas. Cancer Res. 1991;51(23 Pt 1):6393–6396. [PubMed] [Google Scholar]
- Hall MB, Sclar AG, Gardner DF. Albright's syndrome with reactivation of fibrous dysplasia secondary to pituitary adenoma and further complicated by osteogenic sarcoma. Report of a case. Oral Surg Oral Med Oral Pathol. 1984;57:616–619. doi: 10.1016/0030-4220(84)90282-2. [DOI] [PubMed] [Google Scholar]
- Radig K, Schneider–Stock R, Haeckel C, Neumann W, Roessner A. p53 gene mutations in osteosarcomas of low–grade malignancy. Hum Pathol. 1998;29:1310–1316. doi: 10.1016/s0046-8177(98)90263-5. [DOI] [PubMed] [Google Scholar]
- Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP. Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol. 1996;122:559–565. doi: 10.1007/BF01213553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feugeas O, Guriec N, Babin–Boilletot A, et al. Loss of heterozygosity of the RB gene is a poor prognostic factor in patients with osteosarcoma. J Clin Oncol. 1996;14:467–472. doi: 10.1200/JCO.1996.14.2.467. [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Levine MA. Clinical implications of genetic defects in G proteins: oncogenic mutations in G alpha s as the molecular basis for the McCune–Albright syndrome. Arch Med Res. 1999;30:522–531. doi: 10.1016/s0188-4409(99)00075-2. [DOI] [PubMed] [Google Scholar]