Abstract
Endovascular reperfusion therapy is the primary strategy for acute ischemic stroke. No-reflow is a common phenomenon, which is defined as the failure of microcirculatory reperfusion despite clot removal by thrombolysis or mechanical embolization. It has been reported that up to 25% of ischemic strokes suffer from no-reflow, which strongly contributes to an increased risk of poor clinical outcomes. No-reflow is associated with functional and structural alterations of cerebrovascular microcirculation, and the injury to the microcirculation seriously hinders the neural functional recovery following macrovascular reperfusion. Accumulated evidence indicates that pathology of no-reflow is linked to adhesion, aggregation, and rolling of blood components along the endothelium, capillary stagnation with neutrophils, astrocytes end-feet, and endothelial cell edema, pericyte contraction, and vasoconstriction. Prevention or treatment strategies aim to alleviate or reverse these pathological changes, including targeted therapies such as cilostazol, adhesion molecule blocking antibodies, peroxisome proliferator-activated receptors (PPARs) activator, adenosine, pericyte regulators, as well as adjunctive therapies, such as extracorporeal counterpulsation, ischemic preconditioning, and alternative or complementary therapies. Herein, we provide an overview of pathomechanisms, predictive factors, diagnosis, and intervention strategies for no-reflow, and attempt to convey a new perspective on the clinical management of no-reflow post-ischemic stroke.
Keywords: No-reflow, ischemic stroke, pathogenesis, recanalization, therapy
Introduction
The term “no-reflow” refers to the condition wherein a blockage of local blood vessels results in ischemia of the corresponding tissues and organs, despite subsequent reopening of the blood vessels. Although blood flow is restored, the ischemic area receives insufficient blood perfusion. The no-reflow occurrence was first observed in the rabbit brain after transient interruption of blood flow. 1 Subsequently, no reflow has been found in many tissues after ischemic injury, including the brain1 –3 heart, 4 and extremities. 5 The “no-reflow” phenomenon was first described by Ames et al. in 1968, reporting that no-reflow is common following cerebral macrovascular revascularization treatment, especially in longer macrovascular occlusion. 1 Microcirculatory status after thrombolysis is a critical factor in determining the functional recovery of ischemic brain tissue. Although intravenous and mechanical thrombectomy are effective methods for treating acute ischemic stroke, many patients experience poor clinical outcomes.6,7 However, these methods are the keys to reducing ischemic stroke mortality and disability. 8 Recanalization using intravenous (IV) administration of recombinant tissue-type plasminogen activator (rt-PA) was achieved in less than 20% of cases, owing to the hypo-tolerance of brain cells to ischemia and hypoxia, as well as the narrow time frame for thrombolytic therapy.9 –11 Thrombectomy has successfully achieved significant reperfusion in approximately 70–80% of large-vessel occlusion cases. Despite the efficacy of these recanalization techniques, a significant proportion of ischemic stroke patients, ranging from 30–68%, continue to exhibit unfavorable clinical outcomes. 12 Similarly, more than 50% of ischemic stroke patients who received rt-PA thrombolysis did not exhibit any clinical improvement, 13 and it was referred to this condition as “futile recanalization.” Futile recanalization is attributed to no-reflow, which seriously affects the recovery of ischemic stroke patients.
Recent research indicated that microvascular obstruction is a prominent factor in the no-reflow occurrence. 14 Pathological changes in the blood and vascular components may occur owing to oxidative stress, calcium overload, excitatory toxicity, inflammatory response, glial cell activation, and leukocyte adhesion. 15 Consequently, these changes lead to low/no reflow of the microcirculation. High-quality diagnosis and effective interventions for no-reflow will significantly contribute to the no-reflow clinical improvement and ischemic stroke prognosis.
Limited detection means resulted in low and varied clinical detection rates for the no-reflow. According to various studies, the no-reflow detection rates were merely 3%, 16 15%, 17 but some even significantly elevated with ∼25%,18,19 42.5%. 20 Despite the limited detection rate of no-reflow, its prevalence in reperfused patients presents a significant obstacle to recovery. It is crucial to focus on the risk factors of no-reflow and the diagnostic and therapeutic approaches for patients recovering from ischemic stroke.
This review will deliver an updated overview of the pathophysiological mechanisms, risk factors, diagnostic methods, and interventions of no-reflow. Elucidating these issues could provide new perspectives on the no-reflow treatment progress after recanalization.
Pathomechanisms of no-reflow
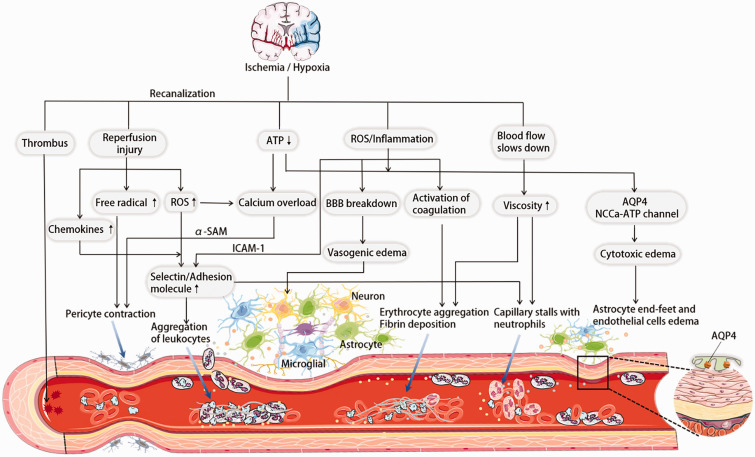
The local metabolic supply is determined by the structural and functional integrity of the cerebrovascular system. The neurovascular unit (NVU) in the brain contains neurons, glial cells, and cerebrovascular components. This intricate network maintains the normal physiological function of neurons and repairs damaged ones. Impaired NVU and microcirculation can directly contribute to no-reflow in ischemia and recanalization. Although no-reflow pathomechanism has been unelucidated entirely, research has indicated several pathological processes involved in its occurrence. These processes include oxidative stress, calcium overload, excitotoxicity, inflammatory response, glial cell activation, and changes in blood flow status. Eventually, these processes contribute to the no-reflow development by causing following changes inside and outside the blood vessels and in the vasculature itself (Figure 1).
Figure 1.
No-reflow phenomenon after recanalization in ischemic stroke. Several pathomechanisms have been postulated. Due to calcium overload and stimulation by oxygen and nitrogen radicals during ischemia and reperfusion, constriction of the pericytes leads to a smaller lumen and low/no reflow. Adhesion and aggregation of blood components (erythrocytes, leukocytes, fibrin, platelets, etc.) and rolling along the endothelium, which may form microthrombi to block the microcirculation. Neutrophils stay in the capillaries and prevent recanalization. Energy deficiency, inflammatory response and oxidative stress induce cytotoxic edema and vasogenic edema after BBB destruction, causing astrocyte end-foot, endothelial cell edema, and brain tissue swelling, which causes mechanical compression of microvasculature. Embolic pieces from the dissolution of the primary thrombus may also block small vessels causing no-reflow.
Adhesion and aggregation of hematological components
Adhesive aggregation and rolling of blood components such as erythrocytes and fibrin are essential causes of no-reflow in the microcirculation. After ischemic stroke, the post-microvascular vein, although still potentially open, has some leukocyte and platelet adhesion or deposition of platelet fibrin aggregates.21 –23
Erythrocyte aggregation and fibrin deposition contributed to the no-reflow, 24 consistent with our histopathological analysis results. In vivo near-infrared fluorescent (NIRF) imaging, a fibrin-targeted NIRF probe revealed fibrin deposition in ipsilateral brain tissue even after successful thrombolytic therapy. Blood is a non-Newtonian fluid, and viscosity increases at low/no-flow rates. The increase in blood viscosity may be related to blood flow deceleration due to ischemia and ion transport disruption. Consequently, this increase results in blood cell aggregation and impaired circulation in the microvasculature. This finding is supported by the fact that tissue perfusion significantly improved in rabbits, where blood was diluted with saline before ischemia. 25 With its increased viscosity, blood in a stagnant state may also increase erythrocyte aggregation to obstruct microvessels. The early ischemic phase triggers the inflammatory response, resulting in arterial occlusion-induced hypoxia. This hypoxia disrupts tissue metabolism, forming reactive oxygen species (ROS) and initiating inflammatory response. 26 ROS contributes to intravascular injury and rapidly activates coagulation and complement cascades. Consequently, this process generates thrombin that converts fibrinogen to fibrin. The resulting fibrin then traps red blood cells, platelets, and polymorphonuclear blood cell elements, provoking irreversible microvessel blockage. 27 Ischemic regions experience exposure to plasma constituents and tissue factors secondary to heightened permeability of the blood-brain barrier (BBB), which further exacerbate fibrinogen and fibrin deposition. 28
Due to ischemia and reperfusion, the upregulation of adhesion molecules on the surface of leukocytes and ECs causes leukocyte accumulation in the microcirculation. This accumulation causes microcirculation blockage and results in no-reflow. An experiment demonstrated that within 48 h, leukocyte aggregation and occlusion of damaged micro arteries occurred in 92 rats with focal cerebral ischemia. 29 The microvascular inflammatory reaction of ischemic foci releases numerous inflammatory factors, damaging the ECs and the microvasculature barrier function. During cerebral ischemia, locally aggregated leukocytes produce massive inflammatory cytokines, activating ECs. This leads to significant overexpression of intercellular cell adhesion molecule-1 (ICAM-1) on their surface, increased cell adhesion, and leukocyte aggregation in the ischemic area. 30 ICAM-1 promotes endothelial cell activation through specific binding to its receptor. It enhances the adhesion between leukocytes, inflammatory cells, and ECs, making it easier for leukocytes to penetrate the endothelium and converge toward the ischemic area. When the leukocytes penetrate the blood vessels and enter the brain tissue, massive inflammatory mediators are re-released, aggravating the endothelial damage of brain tissue and microvasculature. This leads to microthrombi formation, thus resulting in the no-reflow. After ischemia-reperfusion, leukocytes continue to mediate the complex inflammatory response. 31 Oxidative stress and inflammatory factor release induced the expression of selectins and adhesion molecules. Then leukocytes accumulate in blood vessels by adhering to vascular endothelium and blocking capillaries.32 –34 Ischemia-reperfusion overproduces peroxides through pathways such as NADPH oxidase. Moreover, Peroxide-dependent signaling, involved in ICAM-1, VCAM-1, and PECAM-1 expression in vascular ECs, induces leukocyte and endothelial cell adhesion, efflux, and vascular barrier damage. 35 Additionally, leukocytes can adversely affect blood rheology,36,37 and promote thrombosis. 38 Microvascular occlusion may also be triggered by embolic fragments from the original proximal thrombus 39 or local (micro)-thrombosis formed in situ by local platelet activation. 40 Microclots have been found in brain microvessels of cerebral ischemia patients who died within a month after the stroke onset. 41
Overall, a series of pathological changes in blood components, including sludge and aggregation of erythrocytes and fibrin, and leukocyte-released inflammatory factors cause the accumulation in microcirculation and the activation of coagulation and complement cascades. Consequently, all these changes lead to local thrombogenesis of the microcirculation blockage and eventually cause low/no-reflow.
Capillary stalls with neutrophils
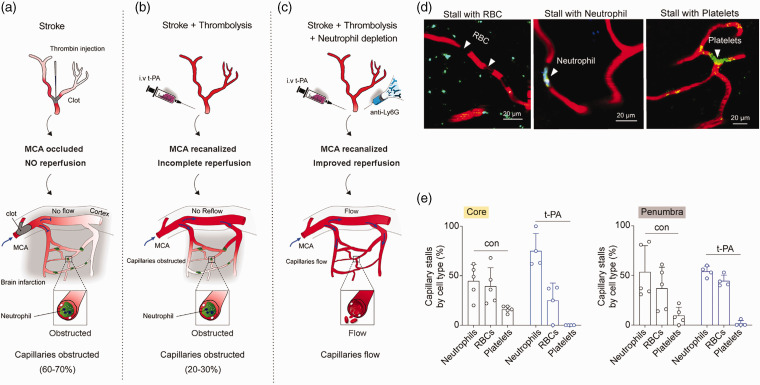
Neutrophils appear to circulate without rotation or margination in the healthy capillary network. 42 It may stay in the capillaries in conditions like Alzheimer’s disease and stroke, obstructing blood flow and aggravating tissue damage. 43 In ischemic stroke, capillary stalls occur in the core of infarction and the penumbral region despite the reopening of occluded middle cerebral artery (MCA). 44 El Amki M’s experiment 45 found that thrombolysis was ineffective in restoring the distal microvascular network. Therefore, using neutrophil-depleting antibodies may restore capillary flow and improve prognosis. This finding is due to the possibility that microvascular dysfunction could be attributed to neutrophil stagnation in the capillaries. By imaging flow in cerebral capillaries using two-photon imaging, it was observed that despite thrombolysis (no obstructions in arterioles), 35% of capillaries in the core and 15% in the penumbra remained stalled. Neutrophils drift along arterioles and venules shortly after ischemia induction, obstructing smaller capillaries and thus causing flow arrest. The diameter of the capillary segment at a cortical depth of 300 mm has been verified to range from 4–10 mm. Since tagging and the direct visualization of stall morphology, platelet aggregates, and RBCs could be separated from stalls caused specifically by neutrophils. This study discovered that neutrophils, RBCs, and even platelet aggregates could be to blame for capillary stalls following cerebral ischemia. However, most stopped capillary segments after thrombolysis were caused by neutrophil congestion (Figure 2). 45 This process involves various factors, including slow blood flow, changes brought on by ischemia in the mural cells (ECs and pericytes), and their interactions with the blood cells (adhesion molecules).46,47
Figure 2.
