Abstract
Purpose:
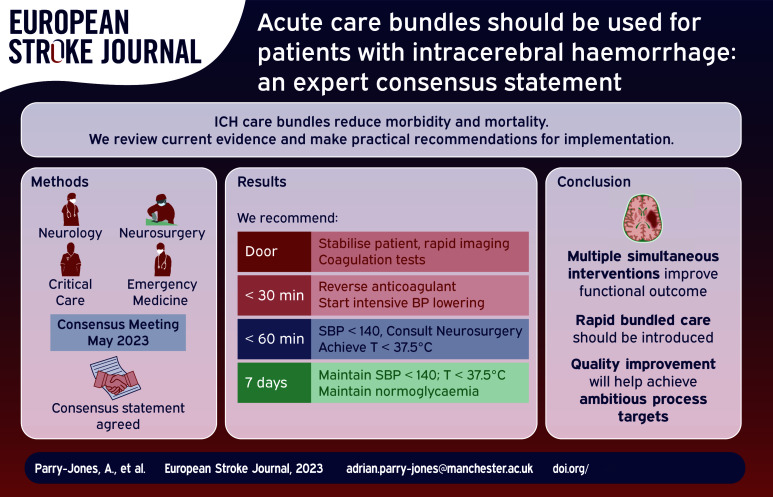
Intracerebral haemorrhage (ICH) is the most devastating form of stroke and a major cause of disability. Clinical trials of individual therapies have failed to definitively establish a specific beneficial treatment. However, clinical trials of introducing care bundles, with multiple therapies provided in parallel, appear to clearly reduce morbidity and mortality. Currently, not enough patients receive these interventions in the acute phase.
Methods:
We convened an expert group to discuss best practices in ICH and to develop recommendations for bundled care that can be delivered in all settings that treat acute ICH, with a focus on European healthcare systems.
Findings:
In this consensus paper, we argue for widespread implementation of formalised care bundles in ICH, including specific metrics for time to treatment and criteria for the consideration of neurosurgical therapy.
Discussion:
There is an extraordinary opportunity to improve clinical care and clinical outcomes in this devastating disease. Substantial evidence already exists for a range of therapies that can and should be implemented now.
Keywords: Intracerebral haemorrhage, oral anticoagulants, blood pressure, haematoma evacuation, hyperglycaemia, fever, care bundles
Graphical abstract.
Introduction
Intracerebral haemorrhage (ICH) represents a major global health burden. 1 Traditionally, the lack of definitive clinical trial data for specific ICH treatments has led to pessimism. 2 However, recent trials have given cause for optimism for the future of ICH care. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3) demonstrated that implementation of a goal-directed care bundle reduces the odds of a poor functional outcome. 3 A similar care bundle approach has been associated with a significant reduction in mortality in a UK hospital. 4 These studies suggest that all hospitals and regional acute care systems should now incorporate a care bundle approach when managing patients with ICH. Here, we consider how a care bundle might be implemented.
Methods
An expert panel with representation from Emergency Medicine (JG, NK, WBG, SJ), Stroke Neurology (APJ, TS, DS, AM, DT, CK), Neurocritical Care (JG, NK, TS), and Neurosurgery (HP, HBB, DM) was convened by the Emergency Medicine Cardiac Research and Education Group (EMCREG)-International and met for a panel discussion in May 2023. Consensus was reached on key components of an ICH care bundle, a writing group was convened, and all members of the panel contributed to the current manuscript.
Initial evaluation of patients with potential ICH
For patients with acute stroke symptoms, the initial evaluation should be rapid and focused. 5 This should include establishing onset time, use of any antithrombotic medications, a focused physical exam including ABCDE algorithm (airway, breathing, circulation, disability, exposure), and rapid neuroimaging. Non-invasive angiography should be considered, using tools such as the DIAGRAM score to select patients with a higher probability of finding a macrovascular cause.5,6 In addition to routine laboratory testing, specialised coagulation tests including thrombin time and anti-factor-Xa levels, if rapidly available, should be considered in patients taking direct oral anticoagulants (DOACs). Point-of-care tests are well-established for the international normalised ratio (INR) and although urine tests for the presence of DOACs are available, they provide only a binary (positive or negative) result and may be positive at very low DOAC concentrations. 7
Components of a care bundle for ICH
Once the patient has been stabilised and diagnosis confirmed, ICH-specific care should be rapidly initiated (Table 1).
