ABSTRACT
We retrospectively studied 18 pregnant women from 600 cases of meningioma treated at this Institution between 1986 and 2001. The variables evaluated included clinical presentation; radiological findings; timing and extent of surgical resection; and an overview of gestational, clinical, and surgical outcomes. Visual impairment was the chief complaint followed by headache and seizures. The tumors involved the tuberculum sella (8), sphenoid wing (4), convexity (2), parasellar (2), cerebellopontine angle (CPA) (1), and anterior falx (1). Gross total resection was achieved in 14, and subtotal resection was achieved in two patients. There were no related fetal or maternal deaths. Of seven patients with advanced or progressive visual impairment who underwent resection during pregnancy, three improved noticeably, two worsened, and two were unchanged. Five other patients with visual disturbance achieved full–term spontaneous delivery with improved vision in two and improved vision after post–confinement surgery in one. Vision remained unchanged in two. Intracranial meningioma during pregnancy challenges the skill of obstetricians and neurosurgeons to secure delivery of the baby and resection of the tumor. Advances in fetal and maternal monitoring, neuroanesthesia, and microsurgical techniques allow safe neurosurgical management of these patients, and pregnancy usually continues successfully to term. Surgical intervention has no major effect on minimal residual vision or advanced optic nerve atrophy.
Keywords: Brain tumor, meningioma, pregnancy
The incidence of meningioma in pregnant women is comparable with that in non–pregnant women of the same age group. However, symptoms may flare during pregnancy and this has been attributed to water retention, engorgement of vessels, and the presence of sex hormone receptors on tumor cells leading to explosive growth of the tumor. The clinical presentation of headache, vomiting, or seizures could be misdiagnosed as hyperemesis gravidarium during early pregnancy or as eclampsia during late pregnancy. An abnormal fundoscopic examination, visual impairment, focal seizures, and lateralizing neurological deficits support the diagnosis of an intracranial mass lesion and should prompt further investigation with magnetic resonance imaging (MRI) to confirm the diagnosis. Advances in noninvasive fetal monitoring using ultrasound, combined with close and regular clinical and neurological evaluation of the mother, allow most such pregnancies to continue safely to term and spontaneous delivery. Urgent neurosurgical intervention is reserved for the management of malignancies, active hydrocephalus, and benign brain tumors associated with signs of impending herniation or progressive neurological deficit.
MATERIALS AND METHODS
A retrospective database study was conducted on 18 pregnant women from a series of 600 cases of meningiomas seen at King Faisal Specialist Hospital and Research Centre between 1986 and 2001. There were 19,000 confinements during this period. All 18 patients were multiparous, and most were diagnosed in the 2nd or 3rd trimester. Clinical presentations included visual impairment as the chief complaint in 12 patients (66.6 %), headache in 8 patients (44.4 %), seizures in 2 (11.11 %), double vision in 1, and exophthalmia in 1. In the earlier cases, diagnosis was supported by computed tomography (CT) scan (with shielding of the fetus). Later MRI became the study methodology of choice because of its high index of diagnosis and no accompanying radiation hazard (Figs. 1, 2, 3, 4).
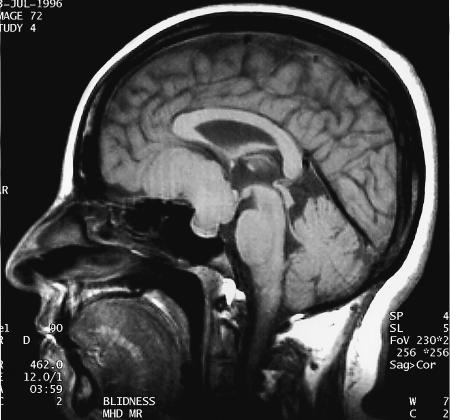
Figure 1.
Sagittal T1–weighted MRI demonstrates isointense skull base mass occupying the suprasellar regions with subfrontal and intrasellar extensions.
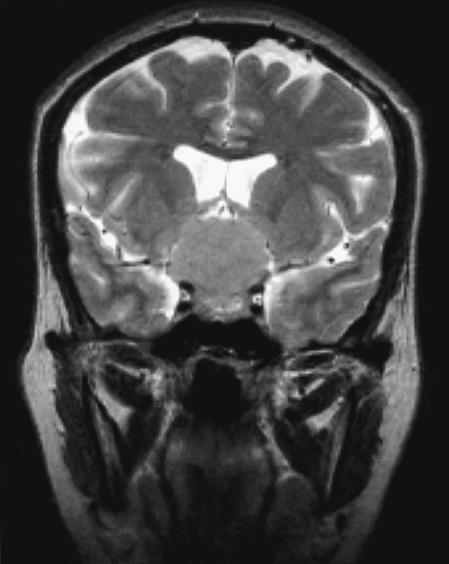
Figure 2.
Coronal T2–weighted MRI shows the same mass in the suprasellar regions displacing the floor of the third ventricle upward.
