Abstract
The Vif protein of human immunodeficiency virus type 1 (HIV-1) is important for virion infectivity. Previous studies have shown that vif-defective virions exhibit structural abnormalities in the virus core and are defective in the ability to complete proviral DNA synthesis in acutely infected cells. We developed novel assays to assess the relative stability of the core in HIV-1 virions. Using these assays, we examined the role of Vif in the stability of the HIV-1 core. The integrity of the core was examined following virion permeabilization or removal of the lipid envelope and treatment with various triggers, including S100 cytosol, deoxynucleoside triphosphates, detergents, NaCl, and buffers of different pH to mimic aspects of the uncoating and disassembly process which occurs after virus entry but preceding or during reverse transcription. vif mutant cores were more sensitive to disruption by all triggers tested than wild-type cores, as determined by endogenous reverse transcriptase (RT) assays, biochemical analyses, and electron microscopy. RT and the p7 nucleocapsid protein were released more readily from vif mutant virions than from wild-type virions, suggesting that the internal nucleocapsid is less stably packaged in the absence of Vif. Purified cores could be isolated from wild-type but not vif mutant virions by sedimentation through detergent-treated gradients. These results demonstrate that Vif increases the stability of virion cores. This may permit efficient viral DNA synthesis by preventing premature degradation or disassembly of viral nucleoprotein complexes during early events after virus entry.
The Vif protein of human immunodeficiency virus type 1 (HIV-1) and other lentiviruses is important for virion infectivity. Vif is required for HIV-1 replication in peripheral blood mononuclear cells, and its function is likely to be essential in vivo (12, 19, 20, 24, 56, 58, 61). vif-defective viruses can enter cells normally and initiate reverse transcription but are defective in the ability to complete proviral DNA synthesis (13, 26, 42, 53, 57, 61). This results from an effect of Vif in the virus-producing cell, most likely during events involved in virus assembly (24, 48, 61). A low level of Vif, estimated at between 1 and 40 molecules per virion, is associated with the core of HIV-1 particles (11, 15, 22, 29, 39, 54). However, virion incorporation may be nonspecific and does not appear to be required for Vif function (15, 54). Although Vif is required for HIV-1 replication in primary T cells and monocytes/macrophages, the requirement for Vif differs among cell lines (19, 24, 48, 57, 61). This finding has led to the classification of cell lines as nonpermissive or permissive for the replication of vif-defective viruses. The cellular or viral targets for Vif function have not been identified. Recent studies suggest that Vif may act to overcome an inhibitory factor present in nonpermissive cells (40, 52). An alternative model is that Vif may compensate for a cellular factor(s) that is required for the production of infectious virus particles but is present only in permissive cells.
Several lines of evidence suggest that the action of Vif affects the virion core. The major structural proteins of the mature HIV-1 virion are proteolytic cleavage products of the HIV-1 p55 Gag precursor protein, matrix (p17), capsid (p24), nucleocapsid (p7), and p6 (reviewed in reference 60). The cone-shaped core contains p24, p7, the pol gene products reverse transcriptase (RT), integrase (IN), and protease, the accessory proteins Vpr and Nef, a small fraction of p17, tRNALys, and the viral RNA genome (2, 33, 62). The p24 capsid protein forms the cone-shaped shell, and the other proteins and RNA genome are localized to the core interior (60). The p7 nucleocapsid protein is required for packaging of genomic RNA and is complexed with the RNA in the internal nucleocapsid. Surrounding the core is a protein layer of the p17 matrix protein apposed to the lipid envelope, which contains the gp120 and gp41 Env glycoproteins. vif-defective virions exhibit structural abnormalities in the virus core. Tightly packed electron-dense material is found only in the broad end of the cone-shaped core, while the material inside the narrow end appears transparent (5, 8, 27). Furthermore, vif-defective virions are impaired in the ability to synthesize viral DNA in the endogenous RT reaction, an in vitro assay in which virions synthesize viral DNA by using endogenous RNA as a template (26, 42). Moreover, vif-defective virions can initiate reverse transcription in acutely infected cells, but the reverse transcripts appear to be prematurely degraded (53). These findings suggest that a component of the virion core is likely to be a target for Vif function. However, most studies have not found a consistent difference between wild-type and vif-defective virions in the virion composition of Env or Gag proteins, viral enzymes, or packaged viral RNA (8, 22, 43).
Little is known about the early postentry steps that lead to uncoating and disassembly of the virion core. Fusion and virus entry are followed by a poorly defined uncoating process in which the p24 shell is released from the core to form the uncoated viral nucleoprotein complex (also called the reverse transcription complex [RT complex]), which contains the viral genome, tRNALys primer, RT, integrase p7, p17, Vpr, and host cell proteins (reviewed in reference 60). After reverse transcription of the viral genome, a preintegration complex is formed that is actively transported to the nucleus. These observations together with previous studies described above suggest that Vif may play a role during virus assembly which affects the stability and uncoating of the internalized HIV-1 core.
In this study, we examined the effect of Vif on the stability of the HIV-1 core in permeabilized virions and purified cores. The integrity of the core was examined following virion permeabilization or removal of the lipid envelope and treatment with various triggers, including S100 cytosol, deoxynucleoside triphosphates (dNTPs), detergents, NaCl, and buffers of different pH, to mimic aspects of the uncoating and disassembly process. Under all conditions tested, vif mutant virions produced in nonpermissive cells released increased amounts of core components (p24, RT, and p7) to the soluble fraction compared to wild-type virions. Purified cores could be isolated from wild-type but not vif mutant virions by sedimentation through detergent-treated gradients. These results demonstrate that Vif increases the stability of virion cores and suggest that the core of vif-defective viruses may be degraded prematurely or disassemble abnormally after virus entry.
MATERIALS AND METHODS
Virus and cell culture.
A cocultivation method was used to produce large quantities of wild-type and vif mutant virus in nonpermissive CEM, HUT78, and H9 cells or permissive SupT1 cells (26). Infection of CEM cells was initiated by cocultivation with 293T cells transfected with 10 μg of wild-type or vif mutant HXB2 DNA by the calcium phosphate method from 24 to 48 h after transfection. Infection of HUT78, H9, and SupT1 cells was initiated by cocultivation with 293T cells cotransfected with 1 μg of pHCMV-G, which expresses the vesicular stomatitis virus envelope glycoprotein, and 10 μg of wild-type or vif mutant HXB2 DNA by the calcium phosphate method from 24 to 48 h after transfection. The HIV-1 vif mutant viral DNA was made by changing the HXB2 sequence encoding Vif amino acids 21 and 22 to two in-frame stop codons (24). Cultures were maintained in RPMI medium plus 10% fetal calf serum, with medium changes every 1 or 2 days. Virions were harvested from 24-h culture supernatants from days 4 to 10 after infection. The culture supernatants were clarified by centrifugation at 2,000 × g for 10 min and filtration through a 0.45-μm-pore-size Millipore filter prior to virion pelleting by centrifugation through 20% sucrose in a phosphate-buffered saline (PBS) cushion at 125,000 × g for 90 min. Pelleted wild-type and vif mutant virions were resuspended in 50 mM Tris (pH 7.4) and normalized for the same amount of exogenous RT activity by incorporation of [3H]dTTP into an artificial poly(A)(dT)15 template as described elsewhere (26).
Endogenous RT assay.
The standard endogenous reaction was performed as described previously (26) in a 50-μl volume containing 500,000 cpm of exogenous RT units of HIV-1, 50 mM Tris-HCl (pH 7.4), 2 mM dithiothreitol, 2 mM magnesium acetate, 0.1 mM three dNTPs (dATP, dCTP, and dGTP), 50 μCi of [3H]dTTP, and the indicated detergent for 20 h at 37°C. In initial experiments, virions were permeabilized with the following concentrations of detergents for 10 min at room temperature prior addition of reaction buffer: 5 to 20 μg of melittin (Sigma) per ml, 0.01 to 0.04% NP-40 (Sigma), 0.01 to 0.04% Cymal-6 (cyclohexyl-hexyl-β-d-maltoside; Anatrace), and 0.01 to 0.04% Triton X-100 (Sigma). For subsequent experiments, 10 μg of melittin per ml was used for virion permeabilization. For some reactions, the reaction buffer contained a final concentration of 50, 150, or 500 mM NaCl or 50 mM Tris-HCl buffer with pH 5.0, 7.0, or 9.0. The reactions were terminated by addition of 1/10 volume of stop buffer (final concentrations, 50 mM Tris-HCl [pH 8] and 1% sodium dodecyl sulfate [SDS]) and spotting onto DE81 filters for quantitation by liquid scintillation counting.