Capillaries remain stalled after recanalization of the MCA. 45 (a, b, c) In ischemic stroke, thrombolysis did not result in successful reperfusion of the distal microvascular network. After thrombolysis, microvascular dysfunction may be caused by neutrophil stagnation in the capillaries. The use of neutrophil-depleting antibodies may restore capillary flow and improve prognosis. (d) After recanalization, representative images of a stall caused by neutrophils, RBCs, or platelets distinguished by fluorescence labels and morphology and (e) Percentages of capillaries stalled specifically with neutrophils, RBCs, or platelets in the core and penumbra ROI for control and t-PA-treated mice. Obviously, it is clear from the figure that neutrophils are always predominant in capillary arrest in the ischemic core and semidark areas after thrombolysis. Copyright 2020, Cell Press.
Cellular and interstitial edema
Post-ischemic swelling in ECs and end feet of adjacent astrocytes result in decreased luminal size within 1 h after reperfusion, which impedes blood flow. 48 An experiment showed that, within 60 min after middle cerebral artery occlusion (MCAO), the mean nucleus diameter of the ischemic astrocytes increased by 23.4% compared to control values. Swelling of astrocyte nuclei was most significant during the period between 3–24 h after MCAO. During the initial 2 h, the cytoplasm of ECs showed moderate swelling. Cerebral edema is associated with two pathophysiological processes: those related to cytotoxic (cellular) edema of neurons and astrocytes, as well as those related to the transcapillary flux of Na+ and other ions, water, and serum macromolecules. Cytotoxic edema results from an unchecked or uncompensated influx of cations, mainly Na+, through cation channels. In ischemic stroke, decreased adenosine triphosphate (ATP) inhibits ATP-dependent transporter proteins (e.g. Na+/K+ ATPase). The resulting influx of osmotic substances, such as Na+, leads to cellular swelling.49,50 Aquaporin 4 (AQP4) has predominantly mediated cytotoxic edema, as water is transported to the central nervous system (CNS) with the cerebral blood barrier intact via AQP4 located in the end feet of astrocytes.51,52 In normal brain tissue under physiological conditions, astrocytes are in contact with microglia in the local microenvironment, maintaining microglia in a dormant state. 53 However, microglial activation induced by ischemic stroke can overexpress neurotoxic mediators, activating neighboring astrocytes. The inflammatory cytokines interleukin 1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) released from microglia induce AQP4 upregulation, leading to the swelling of astrocyte end-feet.54,55 Nonselective cations (NC) channels are also important mediators of cell swelling as they allow the flux of any monovalent cation. 51 The NCCa-ATP channel, a crucial NC channel in ischemic stroke, is not constitutively expressed but is activated in astrocytes, neurons, and endothelium during focal ischemia. 56
Loss of tissue perfusion caused by ischemic stroke can lead to neuronal damage and death. Consequently, this causes an increased pro-inflammatory mediator release, activating ECs, astrocytes, and pericytes. The activated ECs attracts leukocytes, resulting in neurovascular inflammation, thereby breaking the tight junctions of BBB to increase its permeability. 57 Subsequently, the increased permeability will allow the filtration of many molecules and ions from the reperfused blood, such as plasma proteins, salts, and water, to cross BBB into brain tissue. 58 Accumulating this filtration fluid in the tissue interstitial space creates vasogenic edema, 59 increasing intracranial pressure and causing tissue edema. 60 Mathematical models show that these might lead to vessel compression, which may obstruct blood flow to a specific region in the brain leading to secondary ischemia or the no-reflow after recanalization. 61
Pericyte contraction
Pericytes, an essential BBB component with a central position in the NVU, cover around 80% of microvessels in the body, including the CNS, which has the highest pericyte density. 62 Moreover, pericytes receive, coordinate, and interpret signals from their surrounding cells to produce multiple neurovascular functions, including capillary hemodynamic control, BBB permeability, harmful metabolite removal, angiogenesis, and stem cell activity. These diverse functions are essential to maintain healthy brain homeostasis.63 –65
Ischemia and reperfusion can produce the no-reflow by causing pericytes to contract differently. Pericytes are constricted in ischemic stroke models, leading to segmental capillaries constriction and blood flow obstruction (Figure 3). 14 Moreover, the constriction is more prominent after reperfusion, despite the reopening of the occluded artery. 66 Electrophysiological and pharmacological studies have shown that brain pericytes are electrically excitable cells. Thus, the interaction between Ca2+ signals and membrane potentials concertedly modulate pericyte constriction.67,68
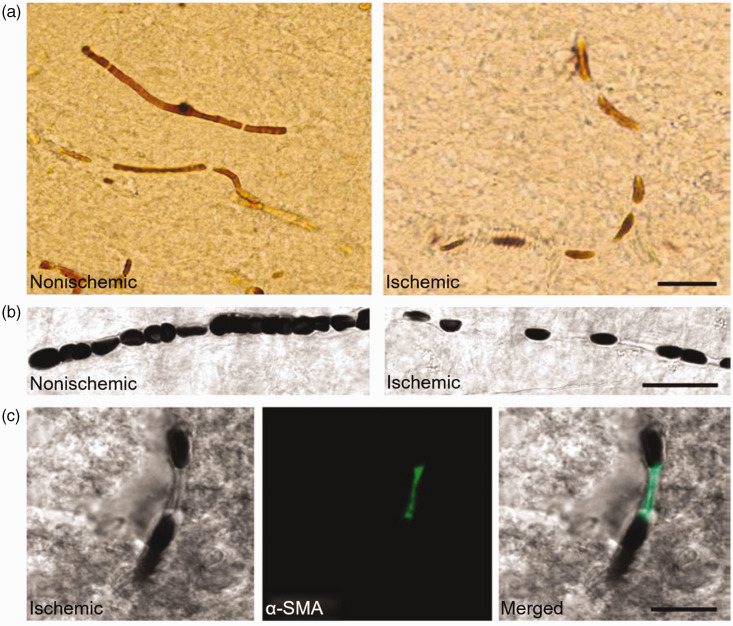
Figure 3.
Ischemia induces persistent pericyte constriction that does not restore even after complete recanalization of the occluded artery. 14 (a) Node-like discontinuities were noted along the course of microvessels in the ischemic hemisphere. (b) The DIC images illustrate frequent interruptions in the erythrocyte column in an ischemic capillary contrary to a continuous row of erythrocytes flowing through an intact capillary. (Green, c) Nodal constrictions on the ischemic capillaries colocalized with α-SMA-positive pericytes. These nodal constrictions might be brought on by pericytes. Indeed, when labeled pericytes with an antibody against α-smooth muscle actin (SMA), it suggested that the constricted microvessels segments colocalized with pericytes. Copyright 2009, Springer Nature.
Additionally, pericyte contraction can result from increased intracellular calcium ion concentration in cerebral ischemia.14,68,69 Pericytes also contract during ischemia due to the uncontrolled rise in intracellular Ca2+ resulting from energy loss triggered by acute cerebral ischemia that disrupts Ca2+ homeostasis. 70 This contraction may be mediated by α-smooth muscle actin (α-SAM). 71 In ischemia-reperfusion, intracellular pro-oxidant enzymes and mitochondria produce massive oxygen radicals with oxygen, resulting in an imbalance of oxidative and antioxidant functions in the body. In the reperfusion process, the significant accumulation of intracellular ROS, the paradox of oxygen, calcium, and pH change contribute to producing free radicals, developing oxidative stress, and other toxic factors. Among these changes, oxidative stress is a potent constriction inducer of pericytes. Oxygen and nitrogen radicals formed in the microvasculature during ischemia/reperfusion cause persistent pericyte constriction. Pericyte contraction occurred one hour after reperfusion in the MCAO model. It was proved that oxidative and nitrative radicals induced pericyte contraction to suppress oxidative and nitrative stress before reperfusion relieved pericyte contraction. 14 This study suggested the importance of pericyte contractility in ischemic stroke pathology.
Moreover, this study indicates that sustained contraction of pericytes might be the reason for the adverse outcome of thrombolysis therapy. The active contraction of pericytes may increase microcirculatory resistance than compression of the lumen by swollen end feet. Consequently, this mechanism may significantly contribute to microvascular clogging. Pericyte contractility may partially be involved in the microcirculation no-reflow. 14
Vasoconstriction
A research group performed a study with MCAO models, revealing that cerebral arteriole constrictions significantly reduce cerebral blood flow (CBF). These constrictions may induce inadequate reperfusion after ischemic stroke. 72 Multiple alternations are involved in this pathological constriction and vascular dysfunction, including increased arterial stiffness, diminished vascular reactivity, and decreased endothelial nitric oxide synthase (eNOS) phosphorylation. 73 Cerebral ischemia affects the vascular ECs, pericytes, and the entire NVU (including astrocytes, microglia, and neurons), leading to several restrictive layers and complicated interactions. 73 A research group conducted an experimental study to determine whether pericyte contraction affects blood flow recovery. They have verified that in pathological constriction of tiny distal vascular segments in ischemic stroke, vascular side branches are sensitive throughout the propagation of pressure gradients and nutrient supply. 74 The changes within the extracellular potassium concentration in the brain during physiological events (e.g. neuronal activity) may be essential for regulating local cerebral blood flow.75,76 High potassium concentrations in the extracellular fluid (<∼20mEq/L) cause a pronounced vascular smooth muscle contraction.77 –81 Cerebrovascular resistance (CVR) changes were assessed by measuring the cerebral perfusion rate (CPR). Following 2 min of ischemia, CVR was decreased to the half control value, while significantly multiplied after 8 and 16 min of ischemia. Therefore, the no-reflow state was approached at 16 min of ischemia. Moreover, cerebral ischemia lasting 8 and 16 min in rats was amid a more significant increase in cis-cerebrospinal fluid potassium concentration and a subsequent increase in CVR. 82 This study did not offer definitive proof for a causal relationship between the rise in the potassium concentration in brain extracellular fluid and the increase in CVR. However, due to the changes in the potassium concentration in cisternal cerebrospinal fluid (CSF), which occur with a delay compared to the changes in brain extracellular fluid, it is necessary for the increase in potassium concentration within the brain interstitial fluid to be greater than the measurement observed in the cisternal CSF. The significant rise in potassium concentration in brain extracellular fluid alone may account for the observed changes in CVR.
Risk factors and predictors for no-reflow
Merely relying on reopening large vessels is inadequate for effectively preventing and managing tissue damage progression. Identifying risk factors and predictors associated with no-reflow prevents its onset and enables timely intervention to improve the prognosis of patients with ischemic stroke.
Clinical risk factors include old age, 83 female sex, diabetes, hypertension, higher baseline National Institutes of Health Stroke Scale (NIHSS) score, lower Alberta stroke project early CT score (ASPECTS), poor collateral status, negative imaging performance, longer endovascular treatment delay, and increased biomarkers of inflammation and microcirculatory disorders. 84 Old age is consistently associated with poor neurological prognosis after stroke, possibly due to more comorbidities, higher complication rates and cardiogenic stroke incidence, and less recovery potential.85 –87 Females are at an elevated risk of experiencing futile recanalization, primarily due to physiological disparities in coagulation, hormonal fluctuations, and immune responses during menopause. 88 Hypertension and hyperglycemia can increase BBB permeability,89,90 impair microvascular cerebrovascular reactivity,91,92 and exacerbate reperfusion injury.93,94 Multifactorial analysis showed that time from onset to endovascular treatment was an independent risk factor for ineffective recanalization.95,96
The predictive factors associated with the occurrence of no-reflow primarily encompass four categories: individual health background and comorbidities, neurologic function scores, imaging manifestations and scores, and molecular markers: (1) Age >70 years, female sex, longer treatment delay and comorbidities (hypertension, diabetes, and atrial fibrillation) could be used as predictors of no-reflow.83,84 (2) Higher NIHSS score ≥10 is a significant predictor in a retrospective multifactorial analysis.97,98 (3) ASPECTS ≤5, 99 moderate-severe leukoaraiosis (van Swieten scale ≥2), 100 a large lesions in the deep white matter (DWM) on pretreatment diffusion-weighted MRI (DWI) which demonstrates a longitudinal hyperintense lesion in >80% of the DWM, 101 collateral score modified Rankin Scale (mRS) ≤ 2, 102 occlusion sites such as internal carotid artery occlusion, pre-stroke brain atrophy, and cerebral edema are shown to be predictors of no-reflow.84,103 (4) Molecular markers such as IL-6, IL-1, 104 the elevated peripheral blood neutrophil-to-lymphocyte ratio (NLR), 105 high sensitivity C-reactive protein, the increased levels of matrix metalloproteinase-9, tenascin-C, thioredoxin, decreased levels of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member13 and gelsolin, 106 and increased neutrophils count 107 are independent predictors of no-reflow.84,108 Due to the cost-effectiveness and ease of measuring biomarkers, the integration of these biomarkers with clinical imaging parameters to develop risk scoring systems has the potential to enhance early screening for no-reflow.
