Abstract
Introduction:
In patients with acute intracerebral haemorrhage (ICH) and elevated systolic blood pressure (BP), guidelines suggest that systolic BP reduction to <140 mmHg should be rapidly initiated. Compared with conventional care, Mobile Stroke Units (MSUs) allow for earlier ICH diagnosis through prehospital imaging and earlier BP lowering.
Patients and methods:
ICH patients were prospectively evaluated as a cohort of the controlled B_PROUD-study in which MSU availability alone determined MSU dispatch in addition to conventional ambulance. We used inverse probability of treatment weighting to adjust for confounding to estimate the effect of additional MSU dispatch in ICH patients. Outcomes of interest were 7-day mortality (primary), systolic BP (sBP) at hospital arrival, dispatch-to-imaging time, largest haematoma volume, anticoagulation reversal, length of in-hospital stay, 3-month functional outcome.
Results:
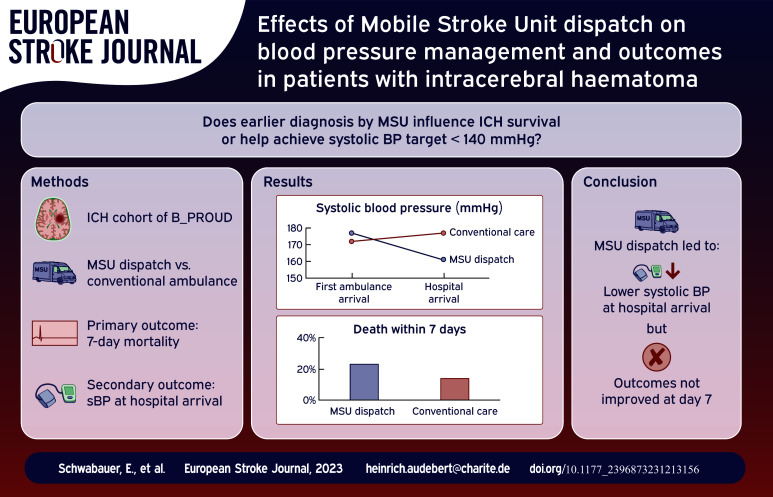
Between February 2017 and May 2019, MSUs were dispatched to 95 (mean age: 72 ± 13 years, 45% female) and only conventional ambulances to 78 ICH patients (mean age: 71 ± 12 years, 44% female). After adjusting for confounding, we found shorter dispatch-to-imaging time (mean difference: -17.75 min, 95% CI: −27.16 to −8.21 min) and lower sBP at hospital arrival (mean difference = −16.31 mmHg, 95% CI: −30.64 to −6.19 mmHg) in the MSU group. We found no statistically significant difference for the other outcomes, including 7-day mortality (adjusted odds ratio: 1.43, 95% CI: 0.68 to 3.31) or favourable outcome (adjusted odds ratio = 0.67, 95% CI: 0.27 to 1.67).
Conclusions:
Although MSU dispatch led to sBP reduction and lower dispatch-to-imaging time compared to conventional ambulance care, we found no evidence of better outcomes in the MSU dispatch group.
Keywords: Stroke, prehospital, mobile stroke unit, intracerebral haemorrhage, blood pressure, anticoagulation reversal
Graphical abstract.
Introduction
Worldwide, annually more than 5 million people are affected by acute intracerebral haemorrhage (ICH) and ICH is responsible for considerable preterm death and disability burden. 1 The majority of ICH patients present with hypertensive BP values. 2 While mechanisms of acute BP elevation are not well understood, the magnitude of BP elevation is positively correlated with admission haematoma volume, 3 haematoma expansion and poor outcome.4,5
Haematoma growth is a major determinant for poorer outcomes 6 and commonly occurs within the first 3 h of symptom onset 7 with highest rates of haematoma enlargement within the first hour of onset.7,8 Avoiding haematoma expansion is therefore an important target for therapeutic strategies. Therapeutic BP lowering in the acute phase of intracerebral haemorrhage leads to a modest reduction in haematoma expansion9,10 and perihaematomal oedema among patients with deep ICH. 11 However, it remains unclear whether intensive BP lowering has a beneficial effect on functional outcomes across patients with acute ICH 12 or if certain patients need individualised BP management. Although randomised controlled trials (RCT) have not shown a significant benefit of BP lowering on pre-specified primary outcomes, the current American Stroke Association (ASA) and European Stroke Organisation (ESO) guidelines recommend lowering of systolic BP to 140 mmHg 13 or to below 140 mmHg as early as possible. 12
Prehospital stroke work-up on Mobile Stroke Units (MSU) allows for earlier diagnosis of stroke subtypes. 14 Consequently, specific treatment not only for ischaemic but also for haemorrhagic stroke can be initiated at the scene – that is, BP management and anticoagulation reversal. 16 The aim of the current investigation was to determine whether MSU dispatch was associated with lower 7-day mortality (primary outcome) after the acute event compared to conventional ambulance dispatch alone among ICH patients in the Berlin_Prehospital Or Usual Delivery of stroke care (B_PROUD) study.
