Abstract
Noninvasive brain stimulation technologies such as transcranial electrical and magnetic stimulation (tES and TMS) are emerging neuromodulation therapies that are being used to target the neural substrates of substance use disorders. By the end of 2022, 205 trials of tES or TMS in the treatment of substance use disorders had been published, with heterogeneous results, and there is still no consensus on the optimal target brain region. Recent work may help clarify where and how to apply stimulation, owing to expanding databases of neuroimaging studies, new systematic reviews, and improved methods for causal brain mapping. Whereas most previous clinical trials targeted the dorsolateral prefrontal cortex, accumulating data highlight the frontopolar cortex as a promising therapeutic target for transcranial brain stimulation in substance use disorders. This approach is supported by converging multimodal evidence, including lesion-based maps, functional MRI-based maps, tES studies, TMS studies, and dose-response relationships. This review highlights the importance of targeting the frontopolar area and tailoring the treatment according to interindividual variations in brain state and trait and electric field distribution patterns. This converging evidence supports the potential for treatment optimization through context, target, dose, and timing dimensions to improve clinical outcomes of transcranial brain stimulation in people with substance use disorders in future clinical trials.
Substance use disorders (SUDs) affect over 1 billion individuals worldwide, and they affect people of every age, race, gender, socioeconomic status, and nationality. Increased reactivity to drug-related cues and disrupted activity in frontal-striatal circuits are commonly observed across all SUDs. Recent technological advances in opto- and chemogenetics have further refined our understanding of the frontal-striatal circuits in reward processing and behavioral control and highlighted their causal role in drug-related behaviors (1). Until recently, however, we had no brain circuit–based intervention that could be applied to people with SUDs.
Mechanistic studies and clinical trials have provided increasing evidence for the effectiveness of noninvasive neuromodulation with transcranial electrical stimulation (tES) or transcranial magnetic stimulation (TMS) in the treatment of SUDs, including alcohol, tobacco, cocaine, cannabis, methamphetamine, and opioid use disorders (2). The enthusiasm for noninvasive neuromodulation approaches to SUD treatment is buoyed by a growing body of work demonstrating a causal relationship between noninvasive stimulation of the frontal-striatal circuits and drug-related behaviors. In a series of studies using interleaved TMS and blood-oxygen-level-dependent (BOLD) imaging, researchers have shown that it is possible to modulate the striatum via TMS to the prefrontal cortex (3, 4). Research has shown that a single session of theta burst stimulation to the frontopolar cortex can dampen cue-evoked BOLD signal in the striatum of individuals with alcohol or cocaine use disorders (5). Moreover, it has been reported that the effectiveness of modulating the striatum in individuals with cocaine use disorder depends on the integrity of the white matter pathways connecting the cortex and striatum (6).
From a mechanistic perspective, there is also a growing appreciation for the relationship between noninvasive stimulation and neurochemistry. One of the possible mechanisms derived from a series of positron emission tomography (PET) studies suggests that TMS modulates striatal dopamine release (7). Given the well-established relationship between dopamine release and drug cue seeking, this suggests that observed effects of TMS on cocaine use behavior (e.g., 8–10) could be through dopaminergic pathways, which are targeted in a variety of SUD treatments (11). However, the dopaminergic mechanism is only one of the potential pathways to modify addictive behaviors through neuromodulation. The glutamatergic pathway between the prefrontal cortex (PFC) and the nucleus accumbens or amygdala, or intracortical GABAergic pathways, can also be modulated with neuromodulation technologies such as tES and TMS (10). However, as mechanisms of noninvasive neuromodulation continue to be conceptualized as a brain circuit–based treatment option that modifies activity in brain networks, the potential for more effective and personalized treatment for SUDs continues to grow (12).
In recent years, there has been rapid growth and expansion of noninvasive neuromodulation as a circuit-based interventional tool in the field of SUDs. At the end of 2018, only 84 reports of tES or TMS trials in the field of SUDs had been published (2). By the end of 2022, 205 tES or TMS trials had been published in the treatment of SUDs (13). Following a decade of rapid growth and expansion of the noninvasive neuromodulation tools into the SUD research field (2), the U.S. Food and Drug Administration (FDA) cleared TMS for smoking cessation in 2020. The supporting multicenter double-blind randomized controlled trial, which included 135 participants with tobacco use disorder, showed that the active repetitive TMS (rTMS) group had significantly higher smoking abstinence rates at weeks 2, 4, and 12 compared with the sham treatment group (14). Since then, the pace of new clinical trials using novel tools and protocols of noninvasive neuromodulation for SUDs has accelerated, and the list of devices and indications that have received CE marking in Europe (European Conformity, indicating compliance with the relevant European Union laws) is growing (15).
However, there is still no consensus on the optimal brain stimulation target in SUDs. As of September 2022, 18 main brain regions have been targeted in SUD trials using tES and TMS. The dorsolateral prefrontal cortex (DLPFC) has been the most commonly targeted stimulation site for SUDs, given its success as a brain stimulation target for depression and top-down models of response control (16). Within the DLPFC, the left DLPFC was by far the most frequent target, followed by the right DLPFC (2) (Figure 1). Anode/cathode electrodes over left/right DLPFC were used in 68 of 76 published tES trials in SUDs, as well as 99 of 116 published excitatory or inhibitory TMS trials. Other brain areas that have been targeted in SUD research include the frontopolar cortex, superior frontal gyrus, inferior frontal gyrus, orbitofrontal cortex, motor cortex, vertex, anterior cingulate cortex, posterior cingulate cortex, insula, temporoparietal cortex, and occipital cortex (Figure 1). However, the physiological and clinical responses to tES or TMS reported by these studies show great variability. It is important to consider that the brain region that is “targeted” (the brain region underneath the electrode or coil) may be very different from the brain region that is actually stimulated (distal areas that might be modulated through a diffuse current flow or interactions between brain regions) for each person.
FIGURE 1. Brain targets for TMS/tES trials in substance use disordersa.
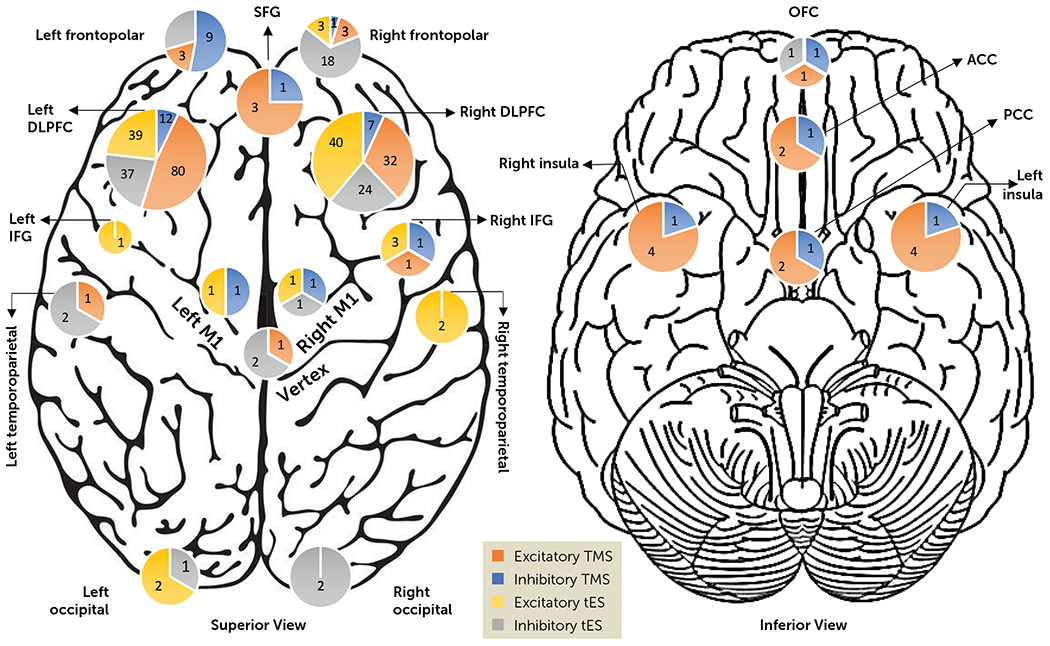
a Studies were categorized according to their assumed stimulation effects. TMS with frequency >5 Hz and iTBS studies were considered “excitatory TMS”; TMS with frequency ≤5 Hz and cTBS studies were considered “inhibitory TMS”; anodal tES was considered “excitatory tES”; and cathodal tES was considered “inhibitory tES.” In 20 tES studies, one of the electrodes was placed on the right or left supraorbital area (counted as frontopolar). The insula and frontopolar cortex were targeted bilaterally in deep TMS studies with both stimulatory and inhibitory frequencies. ACC=anterior cingulate cortex; DLPFC=dorsolateral prefrontal cortex; IFG=inferior frontal gyrus; OFC=orbitofrontal cortex; PCC=posterior cingulate cortex; SFG=superior frontal gyrus; tES=transcranial electrical stimulation; cTBS or iTBS=continuous or intermittent theta burst stimulation; TMS=transcranial magnetic stimulation.
When considering target selection for interventional psychiatry, two main approaches can be taken. The first is to start with the DLPFC as the target, given the extensive data supporting its efficacy and safety in treating depression, and to explore other targets only if targeting the DLPFC is not sufficient. The second approach involves using other levels of evidence, including neuroimaging, to identify specific brain regions that are disrupted in certain psychiatric disorders and have a causal relationship with symptoms. In this approach, the medial prefrontal cortex (mPFC) and frontopolar cortex emerge as a strong target for TMS and tES montages in SUDs, while we also acknowledge the potential effectiveness of targeting the DLPFC.