Diagnosis of no-reflow
Clinical information on low/no-reflow is limited, and there is a lack of clear diagnostic indicators in the clinic. This is partly due to the challenges in accurately assessing vessel status in early studies without relying on invasive diagnostic angiography. 18 The clinical manifestations of no-reflow, observed in patients who have undergone successful restoration, lack distinct characteristics, thereby increasing the likelihood of oversight. In individuals, the proximal arteries of patients with ischemic stroke commonly exhibit a pathological condition characterized by the presence of atherosclerotic plaque and thrombus formation. Pro-inflammatory or pro-coagulant states such as diabetes and hyperlipidemia are common risk factors for stroke patients, which can increase the risk and intensity of the occurrence of no-reflow. The incidence of no-reflow also rises in patients with white matter hyperintensity burden, pre-stroke brain atrophy, cerebral edema, longer ischemia time, poorer neurologic recovery, and surge in interleukins and neutrophils.84,103,109 Hence, further workup for no-reflow should be focused on patients presenting with the above conditions. The following different diagnostic techniques establish criteria for the assessment of no-reflow. (Table 1)
Table 1.
Summarize the available diagnostic methods for no-reflow after recanalization in ischemic stroke.
| Diagnostic method | Characteristic | Refs. |
|---|---|---|
| TCD |
|
19,110 |
| CTP/MRP |
|
18 |
| OCT |
|
114 |
| ASL |
|
115 |
| NIRS |
|
116 –118 |
| PET-MRI |
|
119,120 |
Transcranial Doppler (TCD)
TCD is routinely used in patients with acute ischemic stroke as a regular version of follow-up investigations and outpatients undergoing carotid Doppler examination for transient ischemic attack and carotid stenosis monitoring. The Gosling pulsatility index (PI) and Pourcelot resistance index (RI) derived from routine TCD parameters are established indices of CVR. 110 At a Comprehensive Stroke Center with a high-volume neurovascular laboratory, consecutive patients with acute MCAO by thrombectomy that had been recanalized (Thrombolysis in Cerebral Infarction grade IIb/III) were discovered. Sonographic measures of middle cerebral artery territory microvascular resistance (PI and RI) were compared on the first to the third days of follow-up TCD. The results revealed that elevated microvascular resistance in the ischemic area is commonly present after successful recanalization as measured by the PI on TCD. The no-reflow was operationally defined as a significant increase in the PI of the middle cerebral artery by more than 20% compared to the contralateral side or a PI exceeding 1.2. 19 TCD, being a non-invasive technique, facilitates the convenient monitoring of occlusion progression before, during, and after treatment. The utilization of TCD has demonstrated its efficacy in identifying collateral flow, active microemboli, vessel patency, and occlusion in acute stroke cases, particularly by detecting collateral flow that may have been overlooked in CT angiography. 111 It may be a readily available and clinically relevant biomarker of the no-reflow.
Magnetic resonance perfusion (MRP) and computed tomography perfusion (CTP)
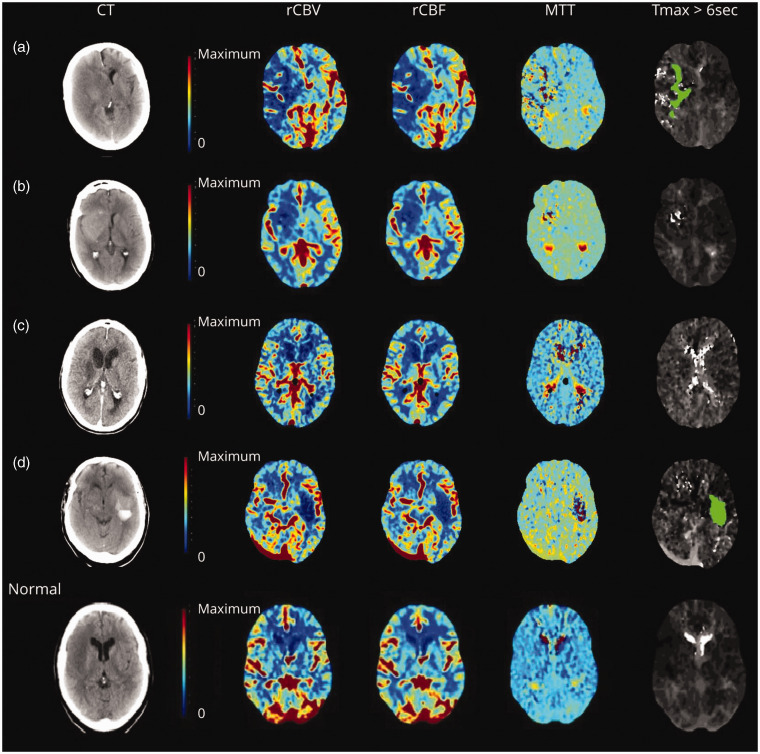
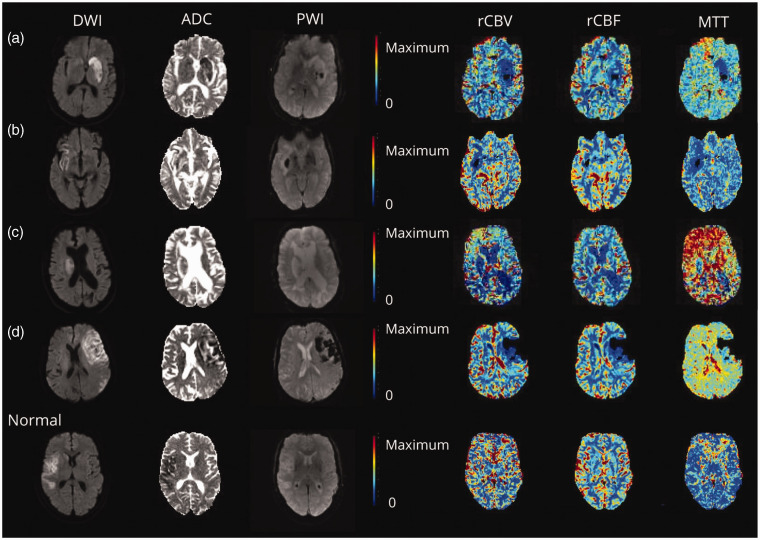
Cerebral no-reflow can be detected by its characteristic perfusion imaging profile using readily available sequences in the clinical setting. Research suggested that regions of no-reflow were identified in 33 patients (25.3%). 18 The definition used relative cerebral blood volume (rCBV) and relative CBF (rCBF). CTP and MRP were processed to generate rCBV, rCBF, mean transit time (MTT) maps, and Tmax > 6 s lesion volume. On the qualitative assessment of perfusion maps, no-reflow appeared as tiny merging areas of reduced rCBV within the infarct lesion. In quantitative interside comparisons, the general median rCBV of infarct foci was reduced by 25.2% and rCBF by 19.1%. Some patients exhibited a reduction in rCBV or rCBF of >40%. Herein, the term “no-reflow” was operationally defined as the sustained occurrence of inadequate blood perfusion in the infarcted region, as evidenced by reduced rCBF or rCBV on maps. Additionally, a reduction of more than 15% in median rCBF or rCBV values within the infarcted area relative to the corresponding contralateral mirror region indicated the no-reflow. The rCBF in the peri-infarct penumbra area of salvaged infarct was also mildly reduced. Most MRP patients experienced MTT reductions exceeding 15%. Between MRP and CTP, an intermodality variation of no-reflow on MTT was detected. MTT was frequently reduced on MRP in the no-reflow. However, on CTP, MTT maps were speckled, with petechial foci of highly extended MTT matching to areas of iodine contrast staining (Figures 4 and 5). 18 Conventionally, prolonged MTT accompanying reduced rCBF is a hallmark of cerebral ischemia from arterial obstruction commonly seen on baseline imaging in acute ischemic stroke and re-occlusions. 112 Contrary, MTT in no-reflow regions exhibited a reduction. This aligns with the proposed pathomechanism of no-reflow, which can be described as a microcirculatory phenomenon with altered flow patterns characterized by segmental capillary obstruction. This obstruction reduces the adequate capillary volume with compensatory dilation of remaining patent vessels to maximize tissue oxygen extraction and local perfusion pressure.18,113
Figure 4.
No-reflow on CT perfusion. 18 No-reflow identified on CT perfusion (a–d) and a patient with right frontal infarct and normal follow-up perfusion imaging for comparison. (c). Quantitative analysis in the patient showed 29% rCBF reduction. Mean transit time (MTT) showed foci of very high values within the infarct on CT perfusion cases of no-reflow (a, b, and d). The corresponding Tmax lesions of these foci differed from that usually seen in arterial occlusions and exhibited a heterogeneous patchy pattern (a), was not present (b), or coincided with areas of contrast leakage (d). Copyright 2022, American Academy of Neurology.
Figure 5.
No-reflow on MR perfusion. 18 No-reflow is manifested by reduction of rCBV or rCBF with darker blue on perfusion maps. Reocclusion was excluded on magnetic resonance (MR) angiography at the time of perfusion imaging. A corresponding well-demarcated area of reduced mean transit time (MTT) was frequently seen in no-reflow cases identified by MR perfusion imaging (a–d). Copyright 2022, American Academy of Neurology.
Optical coherence tomography (OCT)
An investigation applied OCT imaging of CBF to quantify microcirculatory changes. To track changes in the capillary perfusion level (CPL) before, during, and after the stroke, they produced an OCT micro angiography of the cerebral cortex in a mouse cerebral ischemia model. Briefly, researchers implemented a relatively simpler method to estimate the CPL from volumetric OCT angiogram data, revealing that CPL significantly decreased during ischemia but recovered to the baseline after recanalization 1 h after ischemia. However, the CPL was significantly reduced when recanalization was delayed to 2 h after ischemia. The presented data provide evidence that ischemia induces dysfunction in the microcirculation, resulting in a reduction in capillary reperfusion following recanalization. 114 This condition occurs after 2 h of ischemia and may adversely affect the microcirculatory recovery after recanalizing. Advanced optical imaging techniques may assist experimental studies, while clinical trials should examine microcirculatory no-reflow more rigorously.
Arterial spin labeling (ASL)
No-reflow can also be defined as ≥40% reduction in CBF affecting anatomical regions of the affected hemisphere on 24-h ASL maps and the presence of infarcts in areas of hypoperfusion on follow-up MRI. 16 This cut-off was based on a previous ASL study demonstrating that ≥40% CBF reduction relative to contralateral regions best identifies critical hypoperfusion, with a high correlation with standard first-pass gadolinium perfusion-weighted imaging (PWI) sequence. Moreover, an ASL-CBF threshold of <40% was the most accurate predictor of 24 h DWI lesion in patients without reperfusion. 115
Near-infrared spectroscopy (NIRS)
The NIRS, a non-invasive method for assessing brain tissue oxygenation, was first studied in vivo in 1977. 116 In a hemodynamic and structural brain measurements study, the researchers used NIRS to assess and evaluate prefrontal CBF across numerous subjects with varying conditions. 111 The concentration of oxyhemoglobin and deoxyhemoglobin in the human brain was monitored by the distinct absorption spectra of oxidation and deoxygenation states of Hb and the redox state of cytochrome c oxidase in the mitochondria. CBF is indirectly reflected by detecting tissue oxygenation. NIRS exhibits significant promise as a convenient bedside monitoring technique for evaluating the extent of cerebral ischemia, cerebral metabolism, thrombolysis, and tissue reperfusion. 117 Moreover, NIRS can be used as a prognostic indicator for long-term patient prognosis. 118 Therefore, NIRS may provide a reference for whether no-reflow occurs in the reperfused region of patients with ischemic stroke.