Methods
The methods of the B_PROUD study have been detailed in full elsewhere.15,16 B_PROUD was an investigator-initiated, non-randomised study comparing 3-month outcomes of patients with acute stroke in Berlin, Germany with and without dispatch of an MSU in addition to a conventional care ambulance. B_PROUD enrolled patients with acute stroke leading to a code stroke dispatch between February 1, 2017 and May 8, 2019 in Berlin, Germany. The study aimed at investigating the effect of additional MSU dispatch on functional outcomes among adults with ischaemic stroke and TIA patients who had no contraindications to reperfusion therapies (primary study population). 14 B_PROUD was approved by the Charité – Universitätsmedizin Berlin ethics committee (EA4/109/15). An analysis of the effects in all patients with acute stroke or TIA regardless of the aforementioned contraindications to reperfusion therapies including patients with intracranial haemorrhage has recently been published. 15 The present evaluation focuses on reporting procedures, processes and short-term outcomes for the subset of ICH patients. Because of the nature of this substudy and the small sample size, the study had been planned as an exploratory analysis. The B_PROUD study was registered under ClinicalTrials.gov: NCT02869386.
Intervention, study population and study design
During the B_PROUD study period, the number of MSUs increased from one to three, ultimately covering approximately 94% of the Berlin population. 15 MSUs were equipped with a CT scanner (with CT-angiography capability) as well as a point-of-care laboratory and were staffed with an emergency physician trained in neurology, a radiology technician, and a paramedic. 16 These resources allowed for immediate initiation of specific treatments following clinical and imaging-based diagnosis, such as BP lowering or anticoagulation reversal in ICH patients.
Whenever an emergency call led to a code stroke (suspicion of acute stroke within 4 h of symptom onset or unknown time of onset), and an MSU covering the individual’s location was available, an MSU was dispatched together with a conventional ambulance in order to achieve dispatch-to-(first)-ambulance arrival intervals mandated by Berlin EMS legislation. When no MSU was available in the respective area, only a conventional ambulance was dispatched. 14
When an MSU was dispatched, in most cases, the conventional ambulance arrived first to the scene. If the conventional ambulance’s personnel determined that waiting for the MSU was not justified, the MSU could be cancelled. 14 Analogous to the intention-to-treat approach in RCTs, we considered all patients for whom MSUs were dispatched as belonging to the MSU dispatch group.
After arrival at the scene, MSU personnel assessed patients’ conditions. Patients underwent CT imaging if acute stroke was suspected and reperfusion treatment eligibility was determined. If an ICH was diagnosed in CT imaging, the MSU teams followed specific recommendations regarding BP management and anticoagulation reversal or coagulation factor substitution in cases of spontaneous thrombophilia.12,13 Patients presenting with systolic BP higher than 150 mmHg were to receive antihypertensive treatment in the MSU to target a systolic BP below 140 mmHg as rapidly as possible - with the intravenously-administered alpha-blocker Urapidil most commonly used. For patients taking Vitamin K oral antagonist medication and with an international normalised ratio (INR) >1.6 or those having taken direct oral anticoagulants (DOACs) including Rivaroxaban, Apixaban or Edoxaban within 24 h, emergency reversal of oral anticoagulation using prothrombin complex concentrate (PCC) was initiated. Patients on Dabigatran received the reversal agent Idarucizumab.
Berlin EMS recommendations for treatment of suspected acute stroke treatment by conventional ambulances only suggest antihypertensive therapy in cases of acute cardiac decompensation and no concurrent pro- or anticoagulation treatment. Following hospital arrival, acute ICH management followed the standard operating procedures of the individual hospitals.
In the present study, we only included patients with a final discharge diagnosis of primary ICH according to the International Classification of Diseases version 10 (ICD-10) code I61.x. Patients were included if an MSU dispatch code was activated during operating hours (roll-in phases not considered), the site of the emergency was within one of the MSU coverage areas, and if they were transported to one of the 15 Berlin hospitals with a stroke unit.
Data for included patients were obtained from the linked ‘Berlin-SPecific Acute Therapy in Ischemic or hAemorrhagic stroke with Long term follow–up’ (B-SPATIAL) registry (Clinicaltrials.gov identifier: NCT03027453, Ethics approval number: EA1_208_21). This registry contains records of all stroke patients aged 18 years or older, having symptom onset within 6 h of ambulance or hospital arrival, main hospital discharge of acute ischaemic or haemorrhagic stroke including TIA and measurable neurological symptoms at time of EMS or hospital arrival. 14 Details of BP assessment and volumetric measures of brain imaging are described in the Supplement. All analyses only included patients who did not opt-out of data collection.
Outcomes
Although the primary outcome of the original B_PROUD study was functional outcome according to the full range of the modified Rankin Scale (mRS) at 3 months, 7-day mortality was used as the primary outcome for this analysis, because mortality is known to be higher in haemorrhagic stroke patients than in ischaemic stroke patients and outcome assessment is usually complete within this short time frame. All secondary outcomes are listed in Table 2.
Table 2.
Primary and secondary outcomes for ICH patients in B_PROUD.