To use neuroimaging to identify correlates of drug-related behaviors in individuals with SUDs, one can perform functional MRI (fMRI) during behavioral tasks (e.g., drug cue exposure or risky decision making) or during the resting state and identify neuroimaging abnormalities in patients with SUDs that can then potentially be targeted with noninvasive brain stimulation (4, 17–20). An alternative approach is to identify the underlying causal brain circuitry involved in SUDs through lesion studies (21). Understanding causal relationships between the neural substrate and drug-related behavior is critical for guiding interventional therapy (22). To achieve this, causally informative study designs try to identify brain regions that contribute to the cycle of relapse (return to substance use) or addiction remission (an extreme case of reduction of substance use) to inform the intervention efforts or target selection. For instance, it has been reported that lesions involving the insula cause a disruption of tobacco use disorder (23). Additionally, lesions disrupting addiction have been reported in brain regions other than the insula (24, 25). Recently, a new method called lesion network mapping has been applied to study brain lesions that have resulted in addiction remission (26); in the cited study, “remission” was defined as an extreme case of substance use reduction, characterized as “quitting smoking without difficulty immediately after the lesion, without relapse and in the absence of craving since quitting.”
Similar to lesion-based analysis, brain stimulation sites can also help identify therapeutic targets (21). Previous neuromodulation clinical trial results involving tES (including anodal/cathodal stimulation), TMS (including single-pulse, paired-pulse, or repetitive TMS using continuous or intermittent theta burst stimulation [cTBS or iTBS], considering the stimulation frequency [low or high], with both conventional and deep TMS coils), deep brain stimulation (DBS), or transcranial focused ultrasound stimulation can be examined to identify commonly used or novel therapeutic targets, as well as the placement of the electrodes, coils, transducers, and stimulation montages. By leveraging such studies, researchers can gain valuable insights into potential targets for neuromodulation interventions. For example, neuromodulation therapies for depression revealed that functional connectivity maps from lesion-based data and from TMS and DBS studies, as three causal sources of information, converge on the same brain circuits that may serve as a refined therapeutic target to improve neuromodulation outcomes (27, 28).
Functional and structural connectomes derived from fMRI or diffusion tensor imaging data at the group level, referred to as averaged connectome maps or normative connectome, as well as group-level electric field analyses, have been shown to have potential value in target selection and stimulation dose optimization (29–31). However, group-level electric field or connectome maps cannot represent interindividual variability in terms of electric field distribution patterns or functional/structural connectivity. In this regard, personalized computational head models estimate electric field distribution patterns according to each montage/target. This approach accounts for neuroanatomical parameters and estimates the effects of each montage on various brain regions. However, electric field modeling has not been rigorously implemented in SUD studies, where it is commonly assumed that outcomes are associated with the cortical region under stimulating tES electrodes or TMS coils. This assumption is not supported by electric field modeling, however. For example, in tES studies with diffuse current flow, electric field modeling suggests that the peak electric field (as an indicator of stimulation hotspots) may not always be under the electrodes, and the electric field spreads, covering multiple brain regions (32–34). Similar results were also reported in TMS studies, where the peak of the TMS-induced electric field was not always located directly underneath the stimulation coil (35). In addition to personalized head models, patient-specific connectivity maps, rather than a normative connectome, have also been used to identify neuromodulation targets (36, 37). These studies suggest that personalized targeting and stimulation might lead to better treatment outcomes compared with group-averaged targeting. For example, resting-state fMRI data were used to individually target the region of the left DLPFC most functionally anticorrelated with the subgenual ACC in a group of participants with major depressive disorder (36). In that study, with respect to the individualized target and based on computational head models, the depth-corrected intensity was used with the aim of delivering an equivalent stimulation dose to all personalized targets. In a similar approach, two other studies in depression (38, 39) used depth-corrected intensity based on individualized scalp-to-cortex distance measured from each patient’s anatomical MRI and also utilized fMRI targeting with the highest number of sessions per day, total number of sessions, and total number of pulses. Although stimulation intensity and targeting method parameters were not systematically isolated and other variables (e.g., number of sessions per day) simultaneously changed, it remains unclear how electric field intensity and targeting method contribute to treatment response. However, other supporting evidence comes from studies that compared the therapeutic potential of target site personalization to other targeting methods and reported a better antidepressant outcome when the group-average target was closer to the personalized target (40–42).
Research conducted so far has aimed to assess the impact of brain stimulation technologies on clinical outcomes in SUDs. However, given the variability in methodology and population responses to stimulation, it has been challenging to reach a consensus on target selection. Using the approaches described above, we propose the frontopolar cortex as a highly promising target for SUDs, although it has been investigated to a lesser extent than DLPFC stimulation. Here, we synthesize converging evidence from multiple sources, including lesion-based maps, fMRI-based maps, TMS studies, tES studies, and dose-response relationships using electric field modeling, that emphasize the utility of the frontopolar cortex as a treatment target for SUDs (Figure 2).
FIGURE 2. Converging evidence for therapeutic brain stimulation targetsa.
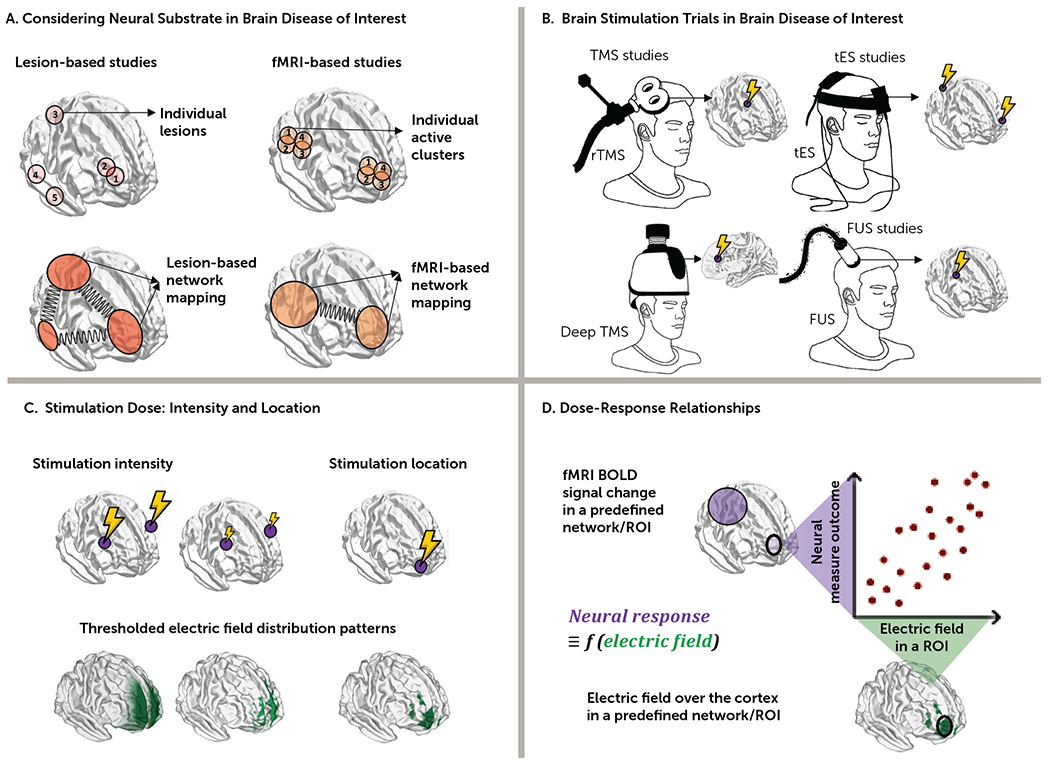
a In panel A, target selection is informed by network-based lesions or fMRI maps at the individual or group level. In panel B, previously reported results in clinical trials with different noninvasive brain stimulation methods inform future experimental designs. In panel C, different factors affect stimulation dose over the cortex, such as stimulation location and intensity; head models can illustrate the effects of these factors on stimulation dose. In panel D, the relationship between outcome measures from neural substrates (e.g., fMRI in panel A) and stimulation dose to a particular network (e.g., electric fields in panel C) aids our understanding of how noninvasive brain stimulation–induced electric fields or magnitude of stimulation site connectivity to a network ultimately modulate brain functions (for example, do larger electric fields in a predefined region of interest or network cause stronger neural response?). BOLD=blood-oxygen-level-dependent; FUS=focused ultrasound stimulation; ROI=region of interest; rTMS=repetitive transcranial magnetic stimulation; tES=transcranial electrical stimulation.
EVIDENCE FROM LESION-BASED MAPPING
Brain lesion studies, which focus on damage that has occurred to a specific part of the brain, are used to localize human brain functions and identify causal links between symptoms and neuroanatomy (21). Combining causal mapping of human brain functions based on brain lesions and brain stimulation with modern neuroimaging techniques like fMRI provides new insights into the role of different brain areas in neuropsychiatric disease. For example, individuals with insula lesions have been shown to be more likely to quit tobacco smoking easily and to remain abstinent (23). This causal knowledge about the functions of specific brain areas (e.g., insular cortex) can be translated into therapeutic targets for brain stimulation treatment programs. In this regard, the recent lesion network mapping study by Joutsa et al. (26) adds growing attention to the frontopolar cortex as a target area for SUD treatment. In that study, addiction remission (i.e., quitting tobacco smoking easily and remaining abstinent) was more likely after strokes in areas that had negative functional connectivity to the medial frontopolar and temporal cortices and positive connectivity to the dorsal cingulate, lateral prefrontal cortex, and insula. These brain regions could be used as neuromodulation treatment targets. The medial frontopolar cortex, the strongest negative peak, can be directly reached with transcranial brain stimulation (43–45). This suggests that high-frequency rTMS, which is usually believed to increase cortical excitability, would be expected to reduce addiction when applied to the frontopolar cortex. Indeed, this peak in the frontopolar cortex overlapped with peak electric fields of TMS coils that were effective for SUDs in multicenter trials, including the coil that is FDA cleared for smoking cessation (14). Although the primary sample of the Joutsa et al. study (26) comprised participants with tobacco use disorder, the results appeared to generalize to other SUDs. Recently, this same network was found to align with neuroimaging abnormalities across all substances of abuse (46). As lesion connectivity has been demonstrated to correlate with treatment effectiveness and has proven beneficial in identifying more successful TMS targets across various disorders (21, 27, 47), these observations support the use of excitatory noninvasive brain stimulation targeting the frontopolar cortex for the treatment of SUDs (Figure 3A).