PET-MRI
PET/MRI is a globally prevalent advanced medical imaging device for early screening and diagnosing malignant tumors and neurological diseases. Integrating PET/MRI is imperative in acute ischemic stroke (AIS). Many studies have been performed on PET/MRI perfusion measurements to optimize low perfusion detection thresholds. PET/MRI can facilitate identifying severely under-perfused tissues by observing two distinct patterns of lesion evolution, symptomatic salvaged tissue (SST) and asymptomatic infarcted tissue (AIT), which provide highly complementary information.119,120 Hence, PET-MRI is recommended as a potential diagnostic tool for no-reflow, warranting further investigation.
Others
Neurovascular coupling, relating changes in neuronal activity to constriction/dilation of microvessels, is dependent on enough blood flow to meet neuronal demands during activation. Cerebral perfusion pressure (CPP) is the best predictor of changes in neurovascular coupling, where changes in CPP substantially affect CBF. 121 Thus, quantifying neurovascular coupling through changes in hemodynamic response function (HRF) synthesized with CPP-induced signaling holds promise as a non-invasive biomarker for assessing changes in CPP and self-regulatory injury. Hence, a potential avenue for future research may involve investigating CBF regulation by assessing neurovascular coupling to ascertain the no-reflow occurrence.
Therapeutic strategies for no-reflow in ischemic stroke
Despite extensive research and development, there is a lack of specific therapies for no-reflow after recanalization. Certain pharmaceutical agents have demonstrated efficacy in mitigating the no-reflow in animal experimentation. It has been suggested that restoring microcirculation, reducing no-reflow, and improving ischemic stroke outcome can be achieved by inhibiting fibrin, platelets, and leukocyte adherence or vascular inflammation, thereby reducing microvascular clogging.46,122 –124 Another suggested treatment for the no-reflow is by enhancing blood rheology and viscosity and facilitating microvascular regeneration. Cilostazol, an antiplatelet agent that functions as a phosphodiesterase inhibitor, has reduced the no-reflow and hemorrhagic transformation induced by rt-PA. This effect is achieved by maintaining microvascular integrity. 125 Adhesion molecule-blocking antibodies that inhibit leukocyte adhesion, including P/E-selectin and ICAM-1, enhance the rt-PA-induced recanalization rates by preventing no-reflow.46,122,124 Administration of pioglitazone, an activator of peroxisome proliferator-activated receptor-gamma (PPAR-γ), reduces the no-reflow in microvessels after MCAO in rats. 126 Adenosine could increase microcirculatory reflow by relaxing contracted pericytes and reducing ischemia-induced erythrocyte entrapment. 123 Moreover, some adjunctive therapies have been found to improve no-reflow. In clinical practice, Traditional Chinese Medicine (TCM) has a long history of preventing and treating ischemic stroke. Its multi-component, multi-pathway, and multi-target action characteristics align effectively with the complex pathomechanism of the no-reflow. Therefore, it has unique characteristics, advantages, and strong potential for new drug development. We retrieve the studies on the intervention of microcirculatory disorders after cerebral ischemia-reperfusion recently to get inspiration for the treatment of low/no-reflow phenomenon (Table 2).
Table 2.
Research advances in medicine for no-reflow after recanalization in ischemic stroke.
| Agents | Targets/Pathway | Functions | Effects | Refs. |
|---|---|---|---|---|
| Cilostazol | ICAM-1/P-selection | Decrease the expression of ICAM-1 and P-selectin. | Prevent microvascular obstruction by leukocytes and microthrombi. | 125 –128 |
| Adhesion molecule blocking antibody | ICAM-1/P-selectin/E-selection | Inhibit expression of ICAM-1, P-selectin, E-selection. | Reduce cell accumulation, infarction size, and improve cerebral blood flow. | 46,122,124,132,133 |
| PPARs activator | IL-6/ ICAM-1 | Block pro-inflammatory IL-6 protein expression, and alleviate neuroinflammation, and apoptosis. | Reduce infarction volume, microglial activation and neutrophil infiltration. Inhibit leukocyte rolling and adhesion, and improve cerebral blood flow recovery. | 135 –140 |
| Adenosine | Receptors on pericytes and astrocyte end-feet | Reduce erythrocyte entrapment and pericyte construction, alleviate endothelial cell and astrocyte edema. | Increase blood flow in the microcirculation and improve no-reflow. | 14,70,123 |
| Superoxide scavengers and NOS inhibitors | Oxidative-nitrosative stress | Relieve pericyte contraction. | Restore microvessels patency. | 14 |
| Iptakalim | K-ATP channel | Relieve pericyte contraction. | Restore microvascular blood flow. | 150 –153 |
| Puerarin | ICAM-1 | Inhibit the expression of ICAM-1, lower blood viscosity. | Improve microcirculation. | 165 |
| Oxymatrine | Lower blood viscosity. | Improve blood rheology, blood hypercoagulability, local microcirculation. | 166 | |
| Flavone glycosides | Decrease blood cell aggregation, lower blood viscosity. | Improve blood rheology and increase blood perfusion to ischemic tissues. | 167 | |
| Tanshinone | ICAM-1/Inflammatory factors | Decrease ICAM-1 expression and release of pro-inflammatory mediators. Inhibits NADPH oxidase and platelet aggregation. | Reduce local infiltration of leukocytes, dilate constricted micro-arteries, and improve microcirculation. | 168 |
| Radix Codonopsis | AKT1/MAPK3/VEGF/TNF | Anti-oxidant, anti-inflammatory, up-regulate VEGF. | Improve blood flow to the brain, promote vascular regeneration, and improve microcirculation. | 169 |
| Paeoniae | PGC-1α/Nrf2 | Up-regulate VEGF, and VEGFR-2. | Increase cerebral microvascular density and promote vascular regeneration. | 170 |
| Buyang Huanwu Decoction | VEGF | Up-regulate VEGF. | Improve cerebral blood flow | 171 |
| Longzhi Decoction | GRP78/CHOP | Inhibit the excessive endoplasmic reticulum stress response. | Promote vascular regeneration | 172 |
| Huoxueshengluo Formula | VEGF | Up-regulate VEGF. | Promote vascular renewal, improve blood supply to tissues and elevate microvascular density in infarcted brain tissue. | 173 |
| Naoluoxintong | Wnt Pathway/β-catenin/VEGF/Ang II | Up-regulate β-catenin, VEGF, Ang II | Promote cerebrovascular regeneration and increases rCBF. | 174,175 |
| Yangxueqingnao granule | bFGF/VEGF | Up-regulate VEGF. | Relieve vascular spasms and increase cerebral blood flow. | 176 |
| Xinnao Shutong capsule | Ang1/Tie2 | Up-regulate Ang1/Tie2. | Mediate vascular angiogenesis. | 177 |
| Erigeron Breviscapus injection | VEGF/Flk-1 | Up-regulate the VEGF and Ang-2, decrease the expression of inflammation. | Induce angiogenesis, increase rCBF in the ischemic area. | 178 |
| Astragalus injection | JNK3 | Decrease the expression of JNK3. | Increase CBF and reduce infarct volume. | 179 |
| Tongxinluo capsule | P-Selectin/ICAM-1/CCL-3/CCL-4/CCL-5/CXCL1 | Decrease the expression of P-Selectin, ICAM-1, CCL-3, CCL-4, CCL-5, and CXCL1. | Reduce interactions between leukocytes and endothelial cells, improve microcirculation. | 180 |
Phosphodiesterase inhibitor
Cilostazol, a phosphodiesterase inhibitor, acts as an antiplatelet agent and has other pleiotropic effects based on phosphodiesterase3-dependent mechanisms. 127 Moreover, cilostazol offers endothelial protection by inhibiting apoptosis in ECs, 128 attenuating the phenotypic modulation of vascular smooth muscle cells, 129 and sustaining blood flow by endothelium-independent vasodilation. 130 This suggests that cilostazol reduces platelet and leukocyte aggregation and maintains vascular integrity, alleviating the no-reflow.
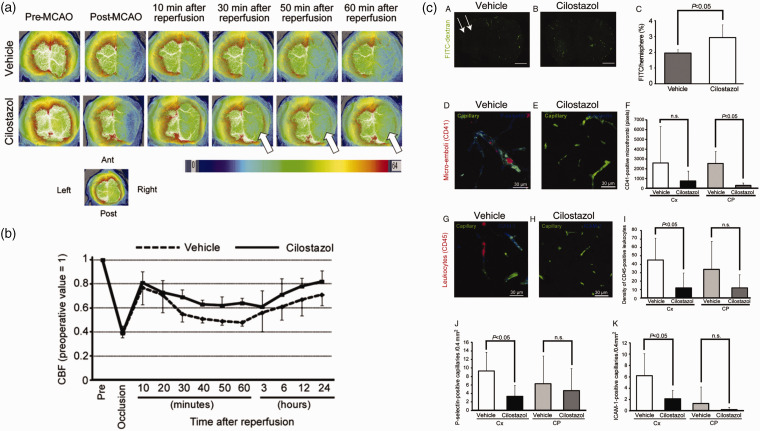
A trial of 79 male mice showed significant benefits from treating the no-reflow. 125 Reperfusion was induced after 45 or 90 min of MCAO, and rt-PA was administered immediately through the tail vein. The effect on the no-reflow was observed by measuring relative CBF, assessing arterial trunk patency and microvascular patency, and performing densitometric analysis of ICAM-1 and P-selectin after immunohistochemistry. The results showed that cilostazol suppressed the no-reflow in 45-min MCAO/R. In cilostazol-treated mice, the no-reflow was significantly suppressed (Figure 6(a) and (b)). 125 Another observation showed that endothelial adhesion molecule expression, such as ICAM-1 and p-selectin, was reduced in mice treated with MCAO/R cilostazol for 45 min in the ischemic cerebral cortex. Cilostazol maintains microvascular blood flow in the ischemic area by inhibiting microvascular platelet aggregation and leukocyte occlusion (Figure 6(c)). 125 Thus, cilostazol, as an antiplatelet aggregating drug, may be more suitable for patients with a hypercoagulable state who have a heavy vascular thrombus load. Simultaneously, cilostazol exhibits antiarrhythmic properties and therapeutic benefits for heart failure. 131 Therefore, we postulate that cilostazol may also be clinically recommended for patients experiencing no-reflow with heart failure. Additionally, it also effectively inhibited the occurrence of edema and hemorrhagic transformation in the rt-PA group, thereby establishing a theoretical basis for the regular utilization of cilostazol in thrombolytic patients in the clinic. 125
Figure 6.
Cilostazol suppressed no-reflow phenomenon. 125 (a) Representative images of CBF at the indicated time points pre- and post-MCAO/R in vehicle- and cilostazol-treated mice. Arrows indicate suppression of no-reflow phenomenon with cilostazol. (b) Temporal profile of CBF of vehicle- and cilostazol-treated mice after 45-minute MCAO/R. CBF was expressed as a ratio to the baseline level and (c) Cilostazol preserved microvascular blood flow in ischemic areas by suppressing platelet aggregation and leukocyte plugging in microvessels. Copyright 2012, Elsevier.
Adhesion molecule blocking antibody
P/E-selectin and ICAM-1 are adhesion molecules primarily expressed on the surface of ECs, facilitating intercellular or extracellular cell matrix (ECM) interactions and binding. The overexpression of the three proteins after ischemia-reperfusion leads to leukocyte adhesion and aggregation in capillaries, impeding blood flow and impairing microcirculatory perfusion.122,132,133
The expression of E-selectin mRNA was analyzed in mice after transient intraluminal MCAO, revealing an upregulation within 4 h of reperfusion and persisting for 24 h. 122 Anti-E-selectin antibody increased ischemic cortical CBF up to 2.6-fold. Furthermore, the administration of anti-E-selectin treatment not only resulted in dose-dependent reductions in neurological deficits, mortality, and infarct volumes, but also effectively decreased cerebral neutrophil accumulation. Even when administered 3 h after ischemia, it exhibited neuroprotective properties. 122
Researchers monitored rCBF using laser Doppler to detect the presence, time course, and P-selectin dependence of cerebrovascular no-reflow after ischemia. In experiments using P-selectin deletional mutant mice, the functional role of P-selectin expression in ischemic stroke was observed. 124 Therefore, subsequent experiments evaluated whether pharmacological inhibition of P-selectin could improve outcomes in mice. The results indicated that administration of a P-selectin blocking antibody before MCAO improved post-reperfusion CBF, reduced cerebral infarction volumes, and trended toward reduced mortality compared to control mice. To enhance the clinical significance of a P-selectin blocking strategy as a novel therapy for ischemic stroke, supplementary experiments were conducted. These experiments involved administering either a control or a blocking antibody after the occlusion of the middle cerebral artery, as most patients typically seek medical attention after the onset of cerebral ischemia. The results revealed a significant reduction in infarct volumes and a trend toward improved CBF. 124
In primates, overexpressed ICAM-1 is found in the lenticulostriate microvasculature after 4 h of ischemia and reperfusion. 132 An autopsy study of recent cerebral infarcts in humans also demonstrated overexpressed ICAM-1. 133 An experiment was performed to explore whether it significantly improved the no-reflow after recanalization. The results showed that ICAM-1 −/− mice were significantly protected from focal cerebral ischemia and reperfusion effects, based on a 3.7-fold reduction in infarct volume compared to ICAM-1 +/+ controls. This reduction in infarct volume was accompanied by reduced neurologic deficit and increased postreperfusion cerebral cortical blood flow. Collectively, their studies indicate that, in a murine model of focal cerebral ischemia and reperfusion, neutrophils accumulate in the infarcted hemisphere and that neutropenic animals demonstrate cerebral protection. Moreover, overexpressed ICAM-1 on cerebral ECs appears to be an essential mechanism driving this neutrophil recruitment, and mice unable to express ICAM-1 demonstrate improved postischemic blood flows, reduced infarction volumes, and reduced mortality. 46 These data indicate that pharmacologic strategies that interfere with neutrophil-endothelial interactions may improve the outcome after ischemic stroke in humans.