| ICH patients with MSU dispatch (n = 95) | ICH patients without MSU dispatch (n = 78) | Effect measure | Unadjusted association (95% confidence interval) | Adjusted association (95% confidence interval) | |
|---|---|---|---|---|---|
| Death within 7 days, n (%) | 22 (23.2) | 11 (14.1) | OR | 1.84 (0.83 to 4.07) | 1.43 (0.68 to 3.31) |
| Systolic BP at hospital arrival, mmHg, mean (SD) | 161 (32) (22 missing) |
177 (36) (7 missing) |
Mean difference | –16.15 (–27.40 to –4.91) | –16.31 (–30.64 to –6.19) |
| Systolic BP ⩽140 mmHg at hospital arrival, n (%) | 14 (19.2) (22 missing) |
9 (12.7) (7 missing) |
OR | 1.63 (0.66 to 4.06) | 1.73 (0.81 to 5.19) |
| Dispatch-to-first cranial imaging, minutes, median (IQR) | 39 (29–58) (2 missing) |
57 (48–72) (1 missing) |
Mean difference | –16.16 (–26.94 to –5.38) | –17.75 (–27.16 to –8.21) |
| Largest measured haemorrhage volume, ml, mean (SD) | 43 (53) (6 missing) |
40 (46) (10 missing) |
Mean difference | 2.59 (–13.14 to 18.32) | –3.93 (–20.58 to 10.72) |
| Anticoagulation reversal treatment or coagulation factor substitution administered, n (%) | 10 (10.5) | 15 (19.7) (2 missing) |
OR | 0.48 (0.20 to 1.14) | 0.45 (0.17 to 1.06) |
| Length of in-hospital stay, days, median (IQR) | 7 (3–12) | 6 (3–11) | Mean difference | 1.28 (–1.06 to 3.62) | 1.82 (–0.55 to 4.10) |
| Modified Rankin Scale (mRS) score at 3 months | Common OR | 1.00 (0.56 to 1.76) | 0.97 (0.55 to 1.62) | ||
| mRS = 0, n (%) | 2 (2.4) | 5 (7.2) | OR for mRS ⩾ 3 | 1.21 (0.56 to 2.61) | 1.20 (0.57 to 2.36) |
| mRS = 1, n (%) | 7 (8.2) | 6 (8.7) | OR for mRS ⩾ 4 | 0.99 (0.50 to 1.98) | 1.06 (0.52 to 1.95) |
| mRS = 2, n (%) | 8 (9.4) | 5 (7.2) | |||
| mRS = 3, n (%) | 9 (10.6) | 5 (7.2) | |||
| mRS = 4, n (%) | 18 (21.2) | 10 (14.5) | |||
| mRS = 5, n (%) | 9 (10.6) | 12 (17.4) | |||
| mRS = 6, n (%) | 32 (37.6) (10 missing) |
26 (37.7) (9 missing) |
|||
| Age-adjusted favourable functional outcome at 3 months a | 10 (11.8) (10 missing) |
12 (17.4) (9 missing) |
OR | 0.63 (0.26 to 1.57) | 0.67 (0.27 to 1.67) |
SD: standard deviation; IQR: interquartile range; OR: odds ratio.
mRS 0–1 among patients aged 80 years or younger, or mRS 0–2 among patients older than 80 years.
Statistical analysis
For all variables, we present descriptive statistics stratified by dispatch group. We aimed at estimating the average causal effect of additional MSU dispatch on the primary and secondary outcomes. We relied on Directed Acyclic Graphs (DAGs) 17 to identify possible open backdoor paths between the exposure and the outcomes based on a priori knowledge about the underlying data generation process (eFigure 1). The assumed data generation process has recently been described in detail. 15 Briefly, since MSU dispatch was solely determined by the MSU availability at the location and index time, the only variable known to influence the exposure and possibly associated with the outcome is ‘MSU coverage’. 15 We operationalised MSU coverage as the number of MSUs covering the postal code in which the index stroke event happened, per quarter-year. 15 We tested the conditional independencies implied by the DAGs using the R package dagitty. 18
We estimated the average causal effects of MSU dispatch using Inverse Probability of Treatment Weighting (IPTW) with stabilised weights.15,17 The causal effect of MSU dispatch on 7-day mortality was estimated by fitting a logistic regression model with 7-day mortality as the dependent variable and MSU dispatch as the independent variable, applying the stabilised weights. The secondary outcomes were analogously analysed using the same stabilised weights using linear regression for the continuous outcomes, logistic regression for the binary ones and ordinal logistic regression for the ordered factors. The mRS distribution of dispatch groups were graphically compared using adjusted Grotta bars. 19 Since systolic BP was not measured at a fixed point in time but rather ‘upon hospital arrival’, we also targeted a conditional causal effect to ensure a fair comparison. In accordance with the causal diagram in eFigure 2, we estimated the conditional effect of MSU dispatch on systolic BP measured at hospital arrival using outcome regression, adjusting for MSU coverage, dispatch-to-hospital arrival time, and the first measurement of stroke severity (using the National Institutes of health stroke scale (NIHSS)). We acknowledge that this conditional effect may also remove part of the MSU effects of interest, since MSU dispatch affects dispatch-to-hospital-arrival time (eFigure 2).
Missing values were imputed by Multiple Imputation by Chained Equations with 10 imputed datasets. We used the BootMI technique 20 to obtain 95% confidence intervals (CI) for the marginal effects. Results were considered statistically significant if the 95% confidence interval did not contain the null value. All analyses were conducted using R version 4.0.3 and RStudio 2021.09.1.
