Abstract
Migraine is a disabling neurologic condition manifesting with attacks of headache, hypersensitivities to visual, auditory, olfactory and somatosensory stimuli, nausea and vomiting. Hypersensitivities to sensory stimuli are most prominent during full-blown migraine attacks, but they often persist with less magnitude between migraine attacks. Furthermore, exposure to sensory stimuli such as certain odors, visual stimuli and sounds commonly trigger migraine attacks. Functional magnetic resonance imaging (fMRI) identification of brain mechanisms that lead to migraine sensory hypersensitivities and the mechanisms that allow for triggering of migraine attacks by sensory stimuli would yield a better understanding of neural dysfunction in migraine, might provide new targets for migraine prevention, and could provide fMRI biomarkers that reflect early responses to migraine preventive therapy. fMRI studies have investigated migraine hypersensitivities by measuring brain responses to visual, olfactory and painful cutaneous stimulation. Other migraine studies have used functional connectivity analyses to investigate the functional organization of specific brain regions and networks that are responsible for sensory processing. Migraine fMRI studies have consistently demonstrated atypical brain responses to sensory stimuli, lack of the normal habituating response when the migraineur is between migraine attacks, andatypical functional connectivity of sensory processing regions. Suggesting a true relationship between migraine and fMRI findings, the extent of fMRI abnormalities often correlates with higher headache frequency and greater number of years with migraine. Herein we discuss published migraine fMRI studies that investigated hypersensitivities in migraine, identify common themes suggested by these studies, and discuss topics in need of further investigations.
Keywords: Migraine, functional magnetic resonance imaging (fMRI), magnetic resonance imaging, headache, functional connectivity
Introduction
Migraine is a disabling and common neurologic disorder with a one-year prevalence of 12% in the general population.1 During a migraine attack there is moderate to severe intensity headache with a combination of nausea, vomiting, and hypersensitivities to visual, auditory, olfactory, and somatosensory stimuli.2, 3 Approximately 1/3 of migraine patients have aura associated with at least some of their migraine attacks.4 Although many different neurologic symptoms might occur during a migraine aura, visual symptoms are the most common.5 Between individual migraine attacks, migraineurs often have less prominent but persistent migraine symptoms including hypersensitivity to visual, auditory, olfactory and somatosensory stimuli.3 In addition, visual, olfactory and auditory stimuli are common migraine attack triggers.6–8
Since migraine is primarily a disorder of brain function, brain functional magnetic resonance imaging (fMRI) studies are especially pertinent for understanding migraine mechanisms. Although the aura and headache associated with migraine were previously attributed primarily to abnormal vasoconstriction and vasodilation of intracranial arteries, it is now known that migraine symptoms are primarily due to brain dysfunction. Although minor and transient changes in the caliber of extracranial and intracranial arteries might occur during a migraine, recent evidence suggests that such changes are not a necessary component.9 fMRI is also useful for studying the sensory hypersensitivities of migraine. The sensory hypersensitivities that are present during and between migraine attacks and the triggering of migraine attacks by sensory stimuli are features that are fairly unique to migraine, not being shared to the same extent by other headache disorders orby other pain disorders. A description of the mechanisms underlying these uniquely migraine features will at least lead to a better understanding of migraine pathophysiology, and might additionally lead to mechanistic dissection of migraine from other headache and pain disorders. Furthermore, fMRI localization of atypical stimulus-induced activations and atypical functional connectivity in migraineurs might provide targets for migraine preventive therapies and normalization of these atypical findings might serve as early biomarkers for response to migraine preventive therapies. The last few years have seen a significant increase in the number of published migraine fMRI studies; studies that contribute to identifying the location and potential significance of migraine-associated brain dysfunction.
In this review of the literature we systematically identified brain fMRI studies that investigate migraine hypersensitivities. Many of thesestudies have examined stimulus-induced brain activations, mostly usingnoxious thermal stimulation of the skin, trigemino-nociceptive stimuli via intranasal ammonia gas, olfactory stimuli, or visual stimuli. Other migraine studies have utilized functional connectivity analyses to investigate functional organization of specific brain regions and functional networks implicated in migraine pathophysiology. The majority of migraine fMRI studies have investigated the migraineur between migraine attacks, in the so-called “interictal” phase, while only a few have been performed during the migraine attack (i.e. in the “ictal” phase). Migraine fMRI studies have enhanced our understanding of migraine hypersensitivities, including identification of brain regions and networks that contribute to atypical processing of sensory stimuli. This atypical processing of sensory stimuli is a key feature of the migraine condition that leads to increased sensitivity to painful touch, non-painful touch, visual stimuli, and olfactory stimuli, and allows for typically non-noxious environmental stimuli such as flashing lights and odors to trigger migraine attacks. In this review of the migraine fMRI literature we summarize published findings, describe how these fMRI studies have helped to clarify our understanding of the anatomy and biology of migraine, discuss their limitations, and propose avenues for future migraine fMRI research.
Search Strategy and Selection Criteria
For this review we searched PubMed for English language articles of human subjects that were published between 1966 and March 25, 2014. The following search terms were used: “migraine and MRI”, “migraine and fMRI”, “migraine andblood oxygen-level dependent”, “migraine and functional connectivity”. Also, the reference lists of included articles and the authors’ own files were searched for additional articles. Articles that utilized fMRI to investigate migraine hypersensitivities were considered for inclusion in this review. Publications were selected for inclusion based upon their relevance to the review topic, their originality, and the extent to which the study findings were deemed to contribute to the migraine neuroimaging field.
Migraine fMRI Studies Investigating Processing of Pain, Odors and Visual Stimuli
Painful Stimuli (Table 1, Figure 1, Figure 2)
Table 1:
fMRI Studies of Migraine UsingPain Stimuli
| Study | Cohorts | Whole Brain or ROI | Timing | Stimulus | Main Findings |
|---|---|---|---|---|---|
| Schwedt 2014 | -EM (n=24) -Control (n=27) |
Whole Brain | Interictal | Heat | EM: Stronger activations in lentiform nuclei, fusiform, subthalamic nucleus, hippocampus, middle cingulate, somatosensory cortex, dorsolateral prefrontal cortex. Weaker activation in precentral gyrus, superior temporal gyrus. |
| Stankewitz 2013 | -EM interictal (n=20) -EM ictal (n=10) -Control (n=20) |
Whole Brain | Interictal Ictal | Ammonia | Interictal EM: Sensitized to pain stimuli while controls habituated -- seen in ant insula, middle cingulate cortex, thalamus. |
| Maleki 2012 | -HF EM (n=10) -LF EM (n=10) |
ROI (hippocampus) | Interictal | Heat | LF EM: Stronger hippocampal deactivation. |
| Maleki 2012 | -Male EM (n=11) -Female EM (n=11) |
Whole Brain | Interictal | Heat | Male EM: Stronger activations in insula, S1 and putamen. Female EM: Stronger activations in caudate, superior temporal, superior frontal, precuneus, posterior cingulate, sensory nucleus and spinal trigeminal nucleus of brainstem. |
| Maleki 2012 | -HF EM (n=10) -LF EM (n=10) |
Results reported as ROIs | Interictal | Heat | HF EM: Stronger activation of S1 and temporal pole. Weaker activation of anterior insula and cingulate. |
| Russo 2012 | -EM (n=16) -Control (n=16) |
Whole Brain | Interictal | Heat | EM: Stronger activation in ACC. Weaker activation in S2 and pons. |
| Maleki 2011 | -HF EM (n=10) -LF EM (n=10) |
Whole Brain | Interictal | Heat | HF EM: Weaker activation in caudate, putamen, pallidum. |
| Moulton 2011 | -EM ictal (n=8) -EM interictal (n=8) -Control (n=8) |
Whole Brain (Interictal vs. Controls) ROI (Ictal vs. Interictal) |
Interictal Ictal | Heat | Interictal EM vs. Controls: Stronger activation in temporal pole and parahippocampal gyrus. Ictal EM vs. Interictal EM: Stronger activation in temporal pole and parahippocampal gyrus. |
| Stankewitz 2011 | -EM interictal (n=20) -EM preictal (n=10) -EM ictal (n=13) -Control (n=20) |
Whole Brain (ictal vs. interictal) ROI (spinal trig nuclei) (preictal vs. ictal vs. interictal vs. control) |
Interictal Ictal | Ammonia | Interictal EM vs. Controls and Ictal EM vs. Controls: Weaker activation of spinal trigeminal nuclei. Preictal EM vs. Interictal EM: Stronger activation in spinal trigeminal nuclei. The stronger the activity within the trigeminal nuclei, the closer the EM was to their next migraine attack. |
| Aderjan 2010 | -EM (n=15) -Control (n=15) |
Whole Brain | Interictal | Ammonia | EM: Decreased activation in prefrontal cortex, ACC, red nucleus, ventral medulla from day 1 to day 8. Controls had increased activation of these regions from day 1 to day 8. |
| Burstein 2010 | -Allodynic EM ictal (n=8) -Allodynic EM interictal (n=8) |
ROI (thalamus) | Interictal Ictal | Heat and Brush | Ictal EM: Stronger activation of numerous thalamic regions. |
| Moulton 2008 | -EM (with ictal allodynia) (n=12) -Controls (n=12) |
ROI (brainstem) | Interictal | Heat | EM: Weaker activation in dorsolateral pons, likely the nucleus cuneiformis. |
ACC = anterior cingulate cortex; EM = episodic migraine; HF = high frequency; LF = low frequency; ROI = region of interest; S1 = primary somatosensory cortex; S2 = secondary somatosensory cortex; trig = trigeminal.