There is a lack of clinical studies using adhesion molecule-blocking antibodies to treat no-reflow. Given the above, it is postulated that adhesion molecule-blocking antibodies may be applied to individuals subjected to no-reflow, particularly with a significant inflammatory reaction and a surge in the neutrophil count after restoration.
PPARs activator
Peroxisome proliferator-activated receptors (PPARs) include three types, PPARα, PPARβ/δ and PPAR-γ. The most often reported feature of PPAR-γ agonists in stroke studies is their ability to lessen inflammation. PPAR-γ activation increased the microvessels area and number, accompanied by reduced infarction, improved functional recovery, and enhanced neuroprotective action of rt-PA. It has also lengthened the time window for initiating rt-PA treatment. 134 Another mechanism by which PPAR-γ can limit inflammation after ischemic stroke is blocking pro-inflammatory IL-6 protein expression. Pioglitazone, a PPAR-γ activation, injected intracerebroventricularly into mice suppressed IL-6 at 24 h after MCAO, which was accompanied by reduced infarction volume and improved neurological sequelae. 135 Endothelial cell-selective PPAR-γ conditional knockout abolished pioglitazone-mediated cerebrovascular protection in mouse MCAO model. 136 Moreover, PPAR-γ activation by pioglitazone significantly inhibited oxygen-glucose deprivation (OGD) induced cerebral vascular endothelial cell death and MCAO-triggered cerebrovascular damage.
Fenofibrate, a group of first-generation synthetic PPAR-α activators, is a lipid-lowering drug. Historically, fenofibrate was the most commonly used PPAR-α agonist in animal ischemic stroke model research providing several protective effects. Fenofibrate can effectively block neuroinflammation and apoptosis, protect the vasculature, and induce neurogenesis. 137 Fenofibrate reduced the risk of thrombolysis-induced hemorrhage during the acute phase of ischemic stroke in a rat MCAO model, decreased the infarct volume, and reduced microglial activation and neutrophil infiltration into the infarct area.
Furthermore, fenofibrate diminished cell death, pro-inflammatory neutrophil infiltration, and microglia activation. Fenofibrate also protected the vasculature by preventing leukocyte rolling and promoted neurogenesis. 138 Additionally, pretreatment with oral fenofibrate in MCAO mice improved CBF recovery following recanalization. It reduced the effects of the enlarged ischemic region, which were demonstrated to be PPAR-α dependent. 139 It has also been demonstrated that a 14-day preventive treatment with fenofibrate reduced susceptibility to stroke and decreased cerebral infarct volume in mice. Moreover, fenofibrate administration was associated with decreased cerebral oxidative stress, increased activity of several antioxidant enzymes, and a reduced expression of adhesion molecules. 140
Both pioglitazone and fenofibrate can improve blood flow recovery by decreasing the inflammatory response, reducing infarct size, and increasing the microvessels number. Pioglitazone is commonly used clinically for the treatment of diabetes mellitus, so we hypothesized that a PPAR agonist such as pioglitazone would be a more appropriate choice in individuals with combined diabetes mellitus and no-reflow. The research findings indicate that PPARs activator decreases plasma triglyceride levels and increases high-density lipoprotein cholesterol levels. 141 Thus, PPARs activator could also potentially be a candidate for the treatment of patients with no-reflow combined with poor lipid control.
Adenosine
Adenosine has various positive benefits, including increasing microvascular flow caused by its vasodilator qualities, inhibiting neutrophil adhesion and migration, acting as an antiplatelet, and preventing generating oxygen radicals, which reduces cellular acidosis. 142 Intravenous and intracoronary adenosine injections are beneficial. Despite the intricate and occasionally contradictory roles of adenosine receptor subtypes in the brain and periphery, its administration through central means or continuous intravenous infusion can effectively protect against cerebral ischemia.143 –145
After 2 h of MCA occlusion, the persistent release of adenosine from nano-assemblies (Nas) lowered ischemia-induced erythrocyte entrapments and increased microcirculatory reflow by relaxing constricted pericytes. 123 The squalenoyl adenosine (SQAd) solution in ethanol was nano-precipitated in a 5% aqueous dextrose solution without adding a surfactant to create the Nas. The results revealed that intravenous bolus administration of SQAd Nas dose-dependently decreased the infarct volume in mice subjected to 2 h of MCAO and 22 h of reperfusion. 123 Furthermore, most ischemic microvessels were patent 6 h after MCA reopening in SQAd Nas administered mice, contrary to untreated animals in whom ischemic capillaries were still clogged with trapped erythrocytes. 123 This phenomenon could potentially be attributed to pericyte contractions induced by ischemia, as has been previously documented. 14 Unlike untreated mice, treated animals did not have swelling astrocyte end-feet around microvessels and endothelial nuclei. After 6 h of reperfusion, when inflammatory response and astrocytes had remained significantly inactivated, the infarct area was much smaller in mice treated with SQAd Nas. Therefore, the endothelium-penetrating adenosine dissociated from Nas may interact with its receptors on pericytes of the cerebral microvessels and astrocyte end-feet, subsequently enhancing microcirculation. 70
Additionally, adenosine is cytoprotective in low intracellular ATP situations by boosting ATP synthesis independently of adenosine receptors, which may have prevented the edema in the endothelium and astrocyte end-feet seen. 146 Thus, the therapeutic effect of adenosine after cerebral ischemia-reperfusion is to increase microcirculatory reflow by relaxing constricted pericytes and reducing ischemia-induced erythrocyte nesting. It may also reduce the edema of endothelial cells and astrocytes to improve the no-reflow.
Nevertheless, the current evidence in support of employing adenosine for the management of cerebral no-reflow is deemed inadequate from an evidence-based medical. In experiments, adenosine has been found to exert a therapeutic effect on no-reflow through different mechanisms. Adenosine has vasodilator qualities and is also used in paroxysmal supraventricular tachycardia, 147 we speculate that adenosine has great potential for the treatment of patients with no-reflow combined hypertension or arrhythmia.
Pericyte regulators
Pericytes are close to capillary intervals and are crucial in stabilizing the blood-brain barrier, regulating blood flow, and immunomodulation. 148 The eventual consequences of sustained pericyte contraction include impaired blood flow and unfavorable clinical outcomes in cases of ischemic stroke. 149 Pericyte constriction causes capillary constriction and microvascular disorders in the penumbra in the acute stage of cerebral ischemia-reperfusion. 69
Experimental evidence suggests that peroxynitrite-induced pericyte contraction occurs. Furthermore, the inhibition of oxidative-nitrosative stress has been found to alleviate pericyte contraction induced by ischemia and reperfusion, consequently improving microcirculation and positively impacting tissue survival. 14 To investigate the possible involvement of oxygen and nitrogen radicals in pericyte contraction induced by ischemia and reperfusion, the researchers administered a low dose of the NOS inhibitor Nω-nitro-L-arginine and the superoxide scavenger N-tertbutyL-α-phenylnitrone to mice just prior to reperfusion. Pericytes were labeled with an anti-α-smooth muscle actin antibody, and contracted microvascular segments were co-localized with pericytes. Contrary to the diffuse staining of non-ischemic pericytes around the microvascular lumen, α-SMA immunostaining of ischemic pericytes was constricted and concentrated. Both agents significantly restored microvessels patency measured on cross sections of α-SMA-labeled capillaries compared to the untreated mice 14 This suggests that superoxide scavengers and NOS inhibitors have a significant alleviating effect on contracted pericytes.
Iptakalim (IPT) is a novel ATP-sensitive potassium channel (K-ATP) opener developed in China, which can protect neurons, astrocytes, and microglia from ischemic damage.150 –152 IPT relieves pericyte contraction and restores microvascular blood flow by reducing SUR2/EPAC1 complex formation, decreasing ET-1 release and calcium influx, and promoting K-ATP channel opening. 153 This result suggests that IPT may be a potential pericyte regulator for treating ischemic stroke and no-reflow.
Pericyte regulators have shown positive therapeutic effects on no-reflow phenomenon in experimental animal studies. However, the clinical evidence necessitates further investigation to validate these findings. This study presents novel insights for the clinical therapeutic agents of no-reflow following recanalization.
Adjunctive therapies
Extracorporeal counterpulsation (ECP)
The ECP device, initially used to treat cardiovascular disorders like coronary heart disease, is a non-invasive method of assisted circulation. During diastole, the heart balloon is inflated and pressurized sequentially, starting distally and then proximally sequentially. This process aims to increase the aortic diastolic pressure and cardiac output. Because cerebral efflux accounts for about 15–20% of cardiac efflux, cerebral flow is also better perfused during diastole, promoting the opening of collateral and anastomotic branches, increasing CBF in ischemic areas, dilating blood vessels, and improving ischemia brain cell metabolism. 154 Enhanced external counterpulsation (EECP) can also change blood rheology 155 and hemodynamic environment, increase the systemic blood circulation rate and venous return, and reduce the whole blood cell aggregation index and plasma viscosity. Moreover, EECP can improve cerebral circulation and the no-reflow by causing the aggregated red blood cells to depolymerize under high-pressure intensity, changing from aggregated to dispersed, accelerating blood flow, and decreasing blood viscosity. 156
ECP is mainly appropriate for patients in the condition of infarct lesions confirmed by head CT or MRI examination, the first onset and within 1–3 months since the disease onset. Nevertheless, this strategy is unrecommended for individuals with bleeding disorders or tendencies, unresolved cerebral edema, severe cardiac, hepatic, and renal dysfunction. 157
Ischemic preconditioning
Ischemic preconditioning is a measure triggering endogenous neuroprotective effects and is extremely sensitive to cerebral ischemic injury. It was demonstrated that remote ischemic preconditioning is one of the neuroprotective modalities. 158 After cerebral ischemic preconditioning, due to the ever-present risk of ischemic brain injury, it is thought that the brain has developed a backup mechanism to defend against the ischemic attack and survive the injury. Over time, specific regenerative processes have evolved in living organisms to protect the body against tissue or organ damage.159 –161 The potential of various preconditioning agents to confer neuroprotection in the brain was examined, revealing that diverse preadaptation experiments offer various avenues through which neuroprotection may be conferred before an impending ischemic event with potentially fatal consequences. 162 Additionally, ischemic preconditioning can enhance microcirculation regulation, facilitate the proportional dilation of capillaries, and expedite blood flow in the microvasculature. Consequently, this process mitigates the inadequate blood flow to tissues during the ischemic phase. It prevents the no-reflow occurrence during the reperfusion phase, facilitating the prompt restoration of efficient tissue perfusion and averting additional cellular harm. 163 Ischemic preconditioning has been shown to improve cognitive brain function in ischemic stroke patients. 164 This intervention may mitigate the prognosis of patients with large vessel occlusion strokes, but it is difficult to be used clinically.
Alternative and complementary therapies
TCM has been studied in rehabilitation treatment for ischemic stroke, revealing significant effectiveness. The ischemic stroke pathogenesis is complex, involving a combination of factors, with a significant role of qi deficiency and blood stasis. Consequently, following the principles of enhancing qi and promoting blood circulation to alleviate collateral obstructions is imperative in treating this condition. No-reflow is an adverse clinical event after cerebral ischemia-reperfusion. Furthermore, TCM exerts a beneficial influence on the enhancement of no-reflow following restoration. Using benefitting qi and invigorating blood can reduce platelet aggregation, increase brain perfusion and cellular hypoxia tolerance, improve blood rheology, promote vascular regeneration and microcirculation, and anti-free radical damage. All these effects are beneficial for alleviating the phenomenon of no-reflow and nerve function after an ischemic stroke. TCM possess multi-target and multi-pathway protective properties in contraposing the complex pathophysiology of no-reflow.