Results
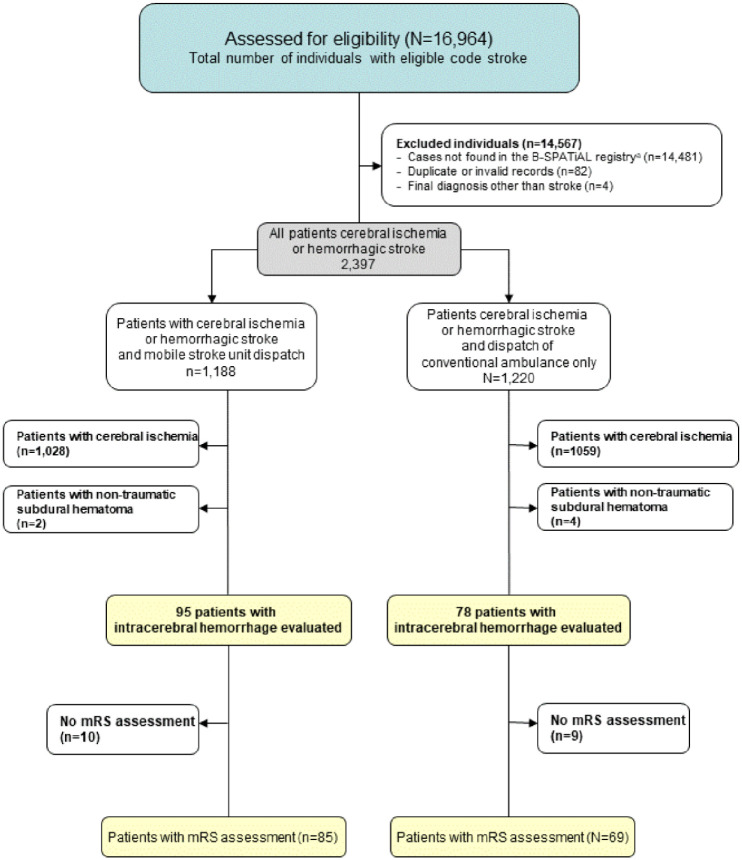
A total of 173 ICH patients fulfilled the inclusion criteria (Figure 1). MSUs were dispatched to 95 patients, of whom 85 actually received MSU care while the other 10 received conventional ambulance care after MSU cancellation (see eTable 1). For 78 patients, only conventional ambulances were dispatched. Baseline characteristics as well as process parameters by group are shown in Table 1. With otherwise similar baseline characteristics between groups, as expected, MSU coverage was higher in the MSU dispatch group. Dispatch-to-imaging time was shorter in the MSU dispatch group (median: 39 (IQR: 29–58) vs 57 (48–72) min). The first mean systolic BP measured in the ambulance was 177 ±34 mmHg in the MSU group and 172 ± 37 mmHg in the non-MSU group.
Figure 1.
Flowchart for patient inclusion during the B_PROUD study recruitment period (February 1, 2017 through May 8, 2019).
mRS, modifiedRankin Scale score.
*To be eligible for inclusion in the *Berlin-SPecificAcute Therapy in Ischemic orhAemorrhagic stroke with Long term follow—up* (B-SPATIAL) registry, patients must have been aged 18 years or older at the time of the index event, had onset of symptoms within 6 h of ambulance or hospital arrival, and a main hospital discharge diagnosis of ischaemic stroke, transientischemcattack(TlA) or hemorrhagic stroke. Patients having no neurological symptoms at the time of EMS arrival nor hospital arrival were not documented in the registry.
Table 1.
Baseline characteristics and process parameters for ICH patients in B_PROUD.
| ICH patients with MSU dispatch (n = 95) | ICH patients without MSU dispatch (n = 78) | |
|---|---|---|
| Age, years, mean (SD) | 72 (13) | 71 (12) |
| Sex, female, n (%) | 43 (45.3) | 34 (43.6) |
| Arterial hypertension, n (%) | 89 (93.7) | 68 (87.2) |
| Atrial fibrillation, n (%) | 23 (24.2) | 25 (32.1) |
| Diabetes mellitus, n (%) | 19 (20.0) | 14 (17.9) |
| Oral anticoagulation within 48 h of event, n (%) | 12 (12.9) (2 missing) |
11 (14.7) (3 missing) |
| Haemorrhage classification | ||
| Deep location, n (%) | 46 (48.4) | 39 (50.0) |
| Lobar location, n (%) | 35 (36.8) | 30 (38.5) |
| Living at home without assistance, n (%) | 69 (72.6) | 49 (62.8) |
| Living in nursing institution, n (%) | 10 (10.5) | 11 (14.1) |
| MSU coverage a at site of emergency at the time of dispatch | ||
| 1 MSU, n (%) | 46 (48.4) | 54 (69.2) |
| 2 MSU, n (%) | 35 (36.8) | 19 (24.4) |
| 3 MSU, n (%) | 14 (14.7) | 5 (6.4) |
| Time from onset or last-seen-well to dispatch, minutes, median (IQR) | 30 (11–100) (4 missing) |
22 (10–64) (2 missing) |
| First assessed b NIHSS score, c median (IQR) | 12 (7–18) (2 missing) |
10 (5–15) (18 missing) |
| NIHSS score assessed at hospital admission, median (IQR) | 11 (7–16) (22 missing) |
10 (5–15) (18 missing) |
| Pre-hospital systolic blood pressure measurement, mmHg, mean (SD) | 177 (34) (1 missing) |
172 (37) (2 missing) |
| Prehospital systolic BP measurement ⩾ 160 mmHg, n (%) | 67 (71.3) (1 missing) |
48 (63.2) (2 missing) |
| Systolic BP difference between prehospital and hospital-arrival measurements, mmHg, mean (SD) | –16 (43) (23 missing) |
5 (37) (9 missing) |
| Dispatch-to-first ambulance arrival, minutes, median (IQR) | 8 (7–11) | 8 (6–11) (1 missing) |
| Dispatch-to-MSU-arrival, minutes, median (IQR) | 17 (14–21) (10 missing) |
|
| First ambulance arrival to hospital arrival, minutes, median (IQR) | 57 (46–63) | 31 (25–37) (1 missing) |
| MSU arrival to hospital arrival, minutes, median (IQR) | 49 (42–57) (10 missing) |
|
| Pre-hospital imaging-to-hospital-arrival, minutes, median (IQR) | 34 (26–43) (29 missing) |
|
| Dispatch-to-hospital arrival, minutes, median (IQR) | 65 (55–74) | 39 (33–46) |
| Number of cranial scans available for haematoma volume measurements per patient, mean (SD) | 2 (1) | 2 (1) |
| Onset or last-seen-well to first cranial imaging, minutes, median (IQR) | 76 (49–160) | 85 (64–136) |
| First measured haemorrhage volume, ml, mean (SD) | 32 (42) (6 missing) |
34 (43) (10 missing) |
| Onset or last seen well to imaging with maximum haematoma volume, minutes, median (IQR) | 248 (99–1342) (9 missing) |
224 (96–762) (12 missing) |
| Surgical ICH treatment, n (%) | 14 (14.7) | 16 (20.5) |
SD: standard deviation; MSU: mobile stroke unit; IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischaemic attack.