High frequency episodic migraine is defined as 8-14 headache days/month. Low frequency episodic migraine is defined as 1-2 headache days/month.
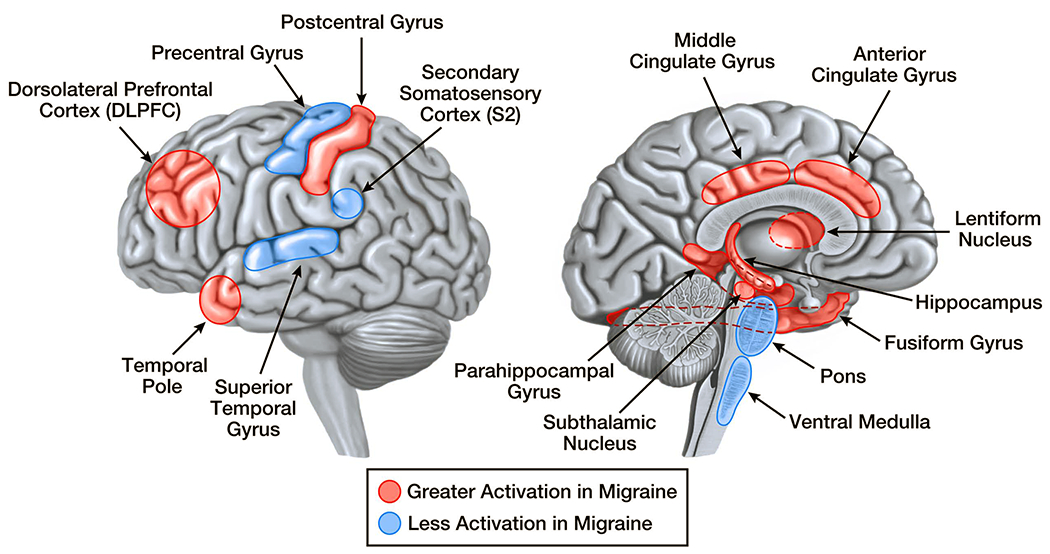
Figure 1: Pain-Induced Brain Activations that Differ in Migraineurs vs. Controls.
Brain regions that have pain-induced activations in interictal migraineurs that differ from activations in healthy controls are demonstrated on the lateral and medial brain surfaces. Areas shaded red are those that have greater activation in migraine compared to controls. Areas shaded blueare those that have less activation in migraine compared to controls. Although shaded areas are within regions of the brain that show differential activation between migraineurs and controls, their placement does not represent the exact location, size, or extent of differential activation.
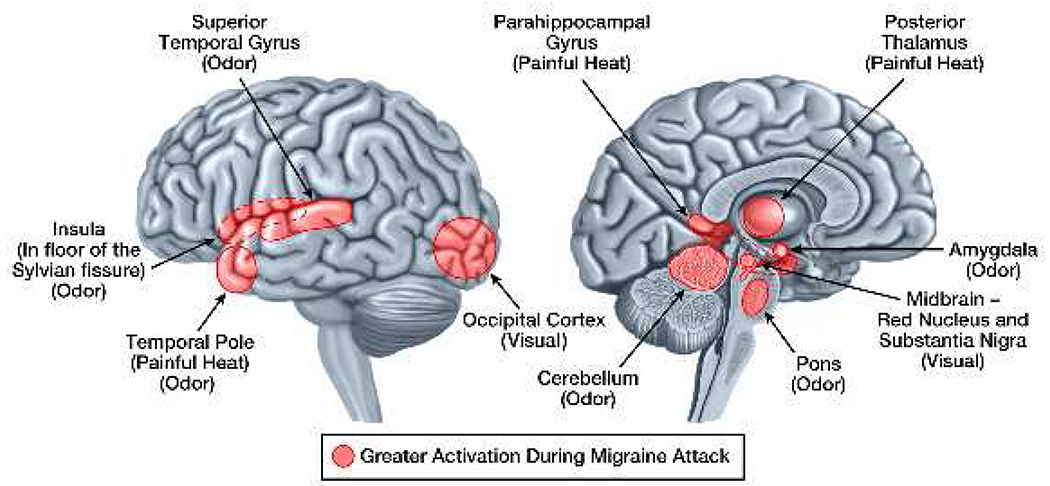
Figure 2: Stimulus-Induced Brain Activations that Differ in Ictal vs. Interictal Migraineurs.
Brain regions that have pain, visual, and odor-induced activations that differ in migraineurs during a migraine vs. between migraines are demonstrated on the lateral and medial brain surfaces. Areas shaded red are those that have greater activation during a migraine attack compared to between migraine attacks. There are no areas that have less activation during a migraine compared to between migraines.Although shaded areas are within regions of the brain that show differential activation between migraineurs during migraine attacks vs. migraineurs between migraine attacks, their placement does not represent the exact location, size, or extent of differential activation.
Suggesting atypical processing of somatosensory stimuli, physiologic studies show that the majority of migraineurs are hypersensitive to potentially noxious stimulation of the skin during a migraine attack and that a smaller proportion of migraineurs maintain this state of hypersensitivity between migraine attacks.10, 11 Implicating a role for central sensitization, hypersensitivity is found in extracephalic regions in addition to within the trigeminal nerve territory.12, 13 Somatosensory hypersensitivity results in the development of cutaneous allodynia in approximately 2/3 of migraineurs during a migraine attack.14–16 The migraineur with cutaneous allodynia finds normally non-noxious stimuli of the skin to be painful, and thus experiences pain or discomfort from stimuli such as light touch of the face or scalp, wearing the hair in a tight ponytail, wearing heavy earrings or eyeglasses, and having a shirt collar buttoned tightly. Atypical processing of pain has been investigated in several migraine fMRI studies.
