ABSTRACT
Primary intracranial melanomas are rare, especially in the primary cerebellopontine angle. We describe a patient with a presumed jugular foramen meningioma that was found to be of melanotic origin at surgery. We followed this 26–year–old woman with mild ataxia with serial imaging for 18 months after the initial discovery of a cerebellopontine angle extra–axial mass. She developed worsening symptoms of ataxia, dysphagia, and right–sided hearing loss. Magnetic resonance imaging showed an interval increase in size of the mass. The lesion was thought to be a meningioma with a dural tail that extended into the jugular foramen and hypoglossal canal. She underwent preoperative angiography and attempted tumor embolization, followed by resection via a transcochlear infratemporal approach. At surgery the lesion was found to be heavily pigmented. Pathological analysis was consistent with a low–grade melanoma. No primary extracranial site was identified. One year after surgery the patient remains free of systemic disease or recurrence.
Keywords: Cerebellopontine angle, melanoma, melanocytoma
Primary intracranial melanoma tumors are very rare. They have been described involving different intracranial locations, including a few cases at the cerebellopontine angle (CPA).1, 2, 3 Histologically, tumors of melanocytic origin range from benign melanocytomas to malignant melanomas.2, 4, 5, 6, 7 This is the first report of a presumed jugular foramen3, 4/CPA meningioma diagnosed as a primary melanoma.
CASE REPORT
History and Examination
A 26–year–old, right–handed Caucasian woman with a history of mental retardation and developmental delay had a longstanding history of mild ataxia, right–sided hearing loss, and dysphagia. The patient's initial magnetic resonance imaging (MRI) study revealed an extra–axial mass involving the right CPA. A dural tail extended into the internal auditory canal, jugular foramen, and hypoglossal canal. The lesion was 3.4 × 2.3 × 2.5 cm and exerted mass effect on the midbrain (Fig. 1). On T1–weighted images the lesion was hyperintense. On T2–weighted images it was homogeneously isointense (Figs. 1A and B). It showed prominent enhancement after gadolinium administration (Figs. 1C and D). A second smaller dural–based lesion in the lateral aspect of the right posterior fossa invaginated into the adjacent cerebellum. It had the same signal characteristics as the dominant lesion. The lesions were presumptively diagnosed as meningiomas.
Figure 1A.
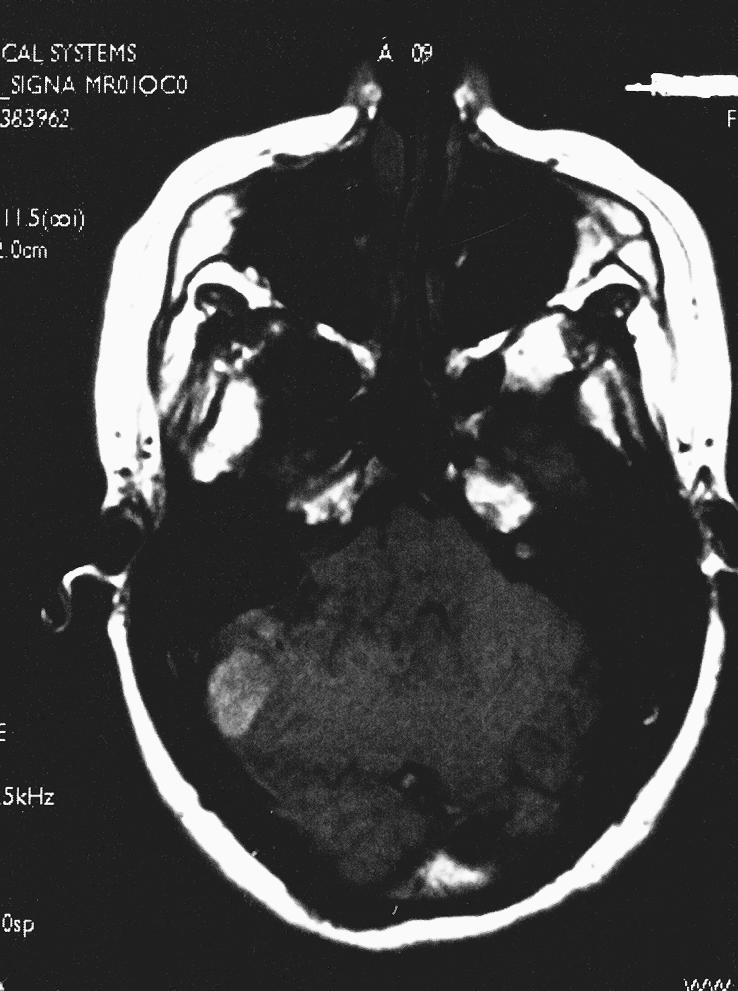
Axial T1–weighted image show an extra–axial mass of the right CPA. A dural tail extends into the internal auditory canal, jugular foramen, and hypoglossal canal. The lesion, 3.4 × 2.3 × 2.5 cm, exerted mass effect on the midbrain. It was hyperintense on T1–weighted images and isointense on T2–weighted images with a homogeneous appearance. After gadolinium administration it enhanced prominently. A second smaller dural–based lesion was found in the lateral aspect of the right posterior fossa, invaginating into the adjacent cerebellum.
