CASE HISTORY
A 40–year–old male had an 8–month history of left nasal congestion and shooting pains in the left face triggered by cold air. He had undergone right orbital exenteration and bilateral orbital radiation at the age of 9 months for bilateral retinoblastoma. The radiation had been administered on an orthovoltage machine to a total dose of about 30 Gy. The dose per fraction was high, but the number of fractions was unknown. The field of radiation was also unknown because simulation was not performed in 1960. Otherwise, his medical history was unremarkable. He was employed as the vice principal of an elementary school and played sports regularly.
On examination he had atrophic midfacial bones and a prosthesis in the right eye. A mass was present in the left side of his nose in the region of the middle meatus. Visual acuity in the left eye was reported as 20/30 with no visual field defects or retinal abnormality. Eye movements were normal and there was no change in facial sensation or facial weakness.
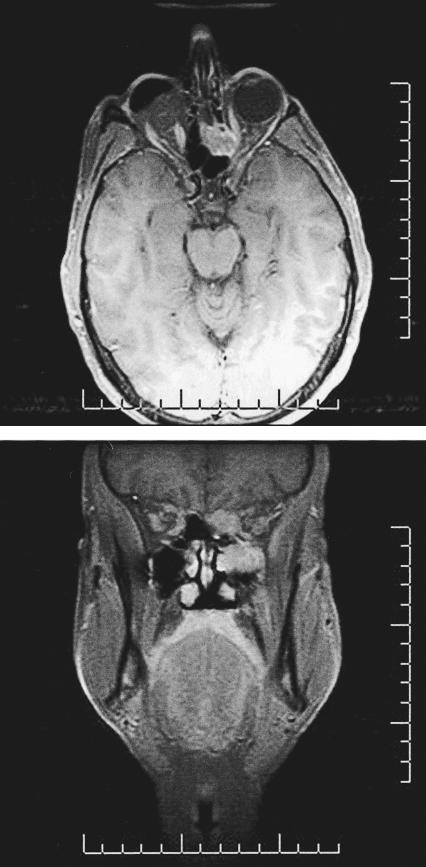
Multiplanar magnetic resonance images (MRIs) obtained before and after gadolinium–contrast enhancement revealed a lobulated soft–tissue mass involving the left inferior orbital wall. Anteroposteriorly, the mass measured 4.3 cm × 2.2 cm (Fig. 1). Within the orbit the lesion appeared extraconal, but the inferior and medial rectus muscles were involved. Superomedially, the mass extended into the ethmoid sinus and up through the cribriform plate. Inferiorly, it extended through the osteomeatal complex as well as through the floor of the orbit into the left maxillary sinus. Posteriorly, it extended through the left inferior orbital fissure and laterally into the pterygopalatine fissure. Abnormal enhancement of the maxillary nerve was consistent with perineural involvement. Transnasal endoscopic biopsy of the mass was reported as consistent with leiomyosarcoma after evaluation by an expert sarcoma panel.
Figure 1.
(Top) Axial and (bottom) coronal T1–weighted magnetic resonance images after gadolinium contrast–enhancement show a lobulated soft–tissue mass involving the left inferior orbital wall.
RESPONSES
Retinoblastomas are particularly fascinating tumors, not only for ophthalmologists but for oncologists in general. It is the most common intraocular malignancy of childhood, but of more interest is that it is one of the first tumors described in which an abnormality in a specific suppressor gene could be demonstrated to be the cause of the tumor. Mutations or deletions at the corresponding locus on chromosome 13 are demonstratively present in each retinoblastoma. This lack of suppressor function has significant implications later in a patient's life. Of patients with retinoblastomas, 95 % survive their disease with an 85 % salvage rate of the globe. However, patients with systemic genetic alterations are at risk of developing secondary malignancies, which most commonly include osteogenic sarcoma. Multiple other tumors have also been described. Previous external beam radiation substantially increases the risk of secondary tumors. Of importance to this particular case, these tumors are often very resistant to treatment and are associated with a 50 % mortality rate. They also tend not to be sensitive to radiation therapy.
I think this young, otherwise healthy patient's only real chance would be a fairly aggressive surgical approach. Although the coronal image suggests that the tumor is contiguous to the medial rectus, most of these lesions, especially if there is no evidence of significant motility problems, have a dissectible plane along the sheath of the medial rectus that is free of tumor. Therefore, I believe it is possible to spare the orbital contents.
The major difficulty would be planning the surgical approach. It might be possible to remove the ethmoidal lesion through an ethmoidectomy and lateral rhinotomy approach. However, the presence of the tumor along the frontal dura suggests that the best chance of free margins would be to perform a minicraniotomy through the frontal sinuses to permit an anterior fossa approach to the medial aspect of the orbit and the area of the cribriform. The mass within the maxillary sinus could be approached with a lateral rhinotomy and inferior orbital dissection.
In this case, the real problem is the tumoral extension through the pterygomaxillary area along the course of the maxillary nerve. Neurotrophic tumors such as squamous cell carcinomas and adenoid cystic carcinomas tend to spread along the trigeminal nerve and enter the cavernous sinus. The most likely site of recurrence would be the cavernous sinus. Therefore, the surgeon should attempt to reach as far posteriorly as possible during the primary operation. This requires a lateral extradural approach, removing the lateral wall of the orbit and identifying the maxillary nerve as it enters the foramen rotundum. To reach this area, an additional subtemporal approach would be necessary. The zygoma would have to be removed to allow the temporalis muscle to be reflected inferiorly enough to access the floor of the middle cranial fossa. This maneuver might be performed more easily through a pterional craniotomy. This approach would be important to identify the lateral aspect of the lesion in the area of the pterygoids and its posterior aspect at the foramen rotundum. The blood supply to this lesion will be through the internal maxillary artery. Preoperative control through neuroradiologic embolization may decrease the amount of bleeding. Ultimately, complete surgical excision will fail in the region of the pterygoids. Reconstruction should be no problem as long as a dural excision is unnecessary.