Table 1.
Individual interventions for possible inclusion in an acute, ICH-specific bundle of care.
| Intervention | Criteria for treatment | Recommended process targets | Supporting guidelines/key evidence |
|---|---|---|---|
| Anticoagulant reversal | PCC and vitamin K (VKA antagonist): INR ⩾ 1.3 Andexanet alfa: currently taking apixaban or rivaroxaban and last dose taken ⩽18 h Idarucizumab: currently taking dabigatran PCC (DOACs): taking a DOAC and specific reversal agent unavailable or unlicenced for specific agent |
Door-to-needle time ⩽ 30 min | 1. ESO anticoagulant- associated ICH guideline (2019)
11
2. AHA/ASA ICH guideline (2022) 5 3. REVERSE-AD 14 4. ANNEXA-415 |
| Intensive blood pressure reduction | ⩽6 h after symptom onset: SBP ⩾ 150 mmHg ⩾6 h after symptom onset or unknown onset: uncertain, consider if SBP ⩾ 150 mmHg |
Treatment target ⩽ 140 mmHg, maintained for 7 days Avoid large (>90 mmHg) initial drops on SBP Door-to-first antihypertensive: ⩽30 min Door-to-target: ⩽60 min |
1. ESO BP guideline (2021)
21
2. AHA/ASA ICH guideline (2022) 5 3. INTERACT247 & 33 |
| Surgical evacuation of haematoma and/or external ventricular drainage | Decision to operate on a case-by-case basis by a multi-disciplinary team. Local criteria should be established to identify patients where a consultation with neurosurgery must occur, for example: Patients with a pre-morbid mRS of ⩽2, reasonable prognosis and one or more of: 1. GCS ⩽ 13 2. Supratentorial ICH volume ⩾ 20 mL 3. Posterior fossa ICH 4. Obstruction of third and fourth ventricle(s) |
100% of patients meeting consultation criteria are discussed with neurosurgery ⩽50% of patients not meeting consultation criteria are discussed with neurosurgery within 60 min of arrival. |
1. ESO ICH guideline (2014)
33
2. AHA/ASA ICH guideline (2022) 5 |
| Control of glucose | Non-diabetic patients: Blood glucose > 7.8 mmol/L in first 7 days Diabetic patients: Blood glucose > 10 mmol/L in first 7 days |
Non-diabetic patients: maintain blood glucose between 6.1 and 7.8 mmol/L for ⩾90% of measurements in first 7 days Diabetic patients: maintain blood glucose between 7.8 and 10 mmol/L for ⩾90% of measurements in first 7 days For both groups, optimise protocols to avoid hypoglycaemia |
1. INTERACT33
2. QASC 37 AHA/ASA guideline (2022) 5 |
| Control of temperature | Monitor body temperature every 4 h for 7 days and initiate anti-pyretic treatment if temperature ⩾ 37.5°C | Achieve normothermia (<37.5°C) within 1 h of starting treatment | 1. INTERACT33
2. QASC 37 3. AHA/ASA guideline (2022) 5 |
AHA/ASA: American Heart Association/American Stroke Association; DOAC: direct oral anticoagulant; ESO: European Stroke Organisation; GCS: Glasgow Coma Scale; ICH: intracerebral haemorrhage; INR: international normalised ratio; PCC: prothrombin complex concentrate; SBP: systolic blood pressure; VKA: vitamin-K antagonist; mRS: modified Rankin Scale.
Anticoagulation reversal
Haematoma expansion (HE) is associated with worse outcomes after ICH and occurs in around 30% of all ICH patients within 3 h of onset. 8 The likelihood of HE increases to 54%in anticoagulated patients 9 ; thus, a critical component of any ICH care bundle is early anticoagulation reversal.