Painful heat applied to the skin of the head, face or upper extremity has been commonly used in migraine fMRI studies. Typically, heat is applied via an MRI compatible contact thermode, with the destination temperature being individualized for each patient in order to elicit moderate or severe intensity pain. In 2010, Stankewitz and colleagues published a new method of eliciting trigeminal pain for fMRI studies utilizing intranasal ammonia gas.17 According to the authors, the ammonia gas “stimulates the nasal mucosa, leading to irritation of the first and second branches of the trigeminal nerve, resulting in short-lasting, stinging or stabbing pain sensations.” Similar to the activations seen in response to noxious heat, pain elicited by intranasal ammonia gas results in activations within several pain processing regions including insula, thalamus, middle cingulate cortex, amygdala, precentral gyrus, calcarine, cerebellum, middle temporal gyrus, rostral medulla, lower pons, caudate, supramarginal gyrus, anterior cingulate cortex, postcentral gyrus, and pallidum.17
Studies comparing thermal pain-induced activations and intranasal ammonia gas-induced activations in interictal migraine subjects to controls consistently find that migraineurs have regional brain activations that differ in their extent.18–24[Figure 1] Enhanced thermal pain-induced activations localize to regions within temporal pole, parahippocampal gyrus, anterior cingulate cortex, lentiform nuclei, fusiform gyrus, subthalamic nucleus, hippocampus, middle cingulate cortex, somatosensory cortex, and dorsolateral prefrontal cortex.19, 21, 24 Reduced thermal pain-induced activations in migraineurs localize to regions within secondary somatosensory cortex, precentral gyrus, superior temporal gyrus, and brainstem.20, 21, 24 Although few migraine patients have been studied during a migraine, such studies have found enhanced thermal pain-induced activation of temporal pole, parahippocampal gyrus and numerous thalamic regions compared to their own interictal activation patterns.19, 25 [Figure 2] Thus, migraineurs have atypical pain-induced activations of regions that participate in different aspects of the pain experience, including sensory-discriminative, affective, cognitive, and modulating processing of pain.
fMRI data suggest that an imbalance of pain facilitation and pain inhibition might contribute to migraine hypersensitivities. A study of interictal migraineurs who reported that they have symptoms of allodynia during migraine attacks found these migraineurs to have less thermal pain-induced activation than controls in the dorsolateral pons, a region containing the nucleus cuneiformis.20 Since the nucleus cuneiformis is predominantly a pain-inhibiting region of the descending pain system, hypoactivation of this region suggests less of a pain-inhibiting response in migraineurs, a muted response that could lead to development of allodynia during a migraine attack. Results from a study of trigemino-nociceptive stimulation with ammonia gas delivered daily over 8 consecutive daysfurther support the notion that migraineurs have inadequate pain inhibition.18 Recurrent stimulation over the 8-day period resulted in decreased activation of prefrontal cortex, rostral anterior cingulate cortex, red nucleus and ventral medulla in migraine subjects, while controls had enhanced activation of these regions over time.18 The decreased activations of these regions involved with endogenous pain control in migraineurs might represent inadequate pain inhibition and suggest that the recurrent pain of migraine leads to progressively less pain inhibition over time.
Perhaps further contributing to atypical pain processing in migraine, physiologic studies have demonstrated interictal migraineurs to have a lack of normal habituation (i.e. migraineurs lack the normal response decrement to repetitive stimuli) to various stimuli, likely related to dysfunctional inhibition or enhanced facilitation of sensory information.26 Deficient habituation has beendemonstrated in an fMRI study that found recurrent painful stimuli with intranasal ammonia gas to result in increased activations in anterior insula, middle cingulate and thalamus in interictal migraineurs, while control subjects habituated over time.23 These fMRI findings further support the notion that recurrent attacks of pain or prolonged pain associated with migraine could lead to increased pain sensitivity due to a lack of habituation and development of sensitization.
Migraine fMRI studies investigating thermal pain-induced brain activations and intranasal ammonia gas pain-induced brain activations suggest that migraineurs have atypical processing of painful stimuli. Although there is no evidence that migraineurs recruit brain regions for pain processing that are not also activated in non-migraineurs, the extent of activation of several pain processing regions differs. For most of these regions there is greater activation in migraineurs, however, with less activation in regions that predominantly inhibit pain transmission. Enhanced sensory-discriminative, cognitive and affective responses to pain in combination with less pain inhibition and lack of habituation, likely correlates with the hypersensitivity to pain that is present in migraineurs.
Olfactory Stimuli(Table 2, Figure 2)
Table 2:
fMRI Studies of Migraine using Visual or Olfactory Stimuli
| Study | Cohorts | Whole Brain or ROI | Timing | Stimulus | Main Findings |
|---|---|---|---|---|---|
| Griebe 2014 | EM with aura (n=18) Control (n=18) |
Whole Brain | Interictal | Visual - Pattern | EM with aura: Stronger activation in V5, V3, precuneus, middle frontal, superior occipital, intraparietal sulcus. |
| Datta 2013 | EM with aura (n=25) EM without aura (n=25) Control (n=25) |
Whole Brain ROI (1° visual cortex, lateral geniculate) |
Interictal | Visual - Pattern | EM with aura: Stronger activation of primary visual cortex and lateral geniculate compared to both other groups. |
| Hougaard 2013 | EM with aura (n=20) Control (n=20) |
Whole Brain (symptomatic vs. asymptomatic hemisphere) | Interictal | Visual - Pattern | Symptomatic Hemisphere vs. Asymptomatic: Stronger activation in inferior frontal, superior parietal/IPS, inferior parietal, inferior frontal. Symptomatic Hemisphere vs. Control: Similar results to symptomatic vs. asymptomatic comparison. Asymptomatic Hemisphere vs. Controls: No differences. |
| Antal 2011 | EM (n=24, 12 with aura) Control (n=12) |
ROI (motion-responsive middle temporal area) | Interictal | Visual – Pattern - Motion | EM: Stronger activation in left superior-anterior portion of the middle temporal complex. Weaker activation in inferior-posterior portion of middle temporal cortex. |
| Huang 2011 | EM (n=11, 7 with aura) Control (n=11) |
ROI (visual cortex) | Interictal | Visual - Pattern | EM: Stronger activation of visual cortex. |
| Martin 2011 | EM (n=19, 7 with aura) Control (n=19) |
ROI (occipital cortex) | Interictal | Visual - Light | EM: Higher number of activated voxels (i.e. a wider photoresponsive area) but no difference in activation intensity. |
| Stankewitz 2011 | EM interictal (n=20) EM ictal (n=13) Control (n=20) |
Whole Brain | Interictal Ictal |
Odor | EM interictal vs. Control: No differences. Ictal EM vs. Interictal EM: Stronger activation of amygdala, insula, temporal pole, superior temporal gyrus, rostral pons and cerebellum. |
| Vincent 2003 | EM with aura (n=5) Control (n=5) |
ROI (specifics not reported) | Interictal | Visual - Pattern | EM: Greater proportion activated the contralateral extrastriate visual cortex. |
| Cao 2002 | Migraineurs with visually triggered attacks (n=12) | ROI (brainstem, occipital cortex) | Interictal Ictal | Visual – Pattern | Ictal Migraineurs: Activations of red nucleus and substantia nigra preceding occipital cortex activation and preceding migraine symptoms. |
EM = episodic migraine; IPS = intraparietal sulcus; ROI = region of interest; V3 = visual area 3; V5 = visual area 5.