FIGURE 3. Evidence from brain imaging maps highlights the role of the frontopolar cortex as an optimal treatment target in addictiona.
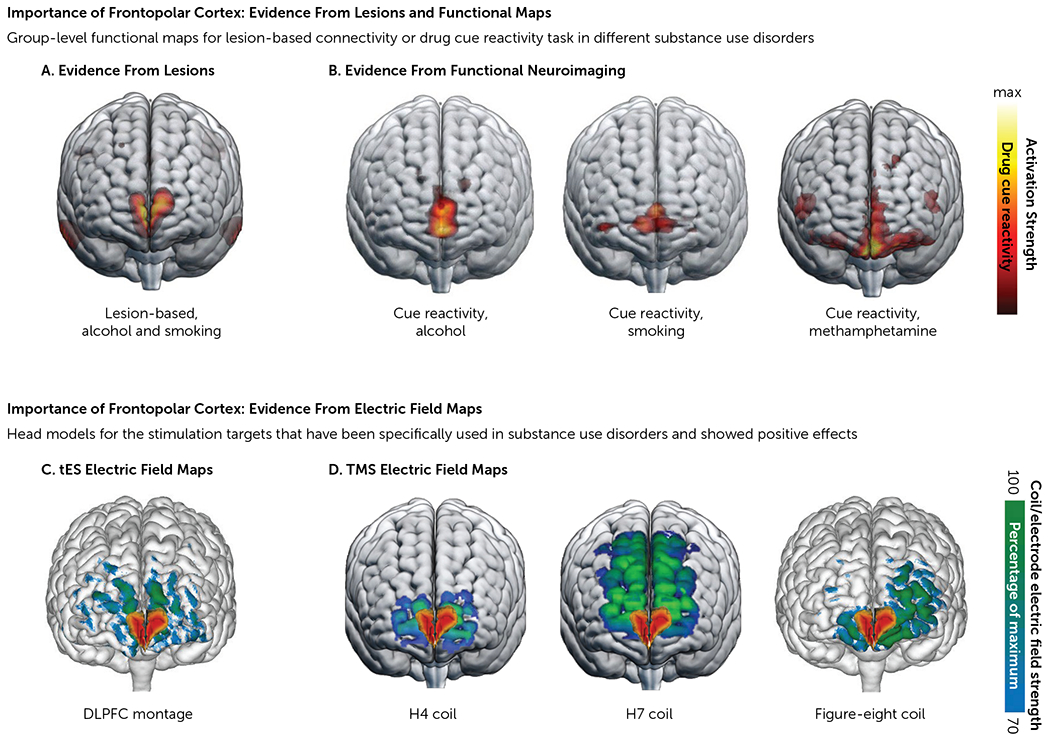
a The upper half of the figure illustrates evidence from lesions and functional mapping. Panel A is lesion-based map illustrating functional connectivity of lesions that lead to addiction remission, as reported by Joutsa et al. (26). Panel B illustrates functional neuroimaging maps showing active voxels obtained from a whole-brain response to a standard fMRI drug cue reactivity task in three studies: in heavy alcohol users (N=53), reported by Hanlon et al. (57); in participants with tobacco use disorder (N=48), reported by Hanlon et al. (57); and in participants with methamphetamine use disorder (N=65), reported by Ekhtiari et al. (119). The lower half of the figure illustrates evidence from electric field maps of transcranial brain stimulation protocols that have been used in substance use disorders with positive outcomes. Note the overlap between the functional map in the lesion-based study in panel A and the electric field distribution patterns in panel C, with the commonly used tES montage (target/reference electrodes: over F3/Fp2, 5×7 cm, with 2 mA intensity [e.g., 72]) and deep TMS (including H4 [e.g., 120] and H7 coils [e.g., 61] and conventional TMS with a figure-eight coil over Fp1 [e.g., 62]). DLPFC=dorsolateral prefrontal cortex; tES=transcranial electric stimulation; TMS=transcranial magnetic stimulation.
EVIDENCE FROM fMRI-BASED MAPS
fMRI data represent a powerful experimental method to identify the optimum stimulation target while considering underlying brain function. According to fMRI findings, the frontopolar cortex is a key region for cognitive flexibility (48) and decision-making procedures (e.g., value-based [49] and unconscious [50] decision making). In addiction research, functional neuroimaging has revealed that the frontopolar cortex, along with other brain regions, such as the inferior frontal gyrus/insula, is reliably activated by drug cues (51). Additionally, its connection to other brain regions, such as the nucleus accumbens, may be altered over the course of different addiction stages (52). These frontal-striatal circuits are critical mediators of drug cue reactivity and habit formation (53), which have a well-replicated relationship with substance use outcomes and relapse (54). The medial frontopolar cortex is one of the primary hubs that mediate the valuation of drug-related stimuli during exposure to drug versus neutral cues. Moreover, modulatory network analysis has shown that the medial frontopolar area facilitates the interaction between default mode, frontoparietal, and salience networks, which are disrupted in SUDs (55, 56).
Numerous fMRI studies have demonstrated that the frontopolar cortex is involved in drug cue reactivity across different drug classes. One of the most extensive studies to date, conducted by Hanlon et al. (57), investigated the spatial topography of drug cue reactivity in a cohort of 156 individuals with tobacco, alcohol, or cocaine use disorders. The study revealed three clusters of cue-reactive activation in response to drug versus neutral cues, including the medial frontopolar cortex (Brodmann area 10) and the left and right insular cortices. Although insular cortex activity was predominantly driven by cocaine users, all three groups exhibited significant clusters of activity in the medial prefrontal cortex that extended anteriorly to the frontopolar cortex (Figure 3B). Projecting these clusters onto the standard EEG 10–20 system that is commonly used for TMS targeting, the medial frontopolar cortex (FPz) was the location closest to the largest percentage of hotspots (Figure 4). This location overlaps strikingly well with the lesion network map identified by Joutsa et al. (4), lending further credence to the notion that the cue-induced activation in the frontopolar aspect of the medial prefrontal cortex may be a transdiagnostic endophenotype of addiction, which can also be reflected in abnormal connectivity in the resting state as well as aberrant activation during disease-relevant tasks (51, 52, 58, 59).
FIGURE 4. Target selection based on brain-state clustering across multiple substance use disordersa.
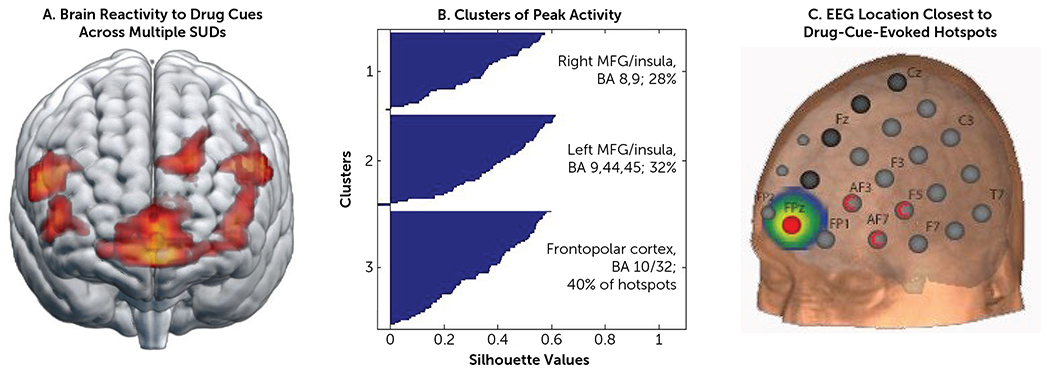
a Brain reactivity to drug cues versus non-drug cues was acquired from 156 non-treatment-seeking chronic cocaine users (N=55), heavy alcohol users (N=53), and participants with current tobacco use disorder (N=48) (57). Analyses were done at the group level (panel A) and at the individual level (panel B). For k-means clustering, the K++ algorithm, 1000 repetitions, and random seeding were used. Of the entire sample of 156 individuals, 103 had at least one cluster that was significantly elevated to the drug versus neutral cues. As illustrated in panel C, for the group as a whole, the EEG 10-10 coordinate FPz had the largest percentage of hotspots within 2 cm (11%), 3 cm (19%), 4 cm (32%), and 5 cm (49%). FPz was also the best location for alcohol cues and tobacco-related cues. The hotspots associated with cocaine cue reactivity were closest to AF3, AF7, and AF5, likely driven by points in the anterior insula. BA=Brodmann area; MFG=middle frontal gyrus; SUD=substance use disorder.
Expanding to other substance use disorders, in a recent study of 65 participants with methamphetamine use disorder, Ekhtiari et al. (60) demonstrated higher fMRI cue reactivity during drug versus neutral cues, which was most prominent in the medial frontopolar cortex (Figure 3B). This pattern overlaps with the drug cue reactivity findings of Hanlon et al. (57), lesion-based peaks reported by Joutsa et al. (26) (Figure 3A), and electric field maps of effective noninvasive brain stimulation targets (Figure 3C, D).