Vitamin K antagonists (VKAs): Rapid VKA reversal requires Vitamin K administration in addition to coagulation factor repletion. Prothrombin complex concentrates (PCCs) reduce the INR quickly and efficiently, 10 and are recommended by the European Stroke Organization and the American Heart Association.5,11 The benefit of this agent may be time dependent; in a large multicentre retrospective study of VKA-ICH, those receiving PCCs within 4 h (and had a systolic blood pressure [SBP] < 160 mmHg) had a rate of HE of 18%, compared to 44% in patients not achieving these values. 12
DOACs have become the first line anticoagulant for most patients, 13 transforming the approach to anticoagulation reversal in ICH.
Factor IIa inhibitors (dabigatran): For this agent, there is a highly specific reversal agent, idarucizumab, a monoclonal antibody fragment that binds dabigatran and appears to be effective for haemostasis. 14
Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban): The specific reversal agent available is andexanet alfa, a recombinant modified version of human Factor X. Of note, the use of andexanet alfa to reverse edoxaban is currently off-label in many countries. Andexanet alfa binds Factor Xa inhibitors and appears to be effective for haemostasis in patients within 18 h of their last DOAC dose. 15 The ANNEXa-I randomised controlled trial (NCT03661528) has compared andexanet alfa with standard care (mainly PCC) in ICH patients within 6 h of symptom onset. Although not yet published, it has been presented at a major international conference and superior haemostatic efficacy with andexanet alfa is reported, at the cost of an increase in thrombotic complications, especially in those with a prior history of stroke or myocardial infarction. 16
Time is critical to anticoagulation reversal. Similar to thrombolysis in ischaemic stroke, 17 door-to-needle (DTN) time metrics should be targeted with ICH, because the risk of HE is highest in the hours following ICH and providing early reversal maximises the benefits. In contrast, the costs and adverse event risks may be the same irrespective of time; therefore, earlier treatment may maximise the risk-benefit ratio. We recommend hospitals implement an aspirational DTN target of under 30 min. This is much shorter than current common practice and a more relaxed target may be needed initially, depending on current performance. However, aspiring to achieve a challenging DTN goal for all cases will likely instil a sense of urgency and ensure that reversal is achieved as quickly as possible. Key steps in reducing DTN time include expediting imaging and IV placement, developing streamlined protocols with key stakeholders, and training staff in drug reconstitution. These components have all been effective steps in reducing DTN time below 30 min in ischaemic stroke. 18
Unlike INR for patients taking VKAs, DOACs do not have a laboratory test that is widely and rapidly available. Therefore, clinicians rely on history, which may be challenging to obtain in ICH. Important information needed for decision-making in patients taking a DOAC includes dose, time of last intake, and kidney function. To avoid delay, we recommend empirical reversal, unless there is a concern about compliance, dosage, timing of last dose, and risks of thrombotic complications.
Blood pressure reduction
Elevated SBP is common in ICH and associated with an increased risk of HE and poor outcome. 19 Control of hypertension in the acute phase reduces the risk of HE and since the majority of HE occurs early, the beneficial effects of intensive SBP reduction are likely to be time-dependent. 8 The recent INTERACT3 trial included a goal SBP < 140 mmHg within the first hour and the number needed to treat (NNT) was 35 (95% CI 15 to infinity) for the care bundle to prevent one patient from death or major disability. 3 It is important to note that many patients did not reach this goal and achieved an average SBP of 150 mmHg, highlighting the value of early SBP reduction even if target SBP is not achieved.
In order to ensure maximum benefit from BP reduction, antihypertensive medications should be initiated within 30 min and target SBP achieved within 60 min.5,20,21 The optimal SBP target in patients with acute ICH remains a matter of debate. Current best evidence suggests:
Patients within 6 h of onset and SBP of 150–220 mmHg: Aiming for SBP < 140 mmHg appears to improve outcome, even if the average achieved SBP is 150 mmHg.