Migraineurs are hypersensitive to odors during and between migraine attacks. During the migraine attack, 25% to 43% of migraineurs report olfactory hypersensitivity, while about one-third of migraineurs have olfactory hypersensitivity between migraine attacks.31–34 Furthermore, one-half of migraineurs report that odors, such as cigarette smoke, perfumes, and certain food smells, can trigger their migraines.31, 34
The processing of olfactory stimuli (rose odor)by migraineurs was investigated in an fMRI study performed during and between migraine attacks.35 No differences in brain activations were found when comparing interictal migraineurs to healthy controls. However, during spontaneous and untreated migraine attacks, migraineurs had greater activation in odor processing regions as well as in a region of the dorsal rostral pons. Compared to their interictal state, migraineurs within a migraine attack had greater activation of amygdala, insula, temporal pole, superior temporal gyrus, rostral pons and cerebellum. Consistent with the frequent reports of osmophobia during the migraine attack, results show that ictal migraineurshave brain hyperresponsiveness to olfactory stimuli. Furthermore, since a region in the rostral pons might be one of the earliest brain regions to activate during the development of migraine (i.e. might be a “migraine generator”), odor-induced activation of the rostral pons in migraineurs might reflect a mechanism by which odors trigger migraine attacks.
Visual Stimuli(Table 2, Figure 3)
Figure 3: Visual Stimuli-Induced Brain Activations that Differ in Migraineurs vs. Controls.
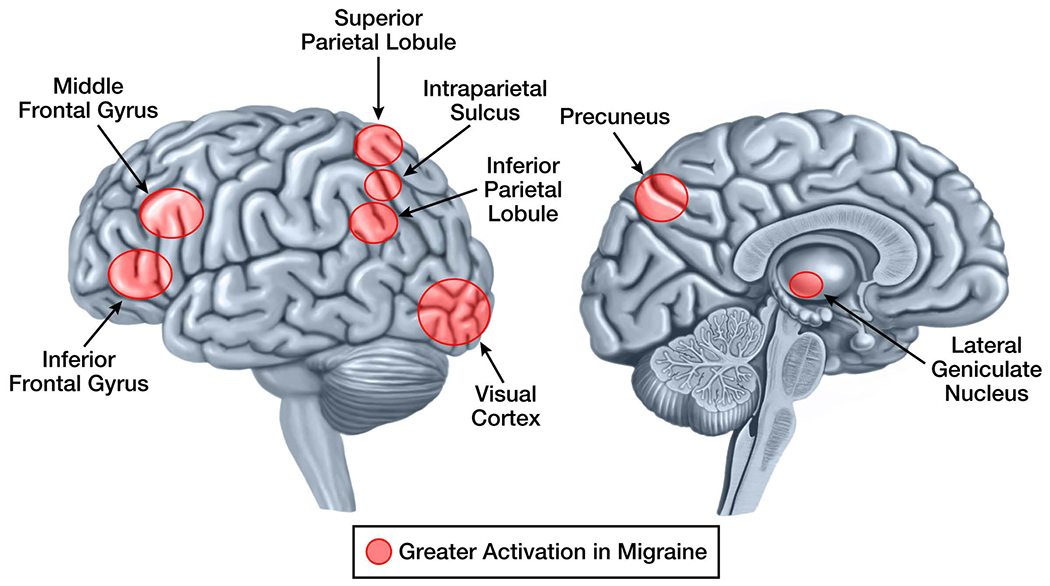
Brain regions that have visual stimuli-induced activations in interictal migraineurs that differ from activations in healthy controls are demonstrated on the lateral and medial brain surfaces. Areas shaded red are those that have greater activation in migraine compared to controls. There are no areas that have less activation in migraine compared to controls. Although shaded areas are within regions of the brain that show differential activation between migraineurs and controls, their placement does not represent the exact location, size, or extent of differential activation.
Migraineurs have increased sensitivity to visual stimuli, such as lights and patterns, during and between migraine attacks. Less intense light is required to cause visual discomfort in interictal migraineurs compared to controls, and even less intense light causes visual discomfort in ictal migraineurs compared to the interictal state.36, 37 Approximately 45% of migraineurs report symptoms of light hypersensitivity when interictal, and up to 90% report such symptoms during a migraine attack.6, 36, 38, 39 In addition, 40% of migraineurs report that visual stimuli can trigger their migraines.7 Given these associations, several fMRI studies have investigated migraineurs responses to visual stimuli. Some of these studies have specifically investigated migraine patients who experience aura since visual cortical hyperexcitability might play a role in predisposing the brain towards development of visual aura and to visual hypersensitivity.40
fMRI studies measuring visual stimuli-induced brain activations find migraineurs to be hyperresponsive. Results from several studies indicate that migraineurs viewing visually stressful patterns (e.g. black and white stripes) have greater activation of striate and extrastriate visual cortex.41–44 Results from a single study in which light was used as the stimulus found that migraineurs had a wider photoresponsive area (i.e. a greater number of voxels activated) compared to controls, but no difference in the intensity of voxels activated.43 A study comparing visual pattern-induced brain activity in migraineurs with aura, migraineurs without aura, and controls, found that the migraineurs with aura had greater activation of primary visual cortex and lateral geniculate compared to both other groups.45 There were no significant differences in activation between the migraineurs without aura and controls. These results support the notion that visual cortex hyperexcitability might associate with having migraine with aura. Finally, one study investigated activation of the motion-responsive middle temporal complex in response to a moving visual stimulus and found that migraineurs had greater activation of the left superior-anterior portion of this motion-processing region.46 These data show that migraine hyperresponsiveness to visual stimuli extends beyond the visual cortex.
Adding to our understanding of how visual stimuli could trigger a migraine attack, brainstem activations occurring with visually triggered migraine attacks were studied in 12 migraineurs. Four subjects developed migraine with visual aura and 8 developed headaches without aura in response to viewing a checkerboard pattern.47 Activations of the red nucleus and substantia nigra were identified in 75% of these migraineurs. Red nucleus and substantia nigra activations preceded onset of visually-triggered migraine symptoms and preceded signal elevations in the occipital cortex. Similar to findings in the study of odor processing, these study findings suggest that brainstem structures play an early or initiating role within a migraine attack and identify a potential path by which exposure to external stimuli could trigger a migraine attack.35
A potential clinical utilization of fMRI is for the measurement of early treatment effects. An fMRI study using visual stimulation showed that the symptoms of visual hypersensitivity and the hyperexcitable occipital cortex that are found in migraineurs are treatable. In this study, migraineurs exposed to a stressful striped pattern had higher ratings of discomfort and greater activation of visual cortex compared to non-migraine controls.42 However, when migraineurs wore precision ophthalmic tints (i.e. eyeglasses with tinted lenses that best reduce visual discomfort for each individual patient) their pattern-induced visual discomfort normalized and there was normalization of visual cortex activation. This study shows that migrainous hypersensitivities can be treated, measured both by subjective patient report of reduced visual discomfort and more objectively by normalization of visual cortex hyperreactivity.
In further support of the notion that interictal migraineurs have a lack of normal habituation to recurrent stimuli, migraineurs were found to have absence of hemodynamic refractory effects in an fMRI study exposing migraineurs to paired visual stimuli.48 Whereas controls had reduced activations to the second stimulation within each pair, hemodynamic refractory effects were not observed in migraineurs.Thus, lack of habituation in migraine goes beyond the processing of pain, including other senses such as vision.