For instance, the efficacy of various monomers, including puerarin and oxymatrine, as well as individual Chinese Materia Medica such as Paeoniae and Radix Codonopsis, alongside patent Chinese medicine and TCM formulas like Naoluoxintong and Buyang Huanwu decoction, have exhibited significant effectiveness. Additionally, acupuncture techniques focused on enhancing qi and promoting blood circulation have yielded favorable results.
Puerarin, 165 Oxymatrine, 166 flavone glycosides, 167 and tanshinone 168 can improve microcirculation and relieve no-reflow by altering blood rheology and lowering blood viscosity. Regarding a single Chinese Materia Medica, odonopsis pilosula can improve behavioral characteristics and CBF in mice 169 and enhance microcirculation to improve no-reflow after recanalization. Paeonia can improve no-reflow by upregulating vascular endothelial growth factor (VEGF), increasing brain microvascular density, and promoting vascular regeneration through the PGC-1α/Nrf2 pathway. 170 Among TCM formulas, Buyang Huanwu decoction improves no-reflow by increasing microvascular density and promoting angiogenesis by upregulating VEGF and angiopoietin-1 (Ang-1). 171 Huoxueshengluo formula promotes vascular regeneration and improves blood supply in no-reflow tissues by upregulating VEGF protein expression level. 172
Additionally, TCM formula Longzhi decoction may inhibit the excessive endoplasmic reticulum stress response in rat brains by down-regulating the expressions of glucose-related protein 78 (GRP78) and C/EBP homologous protein (CHOP), thereby promoting angiogenesis and improving the no-reflow. 173 In the area of patent Chinese medicine, Naoluoxintong can improve no-reflow by overexpressing β-catenin, VEGF, and Ang II, improving the CBF, promoting the blood flow increase in the penumbra, and increasing the number of blood vessel regeneration after cerebral ischemia to a certain extent. 174 Moreover, TCM formula Naoluoxintong can improve the hemorheology indexes and regulate the relevant factors of the fibrinolysis coagulation system in MCAO/R model rats, which significantly affects ischemic cerebrovascular disease. 175 TCM formula Yangxueqingnao granule promotes vascular regeneration and improves blood supply by upregulating the expression levels of VEGF protein. 176 TCM formula Xinnao Shutong capsule overexpresses Ang-1/Tie2, mediates vascular angiogenesis, and improves no-reflow. 177 Erigeron Breviscapus injection raises VEGF and VEGF receptor 2 (VEGFR-2/Flk-1) after focal cerebral ischemia and reperfusion, thereby reducing the extent of vascular endothelial inflammation after cerebral ischemia and reperfusion, inducing ischemic vascular endothelial cell proliferation and angiogenesis, and increasing rCBF in the ischemic area. 178 Astragalus injection, which benefits qi and nourishes energy, can increase CBF and reduce infarct volume by decreasing c-Jun N-terminal kinase-3(JNK3) expression. 179 TCM formula Tongxinluo capsule (TXL) effectively suppresses the overexpression of adhesion molecules (P-Selectin and ICAM-1) and chemokines (CCL-3, CCL-4, CCL-5, and CXCL1) induced by ischemic stroke, thereby reducing interactions between leukocytes and endothelial cells and ultimately improving the microcirculation of no-reflow. 180 Regarding acupuncture, electroacupuncture can effectively increase the local cerebral tissue blood flow and the number of local cerebral cortical microvascular ECs after ischemia-reperfusion to promote microvascular neovascularization in rats. Acupuncture at Baihui acupoint can overexpress VEGF, β-catenin, glycogen synthase kinase-3β (GSK-3β), endostatin (ES) in cerebral tissues of rats with cerebral ischemia-reperfusion, promote the generation of microvessels and improve the microcirculatory status of cerebral tissues in the ischemic area.181,182 The above study provides new perspectives and feasible ideas for the TCM study of the microcirculatory no-reflow phenomenon after recanalization.
In summary, there is a lack of clinically accessible therapeutic interventions to address no-reflow. However, preclinical investigations above have exhibited the effectiveness of certain strategies and we also provide speculations on how to choose a strategy for a given patient. Cilostazol, a phosphodiesterase inhibitor, which can prevent platelet aggregating and arrhythmia are assumed to use in no-reflow patients presenting with a significant burden of vascular thrombosis, heart failure, or arrhythmia. 131 PPAR agonists, such as pioglitazone and fenofibrate that have been shown to directly decrease blood glucose and lipids, 141 could benefit the no-reflow patients particularly when coupled with hypercholesterolemia or diabetes mellitus. Adenosine, another potential therapy targeting no-reflow, which can dilate coronary vessels and slow auriculo-ventricular node conduction. 147 could improve the no-reflow concomitant coronary artery disease or paroxysmal supraventricular tachycardia. However, extracorporeal counterpulsation, an adjuvant treatment, is assumed that could potentially serve as a feasible intervention for patients who exhibit no-reflow with no concurrent cerebral hemorrhage, cerebral edema, or organ dysfunction. 157 TCM has the potential to address various symptoms and manifestations associated with different stages of ischemic stroke via multi-target characterization, thereby reducing the occurrence of no-reflow and enhancing therapeutic outcomes.
Conclusion and future directions
The extensive body of evidence we have unequivocally confirms the existence of the cerebral no-reflow phenomenon in both animal models and humans, following decades of meticulous research. This phenomenon is contingent upon numerous pathomechanisms, which frequently intersect. The available studies manifest that adhesion and aggregation of blood elements to form small thrombi and pericyte contraction contribute significantly to generating no-reflow. NVU, as an important component in maintaining normal physiologic function of the brain, also plays an essential role in the pathogenesis of no-reflow. In the future, the exploration of NVU will also become an important element in the exploration of no-reflow. Constraints in the precision of available equipment, which hinder the monitoring of intracranial microvessels and the precise assessment of their perfusion status. Imaging techniques such as TCD, MRP, and CTP have been validated in experimental studies, but there is still a lack of expert consensus due to limited clinical research on no-reflow. Therefore, more accurate judgments are still necessary in combination with clinical symptoms and risk factors or predictors to delineate the future directions of no-reflow diagnostics.
Due to the favorable application of recanalization therapy for acute ischemic stroke, clinical studies on no-reflow phenomenon are often ignored. With the constant advancement of recanalization treatment, the therapeutic window has been prolonged, and a growing population of patients can access advantages from recanalization treatment. Consequently, it is recommended that clinicians enhance their vigilance regarding the potential escalation of no-reflow. Furthermore, analyzing the risk factors and predictors of no-reflow, it is noticed that metabolic disorders such as hyperglycemia and hyperlipidemia increased the susceptibility to no-reflow.
There is a lack of clinically approved drugs for no-reflow, but in experimental studies it has been revealed the potential of phosphodiesterase inhibitors, adenosine, and PPAR agonists in effectively managing the no-reflow, while simultaneously addressing the severe underlying disease such as diabetes and heart failure. And ongoing research is actively exploring new medicines which have significant clinical efficacy. The contraction of pericytes emerges as a prominent causative factor, thus, relieving contracted pericytes may become the next focus. In the clinic, TCM has effectively prevented and treated low/no-reflow following recanalization, promoted vascular regeneration, and improved microcirculation to distribute blood flow more widely. This will also provide new ideas and approaches for future clinical drug development.
Although some progress in no-reflow has been made, some key issues remain. Therefore, we systematically discuss the pathomechanisms, risk factors, diagnostic criteria, and therapeutic strategies for no-reflow to draw the attention of clinical and scientific researchers to this phenomenon, especially the in-depth research on the mechanisms. With the increased focus on cerebral no-reflow, it is expected that more advanced diagnostic and therapeutic strategies will be developed and implemented, thereby enhancing the efficacy of cerebrovascular recanalization therapy, reducing the lethality and disability caused by reperfusion injury, and improving the quality of life of patients.
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (82174167), the key project of Hunan Province Education Department (20A366, 22A0248), the project of Natural Science Foundation of Hunan Province (2021JJ30499), and the fund for Youth Top Talent Project of Hubei Provincial Health and Family Planning Commission (EWT-2019-48).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ames RA, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437–453. [PMC free article] [PubMed] [Google Scholar]
- 2.Aspey BS, Jessimer C, Pereira S, et al. Do leukocytes have a role in the cerebral no-reflow phenomenon? J Neurol Neurosurg Psychiatry 1989; 52: 526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer EG, Ames RA, Hedley-Whyte ET, et al. Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the “no-reflow phenomenon”. Stroke 1977; 8: 36–39. [DOI] [PubMed] [Google Scholar]
- 4.Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J 2001; 22: 729–739. [DOI] [PubMed] [Google Scholar]
- 5.Law MM, Gelabert HA, Colburn MD, et al. Continuous postoperative intra-arterial urokinase infusion in the treatment of no reflow following revascularization of the acutely ischemic limb. Ann Vasc Surg 1994; 8: 66–73. [DOI] [PubMed] [Google Scholar]
- 6.Group NION. Tissue plasminogen activator for acute ischemic stroke. New Engl J Med 1995; 333: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 9.Rubiera M, Alvarez-Sabin J, Ribo M, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 2005; 36: 1452–1456. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke (1970) 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 11.Meschia JF, Barrett KM, Brott TG. Reperfusion therapy for acute ischemic stroke: how should we react to the third interventional management of stroke (IMS III) trial? Mayo Clin Proc 2013; 88: 653–657. [DOI] [PubMed] [Google Scholar]
- 12.Espinosa DRM, Parrilla G, Manzano-Fernandez S, et al. Combined multimodal computed tomography score correlates with futile recanalization after thrombectomy in patients with acute stroke. Stroke 2015; 46: 2517–2522. [DOI] [PubMed] [Google Scholar]
- 13.Rha J, Saver JL. The impact of recanalization on ischemic stroke outcome a meta-analysis. Stroke (1970) 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 14.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 15.Liang C, Chu H, Wang F. Advances in the pharmacological treatment of cerebral ischemia-reperfusion injury. China Pharmacy 2016; 27: 538–541. [Google Scholar]
- 16.Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab 2021; 41: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks MP, Lansberg MG, Mlynash M, et al. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke 2014; 9: 860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology 2022; 98: e790–e801. [DOI] [PubMed] [Google Scholar]
- 19.Ng FC, Coulton B, Chambers B, et al. Persistently elevated microvascular resistance postrecanalization. Stroke 2018; 49: 2512–2515. [DOI] [PubMed] [Google Scholar]
- 20.Rubiera M, Garcia-Tornel A, Olive-Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke 2020; 51: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 21.Del ZG. Stroke and neurovascular protection. New Engl J Med 2006; 354: 553–555. [DOI] [PubMed] [Google Scholar]
- 22.Choi MH, Park GH, Lee JS, et al. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke (1970) 2018; 49: 652–659. [DOI] [PubMed] [Google Scholar]
- 23.Krajíčková D, Krajina A, Šteiner I, et al. Fibrin clot architecture in acute ischemic stroke treated with mechanical thrombectomy with stent-retrievers-cohort study. Circ J 2018; 82: 866–873. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Wang J, Ge L, et al. A fibrin targeted molecular imaging evaluation of microvascular no-reflow in acute ischemic stroke. Brain Behav 2022; 12: e2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer EG, Ames DA. Studies on mechanisms of impairment of cerebral circulation following ischemia: effect of hemodilution and perfusion pressure. Stroke (1970) 1972; 3: 538–542. [DOI] [PubMed] [Google Scholar]
- 26.Taskiran-Sag A, Yemisci M, Gursoy-Ozdemir Y, et al. Improving microcirculatory reperfusion reduces parenchymal oxygen radical formation and provides neuroprotection. Stroke (1970) 2018; 49: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ZG, Chopp M, Goussev A, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of pai-1 acutely after onset of focal embolic ischemia in rats. J Neurosci 1999; 19: 10898–10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denorme F, Wyseure T, Peeters M, et al. Inhibition of thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 reduces ischemic brain damage in mice. Stroke 2016; 47: 2419–2422. [DOI] [PubMed] [Google Scholar]