For >6 h from onset evidence is limited, but AHA guidelines recommend a SBP target of 130–150 mmHg 5 and ESO guidelines a SBP target of 110–140 mmHg. 21
Target SBP should be achieved smoothly, avoiding SBP fluctuations and large drops (>90 mmHg), especially in the first hour. 21 There is limited evidence to guide SBP treatment in patients with severely impaired consciousness, high volume (e.g. >60 mL) haemorrhages and admission SBP > 220 mmHg.
Once target SBP has been achieved, it should be maintained for the next 7 days. 3 Good BP control is a cornerstone of minimising risk of recurrent ICH, as well as other cardiovascular events. 22
Neurosurgical management
ICH volume is a powerful predictor of functional outcome, and therefore surgical evacuation may improve outcome by reducing mass effect, lowering intracranial pressure, and lessening secondary injury. Many surgical trials have shown trends towards benefit without achieving prespecified primary outcomes, including the STICH trials of craniotomy for supratentorial ICH23,24 and the MISTIE trial of minimally invasive surgery with thrombolysis. 25 However, in recent years, studies have suggested that selected patients can derive substantial benefit from ICH evacuation with a significant effect of time, with earlier surgery appearing to be of greater benefit. 26 Another recent meta-analysis has suggested that minimally invasive haematoma evacuation (rather than full craniotomy) improves functional outcome. 27 The recent ENRICH trial (NCT02880878; not yet published), compared minimally invasive surgery using the BrainPath approach within 24 h to best medical treatment. The BrainPath device has an atraumatic tip and is used through a trans-sulcal approach to allow access and removal of the haematoma. 28 ENRICH investigators recruited 300 patients (around two-thirds lobar ICH, one-third anterior basal ganglia) and have reported a significant reduction in death and dependency, only apparent in lobar ICH.29,30 Meta-analysis of surgical trials with available end of treatment volume measurements suggest that surgery is of significant benefit only when most of the haematoma is removed, highlighting the critical importance of surgical technique.31,32
Ongoing trials (e.g. Dutch ICH Surgery Trial (NCT03608423)) will add to the evidence base for minimally invasive surgery and may allow for robust, evidence-based criteria for haematoma evacuation in ICH. The Decompressive Hemicraniectomy in Intracerebral Hemorrhage (SWITCH, NCT02258919) trial, which recently finished recruitment, will determine whether decompressive hemicraniectomy is beneficial for patients with space-occupying deep ICH. In the meantime, we recommend that each institution develops a consultation process with their local neurosurgical unit based on available experience and resources. ICH severity grading scales provide a standardised means of communicating severity and may be considered as part of local protocols, but caution must be exercised to avoid inappropriate withdrawal of care based on the use of such scales. We provide one example of neurosurgical consultation criteria (Table 1).
Insertion of an external ventricular drain (EVD)
Hydrocephalus after ICH is caused by obstruction of the third or fourth ventricles either by blood or by direct compression from the ICH. Inserting an EVD is a common and relatively low-risk procedure, which is considered lifesaving in patients with acute hydrocephalus and is recommended in both the AHA and ESO guidelines.5,33
Posterior fossa ICH
Cerebellar ICH is associated with increased risks of neurological deterioration due to obstructive hydrocephalus or local mass effect on the brainstem due to its confined anatomical location in the posterior fossa. Data on surgery for posterior fossa ICH, such as suboccipital decompressive craniectomy, haematoma evacuation, or EVD insertion are limited to observational studies. 34 Surgery may be considered for cases of cerebellar ICH causing brainstem compression and/or acute hydrocephalus.