Treatment of virions with chemical triggers or S100 cytosol.
Wild-type and vif mutant virions (200,000 cpm of exogenous RT units) were permeabilized with 5 to 20 μg of melittin per ml, 0.01 to 0.04% NP-40, 0.01 to 0.04% Cymal-6, or 0.01 to 0.04% Triton X-100 for 10 min at room temperature prior to addition of 50 mM Tris-HCl (pH 7.4; to a final reaction volume of 50 μl) and incubation for 1 h at 37°C. The samples were then centrifuged at 14,000 × g in an Eppendorf Microfuge for 1 h. The supernatant and pellet fractions were separated and loaded onto an SDS–16% polyacrylamide gel and analyzed by Western blotting. In separate experiments, 200,000 cpm of exogenous RT units of wild-type or vif mutant virions was permeabilized with 10 μg of melittin per ml for 10 min at room temperature prior to addition of 50 mM Tris-HCl (pH 7.4) with 50 to 500 mM NaCl, 50 mM Tris-HCl buffered to different pH values, or 1 to 10 μg of S100 cytosol proteins and incubated at 37°C for 1 h. The supernatant and pellet fractions were separated and analyzed by Western blotting as described above.
Preparation of S100 cytosol.
H9 cells were resuspended in hypotonic buffer (10 mM Tris-HCl [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol) in the presence of protease inhibitors (leupeptin [5 μg/ml], antipain [50 μg/ml], aprotinin [10 μg/ml], pepstatin [5 μg/ml], and phenylmethylsulfonyl fluoride [100 μg/ml]), incubated on ice for 10 min, homogenized with 20 strokes of a glass Dounce homogenizer (type B), and centrifuged at 500 × g for 5 min to pellet nuclei. A cytosol fraction (S100) was prepared by centrifugation of the supernatant at 100,000 × g for 1 h. Total protein concentration was determined by the Bio-Rad DC assay, and aliquots of S100 fractions were stored at −80°C.
Sucrose gradient analysis.
Wild-type and vif mutant virions (500,000 cpm of exogenous RT units) were permeabilized with 10 μg of melittin per ml, treated with 500 mM NaCl in 50 mM Tris-HCl (pH 7.4), 20 μg of S100 cytosol, and 2 mM dNTPs plus 2 mM magnesium acetate, or 10 μg of RNase (Boehringer Mannheim) per ml, incubated for 1 h at 37°C, and layered over a 1-ml 20 to 60% sucrose gradient. The gradients were centrifuged at 14,000 × g for 3 h at 4°C, and 125-μl fractions were collected from the top. The pellet was resuspended in 125 μl of 20% sucrose. One-third of each fraction was used for SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis.
Western blot analysis.
Samples were separated by SDS-PAGE on 12 or 16% polyacrylamide gels under reducing conditions, transferred to polyvinylidene difluoride membranes (Millipore), and probed with mouse monoclonal anti-p7 antibody 4D1, mouse monoclonal anti-p17 (Advanced Biotechnologies Inc.), rabbit anti-p24 (Intracel), and mouse anti-RT (Intracel) antibodies, and human monoclonal HIV-1 gp41 antibody 2F5 (obtained from Hermann Katinger through the NIH AIDS Research and Reference Reagent Program) (10, 44, 45). Blots used for multiple hybridizations were stripped of antibodies by washing for 30 min at 50°C in stripping buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM β-mercaptoethanol) (22). Blots were visualized by horseradish peroxidase-conjugated anti-human (Cappel), anti-rabbit, or anti-mouse (Amersham) immunoglobulin G antibodies and enhanced chemiluminescence (Renaissance; NEN).
Preparation of virion cores.
Wild-type and vif mutant virion cores were prepared by a modification of the “spin-thru” method previously used for HIV-1 (2, 33) and HIV-2 (30). A sucrose step gradient was prepared by layering 400 μl of 60% sucrose in PBS, 400 μl of 40% sucrose, 200 μl of 20% sucrose containing 0.5% Triton X-100, and 150 μl of 10% sucrose as a barrier between virus and detergent prior to centrifugation; 50 μl of concentrated virions (2 × 106 cpm of exogenous RT units) was layered on top of the gradient and centrifuged at 14,000 × g for 3 h at 4°C. Eight fractions of 150 μl were collected from the top of the gradient, and the pellet was resuspended in 150 μl of 20% sucrose. Fractions were analyzed by SDS-PAGE, Western blot analysis, and electron microscopy.
Electron microscopy.
Virions were permeabilized and treated as described above prior to fixation with 2.5% glutaraldehyde in PBS and postfixation with 1% OsO4. The fixed material was embedded in Epon-Araldite, sectioned, and poststained with 1% uranyl acetate. Virions and cores in sucrose gradient fractions were analyzed by negative staining. The sucrose fractions were diluted with 1 ml of 2.5% glutaraldehyde and centrifuged for 1 h at 14,000 × g. The pellets were resuspended in 0.5% glutaraldehyde, applied onto carbon-coated grids, and stained with 1% uranyl acetate. Sections and negatively stained samples were analyzed at an accelerating voltage of 60 kV with a JEOL 1200EX electron microscope.
RESULTS
Detergent stability of virions.
To examine the effect of Vif on virion core stability, we treated wild-type and vif mutant virions with detergent and performed in vitro assays to assess the functional and structural integrity of virion cores. For endogenous RT assays of detergent-treated virions, we used the nonionic detergents NP-40, Triton X-100, and Cymal-6. We also tested melittin, an amphipathic polypeptide derived from bee venom which forms membrane pores with an average size of 3 nm and can accommodate the release of 50.7-kDa size markers from permeabilized vesicles (34). Melittin has been reported to allow synthesis of more full-length cDNA in the endogenous RT reaction compared to nonionic detergents (4, 6, 14, 66).
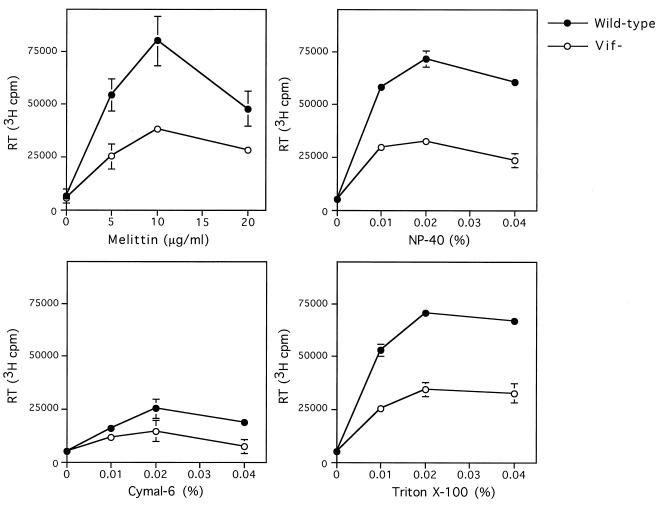
Pelleted wild-type and vif mutant virions produced in nonpermissive CEM cells (24, 26) were normalized for the same quantity of exogenous RT activity. Virions were permeabilized with different concentrations of melittin, Cymal-6, NP-40, or Triton X-100, and endogenous RT activity was determined (Fig. 1). The highest level of endogenous RT activity for wild-type and vif mutant virions was obtained when virions were permeabilized with 10 μg of melittin per ml. High levels of endogenous RT activity were also obtained when virions were permeabilized with 0.01 to 0.04% NP-40 or Triton X-100, whereas permeabilization with Cymal-6 resulted in lower levels of endogenous RT activity. vif mutant virions showed a 40 to 50% reduction in endogenous RT activity compared to wild-type virions when permeabilized with 5 to 10 μg of melittin per ml, 0.01 to 0.04% NP-40, 0.01 to 0.04% Triton X-100, or 0.04% Cymal-6. Similar results were obtained for wild-type and vif mutant virions produced in nonpermissive H9 and HUT78 cells (data not shown). The difference between wild-type and vif mutant virions was less apparent when higher concentrations of melittin or detergents were used (Fig. 1 and data not shown). Therefore, the detergent concentrations shown in Fig. 1 were used for subsequent experiments.
FIG. 1.
Endogenous RT activity in wild-type and vif mutant virions. Wild-type and vif mutant virions produced in CEM cells were used for the endogenous RT reaction. Virions normalized for the same value of exogenous RT activity (500,000 cpm per sample) were permeabilized with different concentrations of melittin, NP-40, Cymal-6, or Triton X-100 prior to addition of reaction buffer, and the endogenous RT activity was measured by incorporation of [3H]dTTP for 20 h at 37°C. Values shown are means ± standard deviations (n = 2) and are representative of three or four independent experiments using different preparations of virions.