Figure 1B.
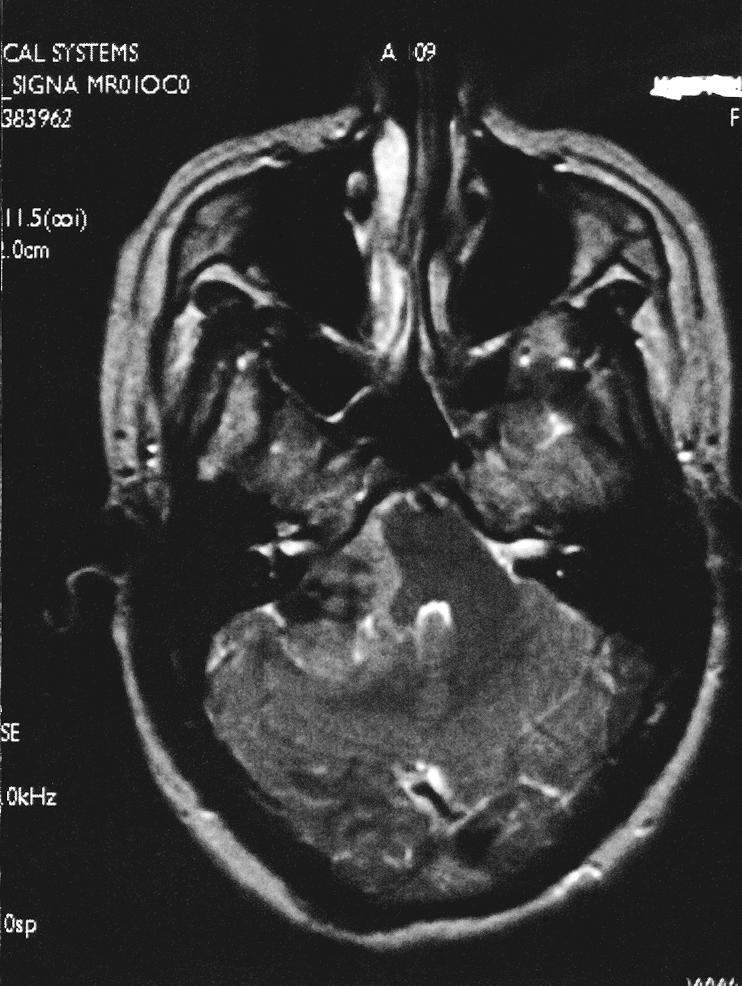
Axial T2–weighted image show an extra–axial mass of the right CPA. A dural tail extends into the internal auditory canal, jugular foramen, and hypoglossal canal. The lesion, 3.4 × 2.3 × 2.5 cm, exerted mass effect on the midbrain. It was hyperintense on T1–weighted images and isointense on T2–weighted images with a homogeneous appearance. After gadolinium administration it enhanced prominently. A second smaller dural–based lesion was found in the lateral aspect of the right posterior fossa, invaginating into the adjacent cerebellum.