Control of glucose
Hyperglycaemia is relatively common in ICH, as in ischaemic stroke, even in patients without diabetes mellitus. 35 This finding is associated with increased risk of HE, perihaematomal oedema, and worse outcome. 35 Arguments suggesting that this is a therapeutic target include a post-hoc analysis of the INTERACT2 trial, finding a linear relationship between elevated serum glucose and worse outcome even when adjusting for disease severity. 36 The best evidence for active treatment comes from cluster-randomised trials, where close glucose management is introduced and compared to standard care. Two trials argue for a benefit: the QASC trial 37 and the INTERACT3 trial. 3 In both cases, introducing a bundle of care including hyperglycaemia management significantly improved outcome. It appears that actively monitoring and normalising glucose may reduce brain injury after stroke. However, there was little difference between groups in terms of adjusted mean glucose concentrations over 24 h (−0.5 mmol/L; 95% CI −0.8 to −0.2) in INTERACT3, so the magnitude of contribution of glucose control is uncertain. 3 Considering the significant resource required to achieve targets and the risk of hypoglycaemia, further evidence is probably needed to support widespread implementation. Pending further studies, we recommend considering the approach used in the INTERACT3 trial:
Target blood glucose level of 6.1–7.8 mmol/L for nondiabetic patients
Target blood glucose level of 7.8–10.0 mmol/L for diabetic patients
Maintain this for 7 days or until hospital discharge.
Control of temperature
Much as with hyperglycaemia, elevated body temperature (pyrexia) is relatively common in ICH 38 and is linked to early HE, early neurologic deterioration and poor outcome. 39 Arguments suggesting that fever is a therapeutic target include a post-hoc analysis of the INTERACT2 trial, finding a linear relationship between pyrexia and worse outcome even when adjusting for disease severity. 36 As a result, ICH guidelines recommend antipyretic treatment.5,33 The best evidence for real-world treatment of pyrexia comes from cluster-randomised trials, where close temperature management is introduced and compared to standard care. Both the QASC trial 37 and the INTERACT3 trial 3 argue for a benefit. However, less than 10% of patients in INTERACT3 required any antipyrexia treatment and there was no significant difference in temperature at 1 and 24 h, so the effect size of this intervention is not yet clear. Pending further studies, we recommend considering the antipyrexia approach used in the INTERACT3 trial, which includes temperature checks every 4 h and treatment of any temperature ⩾37.5°C. 3
Care bundling
The concept of care bundles was developed by the Institute for Healthcare Improvement in 2001 and is described as ‘a small set of evidence-based interventions for a defined patient segment/population and care setting that, when implemented together, will result in significantly better outcomes than when implemented individually’. 40 Care bundles may act as a tool to facilitate implementation of evidence-based practice by ensuring that all components of the bundle are considered and delivered effectively to every patient. They have been deployed in other areas of healthcare and are summarised in a recent systematic review and meta-analysis. 41 The review identified 31 before and after studies suggesting care bundles reduce the risk of negative outcomes but higher level evidence from six randomised trials was less certain. 41
The concept of a care bundle for ICH was tested at a UK Comprehensive Stroke Centre in 2015–16. The ‘ABC’ care bundle consisted of anticoagulant reversal, BP lowering and a care pathway for neurosurgery consultation. The bundle was associated with a reduction in 30-day case fatality of over 33% (from 35.5% to 24.2%) in a before and after study. 4 The QASC trial also provided evidence that the ‘fever, sugar, swallow’ (FeSS) care bundle in all stroke patients led to improved outcomes. 37 Most recently, INTERACT3 has provided higher level evidence for benefit of a care bundle in ICH, incorporating anticoagulant reversal and BP lowering interventions of the ABC bundle and the fever and hyperglycaemia interventions from the FeSS bundle. 3 Overall, it seems clear that a care bundle approach is beneficial in ICH and should be implemented at all centres caring for ICH patients. Quality improvement methodology is required to ensure optimal implementation. 4
Stroke unit care
Determining the optimal environment for ICH patients is critical. Different hospitals may admit such patients to a general medical unit, an intensive care unit, a neurointensive care unit, or a dedicated stroke unit. Stroke unit care is associated with a reduction of the odds of poor outcome, 42 lower hazard of death, and lower odds of death or dependency and is at least as effective in ICH as for ischaemic stroke.43,44 Most recently, a German multicentre retrospective study reported that treatment outside stroke units was associated with higher odds for unfavourable outcome and intrahospital mortality. 45
The apparent benefit of stroke unit admission is likely related to greater staff expertise, better diagnostic procedures, better nursing care, early mobilisation, prevention of complications, and more effective rehabilitation procedures together with multiparametric telemetry. This means that all ICH patients who do not require intensive care should be preferentially admitted to a stroke unit. Since ICH is a dynamic event with risk of early clinical deterioration occurring over the initial 48 h, 46 ICH patients should be admitted to a stroke unit as early as possible for close monitoring of physiological parameters and provision of a range of procedures and treatments. 5
Conclusions
For many years, randomised trials of single interventions in ICH have failed to meet their primary goal of statistically significant improvement in neurologic outcome. This has led to nihilism in the approach to ICH care. 2 However, evidence from numerous sources, including randomised trials of bundled care, consistently argue that delivering multiple simultaneous interventions improves functional outcome, with more widespread improvement in supportive care arising from this active approach. 4 We advocate for the widespread adoption of early bundled care for ICH patients including the optimisation of time-based metrics for BP control and anticoagulation reversal.