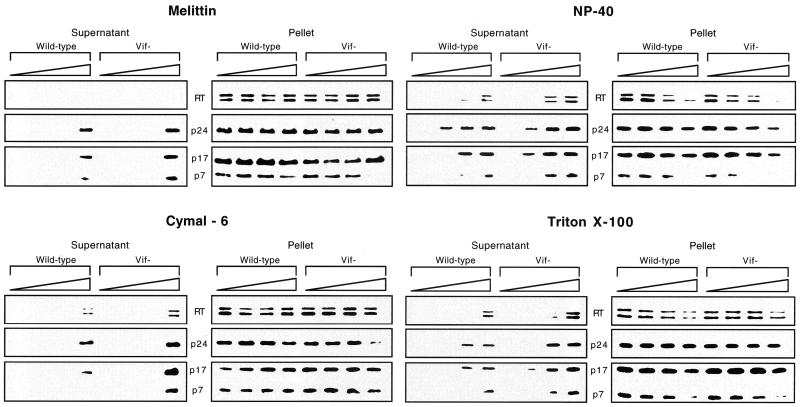
To analyze the integrity of cores in detergent-treated virions, the release of core proteins into the soluble fraction was analyzed by Western blotting. Wild-type and vif mutant virions were permeabilized with melittin or detergents for 10 min prior to addition of buffer and incubation for 1 h at 37°C. Virions were then subjected to centrifugation for 1 h at 14,000 × g, which is sufficient to pellet intact virions but not solubilized free proteins (64). The pellet and supernatant fractions were analyzed by Western blotting. Treatment of wild-type and vif mutant virions with no detergent, 5 to 10 μg of melittin per ml, or 0.01 to 0.02% Cymal-6 resulted in no detectable release of core proteins (Fig. 2). The core proteins p24, p7, and RT and the matrix protein p17 remained associated with the pellet fraction, indicating that virion cores appeared to be intact. Notably, 10 μg of melittin per ml did not solubilize any viral core proteins. Thus, virion cores appear to be grossly intact following treatment with melittin at this concentration. Higher concentrations of melittin or Cymal-6 resulted in partial release of p17, p24, and p7 into the soluble fraction; 0.04% Cymal-6 also released detectable quantities of RT into the soluble fraction. Under these conditions, the release of core proteins was two- to fivefold higher for vif mutant virions than for wild-type virions, as determined by densitometry of the bands obtained by Western blotting. In general, the most notable differences between wild-type and vif mutant virions were observed for RT and p7. In contrast to melittin and Cymal-6, treatment with NP-40 or Triton X-100 resulted in partial release of core proteins even at low detergent concentrations (Fig. 2). The release of core proteins was more pronounced for vif mutant virions than for wild-type virions, with the most notable differences between the wild type and vif mutant observed for RT and p7. Little or no RT and p7 were released from wild-type virions treated with 0.02% NP-40 or 0.02% Triton X-100, whereas vif mutant virions released 10 to 40% of RT and 35 to 100% of p7. In contrast, approximately 25 to 50% of p24 and 15 to 30% of p17 was solubilized from wild-type and vif mutant virions (Fig. 2). Together, the results of the endogenous RT and detergent sensitivity assays provide evidence that the vif mutant core is less stable than the wild-type core. Additionally, 10 μg of melittin per ml was shown to permeabilize virions without solubilizing any virion proteins. Therefore, we used 10 μg of melittin per ml to permeabilize virions in subsequent experiments.
FIG. 2.
Release of core proteins into the soluble fraction after detergent treatment of wild-type and vif mutant virions. Wild-type and vif mutant virions (200,000 cpm of exogenous RT units per sample) produced in CEM cells were permeabilized with melittin (0, 5, 10, and 20 μg/ml), NP-40 (0, 0.01, 0.02, and 0.04%), Cymal-6 (0, 0.01, 0.02, and 0.04%), or Triton X-100 (0, 0.01, 0.02, and 0.04%) prior to addition of buffer and incubation for 1 h at 37°C. The samples were centrifuged, and the supernatant and pellet fractions were separated by SDS-PAGE and analyzed by Western blotting. The blots were used for multiple hybridizations and were initially hybridized with a rabbit anti-p24 antibody. The blots were then stripped and reprobed sequentially with mouse anti-RT followed by a mixture of mouse anti-p17 and anti-p7 monoclonal antibodies. Results are representative of two or three independent experiments using different preparations of virions.
Effects of NaCl, pH, and S100 cytosol on virion cores.
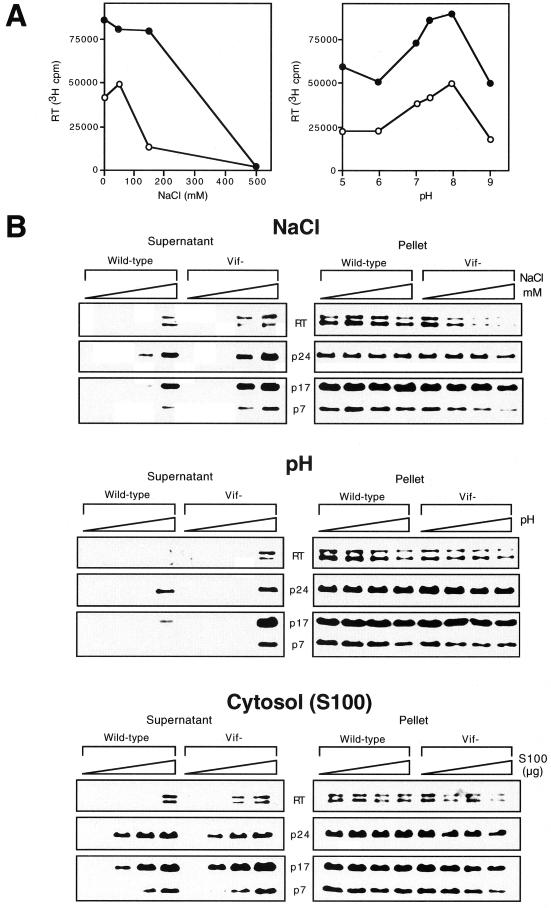
To further examine the effect of Vif on the stability of virion cores, we analyzed the sensitivity of wild-type and vif mutant virions to treatment with NaCl or different pH conditions. Virions produced in nonpermissive HUT78 cells were permeabilized with 10 μg of melittin per ml prior to addition of endogenous RT reaction buffer containing 0 to 500 mM NaCl or 50 mM Tris-HCl buffered to different pH values. For both wild-type and vif mutant virions, optimal endogenous RT activity was detected at 0 to 50 mM NaCl (Fig. 3A). NaCl concentrations above 50 mM consistently decreased endogenous RT activity, consistent with a previous study (66). An increase in NaCl concentration from 50 mM to 150 mM markedly reduced endogenous RT activity for vif mutant but not wild-type virions. Thus, the endogenous RT activity of vif mutant virions is more sensitive to high salt concentrations than that of wild-type virions. The endogenous RT reaction had a narrow optimal range for pH (Fig. 3A). The optimal pH for the endogenous RT reaction was 8, consistent with previous reports (6, 66). Both wild-type and vif mutant virions showed reduced endogenous RT activity at more acidic or basic pH. At neutral or slightly basic pH (pH 7 to 8), the endogenous RT activity of vif mutant virions was 53 to 55% of that of the wild type. At very low (pH 5 and 6) or high (pH 9) pH, the endogenous RT activity of vif mutant virions was 36 to 38% of that of the wild type.
FIG. 3.
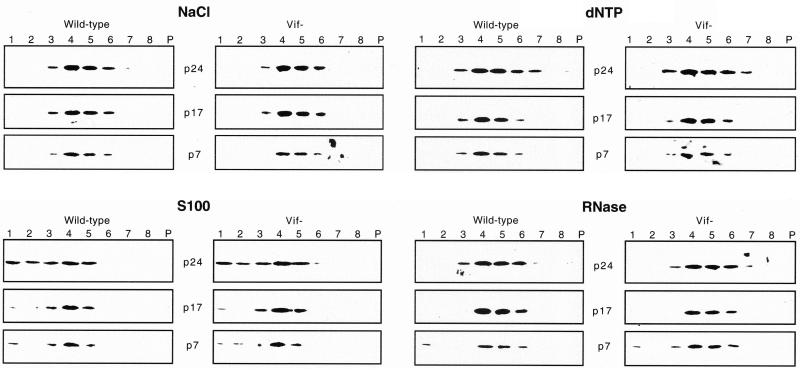
Release of core proteins into the soluble fraction after treatment of wild-type and vif mutant virions with NaCl, different pH conditions, or S100 cytosol. Wild-type and vif mutant virions produced in HUT78 cells were permeabilized with melittin (10 μg/ml) prior to addition of reaction buffer containing 50 mM Tris-HCl (pH 7.4) with 0, 50, 150, or 500 mM NaCl, 50 mM Tris-HCl buffered to 7.4 (control pH), 5, 7, or 9, or 0, 1, 5, or 10 μg of S100 cytosol proteins resuspended in 50 mM Tris-HCl (pH 7.4). (A) Endogenous RT activity of wild-type (closed circles) and vif mutant (open circles) (500,000 cpm of exogenous RT units per sample) was measured as described in the legend to Fig. 1. (B) The supernatant and pellet fractions of treated wild-type and vif mutant virions (200,000 cpm of exogenous RT units per sample) were analyzed by Western blotting as described in the legend to Fig. 2. Results are representative of two or three independent experiments using different preparations of virions.