Figure 1C.
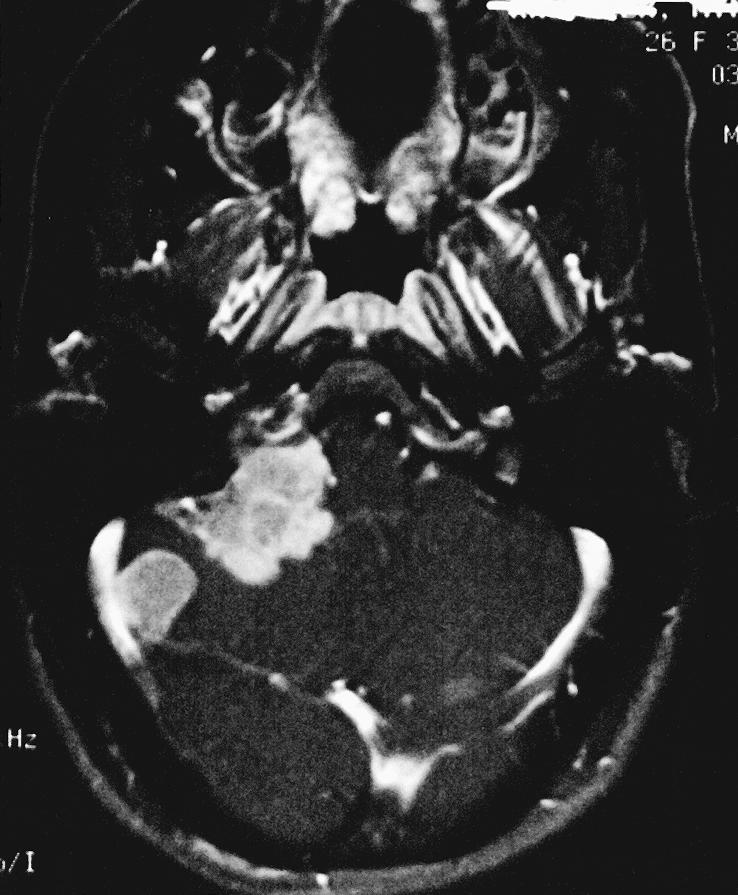
Axial T1–weighted image with gadolinium show an extra–axial mass of the right CPA. A dural tail extends into the internal auditory canal, jugular foramen, and hypoglossal canal. The lesion, 3.4 × 2.3 × 2.5 cm, exerted mass effect on the midbrain. It was hyperintense on T1–weighted images and isointense on T2–weighted images with a homogeneous appearance. After gadolinium administration it enhanced prominently. A second smaller dural–based lesion was found in the lateral aspect of the right posterior fossa, invaginating into the adjacent cerebellum.
Figure 1D.
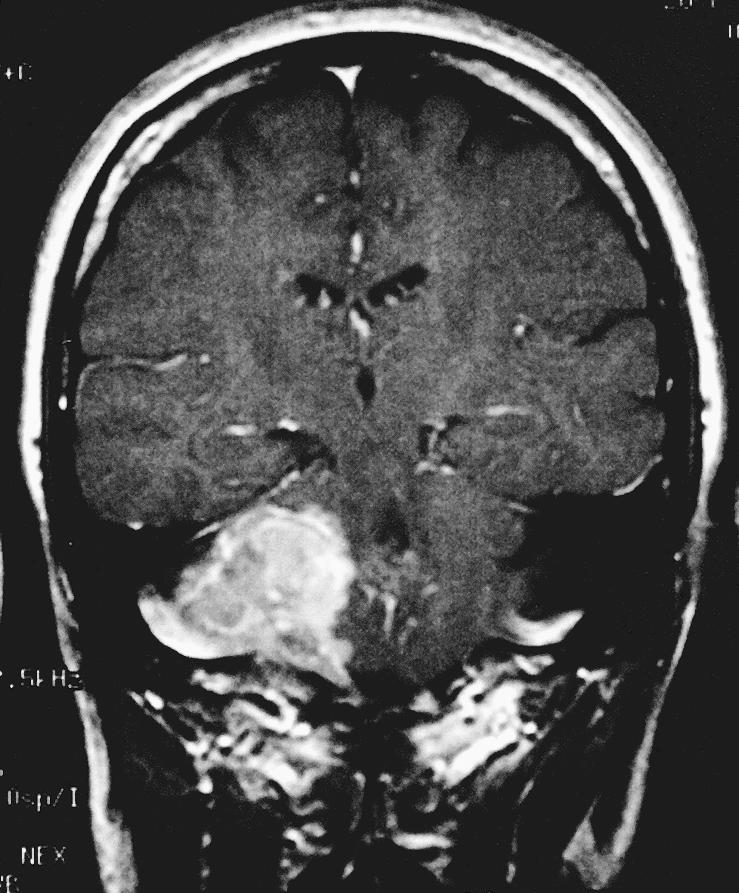
Coronal T1–weighted image with gadolinium show an extra–axial mass of the right CPA. A dural tail extends into the internal auditory canal, jugular foramen, and hypoglossal canal. The lesion, 3.4 × 2.3 × 2.5 cm, exerted mass effect on the midbrain. It was hyperintense on T1–weighted images and isointense on T2–weighted images with a homogeneous appearance. After gadolinium administration it enhanced prominently. A second smaller dural–based lesion was found in the lateral aspect of the right posterior fossa, invaginating into the adjacent cerebellum.
The family was counseled and opted for observation. The patient was followed by serial imaging 3 and 9 months after her initial visit. At the 9–month follow–up, her symptoms had progressed and the size of the tumor had increased on imaging. At that point, the family decided to proceed with resection. Preoperative angiography revealed no tumor blush or neovascularity associated with the lesions. The right inferior petrosal sinus was embolized to minimize intraoperative blood loss during opening of the jugular bulb.
Operation