EVIDENCE FROM PREVIOUS TMS CLINICAL TRIALS
Research increasingly supports the frontopolar aspect of the medial prefrontal cortex as a promising target for transdiagnostic TMS interventions for SUDs. At least 12 TMS studies (with both inhibitory and excitatory stimulation protocols) have demonstrated that stimulating the frontopolar cortex can modulate cortical-striatal circuits involved in drug cue reactivity, leading to reductions in drug craving and/or consumption, and none of them reported negative results (4, 5, 61–70). For example, cTBS applied to the frontopolar cortex for non-treatment-seeking cocaine users and heavy alcohol users reduced neural reactivity to cocaine cues and alcohol cues (5), with the effects influenced by gray and white matter integrity (64). Another study found that cTBS to the frontopolar cortex in non-treatment-seeking chronic cocaine users and alcohol-dependent individuals decreased TMS-evoked BOLD signal in several cortical nodes that are believed to regulate salience processing and are typically activated by drug cues (62). A recent study of 74 inpatients with severe methamphetamine use disorder demonstrated that 10 sessions of active TMS to the frontopolar cortex (cTBS over Fp1), left DLPFC (iTBS over F3), or both targets significantly decreased craving, with the largest effect size observed in the group that received cTBS to the left frontopolar cortex (63). Craving scores in that study were assessed using a visual analogue scale at five time points (at baseline and twice weekly for 2 weeks). During these assessments, participants rated their cravings after being exposed to drug-related images for 5 minutes while recalling their last drug use, and the changes in craving were positively correlated with improvements in ratings of anxiety and withdrawal symptoms (63).
Furthermore, the electric fields induced by figure-eight TMS coils or deep TMS (Figure 3D) with H4 and H7 coils (intended to target the insula and the medial prefrontal/anterior cingulate cortex, respectively) overlap with the lesion locations associated with addiction remission (i.e., quitting tobacco smoking easily with no relapse) in the Joutsa et al. lesion study (Figure 3A) (26, 71). Of note, the deep TMS coil (H4), approved by the FDA for smoking cessation, is typically used to stimulate the insula and lateral prefrontal cortex, but its peak electric field intensity intersects with this medial frontopolar cortex target (Figure 3D).
EVIDENCE FROM PREVIOUS tES CLINICAL TRIALS
Of 89 tES experiments conducted in 76 published studies that successfully modulated drug craving or consumption, 79 used either unilateral (13 trials; anode: F3/F4; cathode: Fp2/Fp1) or bilateral (51 trials; anode/cathode over F3 or F4) electrode montages “over” the DLPFC (Figure 1). However, tES produces a current that flows through different anatomical structures in a complex manner, which means that the peak induced fields may not necessarily be in the cortical areas under the stimulating electrodes (32–34). Research has shown that even when the DLPFC is targeted (with unilateral or bilateral large electrode pads over F3/F4), the frontopolar area receives the strongest electric field in both healthy participants and people with SUDs (72). As a result, modulation of the frontopolar area may mediate the efficacy of tES when “targeting” the DLPFC (Figure 3C).
EVIDENCE FROM DOSE-RESPONSE RELATIONSHIPS
The analysis of the dose-response relationship in brain stimulation studies has not been well established. To the best of our knowledge, no dose-response relationship analysis has been published to explore the association between cortical electric fields and changes in neural response in the application of TMS for SUDs. However, we have investigated the extent to which individualized electric field distribution patterns over the cortex, as an indicator of the received stimulation dose, can explain neurophysiological outcomes of tES (73). The frontopolar cortex was the area where field strength was found to be related to the neurophysiological response to bilateral transcranial direct current stimulation (tDCS) over the DLPFC. Higher electric field strength was found to be correlated with greater BOLD signal change in the drug>neutral contrast in people with methamphetamine use disorder during a standard fMRI drug cue reactivity task (74). In a cohort of 60 inpatients with methamphetamine use disorder (60), unilateral DLPFC stimulation also showed a significant correlation between the normal component of the electric fields and BOLD signal change in the drug>neutral contrast. This finding was specific to the frontopolar area, which was identified as the brain region with maximum electric field strength across the population. A significant positive correlation between the normal component of the electric field and cue reactivity in the frontopolar area (more positive electric field correlated with greater BOLD signal change) indicated that tDCS over the right DLPFC can induce excitatory effects in neural reactivity to drug cues in the frontopolar cortex.
INTERINDIVIDUAL VARIABILITY
Despite converging evidence from lesion-based, fMRI, TMS, and tES studies pointing to the frontopolar cortex as a potential target for transcranial brain stimulation in SUDs, the reliability and generalizability of the individual-level outcomes are still questionable. Accumulating evidence in various clinical populations—for example, patients with major depressive disorder—indicates that responses to neuromodulatory interventions are variable, with a substantial portion of participants considered nonresponders (75, 76). Two main factors contribute to variation: differences in skull and brain anatomy, which affect the current flow and received stimulation dose over the cortex (32, 34, 77), and differences in brain state, function, and connectivity, which can cause variability even for the same current flow pattern (74, 78, 79). The first factor—skull and brain anatomy—is related to the estimation of the electric field magnitude and distribution patterns through the brain that interact with the underlying brain structure and should be carefully simulated using high-resolution structural images and finite element modeling (34, 80). These computational approaches were well suited to addressing anatomical variability between participants in response to the applied brain stimulation technique (77, 81). The other factor—brain state, function, and connectivity—is subject to the impact of the functional organization of local or distributed brain circuits such that the applied brain stimulation interacts with the underlying brain state (78, 82–84). To better understand how brain states affect response variations, brain mapping tools such as fMRI data can be used retrospectively to investigate differences within or between subjects (74). Variations in both brain factors could be quantified in terms of the strength and the location of a relevant measure extracted from the current flow and functional activity maps across a population. Responsiveness to frontopolar cortex stimulation may be changed, for example, according to the location and intensity of the maximum electric field within the frontopolar cortex or brain regions strongly connected to the frontopolar cortex.
At the group level, electric field distribution patterns and functional state in response to different drug-related cues indicate that electric field and functional activity are predominantly concentrated around the frontopolar cortex across different populations with SUDs (e.g., in alcohol use disorder [85]). However, group-level maps, by definition, are unable to represent interindividual variations in electric fields and functional connectivity or activity (86). Although a few clinical trials have implemented the use of individualized data (e.g., group-level electric fields or connectome-based data) to determine the cortical target for brain stimulation studies, findings in the field of depression emphasize the need for the development of personalized target selection and stimulation optimization strategies (36, 40, 42, 87). Two examples of the importance of individual differences in a group of participants with SUDs when targeting the frontopolar cortex are 1) the widely variable location and intensity of connectivity between the frontopolar cortex and the amygdala in response to drug cues (88) (Figure 5A), and 2) the significant variability in the location and intensity of the electric field in the frontopolar cortex during DLPFC tES among individuals with methamphetamine use disorder (88) (Figure 5B). This highlights the importance of precision functional mapping at the individual level in future frontopolar cortex stimulation studies.
FIGURE 5. Interindividual variability in targeting the frontopolar cortexa.
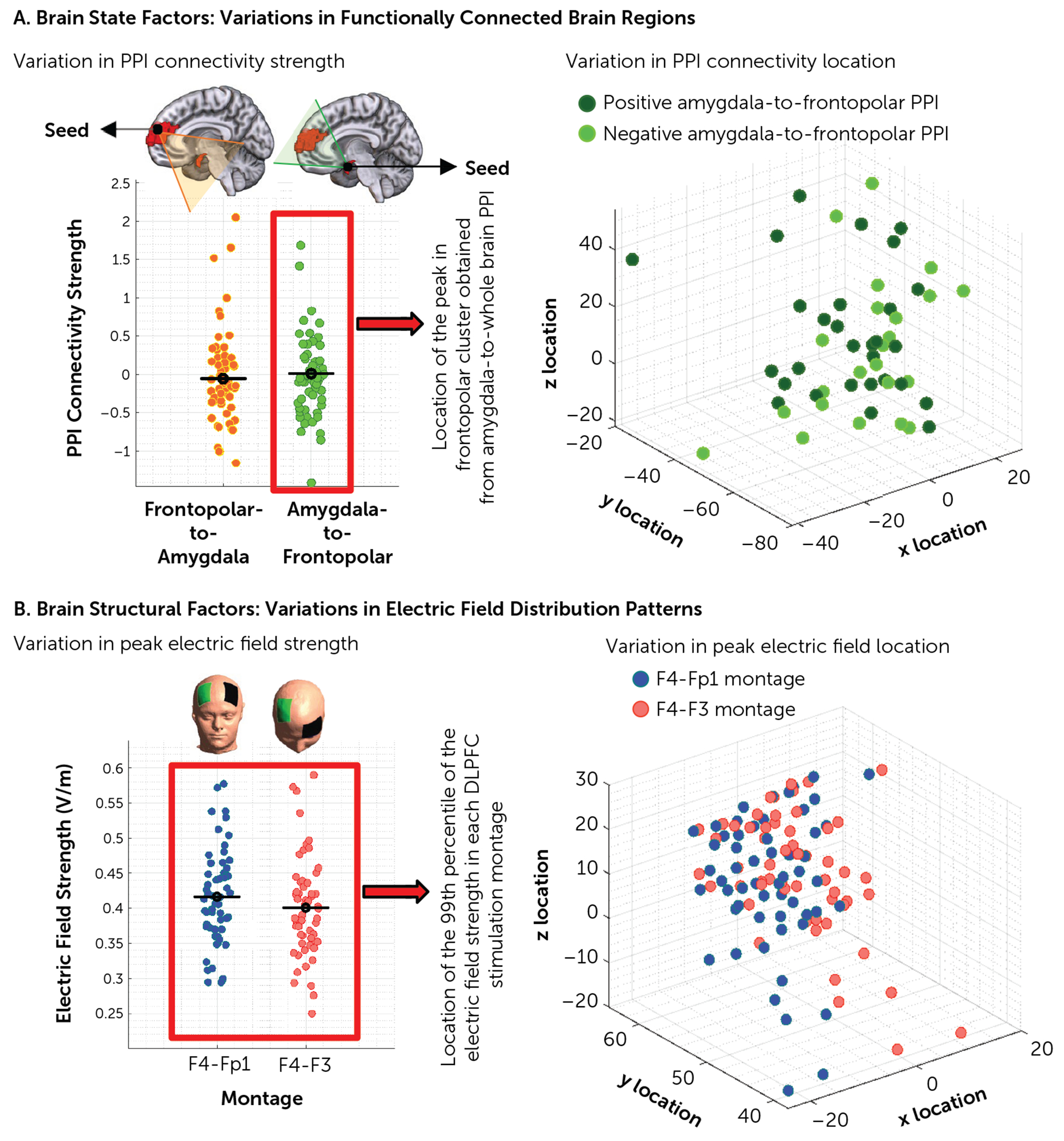
a Between-individual differences are visualized in terms of strength and the location of two main sources of variations (dots represent the data for individual subjects). Panel A illustrates brain state factors. Group-level frontopolar cortex-to-whole brain psychophysiological interaction (PPI) analysis showed a significant cluster in the amygdala, and group-level amygdala-to-whole brain PPI analysis showed a significant cluster in the frontopolar area. In the left-hand panel, PPI strength in each direction is presented for each subject. In the right-hand panel, the amygdala-to-whole brain peak location of the connected brain region in MNI space is represented for each subject; positive PPI connections are in dark green and negative PPI connections are in light green. Panel B illustrates brain structural factors. The left-hand panel shows electric field distribution patterns that were simulated for two of the most commonly used electrode montages, F4-Fp1 and F4-F3. The individualized strength of the 99th percentile of the electric field (which is commonly located in the frontopolar area) is presented for each montage; F4-Fp1 in red and F4-F3 in blue (left-hand panel). The location of the peak electric field in Montreal Neurological Institute space for each subject is also reported (right-hand panel). Results are reported for 60 participants with methamphetamine use disorder. DLPFC=dorsolateral prefrontal cortex.