To determine the effect of NaCl and pH on the integrity of virion cores, the release of core proteins into the soluble fraction was analyzed by Western blotting. Wild-type and vif mutant virions were permeabilized with melittin (10 μg/ml) and then incubated with different concentrations of NaCl. Interestingly, 150 mM NaCl, which markedly reduced endogenous RT activity in vif mutant but not wild-type virions, induced the release of 36 to 58% of RT, p24, p17, and p7 into the soluble fraction from vif mutant virions, whereas only 6 to 33% of p24 and p17 and no detectable RT and p7 were released from wild-type virions (Fig. 3B). Treatment with 500 mM NaCl, which completely inhibited the endogenous RT reaction, resulted in the release of similar quantities of RT, p24, p17, and p7 into the soluble fraction for both vif mutant and wild-type virions. Treatment with buffers of different pH revealed that both wild-type and vif mutant virions were more stable at acidic than at basic pH (Fig. 3B). Wild-type and vif mutant virions showed no evidence of disruption at pH 7.4. However, at pH 9, 40 to 70% of RT, p24, p17, and p7 was released into the soluble fraction from vif mutant virions, whereas only 5 to 40% of p24 and p17 and no detectable RT and p7 were released from wild-type virions (Fig. 3B). To analyze virion core stability under conditions that mimic the cytoplasm, similar experiments were performed to analyze the release of core proteins into the soluble fraction from wild-type and vif mutant virions treated with S100 cytosol (Fig. 3B). One microgram of S100 cytosol was sufficient to induce partial release of viral proteins associated with the matrix and the core shell (p17 and p24) (Fig. 3B). At higher S100 cytosol concentrations, RT and p7 were solubilized in addition to p24 and p17. Treatment with S100 cytosol resulted in release of greater quantities of RT and p7 into the soluble fraction from vif mutant virions compared to wild-type virions (Fig. 3B). These results provide further support for a model in which Vif is important for the stability of virion cores.
Sucrose gradient analysis of virion core stability.
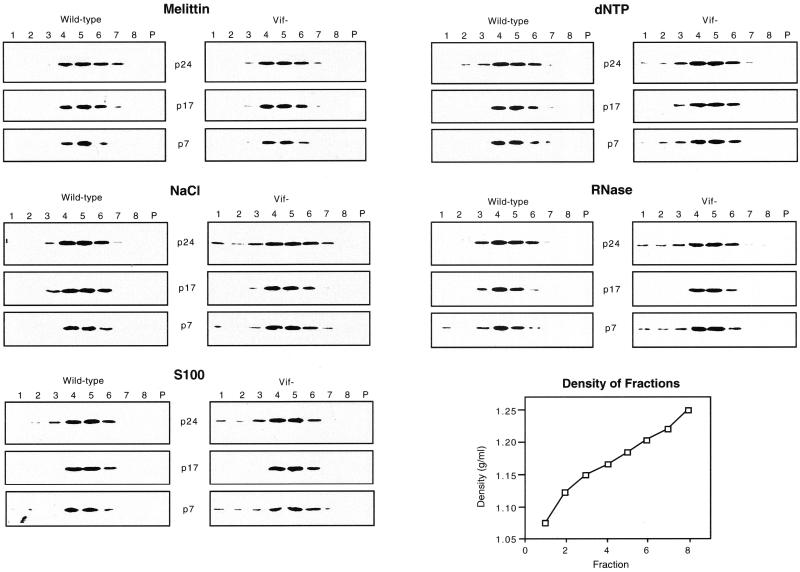
To further investigate the effect of Vif on virion core stability and to detect more subtle alterations in virion integrity, wild-type and vif mutant virions produced in HUT78 cells were analyzed on 20 to 60% sucrose gradients following treatment with melittin and other triggers (Fig. 4). After melittin treatment alone, the gradient distributions of viral proteins were similar for wild-type and vif mutant virions, as determined by analysis of gradient fractions by Western blotting. The peak of p24, p17, and p7 proteins was found in fractions 4 to 6, which correspond to a density of 1.16 to 1.20 g/ml (Fig. 4), similar to the density of intact HIV-1 virions. This finding provides further evidence that treatment of virions with 10 μg of melittin per ml does not grossly disrupt virion integrity. Treatment with 500 mM NaCl resulted in detection of p24 and p7 proteins in the top fractions (1 to 3), most likely corresponding to free or partially solubilized proteins. A larger fraction of p24 and p7 proteins was released from vif mutant virions than from wild-type virions. A small amount of p17, which may represent a minor fraction of partially solubilized viral membranes, was detected in fraction 3 after treatment of virions with NaCl. Treatment of virions with S100 cytosol and dNTPs had similar effects as treatment with NaCl (Fig. 4). Treatment of virions with 10 μg of RNase per ml resulted in release of a small fraction of p7 into the top fractions to similar extents in wild-type and vif mutant virions, whereas release of p24 into the top fractions was greater for the vif mutant (Fig. 4). In endogenous RT assays, 10 μg of RNase per ml resulted in 60 and 100% decreases in endogenous RT activity of wild-type virions and vif mutant virions, respectively (data not shown). To determine the effects of the various treatments on the association of RT with virion cores, the gradient distribution of RT was analyzed. Western blotting with the anti-RT antibody was not sufficiently sensitive to detect minor changes in the quantity of RT, most likely because of the low level of RT in virions. We therefore performed exogenous RT assays of the gradient fractions. After melittin treatment alone, the gradient distribution of exogenous RT activity paralleled that of other virion proteins, with peak levels detected in fractions 4 to 6 (Table 1). Following treatment of virions with NaCl, dNTPs, or S100 cytosol, the amount of exogenous RT activity detected in top fractions 1 to 3 was 1.4- to 3.4-fold higher for vif mutant virions than for wild-type virions under all conditions tested except treatment with RNase. Together with the Western blot analyses, these results demonstrate that a larger fraction of p24, p7, and RT is released from vif mutant virions than from wild-type virions after treatment with NaCl, dNTPs, or S100 cytosol.
FIG. 4.
Sucrose gradient analysis of wild-type and vif mutant virions produced in HUT78 cells. Virions (500,000 cpm of exogenous RT units per sample) were permeabilized with melittin (10 μg/ml), treated with 500 mM NaCl in 50 mM Tris-HCl (pH 7.4), 20 μg of S100 cytosol proteins, and 2 mM dNTPs together with 2 mM magnesium acetate, or 10 μg of RNase per ml, and then analyzed on 20 to 60% sucrose gradients. Fractions were collected from the top of the gradient. Lanes P represent the pellet. The gradient fractions were analyzed by Western blotting as described in the legend to Fig. 2. Results are representative of two or three independent experiments using different preparations of virions.
TABLE 1.
Exogenous RT units of sucrose gradient fractionsa
| Sample | Cpm of exogenous RT units/5-μl sucrose fraction
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Melittin
|
NaCl
|
dNTP
|
S100
|
RNase
|
||||||
| WT | Vif− | WT | Vif− | WT | Vif− | WT | Vif− | WT | Vif− | |
| Fractions | ||||||||||
| 1 | 124 | 226 | 535 | 753 | 175 | 589 | 374 | 789 | 241 | 173 |
| 2 | 148 | 374 | 357 | 683 | 192 | 644 | 591 | 892 | 229 | 356 |
| 3 | 369 | 916 | 832 | 1,337 | 540 | 1,680 | 745 | 1,733 | 1,406 | 1,036 |
| 4 | 2,987 | 4,039 | 4,735 | 3,976 | 3,234 | 3,908 | 4,278 | 4,466 | 3,113 | 4,520 |
| 5 | 5,710 | 5,130 | 3,922 | 4,442 | 5,027 | 4,997 | 5,650 | 4,264 | 5,249 | 4,520 |
| 6 | 2,854 | 1,970 | 1,464 | 1,885 | 2,543 | 2,102 | 2,446 | 1,832 | 1,462 | 1,359 |
| 7 | 809 | 470 | 321 | 561 | 801 | 934 | 896 | 649 | 339 | 539 |
| 8 | 351 | 214 | 111 | 107 | 192 | 300 | 344 | 337 | 168 | 426 |
| Pellet | 131 | 60 | 361 | 90 | 217 | 199 | 590 | 127 | 193 | 348 |
| Total | 13,483 | 13,725 | 13,399 | 13,834 | 12,921 | 15,353 | 15,914 | 15,526 | 12,400 | 13,277 |
Wild-type (WT) and vif mutant virions produced in HUT78 cells were analyzed on sucrose gradients. Fractions were collected from the top of the gradients and correspond to those shown in Fig. 4.