Migraine Functional Connectivity MRI Analyses
Functional connectivity MRI investigates brain functional organization based upon temporal correlations in BOLD signal fluctuations amongst brain regions.49 Most functional connectivity analyses are done when the brain is “at rest”, meaning that the study subject is not performing a task and is not being stimulated. During the resting state there is continuous low-frequency fluctuation of BOLD activity throughout the brain. Brain regions with temporal correlations in BOLD fluctuations are regions considered to be “functionally connected” or “functionally communicating”.50 Functional connections and the strength of such functional connections can be atypical in the presence of neurologic diseases, including migraine.
Migraine functional connectivity studies have consistently shown migraineurs to have aberrant brain functional organization, mostly amongst regions that participate in pain processing.28–30, 51–66 Many studies have identified positive correlations between migraine attack frequency or number of years with migraineand the extent of atypical functional connectivity.52, 54, 55, 57, 59, 61–65 Although these correlations are clearly suggestive of a direct relationship between migraine and atypical functional connectivity, longitudinal studies are required to determine if atypical functional connectivity might predispose the brain to migraine, more atypical connectivity predisposing to more severe migraine, or if atypical functional connectivity is a result of recurrent migraine attacks.
Functional connectivity studies that have compared migraineurs to healthy controls have demonstrated atypical connectivity of numerous pain processing regions.51, 52, 54, 55, 57, 59–66 Regions that have commonly been found to have atypical functional connectivity include those involved in sensory-discriminative pain processing (e.g. somatosensory cortex, posterior insula), affective-emotional processing (e.g. anterior insula, anterior cingulate cortex, amygdala), cognitive processing (e.g. hippocampus, parahippocampal gyrus, orbitofrontal cortex) and pain modulation (e.g. periaqueductal gray, nucleus cuneiformis).51, 52, 54, 55, 58, 59, 61–65 Migraineurs have atypical functional connectivity that involves several brain resting state networks including the salience network, default mode network, executive network, somatomotor network and frontoparietal network.51, 52, 54, 55, 57, 59, 61–66 Two small studies have investigated functional connectivity of brainstem descending pain modulating regions in migraine patients who report symptoms of cutaneous allodynia during their migraine attacks.55, 58 These studies have identified atypical functional connectivity of periaqueductal gray and nucleus cuneiformis in migraineurs with allodynia. Atypical functional connectivity of these brainstem regions suggests that altered functional organization of pain-inhibiting regions is associated with the development of central sensitization and cutaneous allodynia in migraine.
Themes Emerging from Migraine fMRI Studies
Some themes emerging from fMRI studies of migraine include: 1) migraineurs are hypersensitive to external stimuli; 2) patterns of atypical activation and atypical functional connectivity in migraine are multifaceted, involving numerous brain regions and brain networks that participate in processing of different senses; 3) migraineurs lack the normal habituation response to repetitive stimuliwhen they are between migraine attacks; 4) atypical brain activation and functional connectivity are associated with higher headache frequency andgreater number of years with migraine, suggesting a true relationship between migraine and the fMRI findings.
Migraineurs are Hypersensitive to External Stimuli:
When exposed to pain, visual and olfactory stimuli, the migraine brain has hyperactive responses within regions that facilitate the perception of such stimuli. Conversely, regions that primarily inhibit the perception of external stimuli, such as pain-inhibiting regions of the brainstem, have hypoactive responses. Furthermore, functional connectivity studies have demonstrated atypical connectivity of pain inhibiting brainstem regions in migraineurs with allodynia. An imbalance of pain facilitation and pain inhibition could be responsible for the hypersensitivities to external stimuli and lack of interictal habituation that are present in migraine patients, resulting in hypersensitivities to somatosensory, visual, olfactory and auditory stimuli.
Patterns of Atypical Activation and Functional Connectivity in Migraine are Multifaceted:
Consistent with expectations regarding a complex neurologic disorder that consists of headache and hypersensitivities to several different types of external stimuli, migraineurshave atypical brain activations and functional connectivity that involves regions participating in pain, visual, and olfactory processing. Furthermore, migraine is associated with atypical function of numerous subdomains within each of these processing categories. For example, painprocessing regions affected in migraine include those with sensory-discriminative, affective, cognitive and modulating roles.19–21, 24 Visual regions with atypical function in migraine include those with roles in primary visual processing as well as those responsible for processing visual motion.41–46, 67 Resting state functional connectivity studies show that migraine is associated with atypical functional connectivity that involves numerous different resting state networks such as somatomotor, executive, salience, frontoparietal and default mode networks.51, 52, 54, 55, 57, 59, 61–66 Thus, atypical functional activations and atypical functional connectivity patterns in migraine are complex. For the description of migraine mechanisms, the challenge is to identify atypical function that is specific for migraine, as opposed to findings that would be shared by other headache types and other pain disorders. To make fMRI findings useful for managing and treating migraineurs, studies need to determine cause and effect relationships between migraine symptoms and atypical fMRI findings and explore which fMRI findings can predict important clinical outcomes such as increasing frequency of migraine attacks over time and response to specific migraine treatments. Of note, studies comparing migraineurs with low frequency episodic migraine (1-2 headache days/month) to those with higher frequency episodic migraine (8-14 headache days/month) have identified group differences in pain-induced activations and resting state functional connectivity.28–30 Identification of brain biomarkers for frequent migraine might contribute to understanding mechanisms that underlie the transformation from less frequent to more frequent migraine attacks, while discovery of biomarkers for this progression could help identify patients at high risk for transformation and thus in need of more aggressive migraine therapy.
The Interictal Migraine Brain Lacks the Normal Habituating Response:
fMRI studies support what electrophysiologic studies had previously demonstrated: when between migraine attacks, the brains of migraineurs fail to habituate to repetitive stimuli. Thus, as the healthy brain acclimates to repetitive stimuli, the migraine brain remains sensitive to such stimuli. Furthermore, repetitive stimulation can lead to progressively increasing responses in the migraineur. This lack of habituation and a tendency toward sensitization are likely important mechanisms in migraine that lead to frequent pain, cutaneous allodynia, photosensitivity, phonosensitivity, and olfactory hypersensitivity. The lack of habituation and sensitization might lower the threshold for future migraine attacks and enhance the ability of sensory stimuli to trigger migraine, thus playing a role in the transformation from less frequent migraine attacks (i.e. episodic migraine) to more frequent migraine attacks (i.e. chronic migraine).26
The Extent of fMRI Abnormalities in Migraine Correlate with Headache Frequency and Number of Years with Migraine:
Many migraine fMRI studies have found significant correlations between the extent of fMRI abnormalities and headache frequency and/or number of years with migraine. These correlations serve as evidence that the fMRI findings are directly related to migraine, as opposed to other confounding factors. Although it is tempting to conclude that these correlations show that migraine has a cumulative impact on brain function, longitudinal studies are needed to support this assertion.
Limitations to Current fMRI Migraine Studies
There are several limitations of published fMRI studies that inhibit the ability to draw summary conclusions regarding stimulus-induced brain activity and functional connectivity in migraine.
First, migraine fMRI studies have included relatively small numbers of subjects, limiting study power, often resulting in less than optimal statistical methods for determining significance, and limiting the generalizability of study results. Furthermore, there are fewreplication studies that confirm initial fMRI study results. Additional confirmatory studies are needed in order to increase confidence in and determine generalizability of fMRI findings.