The tumor was excised via a transcochlear infratemporal approach. This approach was chosen because tumor was in the temporal bone anterior to the internal auditory canal. By routing the facial nerve posteriorly, the transcochlear approach allowed complete removal of all involved bone and invasive tumor. Intraoperatively, the tumor was pigmented, circumscribed, and avascular. A complete resection was obtained with the sacrifice of the cranial nerves IX, X, and XI, which were completely encased within the tumor.
Histologic Examination
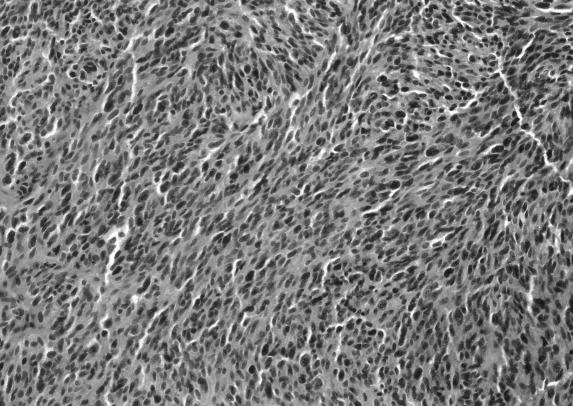
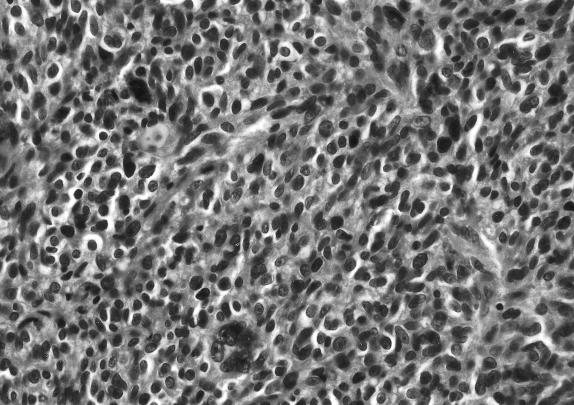
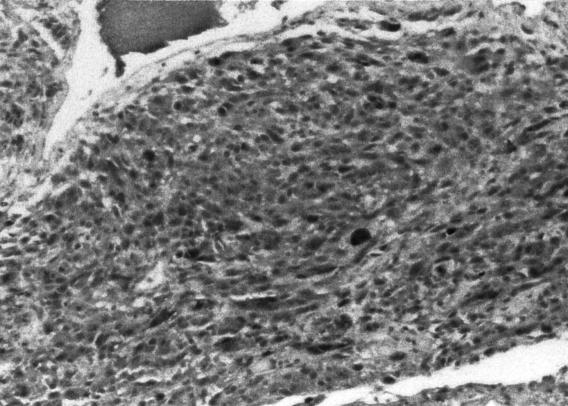
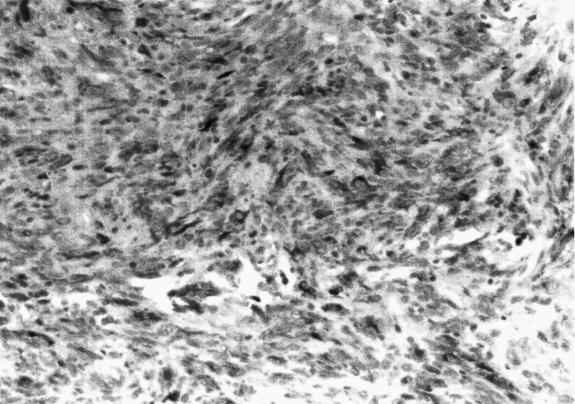
Frozen section revealed a melanotic tumor. Microscopic examination revealed tumor infiltrating the surrounding connective tissue. The nuclei exhibited pleomorphism and hyperchromasia. Brown pigment was seen within the tumor (Figs. 2A and B). Mitoses were difficult to find. The HMB–45 and S–100 markers showed marked positive staining of tumor cells (Figs. 2C and D). The MIB–1 labeled less than 1 % of the nuclei as positive (data not shown). The response to epithelial membrane antigen was negative, and no basal lamina was seen on electron microscopy. A melanoma of the CPA was diagnosed.
Figure 2A.
Hematoxylin and eosin histological sections seen at magnification 10×. Microscopic examination showed a tumor infiltrating into the surrounding connective tissue. The nuclei exhibited pleomorphism and hyperchromasia. Brown pigment was seen within the tumor, but mitoses were difficult to find. A CPA melanoma was diagnosed.
Figure 2B.
Hematoxylin and eosin histological sections seen at magnification 40×. Microscopic examination showed a tumor infiltrating into the surrounding connective tissue. The nuclei exhibited pleomorphism and hyperchromasia. Brown pigment was seen within the tumor, but mitoses were difficult to find. A CPA melanoma was diagnosed.
Figure 2C.
The HMB–45 marker showed marked positive staining, with 40× hematoxylin counterstain. A CPA melanoma was diagnosed.