SupT1 cells are permissive and do not require Vif to produce fully infectious virions (24, 26). To determine whether the effect of Vif on virion core stability is observed when vif mutant virions are produced in nonpermissive but not permissive cells, wild-type and vif mutant virions produced in SupT1 cells were analyzed on sucrose gradients following treatment with melittin and other triggers (Fig. 5). The gradient distribution of p24, p17, and p7 proteins in wild-type virions was very similar to that observed for wild-type virions produced in HUT78 cells (Fig. 4 and 5). However, in contrast to virions produced in HUT78 cells, wild-type and vif mutant virions produced in SupT1 cells showed very similar gradient distributions of p24, p17, and p7 proteins after treatment with NaCl, S100 cytosol, dNTPs, or RNase (Fig. 5). Similar experiments were performed to analyze virions produced in four additional nonpermissive or permissive cell types. A larger fraction of p24 and RT proteins was released into the top fractions (1 to 3) from vif mutant virions than from wild-type virions produced in nonpermissive H9 and CEM cells following treatment with 500 mM NaCl, similar to results shown in Fig. 4 and Table 1 (data not shown). In contrast, the gradient distributions of p24 and RT were similar when virions were produced in permissive COS-1 and 293T cells. These results demonstrate that the effect of Vif on the stability of virion cores is observed when virions are produced in nonpermissive but not permissive cells.
FIG. 5.
Sucrose gradient analysis of wild-type and vif mutant virions produced in permissive SupT1 cells. Virions (500,000 cpm of exogenous RT units per sample) were treated and analyzed as described in the legend to Fig. 4.
Electron microscopy analysis of virion integrity and core structures.
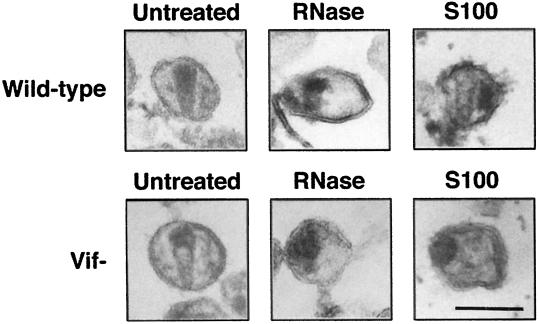
To examine the effect of RNase and S100 cytosol on virion integrity and core structures, wild-type and vif mutant virions produced in HUT78 cells were treated with melittin alone or in combination with RNase and S100 cytosol as described above and then analyzed by electron microscopy (Fig. 6). Untreated morphologically mature wild-type virions exhibited dense cone-shaped or round cores (Fig. 6). In contrast, untreated mature vif mutant virions exhibited nonhomogenous packing of the core (Fig. 6), consistent with previous studies (5, 8, 27). A fraction of untreated wild-type and vif mutant virions exhibited an immature morphology with a dense spherical shell along the inner surface of the envelope. Treatment with melittin alone reduced the frequency of cone-shaped cores by 40% for wild-type and vif mutant virions and increased the frequency of mature wild-type and vif mutant virions with round cores (data not shown). Occasional disruptions of the viral membrane were also detected.
FIG. 6.
Electron microscopy analysis of virions after treatment with RNase or S100 cytosol. Wild-type and vif mutant virions produced in HUT78 cells were resuspended in buffer (50 mM Tris-HCl [pH 7.4]) (Untreated) or treated with melittin (10 μg/ml) in combination with 10 μg of RNase per ml (RNase) or 20 μg of S100 cytosol (S100) for 1 h at 37°C prior to embedding and analysis by electron microscopy. Size bar indicates 100 nm.
Melittin permeabilization in combination with RNase or S100 cytosol had more marked effects on core morphology (Fig. 6). Following RNase treatment, most wild-type cores were round and less dense than untreated virion cores, but a few cone-shaped cores were still detected. No cone-shaped cores were observed after RNase treatment of vif mutant virions (Fig. 6 and data not shown). Instead, the cores either were immature or were round and less dense than untreated virion cores. Similarly, treatment of virions with S100 cytosol reduced the frequency of cone-shaped cores for wild-type virions but completely abolished detection of cone-shaped cores in vif mutant virions. After treatment with S100 cytosol, the majority of wild-type and vif mutant cores either were immature or were round and dense. In contrast to RNase treatment, the cores observed after S100 cytosol treatment were more tightly organized into dense round structures. Wild-type virions treated with S100 cytosol occasionally exhibited round or elongated dense structures outside a conical low-density core (Fig. 6). The viral membrane was occasionally disrupted, similar to virions treated with melittin alone or in combination with RNase (Fig. 6). These results demonstrate that treatment with RNase and S100 cytosol (Fig. 4) is accompanied by structural changes in the virion core and suggest that the structure of vif mutant cores is more sensitive to these treatments than that of wild-type cores.
Stability of purified virion cores.
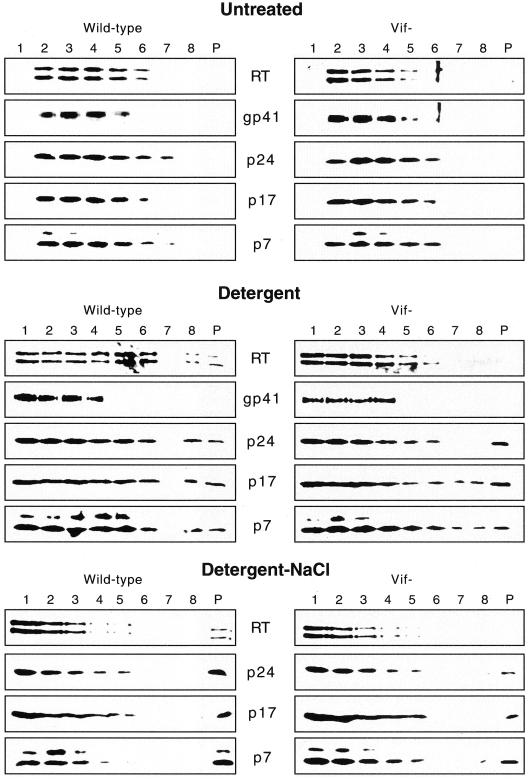
To analyze the effect of Vif on the stability of virion cores in the absence of the surrounding viral envelope, cores from wild-type and vif mutant virions produced in HUT78 cells were purified by a modification of the spin-thru method previously used for wild-type HIV-1 (2, 33) and HIV-2 (30). Concentrated wild-type and vif mutant virions were layered on top of a sucrose gradient containing a detergent layer of 0.5% Triton X-100 and centrifuged for 3 h at 14,000 × g. Eight fractions and the resuspended pellet were analyzed for viral proteins by Western blotting (Fig. 7). The distributions of viral proteins for wild-type and vif mutant virions were very similar after sedimentation through control sucrose gradients without a detergent layer. The peak of viral proteins was detected in fractions 2 to 5, which correspond to densities of 1.08 to 1.19 mg/ml. A 15-kDa band was detected by the anti-p7 antibody in both wild-type and vif mutant preparations, most likely corresponding to the uncleaved p7-p6 Gag protein.
FIG. 7.
Western blot analysis of purified cores. Wild-type and vif mutant virions produced in HUT78 cells were layered on top of 10 to 60% sucrose gradients containing a layer of 20% sucrose without any detergent (Untreated), 20% sucrose with 0.5% Triton X-100 (Detergent), or 20% sucrose with 0.5% Triton X-100 and 500 mM NaCl (Detergent-NaCl). The gradients were centrifuged for 3 h at 14,000 × g, and fractions were collected from the top. Lanes P represent the pellet. The distribution of viral proteins was analyzed by Western blotting of the fractions. Blots were used for multiple hybridizations and were stripped and reprobed as described in the legend to Fig. 2.