MSU coverage varied by geographical location due to partially overlapping operation areas and subsequent roll-out of second and third MSU; Number of MSUs covering the individual’s location.
Assessment in MSU in patients cared for by MSU or in emergency department in patients not cared for by MSU.
The NIHSS is a score ranging from 0 to 42, with higher scores indicating greater neurological deficits.
The mean systolic BP difference between prehospital measurement and measurement at hospital arrival was −16 ± 43 mmHg in patients with MSU dispatch compared to +5 ± 37 mmHg in patients without. For the subset of patients with a systolic BP ⩾ 160 mmHg at ambulance measurement, the mean BP lowering was more pronounced (MSU dispatch: −29 ± 38 mmHg, non-MSU: −7 ± 31 mmHg). In those patients who received MSU care, 54 (63.5%) received antihypertensive treatment on the MSU, while 25 did not (29.4%; six patients without documentation). Among those patients who received antihypertensive treatment on the MSU, the mean first measured systolic BP upon ambulance arrival and upon hospital arrival were 183 ± 32 mmHg and 166 ± 34 mmHg, respectively, with a mean BP reduction of −16 ± 47 mmHg. In patients receiving MSU care who were not treated with antihypertensives on the MSU, the corresponding mean measurements were 154 ± 28 mmHg and 143 ± 25 mmHg, with a mean BP reduction of −13 ± 37 mmHg.
In the MSU dispatch group, 12 individuals had very high systolic BP (⩾220 mmHg) at ambulance arrival compared to 8 without MSU dispatch. Among these individuals, BP measurements at hospital arrival were available for 16, and only two of these patients (both with MSU dispatch and who died within 1 day of hospital arrival) had a systolic BP decline of >90 mmHg, which exceeds the recommended BP reduction in the recent ESO guidelines’ expert consensus statement. 12
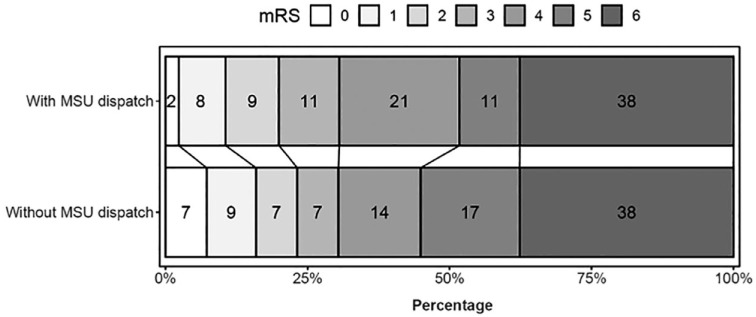
The primary and secondary outcomes are presented in Table 2 with unadjusted and adjusted effect estimates. The observed mRS distribution is represented in Figure 2.
Figure 2.
Stacked proportional bar graph showing the observed modified Rankin Scale (mRS) score distributions at 3 months for those with mobile stroke unit (MSU) dispatch versus those with conventional care only without any confounding adjustment. This figure was created using the rankinPlot R package. The mRS, which measures functional outcomes, has seven categories: 0 (no symptoms), 1 (no significant disability), 2 (slight disability), 3 (moderate disability), 4 (moderately severe disability), 5 (severe disability) and 6 (death). Percentages are rounded and thus may not add up to 100%.
Twenty-two (23.2%) patients died within 7 days in the MSU group compared to 11 patients (14.1%) in the non-MSU group. After adjustment, we found no statistically significant association with 7-day mortality (OR = 1.43, 95% CI: 0.68 to 3.31).
The mean systolic BP at hospital arrival in the MSU group was 161 ± 32 compared to 177 ± 36 in non-MSU group. After confounding adjustment, we found a statistically significant marginal (−16.31, 95% CI: −30.64 to −6.19) and conditional (−13.59, 95% CI: −26.09 to −1.10) association between systolic BP at hospital arrival and MSU dispatch. The proportion of individuals with systolic BP ⩽140 mmHg at hospital arrival was higher in MSU group (19.2%vs 12.7%), but without statistically significant difference after confounding adjustment (marginal OR = 1.73, 95% CI: 0.81–5.19, conditional OR = 1.76 95% CI: 0.57 to 5.45).
After adjustment, time from dispatch-to-imaging was on average 18 min shorter (95% CI: −27 to −8 min) in the MSU group.