Figure 2D.
The S–100 markers also showed marked positive staining of the tumor cells. A CPA melanoma was diagnosed.
Postoperative Course
Postoperatively, the patient developed right vocal cord paralysis and continued to have difficulties swallowing similar to her preoperative status. She received an injection of the right vocal cord and a percutaneous endoscopic gastrostomy (PEG) was placed. Her metastatic evaluation was negative. Ophthalmological evaluation had ruled out an intraocular melanoma, and dermatological evaluation had ruled out dermal melanotic lesion. Computed tomography (CT) of the chest, abdomen, and pelvis failed to reveal a primary melanoma. No adjuvant therapy was given. Over the next 3 months, the patient's swallowing and ataxia improved, and the PEG tube was removed. One year after surgery she remains free of systemic disease or a recurrence at the site of resection. Her facial nerve function has improved to a House–Brackmann level of IV, and her ability to swallow has returned to its level of preoperative function. The second smaller dural–based lesion was not removed and is unchanged on follow–up imaging.
DISCUSSION
Primary intracranial melanocytic lesions are very rare.4 Their estimated incidence is 0.9 per 10 million.8 They originate from melanocytes derived from neural crest cells during early embryonic development. Intracranially, primary melanomas have been found in a variety of locations: lobar, posterior fossa, pineal, and CPA.1, 2, 3, 5, 7, 9, 10 Primary melanocytic tumors of the central nervous system (CNS) can be classified into diffuse or localized diseases. Diffuse disease, also referred to as leptomeningeal melanosis, represents the diffuse infiltration of the subarachnoid space of the brain and spinal cord by melanocytes. The melanocytes are often associated with cutaneous melanosis such as giant pigmented nevi.