Steven A. Newman M.D.
School of Medicine
University of Virginia
Health Sciences Center
Box 800715
Charlottesville, VA 22908–0715
san7a@virginia.edu
This high–grade radiation–associated sarcoma involves the paranasal sinuses, anterior skull base, and orbit of the patient's only remaining eye.
Leiomyosarcomas are exceedingly rare, high–grade tumors of the head and neck with the potential for distant metastases. Before local and regional management is planned, absence of distant disease must be established. At my institution, a bone scan and computed tomography of the chest, abdomen, and pelvis would be performed to rule out distant disease.
For patients with a soft–tissue sarcoma, wide resection and reconstruction followed by radiation offer the best chance of a disease–free survival. In principle, orbital invasion dictates that exenteration be performed in most cases. Unfortunately, this patient has only one eye. The patient must be involved in the decision making, but I would attempt to salvage his vision at the expense of a close surgical margin. However, the patient must be fully prepared for an intraoperative decision to remove the orbit if necessary.
Extension of the tumor through the cribriform plate requires a combined intracranial–transfacial surgical approach. In such situations, my preferred method is an anterior craniofacial resection through a bifrontal craniotomy and lateral rhinotomy with a subciliary extension. The resection would include the anterior skull base, ethmoid sinuses, maxillary suprastructure, and the medial wall and floor of the orbit. The involved intraorbital contents would also be resected en bloc. I would make every attempt to preserve the globe and its neurovascular integrity. The patient will not suffer from diplopia even with limited extraocular movement. The palate would be left intact. The likelihood of cervical metastases is low, and I would perform no neck dissection in the absence of clinical disease.
The reconstructive goals include reconstitution of dural integrity and restoration of the orbital–facial contour. Postoperative complications should be minimized. A pericranial flap based on the supratrochlear and supraorbital vessels can be used to support the anterior duraplasty. The facial contour can be maintained using calvarial bone grafts to replace the bony orbit. A free vascularized myogenous flap (my preference is the latissimus dorsi muscle) would be positioned to fill the soft–tissue defect, to provide further dural protection, and to vascularize the bone grafts.
Postoperative radiation is fundamental to this case, in particular because a close margin is expected if vision is to be preserved. Planning should begin preoperatively in discussion with the radiation oncologist. This patient previously received orthovoltage radiation of 30 Gy, likely delivered using the POP (parallel opposed pair) technique. The dosage to the brain was likely limited, and reradiation is possible. At our center, the patient would be considered for intensity modulated radiation therapy to limit radiation to the retina and optic nerve while delivering more than 60 Gy to the surgical bed. However, postoperative proton–beam radiation has distinct advantages over conventional radiation. In such a case, I would seek an opinion from a center with this capability before surgery.
John Yoo M.D.
Department of Otolaryngology
Head and Neck Oncology–Reconstructive Microsurgery
University of Western Ontario
London Health Sciences Centre
800 Commissioners Road East
London, Ontario, N6A 4G5 Canada
John.Yoo@lhsc.on.ca
This case represents a rare radiation–induced leiomyosarcoma of the ethmoid sinuses. Although I believe I have a wide breadth of experience with most rhinological conditions, I have never come across such a case. With no experience managing this condition, it would be my practice to seek the advice of national and international colleagues with an interest in this area. I would also review the literature and confer with my oncological colleagues. I would then feel better equipped to offer an opinion. Although lack of experience is rarely published in journals, I believe it is important for surgeons to acknowledge that they have not seen a case, to seek the advice of others, and to review the literature.
Nick Jones M.D., F.R.C.S.
Directorate of Otorhinolaryngology & Head and Neck Surgery
Queen's Medical Centre
University Hospital NHS Trust
Nottingham, England
nick.jones@nottingham.ac.uk
ACTUAL MANAGEMENT
Two factors played a major part in this patient's management. The total dose and field of prior radiation to the orbit were uncertain, and the patient expressed a strong desire to retain function in his remaining eye. Given the uncertainty regarding his previous radiation, it was decided to try to shrink the tumor with neoadjuvant chemotherapy (doxorubicin and ifosfamide). He underwent two cycles and showed no evidence of a response after MR imaging.
The patient then underwent anterior craniofacial resection with resection of the anterior skull base, left medial maxillectomy, ethmoid sinusectomy, cribriform plate, clivus, and orbital apex. During surgery the optic chiasm was identified, and the left optic nerve was followed anteriorly. The optic nerve was decompressed at the orbital apex and kept in view during surgery. Although devascularization of the optic nerve during surgery was a concern, vision in his left eye remained intact postoperatively. The floor of the anterior cranial fossa was reconstructed using a fascia lata graft.
Nigel Beasley M.B., F.R.C.S. Patrick Gullane M.B., F.R.C.S.C.
Wharton Head and Neck Centre
Princess Margaret Hospital
Toronto, Ontario, M5G 2M9 Canada
nigel.beasley@doctors.org.uk