- 29.Garcia JH, Liu KF, Yoshida Y, et al. Influx of leukocytes and platelets in an evolving brain infarct (wistar rat). Am J Pathol 1994; 144: 188–199. [PMC free article] [PubMed] [Google Scholar]
- 30.Ritter LS, Orozco JA, Coull BM, et al. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke 2000; 31: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 31.Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl 1996; 66: 27–31. [DOI] [PubMed] [Google Scholar]
- 32.Schmid-Schönbein GW. The damaging potential of leukocyte activation in the microcirculation. Angiology 1993; 44: 45–56. [DOI] [PubMed] [Google Scholar]
- 33.Ritter LS, Wilson DS, Williams SK, et al. Early in reperfusion following myocardial ischemia, leukocyte activation is necessary for venular adhesion but not capillary retention. Microcirculation 1995; 2: 315–327. [DOI] [PubMed] [Google Scholar]
- 34.Han JY, Miura S, Akiba Y, et al. Chronic ethanol consumption exacerbates microcirculatory damage in rat mesentery after reperfusion. Am J Physiol Gastrointest Liver Physiol 2001; 280: G939–G948. [DOI] [PubMed] [Google Scholar]
- 35.Buras JA, Reenstra WR. Endothelial-neutrophil interactions during ischemia and reperfusion injury: basic mechanisms of hyperbaric oxygen. Neurol Res 2007; 29: 127–131. [DOI] [PubMed] [Google Scholar]
- 36.Hallenbeck JM, Dutka AJ, Tanishima T, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke 1986; 17: 246–253. [DOI] [PubMed] [Google Scholar]
- 37.Grogaard B, Schurer L, Gerdin B, et al. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab 1989; 9: 500–505. [DOI] [PubMed] [Google Scholar]
- 38.Grau AJ, Graf T, Hacke W. Altered influence of polymorphonuclear leukocytes on coagulation in acute ischemic stroke. Thromb Res 1994; 76: 541–549. [DOI] [PubMed] [Google Scholar]
- 39.Kaesmacher J, Boeckh-Behrens T, Simon S, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol 2017; 38: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heye N, Paetzold C, Steinberg R, et al. The topography of microthrombi in ischemic brain infarct. Acta Neurol Scand 1992; 86: 450–454. [DOI] [PubMed] [Google Scholar]
- 41.Heye N, Cervos-Navarro J. Microthromboemboli in acute infarcts: analysis of 40 autopsy cases. Stroke 1996; 27: 431–434. [DOI] [PubMed] [Google Scholar]
- 42.Doerschuk CM, Beyers N, Coxson HO, et al. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 1993; 74: 3040–3045. [DOI] [PubMed] [Google Scholar]
- 43.Cruz HJ, Bracko O, Kersbergen CJ, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat Neurosci 2019; 22: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdener SE, Tang J, Kilic K, et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab 2021; 41: 236–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Amki M, Glück C, Binder N, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep 2020; 33: 108260. [DOI] [PubMed] [Google Scholar]
- 46.Connolly JES, Winfree CJ, Springer TA, et al. Cerebral protection in homozygous null ICAM-1 mice after Middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 1996; 97: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amki ME, Wegener S. Reperfusion failure despite recanalization in stroke: new translational evidence. Clinical and Translational Neuroscience 2021; 5: 2514183X2110071. [Google Scholar]
- 48.Garcia JH, Liu KF, Yoshida Y, et al. Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (wistar rat). Am J Pathol 1994; 145: 728–740. [PMC free article] [PubMed] [Google Scholar]
- 49.Simard JMD, Kent TAM, Chen MM, et al. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 2007; 6: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab 2016; 36: 513–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang D, Bhatta S, Gerzanich V, et al. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg Focus 2007; 22: E2–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michinaga S, Koyama Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci 2015; 16: 9949–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019; 10: 5816–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blecharz-Lang KG, Wagner J, Fries A, et al. Interleukin 6-mediated endothelial barrier disturbances can be attenuated by blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Transl Stroke Res 2018; 9: 631–642. [DOI] [PubMed] [Google Scholar]
- 55.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 2004; 45: 545–552. [DOI] [PubMed] [Google Scholar]
- 56.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med 2006; 12: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 58.Greenwood J. Mechanisms of blood-brain barrier breakdown. Neuroradiology 1991; 33: 95–100. [DOI] [PubMed] [Google Scholar]
- 59.Unterberg AW, Stover J, Kress B, et al. Edema and brain trauma. Neuroscience 2004; 129: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 60.Donkin JJ, Vink R. Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments. Curr Opin Neurol 2010; 23: 293–299. [DOI] [PubMed] [Google Scholar]
- 61.Mohamed Mokhtarudin MJ, Payne SJ. Mathematical model of the effect of ischemia–reperfusion on brain capillary collapse and tissue swelling. Math Biosci 2015; 263: 111–120. [DOI] [PubMed] [Google Scholar]
- 62.Winkler EA, Sengillo JD, Bell RD, et al. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 2012; 32: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher M. Pericyte signaling in the neurovascular unit. Stroke 2009; 40: S13–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai W, Liu H, Zhao J, et al. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res 2016; 8: 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burdyga T, Borysova L. Calcium signalling in pericytes. J Vasc Res 2014; 51: 190–199. [DOI] [PubMed] [Google Scholar]
- 68.Kamouchi M, Kitazono T, Ago T, et al. Calcium influx pathways in rat CNS pericytes. Brain Res Mol Brain Res 2004; 126: 114–120. [DOI] [PubMed] [Google Scholar]
- 69.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature (London) 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2010; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, et al. Retinal ischemia induces alpha-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol Com 2019; 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanoke A, Akamatsu Y, Nishijima Y, et al. The impact of native leptomeningeal collateralization on rapid blood flow recruitment following ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freitas-Andrade M, Raman-Nair J, Lacoste B. Structural and functional remodeling of the brain vasculature following stroke. Front Physiol 2020; 11: 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma J, Ma Y, Dong B, et al. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab 2017; 37: 3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cameron IR, Segal MB. The effect on pial arteriole diameter of local changes in potassium concentration. Eur Neurol 1971; 6: 100–106. [DOI] [PubMed] [Google Scholar]
- 76.Kuschinsky W, Wahl M, Bosse O, et al. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res 1972; 31: 240–247. [DOI] [PubMed] [Google Scholar]
- 77.Keatinge WR. Electrical and mechanical responses of vascular smooth muscle to vasodilator agents and vasoactive polypeptides. Circ Res 1966; 18: 641–649. [DOI] [PubMed] [Google Scholar]
- 78.Konold P, Gebert G, Brecht K. The effect of potassium on the tone of isolated arteries. Pflugers Arch Gesamte Physiol Menschen Tiere 1968; 301: 285–291. [DOI] [PubMed] [Google Scholar]
- 79.Baldy-Moulinier M. Cerebral blood flow and membrane ionic pump. Eur Neurol 1971; 6: 107–113. [DOI] [PubMed] [Google Scholar]
- 80.Freeman DJ, Daniel EE. Calcium movement in vascular smooth muscle and its detection using lanthanum as a tool. Can J Physiol Pharmacol 1973; 51: 900–913. [DOI] [PubMed] [Google Scholar]
- 81.Holloway ET, Bohr DF. Reactivity of vascular smooth muscle in hypertensive rats. Circ Res 1973; 33: 678–685. [DOI] [PubMed] [Google Scholar]
- 82.Wade JG, Amtorp O, Sorensen SC. No-flow state following cerebral ischemia. Role of increase in potassium concentration in brain interstitial fluid. Arch Neurol 1975; 32: 381–384. [DOI] [PubMed] [Google Scholar]
- 83.Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol 2010; 31: 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng G, Xiao J, Yu H, et al. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: a meta-analysis. J Neurointerv Surg 2022; 14: 881–885. [DOI] [PubMed] [Google Scholar]
- 85.Groot AE, Treurniet KM, Jansen I, et al. Endovascular treatment in older adults with acute ischemic stroke in the MR clean registry. Neurology 2020; 95: e131–e139. [DOI] [PubMed] [Google Scholar]
- 86.Singer OC, Haring HP, Trenkler J, et al. Age dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis 2013; 36: 437–445. [DOI] [PubMed] [Google Scholar]
- 87.Marini C, Baldassarre M, Russo T, et al. Burden of first-ever ischemic stroke in the oldest old: evidence from a population-based study. Neurology 2004; 62: 77–81. [DOI] [PubMed] [Google Scholar]
- 88.Howe MD, McCullough LD. Prevention and management of stroke in women. Expert Rev Cardiovasc Ther 2015; 13: 403–415. [DOI] [PubMed] [Google Scholar]
- 89.Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab 2007; 27: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 90.Santisteban MM, Ahn SJ, Lane D, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension 2020; 76: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kawai N, Keep RF, Betz AL. Hyperglycemia and the vascular effects of cerebral ischemia. Stroke 1997; 28: 149–154. [DOI] [PubMed] [Google Scholar]
- 92.Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial doppler method. J Hypertens 1994; 12: 191–197. [PubMed] [Google Scholar]
- 93.Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002; 33: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 94.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 2007; 27: 435–451. [DOI] [PubMed] [Google Scholar]
- 95.Hussein HM, Saleem MA, Qureshi AI. Rates and predictors of futile recanalization in patients undergoing endovascular treatment in a multicenter clinical trial. Neuroradiology 2018; 60: 557–563. [DOI] [PubMed] [Google Scholar]
- 96.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. Jama-J Am Med Assoc 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 97.Van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete reperfusion in acute ischemic stroke patients. J Neurointerv Surg 2021; 13: 14–18. [DOI] [PubMed] [Google Scholar]
- 98.Lee SH, Kim BJ, Han MK, et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol 2019; 19: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawiorski MM, Martinez-Sanchez P, Garcia-Pastor A, et al. Alberta stroke program early CT score applied to CT angiography source images is a strong predictor of futile recanalization in acute ischemic stroke. Neuroradiology 2016; 58: 487–493. [DOI] [PubMed] [Google Scholar]
- 100.Gilberti N, Gamba M, Premi E, et al. Leukoaraiosis is a predictor of futile recanalization in acute ischemic stroke. J Neurol 2017; 264: 448–452. [DOI] [PubMed] [Google Scholar]
- 101.Tateishi Y, Wisco D, Aoki J, et al. Large deep white matter lesions may predict futile recanalization in endovascular therapy for acute ischemic stroke. Interv Neurol 2015; 3: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tong E, Patrie J, Tong S, et al. Time-resolved CT assessment of collaterals as imaging biomarkers to predict clinical outcomes in acute ischemic stroke. Neuroradiology 2017; 59: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 103.Nie X, Pu Y, Zhang Z, et al. Futile recanalization after endovascular therapy in acute ischemic stroke. Biomed Res Int 2018; 2018: 5879548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray KN, Girard S, Holmes WM, et al. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia. Stroke 2014; 45: 3412–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olah L, Wecker S, Hoehn M. Relation of apparent diffusion coefficient changes and metabolic disturbances after 1 hour of focal cerebral ischemia and at different reperfusion phases in rats. J Cereb Blood Flow Metab 2001; 21: 430–439. [DOI] [PubMed] [Google Scholar]
- 106.Zang N, Lin Z, Huang K, et al. Biomarkers of unfavorable outcome in acute ischemic stroke patients with successful recanalization by endovascular thrombectomy. Cerebrovasc Dis 2020; 49: 583–592. [DOI] [PubMed] [Google Scholar]
- 107.Boisseau W, Desilles JP, Fahed R, et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology 2019; 93: e467–e475. [DOI] [PubMed] [Google Scholar]
- 108.Li W, Huang K, Wu Y. Predictive factors for futile recanalization of acute anterior circulation ischemic stroke after endovascular treatment. Int J Cerebrov Dis 2021; 3: 201–205. [Google Scholar]
- 109.Li Y, Xu J, Yu T, et al. A labeling strategy for the three-dimensional recognition and analysis of microvascular obstruction in ischemic stroke. Theranostics 2023; 13: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moppett IK, Mahajan RP. Transcranial doppler ultrasonography in anaesthesia and intensive care. Br J Anaesth 2004; 93: 710–724. [DOI] [PubMed] [Google Scholar]
- 111.Maasakkers CM, Thijssen DH, Knight SP, et al. Hemodynamic and structural brain measures in high and low sedentary older adults. J Cereb Blood Flow Metab 2021; 41: 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campbell B, Christensen S, Levi C, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke (1970) 2011; 42: 3435–3440. [DOI] [PubMed] [Google Scholar]