Localized disease suggests the melanocytes are limited to a circumscribed part of the CNS. They range from benign melanocytomas to malignant melanomas. Melanocytoma cells are uniform and well–differentiated, and they have a low nucleus–to–cytoplasm ratio. They also exhibit low cellularity, mild nuclear atypia, low mitotic activity, and a low MIB–1 labeling index, and are not associated with necrosis or invasion. In contrast, melanomas are anaplastic. They exhibit high cellularity and a high nucleus–to–cytoplasm ratio, marked nuclear atypia, high mitotic activity, a high MIB–1 labeling index, and are associated with necrosis or invasion. Histopathologically, intermediate–grade melanocytic tumors are classified between these two extremes. Most melanocytic tumors stain positive for S–100 and HMB–45. S–100 is found in almost all melanocytes. However, it is also present in other neural crest–derived tumors (high sensitivity and low specificity). In contrast, HMB–45 stains many but not all melanomas and seldom stains other tumor types (high specificity and moderate sensitivity). Thus the combination of the two forms a sensitive and specific test for melanoma.
The clinical presentation of primary CNS melanomas is similar to that of other brain tumors, depending on their location. Diagnosis is obtained by exclusion. Other sites of origin for the melanoma must be eliminated through a thorough physical and radiographic examination of other solid–organ sites. Radiographically, melanomas are hyperdense on CT with uniform or ring enhancement. This increased density is associated with intratumoral hemorrhage. MRI often reveals a hyperintense lesion on T1–weighted sequences and a hypointense lesion on T2–weighted sequences.11 The stable free radicals within melanin are responsible for such MRI characteristics. Nevertheless, the MRI signals of these lesions vary widely, depending on the amount of intratumoral hemorrhage.
The differential diagnosis of melanocytic lesions at the CPA includes primary and metastatic lesions. Metastatic melanomas are the third most common CNS metastasis, representing 9 % of all CNS metastases.2 However, metastatic melanoma to the CPA is very rare; only a few cases have been reported to date. Pigmented primary CNS tumors involving the CPA include primary melanocytic tumors, pigmented meningiomas, and pigmented schwannomas.The histopathology of each is different. Pigmented meningiomas often contain psammoma bodies, and they are positive for epithelial membrane antigen. Pigmented schwannomas consist of two cell types: Schwann cells and melanotic cells. Basal lamina can also be seen on electron microscopy. The lesion in our patient had features consistent with a primary melanoma. The diagnosis of melanocytoma was considered but ruled out because of the atypical histological features.
The prognosis for patients with a primary CNS melanoma is poor: 20 % survive more than 12 months and 13.6 % survive less than 1 month.4 The mean length of survival does not seem to be influenced by the duration of symptoms before diagnosis. The mean survival of patients who present with a focal neurological deficit is significantly longer than that of patients who present with signs of intracranial hypertension (11.9 months compared with 7.0 months, respectively).1 Patients with tumors that can be resected completely have the longest survival time. In one study, the mean length of survival of patients who have undergone gross total removal, biopsy and subtotal removal, and no surgery was 19.58 ± 2.3 months, 9.30 ± 2.4 months, and 3.40 ± 0.7 months, respectively, and the difference between each group was significant.4
In the treatment of primary CNS melanomas, the usefulness of radiotherapy, chemotherapy, and immunotherapy has not yet been established.12 In metastatic CNS melanoma, radiosurgery and postoperative radiation has been associated with some success.12, 13 Immunotherapy with agents such as interleukin–2 and IFα (interferon α) has mostly been ineffective because of the difficulty in crossing the blood–brain barrier.2 Chemotherapy with drugs such as temozolamide and dacarbazine has been reported to offer some benefits in treating metastatic melanoma.14 Among CNS tumors, intracranial melanomas represent a challenge for neurosurgeons because their clinical and radiological patterns can mimic those of other tumors, such as meningioma in our case. To date the preferred treatment is surgery and the role of adjuvant therapy is unknown. Our patient has received no adjuvant therapy and remains free of recurrent disease. If a recurrence develops, surgery, stereotactic radiosurgery, or both will be considered.