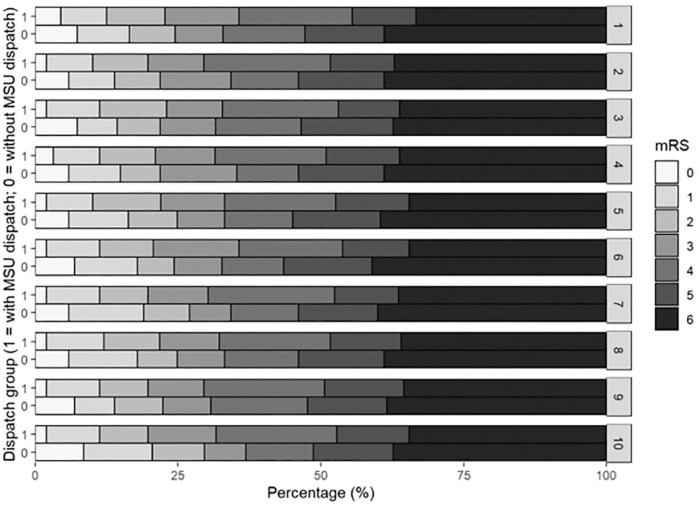
After adjustment, no statistically significant associations were found for largest measured haematoma volume, anticoagulation reversal or coagulation factor substitution, in-hospital stay length, favourable outcome and mRS at 3 months (Figure 3, Table 2).
Figure 3.
Adjusted stacked proportional bar graph showing adjusted 3-month mRS score distributions for both groups (with MSU dispatch = l and without MSU dispatch = 0). mRS distributions by dispatch group are presented for each imputed data set (1–10). Confounding adjustment was performed using inverse probability of treatment weighting with stabilised weights.
Discussion
In this prospective study, we found that among ICH patients, the additional dispatch of an MSU was associated with earlier imaging and lower systolic BP at hospital arrival compared to conventional care. We found no evidence of lower 7-day mortality, improved functional outcomes (3-month mRS), nor lower haematoma volume for these patients.
Our results seem inconsistent with the current guidelines that recommend using medication to lower BP to 140 mmHg 13 or to below 140 mmHg 12 as early as possible in ICH patients. Other studies investigating the effects of BP lowering have not consistently shown a clinical benefit for this practice. The INTERACT-2 study, which included 2,839 ICH patients within 6 h of symptom onset, found no statistically significant benefit of systolic BP lowering on the primary outcome of death or major disability (mRS 3–6) at 90 days. 9 A statistically significant benefit was only found in the secondary analysis of ordinal mRS. 9 However, the median onset-to-randomisation time was around 255 min, compared to 76 min between onset and imaging (allowing immediate start of antihypertensive treatment) in the MSU group in our study. The ATACH-2 trial included 1000 ICH patients within 4.5 h of symptom onset and compared systolic BP targets of 110–139 mmHg and 140–179 mmHg using Nicardipine. 21 In that study, the mean onset-to-randomisation time was about 183 min and mean minimum systolic BP during the first 2 h was 12.2 mmHg lower in the intensive treatment group. 21 No statistically significant difference was observed between the groups for the primary outcome (death or severe disability, mRS 4–6 at 3 months) or for haematoma expansion. 21 In a post-hoc exploratory subgroup analysis in patients who received Nicardipine treatment within 2 h of onset, intensive BP treatment was associated with a statistically significant lower frequency of haematoma expansion and functional independence (mRS 0–2). 22
The RIGHT-2 trial examined the effects of Glyceryl Trinitrate (GTN) in the prehospital setting among patients who had a suspected stroke. 23 In the subgroup of 145 patients with ICH, patients treated with GTN tended to have a lower mean systolic BP at hospital admission as compared to sham-controlled treatment (no statistically significant difference). 24 However, GTN treatment was associated with larger haematoma and growth, higher in-hospital mortality and a trend towards worse global disability according to the mRS at 90 days. 23 Of note, median onset-to-randomisation time was 74 min – hence, very similar to the median onset-to-imaging time in our MSU group.
A post-hoc analysis of the ATACH-2 trial in patients with excessively high initial systolic BP (220 mmHg or higher) found a higher rate of neurological deterioration and kidney adverse events in patients with intensive (vs standard) BP reduction but no evidence of difference in death or severe disability at 90 days. 25 A pooled analysis of the INTERACT-2 and ATACH-2 trials suggested a J-shaped relationship between the magnitude of systolic BP reduction and early neurological deterioration, death, and worse functional outcomes at 3 months. 26 While the most favourable results were seen around a moderate systolic BP reduction of 40 mmHg, large BP reductions over the first hour were associated with worse outcomes. 26
Results from an observational study found more ischaemic lesions on diffusion-weighted MRI-imaging within 2 weeks as well as more acute neurological deterioration among ICH patients who were treated with a more aggressive BP lowering regimen (target <140 mmHg compared to <160 mmHg). 27 No association between intensive acute BP lowering and ischaemic lesions in diffusion-weighted MRI-imaging, however, was found in the post-hoc subgroup analysis of the ATACH-II trial that used Nicardipine. 28
Taken together, the results from the referenced studies suggest that aggressive BP lowering during the hyperacute phase could have harmful effects, particularly in patients with very high BP. It is important to consider that results from our study may also depend on the specific agent used for rapid BP lowering. Urapidil, most commonly used for pre- and in-hospital intravenous BP lowering in Germany, is an alpha-1-blocker that relaxes vascular smooth muscles in arterioles. This medication’s effect may inhibit compensatory rapid vasoconstriction in patients who are repositioned several times during emergency care and lead to unobserved orthostatic BP drops during these manoeuvres.