Sedimentation of virions through detergent-containing gradients resulted in core proteins in the pellet and bottom fractions (Fig. 7). In contrast to wild-type virions, only trace amounts of RT could be detected in the pellet and bottom fraction of vif mutant virions. For both wild-type and vif mutant virions, p24, p17, p7, RT, and gp41 could be detected in the top fraction, probably corresponding to proteins released from solubilized virions. Importantly, gp41 was detected only in fractions 1 to 4, indicating efficient solubilization of the viral membrane. The presence of 500 mM NaCl in the detergent-containing layer resulted in detection of the majority of the viral proteins in the top fractions (Fig. 7), most likely corresponding to completely disrupted and solubilized virions. However, a subset of core proteins could still be detected in the pellets. Interestingly, a larger fraction of the viral proteins could be found in the gradient pellet of wild-type virions compared to vif mutant virions. These results provide evidence that vif mutant cores are less stable than wild-type cores in the absence of the viral envelope or permeabilization with melittin.
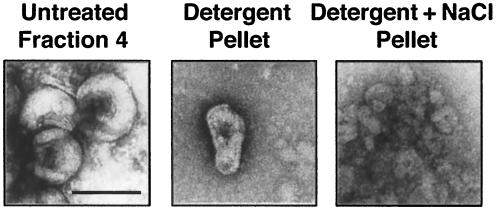
To structurally characterize the viral material in the sucrose gradient fractions, the samples were visualized by electron microscopy after negative staining (Fig. 8). As expected, fraction 4 from gradients without detergent contained large quantities of virions with intact envelopes that excluded the negative stain and therefore did not reveal internal structural details (Fig. 8). In contrast, fraction 4 from gradients with detergent contained large quantities of virions in which the envelope was partially disrupted and internal structures could be visualized (data not shown). For wild-type virions, the pellet and bottom fractions of detergent-containing gradients contained cone-shaped cores devoid of a surrounding lipid envelope and similar in size and structure to the cores of untreated intact virions (Fig. 6 and 8). In contrast to wild-type virions, no cone-shaped cores were observed in the same fractions of parallel gradients layered with vif mutant virions (data not shown). Instead, we observed unstructured protein aggregates, most likely corresponding to remnants of disintegrated virions (62) (data not shown). The pellet and bottom fractions of gradients containing both detergent and NaCl contained large quantities of unstructured protein aggregates, indicating that this treatment completely disrupted the structural integrity of the cores but did not completely dissociate large aggregates of core proteins from disintegrated virions (Fig. 7 and 8). These results provide additional evidence that the core of vif mutant virions is less stable than that of wild-type virions.
FIG. 8.
Electron microscopy analysis of purified wild-type cores. Fraction 4 from the sucrose gradient shown in Fig. 7 without detergent (Untreated Fraction 4) or the combined fraction 8 and pellet from gradients containing detergent (Detergent Pellet), or detergent and NaCl (Detergent+NaCl Pellet) were analyzed by negative staining. Only fractions from gradients layered with wild-type virions are shown. Size bar indicates 100 nm.
DISCUSSION
In this study, we demonstrate that Vif is important for the stability of HIV-1 virion cores. vif mutant cores were more sensitive to disruption by detergents, high salt, basic pH, S100 cytosol, dNTPs, and RNase than wild-type cores, as determined by endogenous RT assays, biochemical analyses, and electron microscopy. We also found that RT and p7 are released more readily from vif mutant virions than from wild-type virions after treatment with detergents, high NaCl and S100 cytosol, and other triggers, indicating that the internal nucleocapsid may be less stably packaged in the absence of Vif. Consistent with this model, previous studies have shown that vif mutant virions exhibit redistribution of electron-dense core material representing the nucleocapsid (5, 8, 27) and are defective in the ability to synthesize viral DNA in the endogenous RT reaction and in acutely infected cells (13, 26, 53, 57, 61). The membrane-associated p17 protein was more readily released from vif mutant virions than from wild-type virions in some experiments, but this finding was inconsistent and was not observed using other methods (Fig. 4 and 7), suggesting that this result may reflect cellular microvesicles that can cosediment with virions (15). These findings suggest that Vif increases the stability of virion cores. This may permit efficient viral DNA synthesis by preventing premature degradation or disassembly of RT complexes during early events after virus entry.
Our studies provide biochemical and structural evidence suggesting that a component of the virion core is likely to be a target for Vif function. One possibility is that Vif may affect the p55 Gag or p160 Gag-Pol precursor proteins, their interactions during virus assembly, or their processing during virus maturation. However, several studies have not demonstrated any difference in the virion composition of Gag or Pol proteins in wild-type or vif mutant virions (8, 22, 43, 48), raising the possibility that Vif function may modify one of these proteins indirectly or may influence interactions between Gag proteins. In vitro studies suggested that Vif may interact with the p55 Gag precursor, possibly by interacting with p7 (7, 28). However, studies thus far have not detected a direct interaction between Vif and Gag proteins in HIV-1-infected cells (50). Other studies have suggested that Vif may affect Gag or Gag-Pol processing (32, 49). However, effects of Vif on Gag or Gag-Pol processing are not consistently observed in vif mutant virions (8, 22, 43, 48), suggesting that this is unlikely to be its major mechanism of action. It is also possible that a component of the core other than a Gag or Pol protein (2, 33, 62) may be a target for Vif function. The Nef and Vpr proteins are unlikely candidates since these genes are defective in the HXB2 viruses used in this and other studies (24, 26, 27, 53). Cyclophilin A, a cellular protein that is associated with HIV-1 cores (23, 59), is also an unlikely candidate, since cores of mutant viruses that are deficient for its incorporation into virions are more stable than those of wild-type virus (25, 65). Moreover, its pattern of cell dependency is different from that of Vif (3, 65). Although Vif does not appear to affect the packaging of HIV-1 RNA into virions (61) or dimerization of the genomic RNA (26), a recent study suggested that Vif may bind to the genomic RNA (70). Further studies are required to identify the target(s) for Vif function in the virion core.
The molecular mechanism for the action of Vif in the virus-producing cell and how this is manifested during early events after virus entry are not known. During assembly, newly synthesized Gag molecules form cytoplasmic complexes prior to transport to the plasma membrane (36, 37). Vif in the cytoplasm might associate with these virus assembly intermediates (50, 51), either directly or via a cellular cofactor (40, 52), and thereby influence virus assembly. Consistent with this model, Vif and Gag have been shown to bind independently of each other to detergent-insoluble cytoplasmic complexes in the virus-producing cell (50). Recent studies suggest that the action of Vif may be mediated through a cellular factor in a species-specific manner (40, 52, 55). In view of this, it will be of interest to determine whether Vif neutralizes an endogenous cellular inhibitor (40, 52) that interferes with the production of stable virion cores. Reverse transcription in vivo takes place after virus entry within a partially disassembled core termed the RT complex (60, 68). The RT complex must allow access of cellular dNTPs for reverse transcription of the viral genome while retaining the association between RT, the viral genome, and other viral and cellular factors (60). The increased stability of virion cores produced in the presence of Vif may be important during steps of the uncoating process which result in release of the internal nucleocapsid and formation of stable RT complexes. Consistent with this model, a recent study showed that treatment of vif mutant virions with high dNTP concentrations in the absence of detergents increases virion infectivity (17), possibly by allowing initiation of reverse transcription in a cell-free environment (69).
Studies on mechanisms involved in HIV-1 virion uncoating and disassembly have been limited by a lack of in vitro assays. One reason these early events are difficult to study is the low fraction of infectious particles in HIV-1 virus preparations, estimated to be 10−4 to 10−7 (35). Furthermore, the rapid fusion between the viral and cellular membranes and the inherent instability of the HIV-1 core (62) provide further obstacles to detailed characterization of early postentry events. Several studies have been published on the purification and biochemical characterization of lentivirus cores (2, 30, 33, 38, 47, 62, 67). In the present study, we found that purified cores contain RT, p24, p17, and p7, but not gp41, consistent with previous studies (2, 33, 62). We further demonstrated that purified HIV-1 cores can be used to demonstrate effects of mutations and various triggers on core stability and disassembly. Interestingly, equine infectious anemia virus lacks a Vif protein (41) and has an unusually stable core (6, 47). However, HIV-1 and other lentivirus cores are unstable and easily disintegrate after removal of the viral membrane (2, 30, 33, 62). Therefore, in vitro assays which are less disruptive yet render virion cores accessible to various triggers are also of interest to facilitate studies on biochemical mechanisms that regulate core stability and disassembly. Early postentry events and viral DNA synthesis can occur in the absence of fusion mediated by the HIV-1 Env (18). The endogenous RT reaction allows for synthesis of viral cDNA without completely removing the viral membrane. In this study, we developed similar assays to study the integrity of virion cores under various conditions. We found that treatment with 10 μg of melittin per ml permeabilizes HIV-1 virions while grossly preserving the structural integrity of virion cores. Permeabilization with melittin was sufficient to allow molecules (dNTP and salt) and enzymes (RNase) to enter virions and for core proteins to be released from virions. These and other in vitro cell-free assays for virion core stability and disassembly may provide new insights into early postentry events and their regulation by viral and cellular factors. Similar approaches may also facilitate identification of the component(s) of the S100 cytosol fraction which induces disassembly of HIV-1 cores.