TYPE OF FRONTOPOLAR CORTEX STIMULATION: EXCITATORY OR INHIBITORY
After identifying a promising target for neuromodulation, the next step is to determine the optimal stimulation protocol, which involves deciding whether to increase or decrease activity in the frontopolar area. The most well-established protocols for increasing cortical excitability using tES/TMS tools are anodal tDCS and iTBS and high-frequency TMS (5–20 Hz). However, these protocols may interact with the underlying brain state or neuronal architecture to result in inhibitory effects (89). While cathodal stimulation, cTBS, single-pulse stimulation, and low-frequency stimulation are commonly assumed to induce inhibitory effects, these protocols can also result in excitatory effects in certain doses, states, or regions. Given the differential effects of these stimulation protocols on neural excitability, it may be challenging to investigate how the type of frontopolar stimulation (e.g., high-frequency vs. low-frequency rTMS) would affect behavioral (e.g., drug consumption) or neural (e.g., BOLD signal change) outcomes.
Functional neuroimaging data are not informative per se on the directionality of neuromodulation in obtaining preferred behavior outcomes even when the basic causality of the targeted area for the preferred behavior is established (90). As an example, fMRI drug cue reactivity data usually do not provide any direction on whether activation is contributing to the craving induction and should be negatively modulated (inhibitory) or is an attempt to control craving and should be positively modulated (excitatory). In the lesion-based network derived by Joutsa et al. (26), the frontopolar cortex showed the opposite connectivity profile of lesions that led to addiction remission in terms of quitting tobacco smoking easily with no relapse (Figure 3A). They hypothesized that regions with the opposite connectivity profile (e.g., frontopolar cortex) should be good targets for excitatory brain stimulation, based on the logic that regions matching the connectivity profile of lesions leading to addiction remission should be good lesion targets (e.g., the paracingulate gyrus and anterior insula). This hypothesis seems to align well with use of the deep TMS coils, including the coil that is FDA cleared for smoking cessation, which use high-frequency stimulation over the medial prefrontal cortex, generally assumed to exert excitatory effects (in all three deep TMS studies for SUDs [61, 65, 66]). However, this proposal does not align with the figure-eight cTBS or single-pulse TMS results, which used cortical inhibition paradigms and led to a significant decrease in BOLD signal and attenuated stimulus-evoked activity in the medial prefrontal cortex (all figure-eight TMS studies for SUDs were cTBS [5, 62–64, 67] or single pulse [4]). It is also unclear whether this aligns with tES results. While the inward current (anodic effects) is thought to increase excitability, and the outward current (cathodic effects) is inhibitory, many tES studies have shown the same effect with anode and cathode switched (91). In TMS studies, as with tES, a certain percentage of participants show effects opposite to the usual direction or no effect at all (e.g., excitatory or neutral effects from an inhibitory stimulation such as 1 Hz rTMS, or showing inhibition rather than excitation in response to 10 Hz rTMS [92, 93]).
Hence, it is still unclear whether we should aim to facilitate or inhibit activity in the frontopolar area. If we increase the local field potential in the medial frontopolar cortex, which houses both glutamatergic pyramidal cells and GABAergic interneurons, the resulting effect on firing at the afferent targets remains uncertain, whether it leads to a net increase or decrease. While functional connectivity can assess the direction and strength of the temporal correlation between brain regions, a more mechanistic neurobiological inquiry is needed to evaluate the activity magnitude at each node independently. The overall effect could be influenced by several factors, including stimulation intensity (with low and high intensities tending to inhibit and facilitate, respectively), number of pulses, electric field direction (inward or outward), stimulation duration, and brain state (94–96). For example, a study on 28 individuals with refractory binge-purge eating disorders found that the outcomes of 30 sessions of 10 Hz rTMS over the dorsomedial prefrontal cortex depended on baseline functional connectivity, such that responders had lower baseline frontal-striatal connectivity than nonresponders (97). Therefore, in future studies, the state dependency of stimulation outcomes and dynamic transitions between brain states during the stimulation periods should be considered in the study design (e.g., by designing a task to optimize target engagement or the stimulation dose at the individual level).
Although brain responses to stimulation have been found to go beyond the expected “excitatory” or “inhibitory” effects of neuromodulatory protocols, the description of excitatory and inhibitory effects of brain stimulation is commonly based on motor-evoked potential (MEP) data (98). However, a direct comparison of MEP and changes in brain circuits regarding resting-state functional connectivity shows no correlation between the two measures (99). At the brain circuit level, regardless of whether excitatory and inhibitory stimulation produce opposite effects, they both have the potential to disrupt or modulate connectivity. For example, resting-state connectivity is measured based on the correlation between two time courses, meaning that excitatory or inhibitory stimulation of one region can alter its time course and decrease its correlation with other brain regions. This disruption in connectivity can occur with both excitatory and inhibitory stimulation, leading to circuit modulation. For instance, a study using deep TMS over the right insula reported disrupted connectivity between the insula and the mPFC for both 1 Hz and 10 Hz single-session rTMS compared with sham stimulation (100). Even at the MEP level, it has been shown that there is significant interindividual variability in response to cTBS; in one study, around 57% of participants showed a decrease in cortical excitability, while others showed an increase or no change (101). Therefore, it is possible that excitatory and inhibitory stimulation may not necessarily have opposite effects on the frontopolar area, and this could be explained by high rates of interindividual variability following both stimulation paradigms.
TOLERABILITY OF FRONTOPOLAR CORTEX STIMULATION
One concern regarding targeting the frontopolar area is related to tolerability and discomfort, particularly in supra-threshold techniques such as TMS. The feasibility and tolerability of TMS over the frontopolar area have been investigated, and the results showed that TMS over the frontopolar area at 110% of resting motor threshold is well tolerated and is not associated with significantly more discomfort than TMS over the DLPFC (55). Of 129 individuals who received multiple sessions of TMS over the frontopolar area, none failed to complete their treatments as a result of pain or discomfort (55). In tES studies over the frontopolar area, no adverse effects were reported by participants. For example, it has been shown that applying 1 or 1.5 mA tDCS with anode/cathode over frontopolar cortex/vertex via a 5×5 cm electrode as anode and a 10×10 cm electrode as the cathode is well tolerated, and applying tDCS via two 5×6 cm electrodes over the frontopolar cortex and forearm is also tolerable (102–104). In sum, frontopolar cortex stimulation is generally well tolerated when the ramping procedure is taken into account. Implementation of novel TMS coils or electrode placement in future studies can help improve the safety and tolerability of frontopolar cortex stimulation.
OTHER APPROACHES AND EFFECTS IN TARGETING THE FRONTOPOLAR CORTEX
While targeting the frontopolar area and circuits will have some direct effects, recent studies have demonstrated the possibility of indirectly targeting subcortical areas through cortico-subcortical connections (105, 106). For example, the ventromedial prefrontal network comprises cortical, subcortical, and striatal nodes, and targeting the frontopolar cortex as a part of this network with transcranial brain stimulation technologies can indirectly modulate other parts of the network (107). In the same vein, indirect targeting of the frontopolar area is also possible through other cortical brain regions that are structurally or functionally connected to it. Previous studies have identified robust connections between the frontopolar area and other cortical regions in the temporal lobe, such as the superior temporal gyrus and the medial temporal cortex (108, 109). The addiction remission circuit derived from lesion studies also included both positively connected (insula, cingulate, DLPFC) and negatively connected (frontopolar) regions (26). Consequently, while the frontopolar cortex is one of the most promising noninvasive brain stimulation targets, it may only serve as a gateway to the entire network, and multisite stimulation might boost the effect further by modulating connected brain areas. For example, it has been found that the combined stimulation of the DLPFC and frontopolar cortex (combination of iTBS over the DLPFC and cTBS over the ventromedial PFC) reduced cravings more effectively than stimulating the DLPFC (using iTBS) or frontopolar cortex (using cTBS) alone in the treatment of patients with severe methamphetamine use disorder (63).