Several limitations should be considered when interpreting our findings: first, MSU care is multifaceted and does not only comprise BP treatment but also includes work-up by a more specialised team, prehospital anticoagulation reversal, routeing patients to specialised facilities, and pre-notification of in-hospital teams. Therefore, readers should not attribute the effect of MSU dispatch to antihypertensive treatment alone. Second, the sample size is limited and B_PROUD was not designed to detect differences in the subset of patients with ICH. Third, functional outcome was not assessed in a blinded manner in most of the included patients because the primary study population of B_PROUD did not include ICH patients. 15 Fourth, despite the used state-of-the-art methods to estimate causal effects and the chosen study design to reduce risks of unmeasured confounding on the effect of MSU dispatch, B_PROUD was not randomised and we cannot absolutely rule-out the presence of residual unmeasured confounding. Fifth, due to data collection limitations some inclusion criteria for the study rely on post-treatment variables, therefore we cannot rule out selection bias. Sixth, the lack of detailed clinical, imaging, and autopsy data does not allow us to draw further conclusions about the specific cascade of events ultimately leading to death.
Having observed a higher number of ICH patients dying within 7 days in the MSU dispatch group than in the conventional ambulance group and also based on the referenced literature published since the initiation of B_PROUD, starting in October 2021, the Berlin MSU teams have adopted the ESO Guideline Expert Consensus Statement to avoid excessive BP reductions of more than 90 mmHg. 12 Consequentially, from this date onwards, Berlin MSU medical teams modified their standard operating procedures accordingly, restricting prehospital BP lowering to those patients with ICH with systolic BP higher than 160 mmHg at first measurement and aiming at a reduction of no >10% within 30 min.
Conclusions
Based on current European recommendations, within the framework of the Berlin-based B_PROUD study, antihypertensive treatment was administered to ICH patients with systolic BP measured above 150 mmHg, which was associated with a relevant reduction in systolic BP during the short duration of prehospital management until hospital arrival. However, despite the earlier imaging, earlier antihypertensive treatment administration, and earlier anticoagulation reversal, we found no evidence of better outcomes in the MSU group. Even within this large intervention study, the overall number of patients with ICH was rather small; data from larger cohorts are urgently needed to draw definitive conclusions about the effectiveness of pre-hospital treatments – in particular BP lowering – in ICH patients.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231213156 for Effects of Mobile Stroke Unit dispatch on blood pressure management and outcomes in patients with intracerebral haematoma: Results from the Berlin_Prehospital Or Usual Care Delivery in acute Stroke (B_PROUD) controlled intervention study by Eugen Schwabauer, Marco Piccininni, Erik Freitag, Martin Ebinger, Frederik Geisler, Peter Harmel, Annegret Hille, Irina Lorenz-Meyer, Ira Rohrpasser-Napierkowski, Tobias Kurth, Jessica L Rohmann, Matthias Endres, Frieder Schlunk, Joachim Weber, Matthias Wendt and Heinrich J Audebert in European Stroke Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MP reports funding from Novartis Pharma and being awarded a research grant from the Center for Stroke Research Berlin (private donations). ME reports grants from Bayer and fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Novartis, Pfizer, all outside the submitted work. HJA reports personal fees from AstraZeneca, Boehringer-Ingelheim, Novo -Nordisk, and Roche. All other authors do not report conflicts of interest related to the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: B_PROUD was funded by the German Research Foundation (DFG) with grant number EB 525/2-1 and under Germany’s Excellence Strategy – EXC-2049 – 390688087. B-SPATIAL was funded by the Federal Ministry of Education and Research via the Center for Stroke Research Berlin.
Ethical approval: The B_PROUD study was approved by the Charité – Universitätsmedizin Berlin ethics committee (EA4/109/15).
Informed consent: Data for included patients were obtained from the linked ‘Berlin-SPecific Acute Therapy in Ischemic or hAemorrhagic stroke with Long term follow–up’ (B-SPATIAL) registry (clinicaltrials.gov identifier: NCT03027453, Ethics Committee number: EA1_208_21). This registry contains records of all stroke patients aged 18 years or older, with symptom onset within 6 hours of ambulance or hospital arrival, main hospital discharge of acute ischaemic or haemorrhagic stroke including TIA and measurable neurological symptoms at time of EMS or hospital arrival. Details of BP assessment and volumetric measures of brain imaging are described in the supplement. All analyses only included patients who did not opt-out of data collection but explicit informed consent was not necessary.
Guarantor: Heinrich J Audebert states that he had full access to the data and takes responsibility for the completeness and the correctness of the analyses.
Contributorship: Eugen Schwabauer contributed in designing the study, data collection, drafting the manuscript. Marco Piccininni contributed in designing the study, data analysis and drafting the manuscript. Martin Ebinger contributed in designing the study, obtaining funding and with critical revisions of the manuscript. Frederik Geisler contributed in data collection and with critical revisions of the manuscript. Peter Harmel contributed in data collection and with critical revisions of the manuscript. Annegret Hille contributed in data collection and with critical revisions of the manuscript. Irina Lorenz-Meyer contributed in data collection and with critical revisions of the manuscript. Ira Rohrpasser-Napierkowski contributed in data collection and with critical revisions of the manuscript. Tobias Kurth contributed with critical revisions of the manuscript. Jessica Rohmann contributed in data collection and with critical revisions of the manuscript. Matthias Endres contributed with critical revisions of the manuscript. Frieder Schlunk contributed in data collection and with critical revisions of the manuscript. Joachim Weber contributed in data collection and with critical revisions of the manuscript. Matthias Wendt contributed in data collection and with critical revisions of the manuscript. Heinrich contributed in designing the study, obtaining funding, data analysis, drafting the manuscript and overall responsibility.