Most replication-defective gag mutants exhibit altered core morphology with round rather than cone-shaped cores (16, 21, 31, 46). Wild-type virion preparations also contain a large fraction of virions with round cores (27, 46). These round cores might represent incorrectly packaged and noninfectious virion cores, or they may be cone-shaped cores viewed in horizontal plane of section (27). The observation that electron-dense cone-shaped cores generally correlate with the ability to perform the early steps of the viral life cycle including the synthesis of full-length cDNA (46) suggests that virions with round cores may largely represent noninfectious particles. Consistent with this prediction, our finding that S100 cytosol and RNase treatment reduced the frequency of cone-shaped but not round cores suggests that the majority of round cores may not be competent to undergo the normal uncoating process. This possibility together with the low infectivity-to-particle ratio (35) may help to explain why our assays did not detect greater differences between wild-type and vif mutant virions. It has been suggested that maturation of the HIV-1 core facilitates the formation of a structure of intermediate stability (1, 63). The mature cone-shaped core is stable enough to prevent premature degradation of the viral reverse transcription machinery but less stable than the immature core to allow for uncoating and disassembly after viral entry. It is possible that during the postentry steps of the viral life cycle, the conical p24 core shell is released and a round or irregularly shaped RT complex is formed (Fig. 6) (17). Previous studies have shown that several p24 gag mutants with reduced infectivity exhibit increased or decreased core stability (9, 21). These observations together with our studies on vif mutant cores provide support for the hypothesis that a cone-shaped core of intermediate stability may be optimal for early events that lead to release of the internal nucleocapsid and the formation of stable RT complexes.
Our results support a model for the action of Vif in which Vif acts in nonpermissive cells to allow formation of a condensed mature viral core. The stable core formed in the presence of Vif may protect the viral genome and prevent premature degradation of the viral nucleoprotein complex during uncoating in a newly infected cell. Understanding the function of Vif may provide new insights into critical steps involved in virus assembly, uncoating, reverse transcription, and other early events required for the establishment of infection and thereby may lead to the identification of potential therapeutic targets.
ACKNOWLEDGMENTS
We thank L. Arthur for providing the p7 monoclonal antibody; the NIH AIDS Research and Reference Reagent Program for the 2F5 gp41 monoclonal antibody (donated by H. Katinger) and for antibodies raised against other HIV-1 proteins that were used for preliminary experiments; and A. Engelman, H. Göttlinger, A. Mehle, C. Zhang, and T. Mirzabekov for helpful discussions and critical reading of the manuscript.
This work was supported by NIH grant AI36186. Core facilities were supported by a Center for AIDS Research grant and a Center for Cancer Research grant. We also acknowledge support from the G. Harold and Leila Mathers Foundation. Å.Ö. was supported in part by the Swedish Medical Research Foundation and the Swedish Institute. D.G. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Accola M, Höglund S, Göttlinger H. A putative α-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J Virol. 1998;72:2072–2078. doi: 10.1128/jvi.72.3.2072-2078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accola M, Öhagen Å, Göttlinger H. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6gag. J Virol. 2000;74:6198–6202. doi: 10.1128/jvi.74.13.6198-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerson B, Rey O, Canon J, Krogstad P. Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J Virol. 1998;72:303–308. doi: 10.1128/jvi.72.1.303-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone L R, Skalka A. Two species of full-length cDNA are synthesized in high yield by melittin-treated avian retrovirus particles. Proc Natl Acad Sci USA. 1980;77:847–851. doi: 10.1073/pnas.77.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman A M, Quillent C, Charneau P, Gauguet C, Clavel F. Human immunodeficiency type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borroto-Esoda K, Boone L. Equine infectious anemia virus and human immunodeficiency virus DNA synthesis in vitro: characterization of the endogenous reverse transcriptase reaction. J Virol. 1991;65:1952–1959. doi: 10.1128/jvi.65.4.1952-1959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouyac M, Courcoul M, Bertoia G, Baudat Y, Gabuzda D, Blanc D, Chazal N, Boulanger P, Sire J, Vigne R, Spire B. Human immunodeficiency virus type 1 Vif protein binds to Pr55Gag precursor. J Virol. 1997;71:9358–9365. doi: 10.1128/jvi.71.12.9358-9365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouyac M, Rey F, Nascimbeni M, Courcoul M, Sire J, Blanc D, Clavel F, Vigne R, Spire B. Phenotypically Vif− human immunodeficiency virus type 1 is produced by chronically infected restrictive cells. J Virol. 1997;71:2473–2477. doi: 10.1128/jvi.71.3.2473-2477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braaten D, Aberham C, Franke E K, Yin L, Phares W, Luban J. Cyclosporin A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J Virol. 1996;70:5170–5176. doi: 10.1128/jvi.70.8.5170-5176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 11.Camaur D, Trono D. Characterization of human immunodeficiency virus type 1 Vif particle incorporation. J Virol. 1996;70:6106–6111. doi: 10.1128/jvi.70.9.6106-6111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury I H, Chao W, Potash M J, Sova P, Gendelman H E, Volsky D J. Vif-negative human immunodeficiency virus type 1 persistently replicates in primary macrophages producing attenuated progeny virus. J Virol. 1996;70:5336–5345. doi: 10.1128/jvi.70.8.5336-5345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courcoul M, Patience C, Rey F, Blanc D, Harmache A, Sire J, Vigne R, Spire B. Peripheral blood mononuclear cells produce normal amounts of defective Vif− human immunodeficiency virus type 1 particles which are restricted for the preretrotranscription steps. J Virol. 1995;69:2068–2074. doi: 10.1128/jvi.69.4.2068-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debyser Z, Vandamme A-M, Pauwels R, Baba M, Desmyter J, De Clerq E. Kinetics of inhibition of endogenous human immunodeficiency virus type 1 reverse transcription by 2′,3′-dideoxynucleoside 5′-triphosphate, tetrahydroimidazo-[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thione, and 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio) thymine derivates. J Biol Chem. 1992;267:11769–11776. [PubMed] [Google Scholar]
- 15.Dettenhofer M, Yu X-F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorfman T, Bukovsky A, Öhagen Å, Höglund S, Göttlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dornadula G, Yang S, Pomerantz R J, Zhang H. Partial rescue of the Vif-negative phenotype of mutant human immunodeficiency virus type 1 strains from nonpermissive cells by intravirion reverse transcription. J Virol. 2000;74:2594–2602. doi: 10.1128/jvi.74.6.2594-2602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres M J, Jaffer S, Haggarty B, Turner J D, Doranz B J, O'Brien P J, Kolson D L, Hoxie J A. Targeting of HIV- and SIV-infected cells by CD4-chemokine receptor pseudotypes. Science. 1997;278:1462–1464. doi: 10.1126/science.278.5342.1462. [DOI] [PubMed] [Google Scholar]
- 19.Fan L, Peden K. Cell-free transmission of Vif mutants of HIV-1. Virology. 1992;190:19–29. doi: 10.1016/0042-6822(92)91188-z. [DOI] [PubMed] [Google Scholar]
- 20.Fisher A G, Ensoli B, Ivanoff L, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 21.Fitzon T, Leschonsky B, Bieler K, Paulus C, Schröder J, Wolf H, Wagner R. Proline residues in the HIV-1 NH2-terminal capsid domain: structure determinants for proper core assembly and subsequent steps of early replication. Virology. 2000;268:294–307. doi: 10.1006/viro.1999.0178. [DOI] [PubMed] [Google Scholar]
- 22.Fouchier R A M, Simon J H M, Jaffe A B, Malim M H. Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins. J Virol. 1996;70:8263–8269. doi: 10.1128/jvi.70.12.8263-8269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franke E K, Yuan H E H, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 24.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble T R, Vajdos F, Yoo S, Worthylake D K, Houseweart M, Sundquist W I, Hill C P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 26.Goncalves J, Korin Y, Zack J, Gabuzda D. Role of Vif in human immunodeficiency type 1 reverse transcription. J Virol. 1996;70:8701–8709. doi: 10.1128/jvi.70.12.8701-8709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höglund S, Öhagen Å, Lawrence K, Gabuzda D. Role of Vif during packing of the core of HIV-1. Virology. 1994;201:349–355. doi: 10.1006/viro.1994.1300. [DOI] [PubMed] [Google Scholar]