BEHAVIOR/BRAIN STATE
There is an increasing recognition of the significance of brain “state” on the directionality and amplitude of tES/TMS effects. This can be seen in the contrast between collecting an active versus resting motor threshold with TMS, where even a slight muscle engagement can significantly enhance the TMS-evoked response. Although this is more difficult to measure outside the motor system, it has also been shown in conditions such as PTSD (110), obsessive-compulsive disorder (OCD) (111), and addiction (112). In such scenarios, participants are often given a provocation script to engage a particular brain state (e.g., imagining their greatest trigger for craving, listening to an audio script with instructions to handle a cigarette and a lighter, and viewing pictures of tobacco-related cues [14]), and the targeted brain regions appear to be more responsive to the induced electric fields. The idea is that certain brain networks are triggered by a particular task, such as provocation, and as a result of their existing activation, they are more susceptible to modulation by tES or TMS (79, 113). For instance, Dinur-Klein et al. (112) found that in a group of participants with tobacco use disorder, TMS with and without provocation was more effective when the stimulation was delivered after the presentation of tobacco-related cues. Similarly, FDA-approved protocols for OCD and smoking cessation also include symptom provocation to elicit a moderate level of obsessional distress or craving before each stimulation session, and it is anticipated that the use of provocation-based brain stimulation studies will continue to increase (14, 114). Thus, designing an appropriate task to optimally target the desired brain region, such as the frontopolar cortex, during neuromodulation may be crucial.
FUTURE DIRECTIONS
Future research directions in neuromodulation for SUDs could include network-level dose-response studies that integrate data at both the individual and group levels. By manipulating intrinsic or extrinsic variables, such as ongoing brain state and stimulation parameters, it may be possible to delineate dose-response relationships and characterize response profiles for the targeted brain networks (115). Furthermore, brain-wide mapping of lesions/fMRI/PET, collection of tES/TMS-evoked BOLD signal changes, and integration of the results with computational head models can help identify network origins of changes in behavioral task performance or clinical outcomes such as drug craving or consumption (116).
Future trials tailored to individuals, such as Stanford Neuromodulation Therapy (36), and closed-loop tES, TMS-fMRI, or EEG (117, 118) can help to rapidly and effectively target specific brain regions with optimal stimulation doses for each person. Addressing between-subject variations in targeting the frontopolar cortex and finding methods to reliably measure brain response/target at the individual level should be prioritized in future studies. Recording the exact location of the stimulation coil or electrode montage for each person in future clinical trials can help map heterogeneity in response/target, ultimately establishing the optimal target site at the individual level and leading to pragmatic improvements in treatment designs for SUDs.
CONCLUSIONS
The evidence based on brain lesion maps, fMRI drug cue reactivity studies, and simulations of the electric field in previously successful transcranial brain stimulation converge and support the frontopolar cortex as a target for transcranial stimulation across various substance use disorders. Additionally, our evidence suggests that the frontopolar cortex may be mediating the observed clinical effects of tES/TMS protocols even when the frontopolar cortex is not necessarily the intended stimulation target. However, further research is needed to pinpoint the optimal individualized frontopolar coordinates and stimulation dose and pattern over the targeted region to maximize clinical benefits at both the individual and group levels.
Acknowledgments
Supported in part by the William K. Warren Foundation, by grant 1P20GM121312 from the National Institute of General Medical Sciences Center, by NIDA grant U01DA050989, by funds from the Laureate Institute for Brain Research and the Medical Discovery Team on Addiction at University of Minnesota, and by a Brain and Behavior Foundation (NARSAD) Young Investigator Award to Dr. Ekhtiari. Dr. Moussawi is funded by NIDA grant DA048085 and NIAAA grant AA030505. Dr. Fox is funded by the Nancy Lurie Marks Foundation, the Kaye Family Research Endowment, the Baszucki Brain Research Fund, and NIH grants R01MH113929, R21MH126271, R56AG069086, R01MH115949, and R01AG060987.
Dr. Joutsa has received grants from the Finnish Foundation for Alcohol Studies, the Finnish Medical Foundation, the Instrumentarium Research Foundation, the Sigrid Juselius Foundation, Turku University Hospital (VTR funds), and University of Turku, congress travel support from Abbott and AbbVie, and lecturer honoraria from Lundbeck and Novartis, and he has served as consultant for Adamant Health and Summaryx. Dr. Siddiqi has served as a scientific consultant for Magnus Medical and as a clinical consultant for Acacia Mental Health, Boston Precision Neurotherapeutics, and Kaizen Brain Center; he has received investigator-initiated research funding from BrainsWay and Neuronetics; he has served as a speaker for BrainsWay and for PsychU.org (sponsored by Otsuka); he owns intellectual property involving the use of functional connectivity to target TMS; and he owns stock in BrainsWay and Magnus Medical. Dr. Bikson has served as a consultant for, received grants from, served on scientific advisory boards, or has invention assignment agreements with Allergan (AbbVie), Apple, Biovisics, Boston Scientific, Ceragem, GlaxoSmithKline, Google X, Halo Neuroscience, Humm, i-Lumen, Lumenis, Mecta, SafeToddles, and Ybrain; he is an inventor on a patent on brain stimulation held by City University of New York; and he has equity in Soterix Medical. Dr. Paulus has served as an adviser for Hoffmann–La Roche and Spring Care, and he has received royalties from UpToDate. Dr. Fox holds intellectual property on the use of brain connectivity imaging to analyze lesions and guide brain stimulation; he has served as a consultant for Abbott, Boston Scientific, Magnus Medical, and Soterix; and he has received investigator-initiated research funding from Neuronetics. Dr. Hanlon is employed by and has financial interests in BrainsWay. Dr. Ekhtiari has received honoraria from Indivior for speaking at educational events. The other authors report no financial relationships with commercial interests.
Continuing Medical Education
You can earn CME credits by reading this article. Three articles in every American Journal of Psychiatry issue comprise a short course for up to 1 AMA PRA Category 1 Credit™ each. The course consists of reading the article and answering three multiple-choice questions with a single correct answer. CME credit is issued only online. Readers who want credit must subscribe to the AJP Continuing Medical Education Course Program (psychiatryonline. org/cme), select The American Journal of Psychiatry at that site, take the course(s) of their choosing, complete an evaluation form, and submit their answers for CME credit. A certificate for each course will be generated upon successful completion. This activity is sponsored by the American Psychiatric Association.
Examination Questions for “Converging Evidence for Frontopolar Cortex as a Target for Neuromodulation in Addiction Treatment”
- What are the converging levels of evidence provided in this paper to support frontopolar cortex as a target for neuromodulation in addiction treatment?
- Pharmacological trials, quantitative EEG mapping, animal models, gene manipulation
- Lesion based maps, functional maps, brain stimulation trials, dose-response relationship
- Epidemiologic studies, quasi-causal modeling, electric field manipulation, effective connectivity
- Optimization trials, safety studies, adherence measurement, gene linkage mapping
- What are the two main factors that contribute to the variations in response to a neuromodulation in the individual level (making some patients response and some non-responsive)?
- Differences in skull and brain anatomy and differences in brain state, function, and connectivity
- Differences in the neuromodulation technology and differences in duration of stimulation
- Differences in the level of education in the staff and differences in patients’ adherence
- Differences in selected targets and differences in post stimulation management
- To address “state dependency” in response to brain stimulation in people with substance use disorders and to increase the efficacy of the intervention, which of the following strategies were most effectively implemented:
- Structural MRI
- Priming with medications
- Drug cue provocation
- Electric field modeling
Contributor Information
Ghazaleh Soleimani, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis
Juho Joutsa, Turku Brain and Mind Center, Clinical Neurosciences, University of Turku, and Neurocenter and Turku PET Center, Turku University Hospital, Turku, Finland
Khaled Moussawi, Department of Psychiatry, University of Pittsburgh, Pittsburgh
Shan H. Siddiqi, Center for Brain Circuit Therapeutics and Departments of Neurology, Psychiatry, Neurosurgery, and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston
Rayus Kuplicki, Laureate Institute for Brain Research, Tulsa, Okla
Marom Bikson, Department of Biomedical Engineering, City College of New York, New York
Martin P. Paulus, Laureate Institute for Brain Research, Tulsa, Okla
Michael D. Fox, Center for Brain Circuit Therapeutics and Departments of Neurology, Psychiatry, Neurosurgery, and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston
Colleen A. Hanlon, Department Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, N.C.
Hamed Ekhtiari, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis; Laureate Institute for Brain Research, Tulsa, Okla
Data availability:
fMRI data and computational head models related to the 65 participants with methamphetamine use disorder are available on request from the corresponding author. The fMRI drug cue reactivity task and its codes are available at https://github.com/rkuplicki/LIBR_FDCR_Dynamic. The data associated with cue reactivity in participants with cocaine, alcohol, and tobacco use disorders are available on request from Dr. Hanlon. More details on lesion maps can be found in the supplement to reference 26.