Substantial variability in data collection and analysis techniques amongst migraine fMRI studies results in an inability to develop a cohesive model of specific brain regions and networks that are altered in migraine. For example, variability in techniques does not allow for a proper meta-analysis of regions that atypically activate in response to painful stimuli in people with migraine. The use of region-of-interest based analysesrather than whole-brain analysesis a substantial limitation in this regard. Additional whole brain analyses are needed in order to obtain a global picture of atypical stimulus-induced activations and atypical functional connectivity in migraine.Furthermore, there might be a need to consider the usual laterality of headache pain when analyzing fMRI data. A migraine study comparing the symptomatic brain hemisphere (i.e. brain hemisphere contralateral to side of usual headache pain), asymptomatic brain hemisphere, and controls found the symptomatic hemisphere to be hyperreactive to visual stimuli compared to the asymptomatic hemisphere and compared to controls.67 However, there were no activation differences between the asymptomatic hemisphere and controls. Thus, migraine fMRI activation studies might need to consider the usual side of headache pain when performing analyses. Additional variability amongst migraine fMRI studies is attributed to use of different techniques of providing stimuli, varying MRI resolution, and different statistical thresholds for significance. Functional connectivity analyses of migraine have additional heterogeneity due to use of several different analysis techniques: 1) region-of-interest (measuring functional connectivity amongst chosen regions or between chosen regions and the rest of the brain); 2) independent components analysis (computational blind separation of the whole brain into functional networks or functional subcomponents); 3) voxel-mirrored homotopic connectivity (measuring functional connectivity between voxels in one hemisphere of the brain and their symmetric counterparts on the other hemisphere as a measure of interhemispheric functional connectivity); and 4) regional homogeneity (measuring synchronization of BOLD fluctuations amongst local voxels). The use of these different analysis techniques makes it difficult to compare results across studies. Also, the use of medications and the potential effects of migraine comorbidities (e.g. anxiety, depression, myofascial pain) on study results have been inadequately considered in several studies.
Differences in the timing of data collection are another limitation to existing migraine fMRI studies.Migraineurs can be studied during a migraine attackor between migraine attacks. However, the exact time intervals between fMRI, the preceding migraine and the next migraine likely impact the fMRI findings.A study delivering trigemino-nociceptive stimuli via intranasal ammonia gas investigated migraine subjects at multiple time points within and between migraine attacks. 22 Spinal trigeminal nuclei activation was dependent upon the timing of data collection: interictal migraineurs had weaker activation compared to controls, but time to next migraine attack was positively correlated with stronger spinal trigeminal nucleus activation. Similarly, one would presume that brain activations change as a patient progresses through a migraine attack. Thus, the exact timing of fMRI in relation to migraine attacks likely has a substantial effect on study results.
Most studies do not explicitly account for sex-specific differences between men and women migraineurs. However, sex-specific differences have been found in migraine fMRI studies.53, 56 A study comparing male migraineurs to female migraineurs found females to have greater pain-induced activations in caudate, superior temporal, superior frontal, precuneus, posterior cingulate, sensory nucleus and spinal trigeminal nucleus of brainstem, while males had greater activations in insula, primary somatosensory cortex, and putamen. 56 Studies that have investigated functional connectivity differences between male and female migraineurs have found differences in functional connectivity according to sex.53, 56 Sex differences have been identified for the topological organization of resting state networks and in the functional connectivity of the insula and of the precuneus. Thus, studies should carefully match according to sex or investigate men and women migraineurs separately.
Longitudinal studies are lacking and it is thus impossible to determine if the fMRI differences identified in migraine subjects predispose the person to migraine or if they result from recurrent migraine attacks. Prospective longitudinal studies that correlate changes in migraine patterns with changes in fMRI measured activations and changes in functional connectivity will help elucidate the direction of the relationship and will perhaps identify early biomarkers that predict improvements or worsening of migraine patterns. Such studies should also account for the effects of using migraine medications, aging, and development of migraine comorbidities. The large sample size needed for this type of analysis would almost certainly require a multicenter collaborative effort.
The inability to determine if study findings are specific to migraine or if they are representative of any type of pain is a major shortcoming in the migraine fMRI literature. One functional connectivity study compared migraineurs not only to healthy controls, but also to patients with carpal tunnel syndrome and to patients with trigeminal neuralgia.51 In this study, migraineurs were found to have stronger amygdala functional connectivity with visceroceptive insula compared to all other groups. Future studies comparing migraineurs to patients with other headache types and other pain types are needed to determine the specificity of fMRI findings for migraine.These studies might focus on brain regions and networks that could contribute to unique features of migraine,like the co-occurrence and interaction of headache with visual, olfactory and auditory symptoms. Studies showing that the presence and intensity of stimuli within one sensory modality (e.g. visual stimuli) mediates the presence and intensity of symptoms within other sensory realms (e.g. intensity of migraine headache pain) suggests that multisensory integration might play a key role in migraine pathophysiology.3 Thus, fMRI studies of multisensory integration regions of the brain might be useful in differentiating migraine from other headache types and other pain disorders. Of note, the temporal pole participates in multisensory integration and has been found to have atypical activation or atypical functional connectivity in several migraine fMRI studies.19, 28–30, 35, 51, 54, 60, 61, 66
Conclusions and Views for the Future
fMRI studies consistently show that migraine is associated with atypical brain activations in response to painful, olfactory and visual stimuli and that migraine is associated with atypical functional connectivity. Atypical brain activity and functional connectivity involve diffuse areas of the brain, a finding consistent with migraine being a complex neurologic disorder involving atypical processing of several types of sensory stimuli (somatosensory, visual, olfactory). Migraine fMRI studies demonstrate a combination of enhanced sensory facilitation, reduced sensory inhibition, and a lack of interictal habituation. Correlations between the extent of fMRI abnormalities and headache frequency or number of years with migraine suggest that migraine has cumulative effects on brain function or that the extent of underlying fMRI abnormalities positively correlate with the risk of more severe migraine disease.
There are several avenues for future migraine fMRI research that will improve the quality and clinical relevance of collected data. fMRI studies that include larger sample sizes, more stringent statistical thresholds, and replication studies would increase confidence in the validity of study results.Large, multicenter, longitudinal studies are needed to investigate associations between changes in headache patterns and corresponding changes in fMRI measures. Such studies would advance our understanding of mechanisms for migraine transformation (moving from less frequent headaches to more frequent headaches) and migraine reversion (moving from more frequent to less frequent headaches) and might identify baseline biomarkers that predict transformation and reversion. Such studies should investigate the effects of migraine therapies on fMRI measures. Doing so could identify baseline biomarkers that predict treatment responsiveness and biomarkers that reflect early treatment response. Neuroimaging studies that successfully differentiate migraine from other headache types and from other types of pain are also needed. To date, it is not clear if the findings from migraine fMRI studies are specific for migraine or if they are shared by other headache types and other types of chronic recurrent pain. Determining specificity of findings is a crucial step before fMRI could potentially be used for assisting in the diagnosis and management of migraine. Although migraine is a symptom-based diagnosis and neuroimaging would typically not be needed for assigning a migraine diagnosis, there are clinical situations when the addition of a diagnostic test would be very useful. Resting state functional connectivity analyses might be particularly useful in this regard since collection of resting state data does not require equipment beyond the MRI machine, does not require patients to participate in tasks or to be stimulated, and does not require significant acquisition time.
Table 3: Functional Connectivity MRI Studies of Migraine.