- 28.Huvent I, Hong S S, Fournier C, Gay B, Tournier J, Carriere C, Courcoul M, Vigne R, Spire B, Boulanger P. Interaction and co-encapsidation of human immunodeficiency virus type 1 Gag and Vif recombinant proteins. J Gen Virol. 1998;79:1069–1081. doi: 10.1099/0022-1317-79-5-1069. [DOI] [PubMed] [Google Scholar]
- 29.Karczewski M K, Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein. J Virol. 1996;70:494–507. doi: 10.1128/jvi.70.1.494-507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kewalramani V N, Emerman M. Vpx association with mature core structures of HIV-2. Virology. 1996;218:159–168. doi: 10.1006/viro.1996.0176. [DOI] [PubMed] [Google Scholar]
- 31.Kiernan R E, Ono A, Englund G, Freed E. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotler M, Simm M, Zhao Y S, Sova P, Chao W, Ohnona S-F, Roller R, Krachmarov C, Potash M J, Volsky D J. Human immunodeficiency virus type 1 (HIV-1) protein Vif inhibits the activity of HIV-1 protease in bacteria and in vitro. J Virol. 1997;71:5774–5781. doi: 10.1128/jvi.71.8.5774-5781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladokhin A S, Selsted M E, White S H. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. J Biophys. 1997;72:1762–1766. doi: 10.1016/S0006-3495(97)78822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H G, Nara P L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y-M, Yu X-F. Identification and characterization of virus assembly intermediate complexes in HIV-1-infected CD4+ T cells. Virology. 1998;243:78–93. doi: 10.1006/viro.1998.9064. [DOI] [PubMed] [Google Scholar]
- 37.Lingappa J R, Hill R L, Wong M L, Hegde R S. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell-free system. J Cell Biol. 1997;136:567–581. doi: 10.1083/jcb.136.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liska V, Spehner D, Mehtali M, Schmitt D, Kirn A, Aubertin A M. Localization of viral protein X in simian immunodeficiency virus macaque strain and analysis of its packaging requirements. J Gen Virol. 1994;75:2955–2962. doi: 10.1099/0022-1317-75-11-2955. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madani N, Kabat D. An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein. J Virol. 1998;72:10251–10255. doi: 10.1128/jvi.72.12.10251-10255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers G, Josephs S F, Rabson A B, Smith T I, Wong-Staal F, editors. Human retroviruses and AIDS. Los Alamos, N.Mex: Los Alamos National Laboratory; 1988. [Google Scholar]
- 42.Nascimbeni M, Bouyac M, Rey F, Spire B, Clavel F. The replicative impairment of Vif- mutants of human immunodeficiency virus type 1 correlates with an overall defect in viral DNA synthesis. J Gen Virol. 1998;79:1945–1950. doi: 10.1099/0022-1317-79-8-1945. [DOI] [PubMed] [Google Scholar]
- 43.Ochsenbauer C, Wilk T, Bosch V. Analysis of vif-defective human immunodeficiency virus type 1 (HIV-1) virions synthesized in “non-permissive” T lymphoid cells stably infected with selectable HIV-1. J Gen Virol. 1997;78:627–635. doi: 10.1099/0022-1317-78-3-627. [DOI] [PubMed] [Google Scholar]
- 44.Purtscher M, Trkola A, Grassauer A, Schilz P M, Klima A, Dopper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer C, Berger R, Barrett N, Jungbauer A, Katinger H. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 46.Reicin A S, Ohagen A, Yin L, Hoglund S, Goff S P. The role of Gag in human immunodeficiency virus type 1 virion morphogenesis and early steps of the viral life cycle. J Virol. 1996;70:8645–8652. doi: 10.1128/jvi.70.12.8645-8652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts M M, Oroszlan S. The preparation and biochemical characterization of intact capsids of equine infectious anemia virus. Biochem Biophys Res Commun. 1989;160:486–494. doi: 10.1016/0006-291x(89)92459-5. [DOI] [PubMed] [Google Scholar]
- 48.Sakai H, Shibata R, Sakuragi J-I, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simm M, Shahabuddin M, Chao W, Allan J S, Volsky D J. Aberrant Gag protein composition of human immunodeficiency virus type 1 vif mutants produced in lymphocytes. J Virol. 1995;69:4582–4586. doi: 10.1128/jvi.69.7.4582-4586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simon J H M, Carpenter E A, Fouchier R A M, Malim M H. Vif and the p55Gag polyprotein of human immunodeficiency virus type 1 are present in colocalizing membrane-free cytoplasmic complexes. J Virol. 1999;73:2667–2674. doi: 10.1128/jvi.73.4.2667-2674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon J H M, Fouchier R A M, Southerling T E, Guerra C B, Grant C K, Malim M H. The Vif and Gag proteins of human immunodeficiency virus type 1 colocalize in infected human T cells. J Virol. 1997;71:5259–5267. doi: 10.1128/jvi.71.7.5259-5267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon J H M, Gaddis N C, Fouchier R A M, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 53.Simon J H M, Malim M H. The human immunodeficiency virus type 1 Vif protein modulates the postpenetration stability of viral nucleoprotein complexes. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon J H M, Miller D L, Fouchier R A M, Malim M H. Virion incorporation of human immunodeficiency virus type 1 Vif is determined by intracellular expression level and may not be necessary for function. Virology. 1998;248:182–187. doi: 10.1006/viro.1998.9296. [DOI] [PubMed] [Google Scholar]
- 55.Simon J H, Miller D L, Fouchier R A M, Soares M A, Peden K W C, Malim M H. The regulation of primate immunodeficiency virus infectivity by Vif is cell species restricted: a role for Vif in determining virus host range and cross-species transmission. EMBO J. 1998;17:1259–1267. doi: 10.1093/emboj/17.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sodroski J, Goh W C, Rosen C, Tartar A, Portetelle D, Burny A, Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986;231:1549–1551. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- 57.Sova P, Volsky D J. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with Vif-negative human immunodeficiency virus type 1. J Virol. 1993;67:6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strebel K, Daugherty D, Clouse K, Cohen D, Folks T, Martin M A. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature. 1987;328:728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- 59.Thali M, Bukovsky A A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 60.Turner B G, Summers M F. Structural biology of HIV. J Mol Biol. 1999;285:1–32. doi: 10.1006/jmbi.1998.2354. [DOI] [PubMed] [Google Scholar]
- 61.von Schwedler U, Song J, Aiken C, Trono D. Vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welker R, Hohenberg H, Tessmer U, Huckhagel C, Krausslich H-G. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74:1168–1177. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiegers K, Rutter G, Kottler H, Tessmer U, Hohenberg H, Krausslich H-G. Sequential steps in human immunodeficiency virus particle maturation revealed by alterations of individual Gag polyprotein cleavage sites. J Virol. 1998;72:2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willey R L, Martin M A, Peden K W C. Increase in soluble CD4 binding to and CD4-induced dissociation of gp120 from virions correlate with infectivity of human immunodeficiency virus type 1. J Virol. 1994;68:1029–1039. doi: 10.1128/jvi.68.2.1029-1039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin L, Braaten D, Luban J. Human immunodeficiency virus type 1 replication is modulated by host cyclophilin A expression levels. J Virol. 1998;72:6430–6436. doi: 10.1128/jvi.72.8.6430-6436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yong W H, Wyman S, Levy J A. Optimal conditions for synthesizing complementary DNA in the HIV-1 endogenous reverse transcriptase reaction. AIDS. 1990;4:199–206. doi: 10.1097/00002030-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Yu X, Matsuda Z, Yu Q C, Lee T H, Essex M. Vpx of simian immunodeficiency virus is located primarily outside the virus core in mature virions. J Virol. 1993;67:4386–4390. doi: 10.1128/jvi.67.7.4386-4390.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H, Dornadula G, Pomerantz R J. Endogenous reverse transcription of human immunodeficiency type 1 in physiological microenvironments: an important stage for viral infection of nondividing cells. J Virol. 1996;70:2809–2824. doi: 10.1128/jvi.70.5.2809-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Pomerantz R J, Dornadula G, Sun Y. Human immunodeficiency virus type 1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process. J Virol. 2000;74:8252–8261. doi: 10.1128/jvi.74.18.8252-8261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]