REFERENCES
- 1.Saunders BT, Richard JM, Janak PH: Contemporary approaches to neural circuit manipulation and mapping: focus on reward and addiction. Philos Trans R Soc Lond B Biol Sci 2015; 370:20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekhtiari H, Tavakoli H, Addolorato G, et al. : Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev 2019; 104:118–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanlon CA, Canterberry M, Taylor JJ, et al. : Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One 2013; 8:e67917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanlon CA, Dowdle LT, Moss H, et al. : Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: an interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology 2016; 41:3032–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney-Ramos TE, Dowdle LT, Lench DH, et al. : Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney-Ramos TE, Lench DH, Hoffman M, et al. : Gray and white matter integrity influence TMS signal propagation: a multimodal evaluation in cocaine-dependent individuals. Sci Rep 2018; 8:3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinney KR, Hanlon CA: Changing cerebral blood flow, glucose metabolism, and dopamine binding through transcranial magnetic stimulation: a systematic review of transcranial magnetic stimulation-positron emission tomography literature. Pharmacol Rev 2022; 74:918–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diana M: The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2011; 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steele VR: Transcranial magnetic stimulation as an interventional tool for addiction. Front Neurosci 2020; 14:592343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moretti J, Poh EZ, Rodger J: rTMS-induced changes in glutamatergic and dopaminergic systems: relevance to cocaine and methamphetamine use disorders. Front Neurosci 2020; 14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND: Personalizing the treatment of substance use disorders. Am J Psychiatry 2020; 177:113–116 [DOI] [PubMed] [Google Scholar]
- 12.Ekhtiari H, Nasseri P, Yavari F, et al. : Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog Brain Res 2016; 223:115–141 [DOI] [PubMed] [Google Scholar]
- 13.Soleimani G, Ekhtiari H: INTAM Live Systematic Review. 2023. https://osf.io/sv8ky/
- 14.Zangen A, Moshe H, Martinez D, et al. : Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 2021; 20:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.magVenture: MagVenture receives CE approvals for noninvasive brain treatment of addiction, OCD, and depression with anxiety symptoms. 2021. https://www.prnewswire.com/news-releases/magventure-receives-ce-approvals-for-noninvasive-brain-treatment-of-addiction-ocd-and-depression-with-anxiety-symptoms-301278708.html
- 16.Liu Q, Yuan T: Noninvasive brain stimulation of addiction: one target for all? Psychoradiology 2021; 1:172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahbabaie A, Ebrahimpoor M, Hariri A, et al. : Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav 2018; 8:e00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Du L, Sahlem GL, et al. : Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend 2017; 174:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen JM, van Wingen G, van den Brink W, et al. : Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur Neuropsychopharmacol 2015; 25:2230–2239 [DOI] [PubMed] [Google Scholar]
- 20.Ekhtiari H, Faghiri A, Oghabian MA, et al. : Functional neuroimaging for addiction medicine: from mechanisms to practical considerations. Prog Brain Res 2016; 224:129–153 [DOI] [PubMed] [Google Scholar]
- 21.Siddiqi SH, Kording KP, Parvizi J, et al. : Causal mapping of human brain function. Nat Rev Neurosci 2022; 23:361–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson S: Commentary: substance use and the brain: it is not straightforward to differentiate cause from consequence: a commentary on Kim-Spoon et al (2020). J Child Psychol Psychiatry 2021; 62:437–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi NH, Rudrauf D, Damasio H, et al. : Damage to the insula disrupts addiction to cigarette smoking. Science 2007; 315:531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muskens J, Schellekens AFA, de Leeuw FE, et al. : Damage in the dorsal striatum alleviates addictive behavior. Gen Hosp Psychiatry 2012; 34:702.e9–e702.e11 [DOI] [PubMed] [Google Scholar]
- 25.Gaznick N, Tranel D, McNutt A, et al. : Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine Tob Res 2014; 16:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joutsa J, Moussawi K, Siddiqi SH, et al. : Brain lesions disrupting addiction map to a common human brain circuit. Nat Med 2022; 28:1249–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqi SH, Schaper FLWVJ, Horn A, et al. : Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav 2021; 5:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD: Mapping symptoms to brain networks with the human connectome. N Engl J Med 2018; 379:2237–2245 [DOI] [PubMed] [Google Scholar]
- 29.Lee WH, Kennedy NI, Bikson M, et al. : A computational assessment of target engagement in the treatment of auditory hallucinations with transcranial direct current stimulation. Front Psychiatry 2018; 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox MD, Liu H, Pascual-Leone A: Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 2013; 66:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash RF, Zalesky A, Thomson RH, et al. : Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry 2019; 86:e5–e7 [DOI] [PubMed] [Google Scholar]
- 32.Opitz A, Paulus W, Will S, et al. : Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015; 109:140–150 [DOI] [PubMed] [Google Scholar]
- 33.Miranda PC, Faria P, Hallett M: What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol 2009; 120:1183–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta A, Bansal V, Diaz J, et al. : Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2009; 2:201–207, 207.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thielscher A, Opitz A, Windhoff M: Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 2011; 54:234–243 [DOI] [PubMed] [Google Scholar]
- 36.Cole EJ, Phillips AL, Bentzley BS, et al. : Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry 2022; 179:132–141 [DOI] [PubMed] [Google Scholar]
- 37.Menardi A, Ozdemir RA, Momi D, et al. : Effect of group-based vs individualized stimulation site selection on reliability of network-targeted TMS. NeuroImage 2022; 264:119714. [DOI] [PubMed] [Google Scholar]
- 38.Williams NR, Sudheimer KD, Bentzley BS, et al. : High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain 2018; 141:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole EJ, Stimpson KH, Bentzley BS, et al. : Stanford Accelerated Intelligent Neuromodulation Therapy for treatment-resistant depression. Am J Psychiatry 2020; 177:716–726 [DOI] [PubMed] [Google Scholar]
- 40.Cash RF, Cocchi L, Lv J, et al. : Functional magnetic resonance imaging–guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry 2021; 78:337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqi SH, Weigand A, Pascual-Leone A, et al. : Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: an independent replication. Biol Psychiatry 2021; 90:e55–e56 [DOI] [PubMed] [Google Scholar]
- 42.Kong G, Wei L, Wang J, et al. : The therapeutic potential of personalized connectivity-guided transcranial magnetic stimulation target over group-average target for depression. Brain Stimul 2022; 15:1063–1064 [DOI] [PubMed] [Google Scholar]
- 43.Csifcsák G, Boayue NM, Puonti O, et al. : Effects of transcranial direct current stimulation for treating depression: a modeling study. J Affect Disord 2018; 234:164–173 [DOI] [PubMed] [Google Scholar]
- 44.McCalley DM, Hanlon CA: Regionally specific gray matter volume is lower in alcohol use disorder: implications for noninvasive brain stimulation treatment. Alcohol Clin Exp Res 2021; 45:1672–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soleimani G, Saviz M, Bikson M, et al. : Group and individual level variations between symmetric and asymmetric DLPFC montages for tDCS over large scale brain network nodes. Sci Rep 2021; 11:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stubbs JL, Taylor JJ, Siddiqi SH, et al. : Heterogeneous neuroimaging findings across substance use disorders localize to a common brain network. Nat Mental Health 2023; 1:772–781 ( 10.1038/s44220-023-00128-7) [DOI] [Google Scholar]
- 47.Kim NY, Taylor JJ, Kim YW, et al. : Network effects of brain lesions causing central poststroke pain. Ann Neurol 2022; 92:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koechlin E, Hyafil A: Anterior prefrontal function and the limits of human decision-making. Science 2007; 318:594–598 [DOI] [PubMed] [Google Scholar]
- 49.Gläscher J, Adolphs R, Damasio H, et al. : Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci U S A 2012; 109:14681–14686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bode S, He AH, Soon CS, et al. : Tracking the unconscious generation of free decisions using ultra-high field fMRI. PloS One 2011; 6:e21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schacht JP, Anton RF, Myrick H: Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 2013; 18:121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camchong J, Macdonald AW IIIrd, Mueller BA, et al. : Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend 2014; 139:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riga D, Matos MR, Glas A, et al. : Optogenetic dissection of medial prefrontal cortex circuitry. Front Syst Neurosci 2014; 8:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vafaie N, Kober H: Association of drug cues and craving with drug use and relapse: a systematic review and meta-analysis. JAMA Psychiatry 2022; 79:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith HR, Imperatore JP, Hanlon CA: The frontal pole as a target for transcranial magnetic stimulation: a retrospective analysis of feasibility and tolerability. Brain Stimul 2021; 14:655–657 [DOI] [PubMed] [Google Scholar]
- 56.Sridharan D, Levitin DJ, Menon V: A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008; 105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanlon CA, Dowdle LT, Gibson NB, et al. : Cortical substrates of cue-reactivity in multiple substance dependent populations: transdiagnostic relevance of the medial prefrontal cortex. Transl Psychiatry 2018; 8:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Killen JD, Fortmann SP: Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol 1997; 5:137–142 [DOI] [PubMed] [Google Scholar]
- 59.Courtney KE, Schacht JP, Hutchison K, et al. : Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol 2016; 21:3–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ekhtiari H, Soleimani G, Kuplicki R, et al. : Transcranial direct current stimulation to modulate fMRI drug cue reactivity in methamphetamine users: a randomized clinical trial. Hum Brain Mapp 2022; 43:5340–5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harel M, Perini I, Kämpe R, et al. : Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry 2022; 91:1061–1069 [DOI] [PubMed] [Google Scholar]
- 62.Hanlon CA, Dowdle LT, Correia B, et al. : Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend 2017; 178:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen T, Su H, Li R, et al. : The exploration of optimized protocol for repetitive transcranial magnetic stimulation in the treatment of methamphetamine use disorder: a randomized sham-controlled study. Ebiomedicine 2020; 60:103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanlon CA, Lench DH, Dowdle LT, et al. : Neural architecture influences repetitive transcranial magnetic stimulation–induced functional change: a diffusion tensor imaging and functional magnetic resonance imaging study of cue-reactivity modulation in alcohol users. Clin Pharmacol Ther 2019; 106:702–705 [DOI] [PubMed] [Google Scholar]