- 113.Ostergaard L, Jespersen SN, Mouridsen K, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab 2013; 33: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee J, Gursoy-Ozdemir Y, Fu B, et al. Optical coherence tomography imaging of capillary reperfusion after ischemic stroke. Appl Opt 2016; 55: 9526–9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bivard A, Krishnamurthy V, Stanwell P, et al. Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke 2014; 45: 127–133. [DOI] [PubMed] [Google Scholar]
- 116.Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977; 198: 1264–1267. [DOI] [PubMed] [Google Scholar]
- 117.Fan JL, Nogueira RC, Brassard P, et al. Integrative physiological assessment of cerebral hemodynamics and metabolism in acute ischemic stroke. J Cereb Blood Flow Metab 2022; 42: 454–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hametner C, Stanarcevic P, Stampfl S, et al. Noninvasive cerebral oximetry during endovascular therapy for acute ischemic stroke: an observational study. J Cereb Blood Flow Metab 2015; 35: 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Werner P, Saur D, Zeisig V, et al. Simultaneous PET/MRI in stroke: a case series. J Cereb Blood Flow Metab 2015; 35: 1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Debatisse J, Wateau O, Cho TH, et al. A non-human primate model of stroke reproducing endovascular thrombectomy and allowing long-term imaging and neurological read-outs. J Cereb Blood Flow Metab 2021; 41: 745–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Acharya D, Ruesch A, Schmitt S, et al. Changes in neurovascular coupling with cerebral perfusion pressure indicate a link to cerebral autoregulation. J Cereb Blood Flow Metab 2022; 42: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang J, Choudhri TF, Winfree CJ, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke 2000; 31: 3047–3053. [PubMed] [Google Scholar]
- 123.Gaudin A, Yemisci M, Eroglu H, et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol 2014; 9: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Connolly JES, Winfree CJ, Prestigiacomo CJ, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res 1997; 81: 304–310. [DOI] [PubMed] [Google Scholar]
- 125.Hase Y, Okamoto Y, Fujita Y, et al. Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol 2012; 233: 523–533. [DOI] [PubMed] [Google Scholar]
- 126.Collino M, Patel NSA, Thiemermann CR. PPARs as new therapeutic targets for the treatment of cerebral ischemia/reperfusion injury. Ther Adv Cardiovasc Dis 2008; 2: 179–197. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y, Shakur Y, Yoshitake M, et al. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev 2001; 19: 369–386. [DOI] [PubMed] [Google Scholar]
- 128.Kim KY, Shin HK, Choi JM, et al. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther 2002; 300: 709–715. [DOI] [PubMed] [Google Scholar]
- 129.Fujita Y, Lin J, Takahashi R, et al. Cilostazol alleviates cerebral small-vessel pathology and white-matter lesions in stroke-prone spontaneously hypertensive rats. Brain Res 2008; 1203: 170–176. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka K, Gotoh F, Fukuuchi Y, et al. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke (1970) 1989; 20: 668–673. [DOI] [PubMed] [Google Scholar]
- 131.Kanlop N, Chattipakorn S, Chattipakorn N. Effects of cilostazol in the heart. J Cardiovasc Med (Hagerstown) 2011; 12: 88–95. [DOI] [PubMed] [Google Scholar]
- 132.Okada Y, Copeland BR, Mori E, et al. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke 1994; 25: 202–211. [DOI] [PubMed] [Google Scholar]
- 133.Sobel RA, Mitchell ME, Fondren G. Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human Central nervous system. Am J Pathol 1990; 136: 1309–1316. [PMC free article] [PubMed] [Google Scholar]
- 134.Gamdzyk M, Lenahan C, Tang J, et al. Role of peroxisome proliferator‐activated receptors in stroke prevention and therapy-The best is yet to come? J Neurosci Res 2020; 98: 2275–2289. [DOI] [PubMed] [Google Scholar]
- 135.Patzer A, Zhao Y, Stock I, et al. Peroxisome proliferator-activated receptorsgamma (PPARgamma) differently modulate the interleukin-6 expression in the peri-infarct cortical tissue in the acute and delayed phases of cerebral ischaemia. Eur J Neurosci 2008; 28: 1786–1794. [DOI] [PubMed] [Google Scholar]
- 136.Yin KJ, Fan Y, Hamblin M, et al. KLF11 mediates PPARgamma cerebrovascular protection in ischaemic stroke. Brain 2013; 136: 1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gautier S, Ouk T, Pétrault M, et al. PPAr-alpha agonist used at the acute phase of experimental ischemic stroke reduces occurrence of thrombolysis-induced hemorrhage in rats. Ppar Res 2015; 2015: 246329–246329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ouk T, Gautier S, Petrault M, et al. Effects of the PPAR-alpha agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab 2014; 34: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 2010; 30: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deplanque D, Gele P, Petrault O, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci 2003; 23: 6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Botta M, Audano M, Sahebkar A, et al. PPAR agonists and metabolic syndrome: an established role? International Journal of Molecular Sciences 2018; 19: 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marzilli M, Orsini E, Marraccini P, et al. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 2000; 101: 2154–2159. [DOI] [PubMed] [Google Scholar]
- 143.Kitagawa H, Mori A, Shimada J, et al. Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res 2002; 24: 317–323. [DOI] [PubMed] [Google Scholar]
- 144.Tatlisumak T, Takano K, Carano RA, et al. Delayed treatment with an adenosine kinase inhibitor, GP683, attenuates infarct size in rats with temporary Middle cerebral artery occlusion. Stroke 1998; 29: 1952–1958. [DOI] [PubMed] [Google Scholar]
- 145.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab 2007; 27: 1–5. [DOI] [PubMed] [Google Scholar]
- 146.Li S, Li X, Guo H, et al. Intracellular ATP concentration contributes to the cytotoxic and cytoprotective effects of adenosine. Plos One 2013; 8: e76731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alghadeer SM, Almohammed OA, Alshaya AI, et al. Adenosine response and failure to convert paroxysmal supraventricular tachycardia in the emergency department. Eur J Emerg Med 2023; 30: 341–346. [DOI] [PubMed] [Google Scholar]
- 148.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 149.Qiu B, Zhao Z, Wang N, et al. A systematic observation of vasodynamics from different segments along the cerebral vasculature in the penumbra zone of awake mice following cerebral ischemia and recanalization. J Cereb Blood Flow Metab 2023; 43: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ran YH, Wang H. Iptakalim, an ATP-sensitive potassium channel opener, confers neuroprotection against cerebral ischemia/reperfusion injury in rats by protecting neurovascular unit cells. J Zhejiang Univ Sci B 2011; 12: 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ji J, Yan H, Chen ZZ, et al. Iptakalim protects against ischemic injury by improving neurovascular unit function in the mouse brain. Clin Exp Pharmacol Physiol 2015; 42: 766–771. [DOI] [PubMed] [Google Scholar]
- 152.Chen H, Yang Y, Yao HH, et al. Protective effects of iptakalim, a novel ATP-sensitive potassium channel opener, on global cerebral ischemia-evoked insult in gerbils. Acta Pharmacol Sin 2006; 27: 665–672. [DOI] [PubMed] [Google Scholar]
- 153.Guo RB, Dong YF, Yin Z, et al. Iptakalim improves cerebral microcirculation in mice after ischemic stroke by inhibiting pericyte contraction. Acta Pharmacol Sin 2022; 43: 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiujuan F, Chen W, Liwei Q. The effects and mechanistic research of external counter pulsation in the rehabilitation of cerebral infarction. J Apoplexy Nervous Dis 2019; 36: 732–734. [Google Scholar]
- 155.Chunlin X, Musheng Y, Tongwen X. Clinical effect of external counterpulsation in the adjuvant therapy of brain infarction and the impact on hemodynamic index. J Pract Cardiocerebr Pulmon Vasculopathy 2016; 24: 144–146. [Google Scholar]
- 156.Xiao J, Cai X, Huang Z, et al. Clinical effect of enhanced external counterpulsation on progressive ischemic stroke and its impact on neurological function. J Pract Cardiocerebr Pulmon Vasculopathy 2015; 23: 102–104. [Google Scholar]
- 157.Zhou M, Qin J, Pang G. Effect of external counterpulsation combined with rehabilitation on early rehabilitation of patients with ischemic stroke. Chinese Journal of Geriatric Care 2020; 18: 24–26. [Google Scholar]
- 158.Stetler RA, Leak RK, Gan Y, et al. Preconditioning provides neuroprotection in models of cns disease: paradigms and clinical significance. Prog Neurobiol 2014; 114: 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Obrenovitch TP. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol Rev 2008; 88: 211–247. [DOI] [PubMed] [Google Scholar]
- 160.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 2009; 8: 398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 2011; 14: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thushara Vijayakumar N, Sangwan A, Sharma B, et al. Cerebral ischemic preconditioning: the road so far…. Mol Neurobiol 2016; 53: 2579–2593. [DOI] [PubMed] [Google Scholar]
- 163.Wang JH, Liu XH, Liu FY, et al. Protection of ischemic/reperfused brain by ischemic preconditioning and its relationship with microcirculatory regulation. Chinese J Pathophysiol 2003; 4: 102–105. [Google Scholar]
- 164.Yuan HJ, Huang XX, Lan ZJ, et al. Effects of different phase remote ischemic preconditioning on postoperative cognitive function and brain injury in patients with carotid endarterectomy. Chinese J Gen Pract 2022; 20: 1328–1331, 1335. [Google Scholar]
- 165.Yang L, He S. Effect of puerarin on expression of intercellular adhesion molecule-1 in cerebral ischemia/reperfusion rats. Chinese J Integr Trad Western Med 2003; S1: 42–44. [Google Scholar]
- 166.Han YF, Yu T. Effects of oxymatrine on hemorheology after acute cerebral ischemia-reperfusion in rats. J Emerg Tradition Chinese Med 2018; 27: 992–995. [Google Scholar]
- 167.Zhou LL, Ming L, Zou CW. Effect of extracts of ginkgo biloba leaves on hemorheology in repeated cerebral transient ischemia-reperfusion in mice. Chinese J Modern Appl Pharm 2000; 2: 91–93. [Google Scholar]
- 168.Han JY, Fan JY, Horie Y, et al. Ameliorating effects of compounds derived from salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Therapeut 2008; 117: 280–295. [DOI] [PubMed] [Google Scholar]
- 169.Yang PF, Chu SF, Chen NH. Advances in pharmacological researches of Dangshen and the protective mechanisms of Dangshenon brain lschemia reperfusion injury. J Hunan Univ Chinese Med 2015; 35: 5–10. [Google Scholar]
- 170.Chen M, Yan K, Cao Q. Study on the promoting angiogenesis of paeoniae in rats with cerebral ischemic stroke by PGC-1α/Nrf2. Res Practice Chinese Med 2021; 35: 36–40. [Google Scholar]
- 171.Zhou Y, Zhang YX, Yang KL, et al. Connexin 43 mediated the angiogenesis of buyang huanwu decoction via vascular endothelial growth factor and angiopoietin-1 after ischemic stroke. Chin J Physiol 2022; 65: 72–79. [DOI] [PubMed] [Google Scholar]
- 172.Wei Y, Wang Y, Wang Y, et al. The influence of hexue shengluo recipe to vascular endothelial growth factor and angiogenin of the cerebral ischemia/reperfusion rats. J Basic Chinese Med 2008; 14: 840–842. [Google Scholar]
- 173.Li N, Chen Y, Liu Q, et al. Effect of longzhi decoction on angiogenesis in rats with cerebral ischemia-reperfusion injury. Liaoning J Trad Chinese Med 2022; 49: 195–198. [Google Scholar]
- 174.Tan H, Yin T, Deng Y, et al. Mechanisms of yiqihuoxue herb naoluoxintong promotes cerebral vascular regeneration in rats with cerebral ischemia syndrome of Qi deficiency accompanied by blood stasis. Chinese J Cell Mol Immunol 2020; 36: 712–718. [PubMed] [Google Scholar]
- 175.Wang J, Tan H, Jian W, et al. Effects of naoluoxintong on hemorheology and related regulatory factors in focal cerebral ischemia-reperfusion rats. Chinese J Cerebrovasc Dis 2012; 9: 590–594. [Google Scholar]
- 176.Ding X, Wang J, Chen S. Effects of yangxueqingnao granule on the expression of vascular growth factors in rats with focal cerebral ischemia-reperfusion. Chinese J Clin Pharmacol 2016; 32: 1411–1413. [Google Scholar]
- 177.He Q, Yang H, Hu L. The effects of xinnao shutong capsule on the expressions of Angiopoietin1/Tie2 and angiogenesis after cerebral ischemia-reperfusion injury in rats. Chinese J Gerontol 2015; 35: 3203–3206. [Google Scholar]
- 178.Guo X, Zhang H, Chen W. Research of fleabane raising VEGF after focal cerebral ischemia and reperfusion in rats. J Emerg Trad Chinese Med 2016; 25: 985–987. [Google Scholar]
- 179.Liu G, Song J, Guo Y, et al. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct 2013; 9: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu S, Zhang Z, He Y, et al. Inhibiting leukocyte-endothelial cell interactions by Chinese medicine tongxinluo capsule alleviates no-reflow after arterial recanalization in ischemic stroke. Cns Neurosci Ther 2023; 29: 3014–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shi S, Tong X, Han S, et al. Effect of surrounding scalp acupuncture combined with exercise therapy on angiogenesis mechanism in rats with cerebral infarction. J Clin Acupuncture Moxibustion 2020; 36: 66–69. [Google Scholar]
- 182.Yang M, Chen B, Liang C. Effect of head needle on VEGF and ES expressions in rats with focal cerebral ischemia reperfusion. J Emergency Trad Chinese Med 2014; 23: 1012–1013, 1052. [Google Scholar]