Acknowledgments
We would like to thank Dr Judy Racdaio for her expert assistance in editing and formatting the manuscript.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: APJ was an independent member of the INTERACT3 trial steering committee and has received honoraria for advisory boards and speaking engagements from AstraZenenca. NPK has received speaker’s honoraria from AstraZeneca, and consulting with the National Football League. JNG has received research and consulting from AstraZeneca, CSL Behring, Octapharma, Takeda, NControl, Cayuga, and Prothya.CJMK is involved in the Netherlands Cardiovascular Research Initiative, which is supported by the Dutch Heart Foundation, CVON2015–01: CONTRAST, and the Brain Foundation Netherlands (HA2015·01·06). The collaboration project is additionally financed by the Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public- private partnerships (LSHM17016). This work was funded in part through unrestricted funding by Stryker, Medtronic and Cerenovus. The funding sources were not involved in study design, monitoring, data collection, statistical analyses, interpretation of results, or manuscript writing.; Radboud UMC and Erasmus MC received additional unrestricted funding on behalf of CONTRAST, for the execution of the Dutch ICH Surgery Trial pilot study and for the Dutch ICH Surgery Trial from Penumbra Inc. For the Dutch ICH Surgery Trial they also received a grant from ZonMw / Promising care (grant 80- 86200-08-25001). DT has received speaker’s honoraria and consulting from AstraZeneca. TS has received speaker’s honoraria and consulting from AstraZeneca, Bayer, Boehringer Ingelheim, BMS Pfizer, DaiichySankyo. All other authors of this paper have no conflicts of interest related to this review and nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EMCREG-International is a global educational consortium, led by WBG, which organised and funded the consensus panel meeting. EMCREG-International secured funding through an unrestricted educational grant from AstraZeneca. NPK receives funding from the National Institutes of Health, K23HD102555.
Ethical approval: Not applicable.
Informed consent: Not applicable.
Guarantor: APJ
Trial registration: Not applicable.
Contributorship: All authors contributed to the writing of the first draft of the manuscript and critically reviewed the final draft submitted for submission.