- 65.Martinez D, Urban N, Grassetti A, et al. : Transcranial magnetic stimulation of medial prefrontal and cingulate cortices reduces cocaine self-administration: a pilot study. Front Psychiatry 2018; 9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceccanti M, Inghilleri M, Attilia ML, et al. : Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation: a pilot study. Can J Physiol Pharmacol 2015; 93:283–290 [DOI] [PubMed] [Google Scholar]
- 67.McCalley DM, Kaur N, Wolf JP, et al. : Medial prefrontal cortex theta burst stimulation improves treatment outcomes in alcohol use disorder: a double-blind, sham-controlled neuroimaging study. Biol Psychiatry Glob Open Sci 2022; 3:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marques RC, Marques D, Vieira L, et al. : Left frontal pole repetitive transcranial magnetic stimulation reduces cigarette cue-reactivity in correlation with verbal memory performance. Drug Alcohol Depend 2022; 235:109450. [DOI] [PubMed] [Google Scholar]
- 69.Kearney-Ramos TE, Dowdle LT, Mithoefer OJ, et al. : State-dependent effects of ventromedial prefrontal cortex continuous thetaburst stimulation on cocaine cue reactivity in chronic cocaine users. Front Psychiatry 2019; 10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ankit A, Das B, Dey P, et al. : Efficacy of continuous theta burst stimulation-repetitive trancranial magnetic stimulation on the orbito frontal cortex as an adjunct to naltrexone in patients of opioid use disorder and its correlation with serum BDNF levels: a sham-controlled study. J Addict Dis 2022; 40:373–381 [DOI] [PubMed] [Google Scholar]
- 71.Hanlon CA, Philip NS, Price RB, et al. : A case for the frontal pole as an empirically derived neuromodulation treatment target. Biol Psychiatry 2019; 85:e13–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soleimani G, Kuplicki R, Camchong J, et al. : Are we really targeting and stimulating DLPFC by placing transcranial electrical stimulation (tES) electrodes over F3/F4? Hum Brain Mapp (Online ahead of print, Sep 26, 2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soleimani G, Kupliki R, Paulus M, et al. : Dose-response in modulating brain function with transcranial direct current stimulation: from local to network levels. PLoS Comput Biol 2023; 19:e1011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esmaeilpour Z, Shereen AD, Ghobadi-Azbari P, et al. : Methodology for tDCS integration with fMRI. Hum Brain Mapp 2020; 41:1950–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’Urso G, Dell’Osso B, Rossi R, et al. : Clinical predictors of acute response to transcranial direct current stimulation (tDCS) in major depression. J Affect Disord 2017; 219:25–30 [DOI] [PubMed] [Google Scholar]
- 76.Bailey N, Hoy KE, Rogasch NC, et al. : Differentiating responders and non-responders to rTMS treatment for depression after one week using resting EEG connectivity measures. J Affect Disord 2019; 242:68–79 [DOI] [PubMed] [Google Scholar]
- 77.Laakso I, Mikkonen M, Koyama S, et al. : Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex? Sci Rep 2019; 9:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li LM, Violante IR, Leech R, et al. : Brain state and polarity dependent modulation of brain networks by transcranial direct current stimulation. Hum Brain Mapp 2019; 40:904–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bikson M, Name A, Rahman A: Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013; 7:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laakso I, Tanaka S, Koyama S, et al. : Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul 2015; 8:906–913 [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Tames J, Asai A, Mikkonen M, et al. : Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J Neural Eng 2019; 16:036001. [DOI] [PubMed] [Google Scholar]
- 82.Chrysikou EG, Berryhill ME, Bikson M, et al. : Revisiting the effectiveness of transcranial direct current brain stimulation for cognition: evidence, challenges, and open questions (editorial). Front Hum Neurosci 2017; 11:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esmaeilpour Z, Marangolo P, Hampstead BM, et al. : Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul 2018; 11:310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jamil A, Batsikadze G, Kuo HI, et al. : Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: an fMRI study. Hum Brain Mapp 2020; 41:1644–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCalley DM, Hanlon CA: The importance of overlap: a retrospective analysis of electrical field maps, alcohol cue-reactivity patterns, and treatment outcomes for alcohol use disorder. Brain Stimul 2023; 16:724–726 [DOI] [PubMed] [Google Scholar]
- 86.Soleimani G, Kupliki R, Bodurka J, et al. : How structural and functional MRI can inform dual-site tACS parameters: a case study in a clinical population and its pragmatic implications. Brain Stimul 2022; 15:337–351 [DOI] [PubMed] [Google Scholar]
- 87.Siddiqi SH, Trapp NT, Hacker CD, et al. : Repetitive transcranial magnetic stimulation with resting-state network targeting for treatment-resistant depression in traumatic brain injury: a randomized, controlled, double-blinded pilot study. J Neurotrauma 2019; 36:1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soleimani G, Conelea C, Kuplicki R, et al. : Optimizing individual targeting of fronto-amygdala network with transcranial magnetic stimulation (TMS): biophysical, physiological, and behavioral variations in people with methamphetamine use disorder. medRxiv 2023. (doi: 10.1101/2023.04.02.23288047v2 [DOI] [Google Scholar]
- 89.Waldvogel D, van Gelderen P, Muellbacher W, et al. : The relative metabolic demand of inhibition and excitation. Nature 2000; 406:995–998 [DOI] [PubMed] [Google Scholar]
- 90.Bestmann S, Ruff CC, Blankenburg F, et al. : Mapping causal interregional influences with concurrent TMS-fMRI. Exp Brain Res 2008; 191:383–402 [DOI] [PubMed] [Google Scholar]
- 91.Wiethoff S, Hamada M, Rothwell JC: Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 2014; 7:468–475 [DOI] [PubMed] [Google Scholar]
- 92.Maeda F, Keenan JP, Tormos JM, et al. : Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 2000; 133:425–430 [DOI] [PubMed] [Google Scholar]
- 93.Eldaief MC, Halko MA, Buckner RL, et al. : Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A 2011; 108:21229–21234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parkin BL, Ekhtiari H, Walsh VF: Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron 2015; 87:932–945 [DOI] [PubMed] [Google Scholar]
- 95.Bestmann S, Swayne O, Blankenburg F, et al. : Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb Cortex 2008; 18:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCalley DM, Lench DH, Doolittle JD, et al. : Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep 2021; 11:8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunlop K, Woodside B, Lam E, et al. : Increases in frontostriatal connectivity are associated with response to dorsomedial repetitive transcranial magnetic stimulation in refractory binge/purge behaviors. Neuroimage Clin 2015; 8:611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fitzgerald PB, Fountain S, Daskalakis ZJ: A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006; 117:2584–2596 [DOI] [PubMed] [Google Scholar]
- 99.Nettekoven C, Volz LJ, Kutscha M, et al. : Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci 2014; 34:6849–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Addicott MA, Luber B, Nguyen D, et al. : Low-and high-frequency repetitive transcranial magnetic stimulation effects on resting-state functional connectivity between the postcentral gyrus and the insula. Brain Connect 2019; 9:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jannati A, Block G, Oberman LM, et al. : Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin Neurophysiol 2017; 128:2268–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ren P, Ma M, Wu D, et al. : Frontopolar tDCS induces frequency- dependent changes of spontaneous low-frequency fluctuations: a resting-state fMRI study. Cereb Cortex 2022; 32:3542–3552 [DOI] [PubMed] [Google Scholar]
- 103.Raja Beharelle A, Polanía R, Hare TA, et al. : Transcranial stimulation over frontopolar cortex elucidates the choice attributes and neural mechanisms used to resolve exploration-exploitation trade-offs. J Neurosci 2015; 35:14544–14556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soutschek A, Kang P, Ruff CC, et al. : Brain stimulation over the frontopolar cortex enhances motivation to exert effort for reward. Biol Psychiatry 2018; 84:38–45 [DOI] [PubMed] [Google Scholar]
- 105.Philip NS, LaBar KS: Mapping a pathway to improved neuropsychiatric treatments with precision transcranial magnetic stimulation. Sci Adv 2022; 8:eabq7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oathes DJ, Zimmerman JP, Duprat R, et al. : Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Exp Brain Res 2021; 239:1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dunlop K, Hanlon CA, Downar J: Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci 2017; 1394:31–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Markov NT, Ercsey-Ravasz MM, Ribeiro Gomes AR, et al. : A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex 2014; 24:17–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petrides M, Pandya DN: Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 2007; 27:11573–11586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crowley JJ, Collins AL, Lee RJ, et al. : Disruption of the microRNA 137 primary transcript results in early embryonic lethality in mice. Biol Psychiatry 2015; 77:e5–e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carmi L, Tendler A, Bystritsky A, et al. : Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry 2019; 176:931–938 [DOI] [PubMed] [Google Scholar]
- 112.Dinur-Klein L, Dannon P, Hadar A, et al. : Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 2014; 76:742–749 [DOI] [PubMed] [Google Scholar]
- 113.Jackson MP, Rahman A, Lafon B, et al. : Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol 2016; 127:3425–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.US Food and Drug Administration: News release: FDA permits marketing of transcranial magnetic stimulation for treatment of obsessive compulsive disorder. 2022. https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-transcranial-magnetic-stimulation-treatment-obsessive-compulsive-disorder
- 115.Bergmann TO, Varatheeswaran R, Hanlon CA, et al. : Concurrent TMS-fMRI for causal network perturbation and proof of target engagement. NeuroImage 2021; 237:118093. [DOI] [PubMed] [Google Scholar]
- 116.Bergmann TO, Hartwigsen G: Inferring causality from noninvasive brain stimulation in cognitive neuroscience. J Cogn Neurosci 2021; 33:195–225 [DOI] [PubMed] [Google Scholar]
- 117.Mulyana B, Tsuchiyagaito A, Smith J, et al. : Online closed-loop real-time tES-fMRI for brain modulation: feasibility, noise/safety, and pilot study. bioRxiv 2021. (doi: 10.1101/2021.04.10.439268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soleimani G, Nitsche MA, Bergmann TO, et al. : Closing the loop between brain and electrical stimulation: towards precision neuromodulation treatments. Transl Psychiatry 2023; 13:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ekhtiari H, Kuplicki R, Aupperle RL, et al. : It is never as good the second time around: brain areas involved in salience processing habituate during repeated drug cue exposure in treatment engaged abstinent methamphetamine and opioid users. NeuroImage 2021; 238:118180. [DOI] [PubMed] [Google Scholar]
- 120.Fiocchi S, Chiaramello E, Luzi L, et al. : Deep transcranial magnetic stimulation for the addiction treatment: electric field distribution modeling. IEEE J Electromagn RF Microw Med Biol 2018; 2:242–248 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
fMRI data and computational head models related to the 65 participants with methamphetamine use disorder are available on request from the corresponding author. The fMRI drug cue reactivity task and its codes are available at https://github.com/rkuplicki/LIBR_FDCR_Dynamic. The data associated with cue reactivity in participants with cocaine, alcohol, and tobacco use disorders are available on request from Dr. Hanlon. More details on lesion maps can be found in the supplement to reference 26.