All studies were conducted when migraine patients were interictal (i.e. between migraine attacks).
| Study | Cohorts | Analysis Technique | Main Findings |
|---|---|---|---|
| Schwedt 2014 | Migraine severe allodynia (n=8) Migraine no allodynia (n=8) |
ROI (PAG, NCF) | Migraine severe allodynia: Stronger PAG and NCF fc with other sensory discriminative regions in brainstem, thalamus, insula and cerebellum as well as with higher order pain regions in frontal and temporal lobes. |
| Hadjikhani 2013 | Migraine (n=22) Control (n=20) Carpal Tunnel (n=11) Trigeminal Neuralgia (n=9) |
ROI (amygdala) | Migraine vs. All Other Cohorts: Stronger fc to visceroceptive insula. Migraine vs. Control: Stronger fc to insula, SII, Thalamus, Heschl’s gyrus, temporal pole |
| Jin 2013 | EM (n=21) Control (n=21) |
ROI (ACC) | EM: Stronger fc to middle temporal, orbitofrontal cortex, DLPFC. |
| Maleki 2013 | HF EM (n=10) LF EM (n=10) |
ROI (hippocampus) | HF EM: Weaker fc with supramarginal gyrus, temporal pole, fronto-orbital, nucleus accumbens, anterior insula, middle frontal, paracingulate. |
| Schwedt 2013 | CM (n=20) Control (n=20) |
ROI (ACC, anterior insula, amygdala) | CM: Atypical fc with pain-facilitating and pain-inhibiting regions that have sensory discriminative, cognitive, and integrative roles. (ant insula, amygdala, pulvinar, MD thalamus, middle temporal, PAG) |
| Tessitore 2013 | EM (n=20) Control (n=20) |
ICA of DMN | EM: Weaker fc with superior prefrontal gyrus and temporal pole. |
| Xue 2013 | EM (n=18) Control (n=18) |
ALFF and fc ROI (ACC, thalamus, PFC, insula) |
ALFF EM: decreased ALFF PFC, rACC and increased ALFF thalamus. fc EM: stronger fc rACC with frontal lobe, parietal lobe; thalamus, with caudate, temporal lobe, putamen; PFC with precuneus, parietal lobe and temp lobe; insula with temporal pole, frontal lobe and parietal lobe. |
| Yuan 2013 | EM (n=40) Control (n=40) |
ROI (basal ganglia) | EM: Stronger fc caudate with parahippocampal gyrus, amygdala, insula, putamen; nucleus accumbens with parahippocampal, ACC, OFC, and PCC. |
| Zhao 2013 | EM (n=40) Control (n=20) |
Regional homogeneity (whole brain) | EM: Abnormal regional homogeneity in thalamus, inferior frontal, middle occipital, insula, caudate, middle frontal, middle temporal, inferior occipital, ACC, medial frontal, superior temporal, amygdala, lentiform nucleus, uncus, superior frontal, temporal pole, cerebellum, pons, medulla, midbrain, hippocampus, lingual, cuneus, inferior parietal, postcentral, precuneus, fusiform, PCC. |
| Liu 2012 | EM (n=43) Control (n=43) |
ROI (90 ROIs) | EM: brain hubs related to pain-processing have abnormal nodal centrality (precentral gyrus, inferior frontal gyrus, parahippocampal gyrus, ACC, thalamus, temporal pole, inferior parietal). |
| Maleki 2012 | Male EM (n=11) Female EM (n=11) |
ROI (insula, precuneus) | Female EM: Stronger negative fc insula with S1, PCC, precuneus, temporal pole; Stronger fc precuneus with amygdala and S1. |
| Maleki 2012 | HF EM (n=10) LF EM (n=10) |
ROI (postcentral, ant insula, temp pole, ACC) | HF EM: Stronger fc postcentral gyrus to ACC, post insula, pulvinar, parahippocampus, hypothalamus, putamen, frontal pole and weaker fc to substantia nigra; Stronger ACC fc with frontal pole, temporal pole, inf temp gyrus, pulvinar, parahippocampal gyrus; Stronger ant insula fc with ACC, putamen, parahippocampal gyrus, hippocampus; stronger temporal pole fc with postcentral gyrus, middle and superior temp gyrus, frontal pole. |
| Russo 2012 | EM (n=14) Control (n=14) |
Frontoparietal Network | EM: Weaker fc within right frontoparietal network, specifically within middle frontal gyrus and dorsal ACC. |
| Xue 2012 | EM (n=23) Control (n=23) |
ICA of DMN, CEN, SN | EM: intrinsic connectivity differed for the 3 ICNs; greater intranetwork fc within middle frontal gyrus for the right CEN and inf frontal gyrus for the leftCEN and decreased intranetwork fc within the SMA for the SN; greater intrinsic DMN and rCEN connectivity to right anterior insula. |
| Yu 2012 | EM (n=26) Control (n=26) |
Regional homogeneity (whole brain) | EM: Decreased regional homogeneity in ACC, PFC, OFC, SMA. |
| Yuan 2012 | EM (n=21) Control (n=21) |
Voxel-mirrored homotopic connectivity and ROI (ACC) | EM: decreased interhemispheric fc of ACC. Bilat ACC then used as seeds and migraineurs had stronger fc between left ACC and bilat OFC and right DLPFC and stronger fc between right ACC and bilateral OFC. |
| Liu 2011 | Male EM (n=18) Female EM (n=20) Controls (n=38) |
Graph Theory Analysis (whole brain 90 ROIs) | EM: abnormal topological organization including small world properties, resilience, nodal centrality, and interregional connections. Gender-related differences in migraineurs exist |
| Mainero 2011 | EM (n=17) Controls (n=17) EM allodynia (n=5) EM no allodynia (n=5) |
ROI (PAG) | EM: stronger PAG fc to VLPFC, supramarginal gyrus, ant insula, precentral, postcentral, thalamus. EM Allodynia: Weaker fc PAG with prefrontal, ACC, anterior insula. |
| Maleki 2011 | HF EM (n=10) LF EM (n=10) |
ROI (basal ganglia, PAG, pulvinar, hypothalamus) | HF EM: Weaker fc caudate with middle frontal, insula, temporal pole, parahippocampus; weaker fc nucleus accumbens with PCC, superior parietal, hippocampus; stronger fc putamen with hippocampus, caudate, middle frontal, ant insula; stronger fc globus pallidus with middle temporal, supramarginal, thalamus, hippocampus, insula, temporal pole. |
ACC = anterior cingulate cortex; ALFF = amplitude of low-frequency fluctuation; ant = anterior; bilat = bilateral; CEN = central executive network; CM = chronic migraine; DLPFC = dorsolateral prefrontal cortex; DMN = default mode network; EM = episodic migraine; fc = functional connectivity; HF = high frequency; ICA = independent components analysis; ICN = intrinsic connectivity networks; inf = inferior; LF = low frequency; MD = mediodorsal; NCF = nucleus cuneiformis; OFC = orbitofrontal cortex; PAG = periaqueductal gray; PCC = posterior cingulate cortex; PFC = prefrontal cortex; post = posterior; rACC = rostral anterior cingulate cortex; ROI = region of interest; temp = temporal; S1 = primary somatosensory cortex; SII = secondary somatosensory cortex; SMA = supplementary motor area; SN = salience network; VLPFC = ventrolateral prefrontal cortex.
Acknowledgement:
We would like to acknowledge Marv Ruona, Scientific Illustrator, Media Support Services, Mayo Clinic, Arizona for creating the figures for this manuscript.
Funding:
Time writing this manuscript was partially funded by a grant from the National Institutes of Health (K23NS070891) to TJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors state no potential conflicts of interest regarding the contents of this manuscript.