ORCID iDs: Kurth T  https://orcid.org/0000-0001-7169-2620
https://orcid.org/0000-0001-7169-2620
Rohmann JL  https://orcid.org/0000-0003-2420-5716
https://orcid.org/0000-0003-2420-5716
Audebert HJ  https://orcid.org/0000-0002-4785-0366
https://orcid.org/0000-0002-4785-0366
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al.; Global Burden of Diseases, Injuries, Risk Factors Study 2010 (GBD 2010) and GBD Stroke Experts Group. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013; 1(5): e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007; 25(1): 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Luna D, Rodriguez-Villatoro N, Juega JM, et al. Prehospital systolic blood pressure is related to intracerebral hemorrhage volume on admission. Stroke 2018; 49(1): 204–206. [DOI] [PubMed] [Google Scholar]
- 4. Fogelholm R, Avikainen S, Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke 1997; 28(7): 1396–1400. [DOI] [PubMed] [Google Scholar]
- 5. Ohwaki K, Yano E, Nagashima H, et al. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 2004; 35(6): 1364–1367. [DOI] [PubMed] [Google Scholar]
- 6. Davis SM, Broderick J, Hennerici M, et al.; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66(8): 1175–1181. [DOI] [PubMed] [Google Scholar]
- 7. Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997; 28(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 8. Bowry R, Parker SA, Bratina P, et al. Hemorrhage enlargement is more frequent in the first 2 hours: a prehospital mobile stroke unit study. Stroke 2022; 53(7): 2352–2360. [DOI] [PubMed] [Google Scholar]
- 9. Anderson CS, Heeley E, Huang Y, et al.; INTERACT2 Investigators. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368(25): 2355–2365. [DOI] [PubMed] [Google Scholar]
- 10. Leasure AC, Qureshi AI, Murthy SB, et al. Association of intensive blood pressure reduction with risk of hematoma expansion in patients with deep intracerebral hemorrhage. JAMA Neurol 2019; 76(8): 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leasure AC, Qureshi AI, Murthy SB, et al. Intensive blood pressure reduction and perihematomal edema expansion in deep intracerebral hemorrhage. Stroke 2019; 50(8): 2016–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: II–L89. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. 3rdHemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46(7): 2032–2060. [DOI] [PubMed] [Google Scholar]
- 14. Ebinger M, Siegerink B, Kunz A, et al.; Berlin_PRehospital Or Usual Delivery in stroke care (B_PROUD) Study Group. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA 2021; 325(5): 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rohmann JL, Piccininni M, Ebinger M, et al. Effect of mobile stroke unit dispatch in all patients with acute stroke or TIA. Ann Neurol 2023; 93(1): 50–63. [DOI] [PubMed] [Google Scholar]
- 16. Ebinger M, Harmel P, Nolte CH, et al. Berlin prehospital or usual delivery of acute stroke care - Study protocol. Int J Stroke 2017; 12(6): 653–658. [DOI] [PubMed] [Google Scholar]
- 17. Hernán MR, Robins JM. Causal inference: What if. Boca Raton, FL: Chapman & Hall/CRC, 2020. [Google Scholar]
- 18. Ankan A, Wortel IMN, Textor J. Testing graphical causal models using the R package “dagitty.” Curr Protoc 2021; 1(2): e45. [DOI] [PubMed] [Google Scholar]
- 19. Rohmann JL, Huerta-Gutierrez R, Audebert HJ, et al. Adjusted horizontal stacked bar graphs (“Grotta bars”) for consistent presentation of observational stroke study results. Eur Stroke J 2023; 8(1): 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med 2018; 37(14): 2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qureshi AI, Palesch YY, Barsan WG, et al.; ATACH-2 Trial Investigators and the Neurological Emergency Treatment Trials Network. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016; 375(11): 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, Warren AD, Qureshi AI, et al. Ultra-early blood pressure reduction attenuates hematoma growth and improves outcome in intracerebral hemorrhage. Ann Neurol 2020; 88(2): 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Right-Investigators; RIGHT-2 Investigators. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 2019; 393(10175): 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bath PM, Woodhouse LJ, Krishnan K, et al. Prehospital transdermal glyceryl trinitrate for ultra-acute intracerebral hemorrhage: Data from the RIGHT-2 Trial. Stroke 2019; 50(11): 3064–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qureshi AI, Huang W, Lobanova I, et al.; For ATACH-II Trial Investigators. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: post hoc analysis of a randomized clinical trial. JAMA Neurol 2020; 77(11): 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Di Tanna GL, Moullaali TJ, et al. J-shape relation of blood pressure reduction and outcome in acute intracerebral hemorrhage: a pooled analysis of INTERACT2 and ATACH-II individual participant data. Int J Stroke 2022; 17: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 27. Buletko AB, Thacker T, Cho SM, et al. Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology 2018; 91(11): e1058–e66. [DOI] [PubMed] [Google Scholar]
- 28. Shoamanesh A, Cassarly C, Morotti A, et al.; ATACH-2 and NETT Investigators. Intensive blood pressure lowering and DWI lesions in intracerebral hemorrhage: exploratory analysis of the ATACH-2 randomized trial. Neurocrit Care 2022; 36(1): 71–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231213156 for Effects of Mobile Stroke Unit dispatch on blood pressure management and outcomes in patients with intracerebral haematoma: Results from the Berlin_Prehospital Or Usual Care Delivery in acute Stroke (B_PROUD) controlled intervention study by Eugen Schwabauer, Marco Piccininni, Erik Freitag, Martin Ebinger, Frederik Geisler, Peter Harmel, Annegret Hille, Irina Lorenz-Meyer, Ira Rohrpasser-Napierkowski, Tobias Kurth, Jessica L Rohmann, Matthias Endres, Frieder Schlunk, Joachim Weber, Matthias Wendt and Heinrich J Audebert in European Stroke Journal