ORCID iDs: Adrian R Parry-Jones  https://orcid.org/0000-0002-4462-3846
https://orcid.org/0000-0002-4462-3846
Andrea Morotti  https://orcid.org/0000-0002-6558-1155
https://orcid.org/0000-0002-6558-1155
David Seiffge  https://orcid.org/0000-0003-3890-3849
https://orcid.org/0000-0003-3890-3849
Thorsten Steiner  https://orcid.org/0000-0002-5080-8222
https://orcid.org/0000-0002-5080-8222
References
- 1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parry-Jones AR, Paley L, Bray BD, et al. Care-limiting decisions in acute stroke and association with survival: analyses of UK national quality register data. Int J Stroke 2016; 11: 321–331. [DOI] [PubMed] [Google Scholar]
- 3. Ma L, Hu X, Song L, et al. The third intensive care bundle with blood pressure reduction in acute cerebral haemorrhage trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet 2023; 402: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parry-Jones AR, Sammut-Powell C, Paroutoglou K, et al. An intracerebral hemorrhage care bundle is associated with lower case fatality. Ann Neurol 2019; 86: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke 2022; 53: e282–e361. [DOI] [PubMed] [Google Scholar]
- 6. Hilkens NA, van Asch CJJ, Werring DJ, et al. Predicting the presence of macrovascular causes in non-traumatic intracerebral haemorrhage: the DIAGRAM prediction score. J Neurol Neurosurg Psychiatry 2018; 89: 674–679. [DOI] [PubMed] [Google Scholar]
- 7. Tan PS, Park PSW, Cody R, et al. Assessment of direct oral anticoagulant status using the DOASENSE dipstick in thrombolysis eligible patients with stroke: proof-of-concept study. Stroke 2023; 54: e142–e144. [DOI] [PubMed] [Google Scholar]
- 8. Al-Shahi Salman R, Frantzias J, Lee R, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol 2018; 17: 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flibotte JJ, Hagan N, O'Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 10. Steiner T, Poli S, Griebe M, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol 2016; 15: 566–573. [DOI] [PubMed] [Google Scholar]
- 11. Christensen H, Cordonnier C, Kõrv J, et al. European stroke organisation guideline on reversal of oral anticoagulants in acute intracerebral haemorrhage. Eur Stroke J 2019; 4: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015; 313: 824–836. [DOI] [PubMed] [Google Scholar]
- 13. Connors JM. Testing and monitoring direct oral anticoagulants. Blood 2018; 132: 2009–2015. [DOI] [PubMed] [Google Scholar]
- 14. Pollack CV, Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017; 377: 431–441. [DOI] [PubMed] [Google Scholar]
- 15. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019; 380: 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connolly SJ for the ANNEXa-I Investigators. Randomized trial of andexanet alfa versus usual care in patients with acute intracranial hemorrhage while on an oral factor Xa inhibitor: ANNEXA-I. In: World Stroke Conference, Toronto, CA, October 2023. [Google Scholar]
- 17. Howard G, Schwamm LH, Donnelly JP, et al. Participation in get with the guidelines-stroke and its association with quality of care for stroke. JAMA Neurol 2018; 75: 1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binning MJ, Sanfillippo G, Rosen W, et al. The neurological emergency room and prehospital stroke alert: the whole is greater than the sum of its parts. Neurosurg 2014; 74: 281–285; discussion 285. [DOI] [PubMed] [Google Scholar]
- 19. Morotti A, Boulouis G, Dowlatshahi D, et al. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol 2023; 22: 159–171. [DOI] [PubMed] [Google Scholar]
- 20. Shoamanesh A, Patrice Lindsay M, Castellucci LA, et al. Canadian stroke best practice recommendations: management of spontaneous intracerebral hemorrhage, 7th Edition Update 2020. Int J Stroke 2021; 16: 321–341. [DOI] [PubMed] [Google Scholar]
- 21. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: XLVIII–LXXXIX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teo KC, Keins S, Abramson JR, et al. Blood pressure control targets and risk of cardiovascular and cerebrovascular events after intracerebral hemorrhage. Stroke 2023; 54: 78–86. [DOI] [PubMed] [Google Scholar]
- 23. Mendelow A, Gregson B, Fernandes H, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005; 365: 387–397. [DOI] [PubMed] [Google Scholar]