Figure 1E.
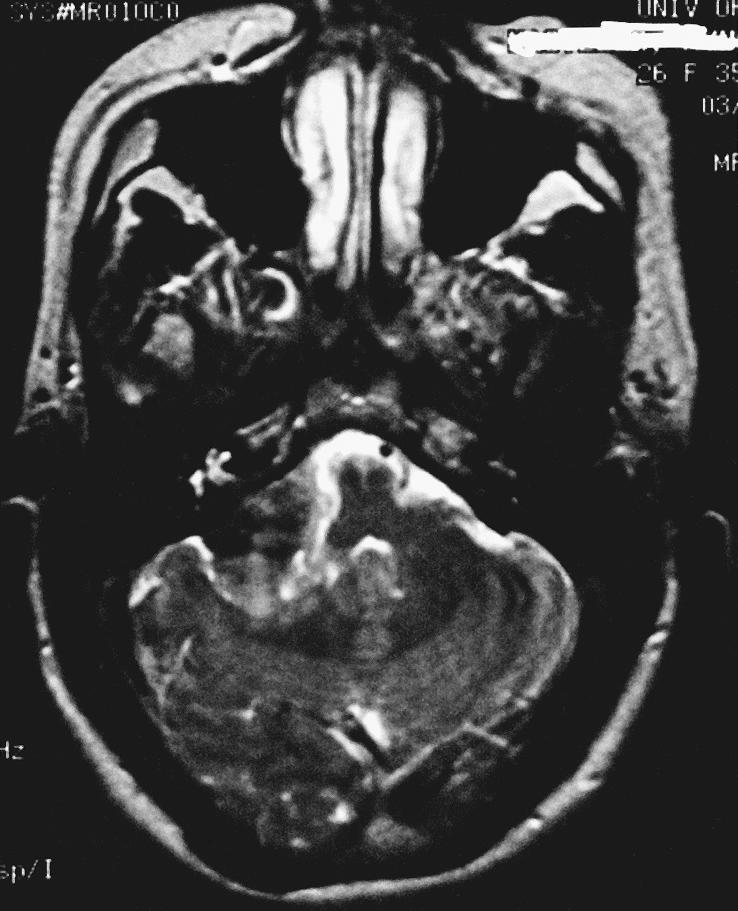
Axial fast–spin echo image show little surrounding edema.
Figure 1F.
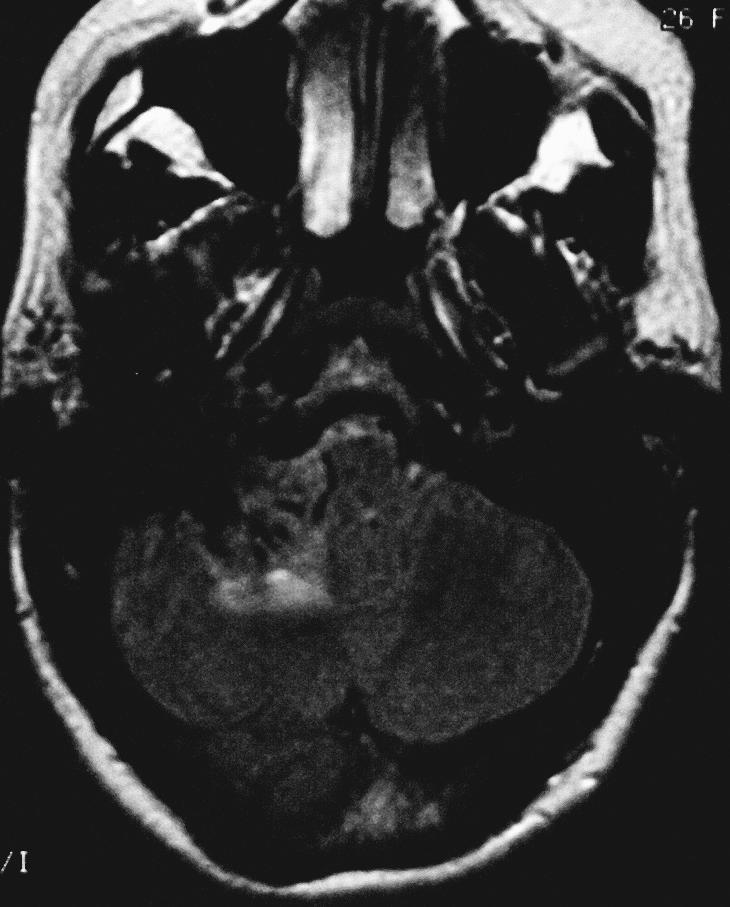
Axial fluid–attenuated inverted recovery image show little surrounding edema.