References
- 1.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007; 68(5): 343–9. [DOI] [PubMed] [Google Scholar]
- 2.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33(9): 629–808. [DOI] [PubMed] [Google Scholar]
- 3.Schwedt TJ. Multisensory integration in migraine. Curr Opin Neurol. 2013; 26(3): 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999; 53(3): 537–42. [DOI] [PubMed] [Google Scholar]
- 5.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996; 119 (Pt 2): 355–61. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DI, De ver Dye T. Migraine and the environment. Headache. 2009; 49(6): 941–52. [DOI] [PubMed] [Google Scholar]
- 7.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007; 27(5): 394–402. [DOI] [PubMed] [Google Scholar]
- 8.Martin PR, Reece J, Forsyth M. Noise as a trigger for headaches: relationship between exposure and sensitivity. Headache. 2006; 46(6): 962–72. [DOI] [PubMed] [Google Scholar]
- 9.Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain. 2008; 131(Pt 8): 2192–200. [DOI] [PubMed] [Google Scholar]
- 10.Schwedt TJ, Krauss MJ, Frey K, Gereau RWt. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011; 31(1): 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003; 104(3): 693–700. [DOI] [PubMed] [Google Scholar]
- 12.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000; 47(5): 614–24. [PubMed] [Google Scholar]
- 13.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004; 63(5): 848–52. [DOI] [PubMed] [Google Scholar]
- 14.Ashkenazi A, Silberstein S, Jakubowski M, Burstein R. Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia. 2007; 27(4): 325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008; 70(17): 1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008; 63(2): 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankewitz A, Voit HL, Bingel U, Peschke C, May A. A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia. 2010; 30(4): 475–85. [DOI] [PubMed] [Google Scholar]
- 18.Aderjan D, Stankewitz A, May A. Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain. 2010; 151(1): 97–103. [DOI] [PubMed] [Google Scholar]
- 19.Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex. 2011; 21(2): 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008; 3(11): e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo A, Tessitore A, Esposito F, Marcuccio L, Giordano A, Conforti R, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012; 259(9): 1903–12. [DOI] [PubMed] [Google Scholar]
- 22.Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011; 31(6): 1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia. 2013; 33(4): 256–65. [DOI] [PubMed] [Google Scholar]
- 24.Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010; 68(1): 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain. 2013; 14(1): 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008; 48(8): 1157–68. [DOI] [PubMed] [Google Scholar]
- 28.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012; 32(8): 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2013; 218(4): 903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, et al. Migraine attacks the Basal Ganglia. Mol Pain. 2011; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Carlo D, Dal Zotto L, Perissinotto E, Gallo L, Gatta M, Balottin U, et al. Osmophobia in migraine classification: a multicentre study in juvenile patients. Cephalalgia. 2010; 30(12): 1486–94. [DOI] [PubMed] [Google Scholar]
- 32.Demarquay G, Royet JP, Giraud P, Chazot G, Valade D, Ryvlin P. Rating of olfactory judgements in migraine patients. Cephalalgia. 2006; 26(9): 1123–30. [DOI] [PubMed] [Google Scholar]
- 33.Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache. 2004; 44(10): 1019–23. [DOI] [PubMed] [Google Scholar]
- 34.Zanchin G, Dainese F, Trucco M, Mainardi F, Mampreso E, Maggioni F. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007; 27(9): 1061–8. [DOI] [PubMed] [Google Scholar]
- 35.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011; 77(5): 476–82. [DOI] [PubMed] [Google Scholar]
- 36.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997; 37(8): 492–5. [DOI] [PubMed] [Google Scholar]
- 37.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997; 17(7): 733–41. [DOI] [PubMed] [Google Scholar]
- 38.Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996; 16(4): 239–45. [DOI] [PubMed] [Google Scholar]
- 39.Wober-Bingol C, Wober C, Karwautz A, Auterith A, Serim M, Zebenholzer K, et al. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia. 2004; 24(1): 12–7. [DOI] [PubMed] [Google Scholar]
- 40.Aurora SK, Ahmad BK, Welch KM, Bhardhwaj P, Ramadan NM. Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology. 1998; 50(4): 1111–4. [DOI] [PubMed] [Google Scholar]
- 41.Griebe M, Flux F, Wolf ME, Hennerici MG, Szabo K. Multimodal Assessment of Optokinetic Visual Stimulation Response in Migraine With Aura. Headache. 2014; 54(1): 131–41. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia. 2011; 31(8): 925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin H, Sanchez del Rio M, de Silanes CL, Alvarez-Linera J, Hernandez JA, Pareja JA. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache. 2011; 51(10): 1520–8. [DOI] [PubMed] [Google Scholar]
- 44.Vincent M, Pedra E, Mourao-Miranda J, Bramati IE, Henrique AR, Moll J. Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia. 2003; 23(9): 860–8. [DOI] [PubMed] [Google Scholar]
- 45.Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013; 33(6): 365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antal A, Polania R, Saller K, Morawetz C, Schmidt-Samoa C, Baudewig J, et al. Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalalgia. 2011; 31(3): 338–45. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y, Aurora SK, Nagesh V, Patel SC, Welch KM. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002; 59(1): 72–8. [DOI] [PubMed] [Google Scholar]
- 48.Descamps B, Vandemaele P, Reyngoudt H, Deblaere K, Leybaert L, Paemeleire K, et al. Absence of haemodynamic refractory effects in patients with migraine without aura: an interictal fMRI study. Cephalalgia. 2011; 31(11): 1220–31. [DOI] [PubMed] [Google Scholar]
- 49.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005; 102(27): 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen AL, Fair DA, Dosenbach NU, Miezin FM, Dierker D, Van Essen DC, et al. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 2008; 41(1): 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadjikhani N, Ward N, Boshyan J, Napadow V, Maeda Y, Truini A, et al. The missing link: Enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013; 33(15): 1264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin C, Yuan K, Zhao L, Yu D, von Deneen KM, Zhang M, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013; 26(1): 58–64. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Qin W, Nan J, Li J, Yuan K, Zhao L, et al. Gender-related differences in the dysfunctional resting networks of migraine suffers. PLoS One. 2011; 6(11): e27049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, et al. Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One. 2012; 7(12): e51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011; 70(5): 838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012; 135(Pt 8): 2546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russo A, Tessitore A, Giordano A, Corbo D, Marcuccio L, De Stefano M, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia. 2012; 32(14): 1041–8. [DOI] [PubMed] [Google Scholar]
- 58.Schwedt TJ, Larson-Prior L, Coalson RS, Nolan T, Mar S, Ances BM, et al. Allodynia and Descending Pain Modulation in Migraine: A Resting State Functional Connectivity Analysis. Pain Med. 2014; 15(1): 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwedt TJ, Schlaggar BL, Mar S, Nolan T, Coalson RS, Nardos B, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013; 53(5): 737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013; 14(1): 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue T, Yuan K, Cheng P, Zhao L, Yu D, Dong T, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013; 26(9): 1051–8. [DOI] [PubMed] [Google Scholar]
- 62.Xue T, Yuan K, Zhao L, Yu D, Dong T, Cheng P, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012; 7(12): e52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu D, Yuan K, Zhao L, Dong M, Liu P, Wang G, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012; 25(5): 806–12. [DOI] [PubMed] [Google Scholar]
- 64.Yuan K, Qin W, Liu P, Zhao L, Yu D, Dong M, et al. Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS One. 2012; 7(9): e45476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan K, Zhao L, Cheng P, Yu D, Dong T, Xing L, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013; 14(8): 836–44. [DOI] [PubMed] [Google Scholar]
- 66.Zhao L, Liu J, Dong X, Peng Y, Yuan K, Wu F, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. 2013; 14: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hougaard A, Amin FM, Hoffmann MB, Rostrup E, Larsson HB, Asghar MS, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