- 24. Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanley DF, Thompson RE, Rosenblum M, et al. Minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label phase 3 trial with blinded endpoint. Lancet 2019; 393: 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sondag L, Schreuder FHBM, Boogaarts HD, et al. Neurosurgical intervention for supratentorial intracerebral hemorrhage. Ann Neurol 2020; 88: 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hallenberger TJ, Guzman R, Bonati LH, et al. Endoscopic surgery for spontaneous supratentorial intracerebral haemorrhage: A systematic review and meta-analysis. Front Neurol 2022; 13: 1054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labib MA, Shah M, Kassam AB, et al. The safety and feasibility of image-guided brainpath-mediated transsulcul hematoma evacuation: a multicenter study. Neurosurg 2017; 80: 515–524. [DOI] [PubMed] [Google Scholar]
- 29. Ratcliff JJ, Hall AJ, Porto E, et al. Early minimally invasive removal of intracerebral hemorrhage (ENRICH): Study protocol for a multi-centered two-arm randomized adaptive trial. Front Neurol 2023; 14. 10.3389/fneur.2023.1126958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratcliff JJR, Hall AJ, Porto E, et al. Efficacy and safety of early minimally invasive removal of intracerebral hemorrhage (ENRICH): a multicenter randomized adaptive trial. In: AANS Annual Scientific Meeting, Los Angeles, 2023. [Google Scholar]
- 31. Gregson BA, Metcalfe S, Iqbal A, et al. Volume reduction with surgery for ICH: when is it effective? Analysis of the CT scans from the STICH II trial. Br J Neurosurg 2023; 37: 1635–1642. [DOI] [PubMed] [Google Scholar]
- 32. Polster SP, Carrión-Penagos J, Lyne SB, et al. Intracerebral hemorrhage volume reduction and timing of intervention versus functional benefit and survival in the MISTIE III and STICH trials. Neurosurg 2021; 88: 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–855. [DOI] [PubMed] [Google Scholar]
- 34. Singh SD, Brouwers HB, Senff JR, et al. Haematoma evacuation in cerebellar intracerebral haemorrhage: systematic review. J Neurol Neurosurg Psychiatry 2020; 91: 82–87. [DOI] [PubMed] [Google Scholar]
- 35. Kimura K, Iguchi Y, Inoue T, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci 2007; 255: 90–94. [DOI] [PubMed] [Google Scholar]
- 36. Song L, Wang X, Ouyang M, et al. Associations of an abnormal physiological score with outcomes in acute intracerebral hemorrhage: INTERACT2 Study. Stroke 2021; 52: 722–725. [DOI] [PubMed] [Google Scholar]
- 37. Middleton S, McElduff P, Ward J, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet 2011; 378: 1699–1706. [DOI] [PubMed] [Google Scholar]
- 38. Greer DM, Funk SE, Reaven NL, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008; 39: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 39. Rincon F, Lyden P, Mayer SA. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocrit Care 2013; 18: 45–53. [DOI] [PubMed] [Google Scholar]
- 40. Resar R, Griffin F, Haraden C, et al. Using care bundles to improve health care quality. Institute for Healthcare Improvement Innovation Series white paper. [Google Scholar]
- 41. Lavallée JF, Gray TA, Dumville J, et al. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci 2017; 12: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Langhorne P, Ramachandra S. Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev 2020; 4: CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Terent A, Asplund K, Farahmand B, et al. Stroke unit care revisited: who benefits the most? A cohort study of 105 043 patients in Riks-Stroke, the Swedish Stroke Register. J Neurol Neurosurg Psychiatry 2009; 80: 881–887. [DOI] [PubMed] [Google Scholar]
- 44. Langhorne P, Fearon P, Ronning OM, et al. Stroke unit care benefits patients with intracerebral hemorrhage: systematic review and meta-analysis. Stroke 2013; 44: 3044–3049. [DOI] [PubMed] [Google Scholar]
- 45. Ungerer MN, Ringleb P, Reuter B, et al. Stroke unit admission is associated with better outcome and lower mortality in patients with intracerebral hemorrhage. Eur J Neurol 2020; 27: 825–832. [DOI] [PubMed] [Google Scholar]
- 46. Kuohn LR, Witsch J, Steiner T, et al. Early deterioration, hematoma expansion, and outcomes in deep versus lobar intracerebral hemorrhage: the FAST Trial. Stroke 2022; 53: 2441–2448. [DOI] [PubMed] [Google Scholar]
- 47. Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013; 368: 2355–2365. [DOI] [PubMed] [Google Scholar]