REFERENCES
- Rodriguez y Baena R, Gaetani P, Danova M, Bosi F, Zappoli F. Primary solitary intracranial melanoma: case report and review of the literature. Surg Neurol. 1992;38:26–37. doi: 10.1016/0090-3019(92)90208-5. [DOI] [PubMed] [Google Scholar]
- Desai K, Dindorkar K, Goel A, et al. Primary cerebello–pontine angle malignant melanoma: a case report. Neurol India. 2001;49:200–202. [PubMed] [Google Scholar]
- Whinney D, Kitchen N, Revesz T, et al. Primary malignant melanoma of the cerebellopontine angle. Otol Neurotol. 2001;22:218–222. doi: 10.1097/00129492-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Ater JL, Rytting M. Rare malignant brain tumors. Malden, MA: Blackwell Science Inc. 1997:626–654. In: Black PMcL, ed. Cancer of the Nervous System. [Google Scholar]
- Brat DJ, Giannini C, Scheithauer BW, et al. Primary melanocytic neoplasm of the central nervous system. Am J Surg Pathol. 1999;23:745–754. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Crasto SC, Soffietti R, Bradac GB, et al. Primitive cerebral melanoma: case report and review of the literature. Surg Neurol. 2001;55:163–168. doi: 10.1016/s0090-3019(01)00348-2. [DOI] [PubMed] [Google Scholar]
- Prabhu SS, Lynch P, Keogh AJ, et al. Intracranial meningeal melanocytoma: a report of two cases and a review of the literature. Surg Neurol. 1992;40:516–521. doi: 10.1016/0090-3019(93)90058-9. [DOI] [PubMed] [Google Scholar]
- Schuchter LM, Haluska F, Fraker D, Elenitsas R. Skin: malignant melanoma. PA: Churchill Livingstone. 2000:1326–1327. In: Abeloff MD, ed. Clinical Oncology. [Google Scholar]
- Yamane K, Shima T, Okada Y, et al. Primary pineal melanoma with long–term survival: case report. Surg Neurol. 1994;42:433–437. doi: 10.1016/0090-3019(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Kiel FW, Starr LB, Hansen JL. Primary melanoma of the spinal cord. J Neurosurg. 1961;18:616–629. doi: 10.3171/jns.1961.18.5.0616. [DOI] [PubMed] [Google Scholar]
- Woodruff WW Jr, Djang WT, McLendon R, et al. Intracerebral malignant melanoma: high–field strength MR imaging. Radiology. 1987;165:209–213. doi: 10.1148/radiology.165.1.3628773. [DOI] [PubMed] [Google Scholar]
- Hagen NA, Cirrincione C, Thaler HT, et al. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40:158–160. doi: 10.1212/wnl.40.1.158. [DOI] [PubMed] [Google Scholar]
- Mingione V, Oliveira M, Prasad D, et al. Gamma surgery for melanoma metastases in the brain. J Neurosurg. 2002;96:544–551. doi: 10.3171/jns.2002.96.3.0544. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Summers Y, Clavert AH, et al. Effect of temozolomide on central nervous system relapse in patients with advanced melanoma. Melanoma Res. 2002;12:175–178. doi: 10.1097/00008390-200204000-00011. [DOI] [PubMed] [Google Scholar]