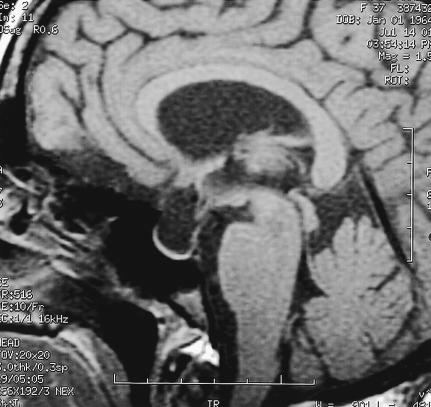
Figure 3.
Postoperative sagittal T1–weighted MRI confirms complete removal of the tumor, which has been replaced with CSF–like signal intensity.
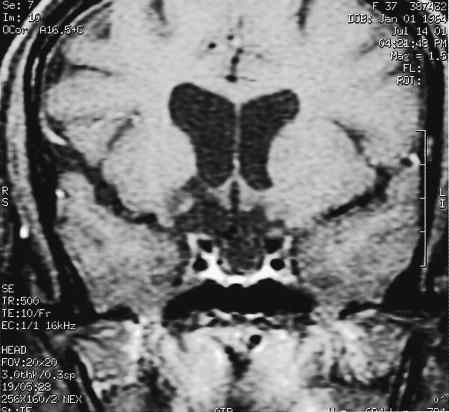
Figure 4.
Postoperative coronal T1–weighted MRI with contrast reveals no residual tumor and a patent suprasellar cistern.
The anatomical distribution of the meningiomas was as follows: tuberculum sella/suprasellar (8), sphenoid wing (4), parasellar (2), convexity (2), cerebellopontine angle (CPA) (1) and anterior falx cerebri (1). Most patients underwent noninvasive fetal–monitoring (Table 1) using ultrasound as well as regular clinical and neurological follow–ups, including examination of fundi, visual acuity, and visual fields.
Table 1.
Fetal Biophysical Profile (Maximum Score =10)
| Variable | Normal =2 | Abnormal =0 |
| AFV | 1 pocket > 3 cm | reduced or absent |
| FBM | >1 FBM / 30 minute, hiccup | continuous FBM, absent FBM |
| FM | > 4 FM / 30 minute | < 3 FM / 30 minute |
| FT | active extension, flexion, opening–closing hands | low velocity movement, flaccid posture |
| CTG | >2 accelerations + palpated fetal movement / 20 minute | absent acceleration or CTG deceleration |
AFV, amniotic fluid volume; FBM, fetal breathing movements; FM, fetal body/limb movements; FT, fetal tone; CTG, cardiotocography (fetal heart rate).The Fetal Biophysical Profile Scoring System is a composite of variables and should be used as part of comprehensive fetal assessment in association with other available information. It has the lowest false–negative rate of any contemporary ante–partum testing scheme.22
Seven patients with progressive impairment of their visual acuity or fields underwent neurosurgical intervention during pregnancy. Nine were managed surgically postconfinement. One patient refused surgery and one was lost to follow–up. A unilateral pterional or fronto–orbitozygomatic approach was used to expose the suprasellar, sphenoid wing, and parasellar meningiomas. After extensive drilling of the lateral and medial sphenoid wing and interruption of basal blood supply to the tumor, a central or segmental resection was performed using ultrasonic aspiration and microsurgical techniques. Finally, sharp dissection of the capsule allowed gross total resection of the tumor.
RESULTS
Gross resection was total in 14, subtotal in one, and partial in one. One patient refused surgery and one was lost to follow–up. All surgical samples were verified as typical meningioma by histopathological examination. There were no related maternal or fetal deaths. Any seizure activity was controlled with monotherapy. The visual outcome in seven patients with advanced or progressive visual impairment that prompted surgical resection during pregnancy showed noticeable improvement in three patients (42.6 %), worsened in two (28.57 %), and no change in two (28.57 %). Postoperative impairment was attributed to a potential ischemic event induced by surgical manipulation. These patients had had severe optic atrophy, were already blind in one eye, and retained only minimal functioning vision of hand motion, or their acuity was less than 20/100. Of five patients with some degree of visual impairment who reached full–term spontaneous delivery, recovery was spontaneous in two patients (40 %), one patient (20 %) improved later after postconfinement surgical resection of the tumor, and two patients (40 %) remained unchanged.
The patient with the CPA meningioma and double vision improved spontaneously after delivery and eventually underwent surgery without incident. The patient with exophthalmia and ptosis took nine months to recover after postpartum surgery.
DISCUSSION
In 1898 Bernard1 described the first case of a brain tumor during pregnancy. The incidence is rare and does not exceed that of nonpregnant women in the same age group.2, 3, 4 However, symptoms of a pre–existing tumor such as a meningioma can flare during gestation and improve spontaneously postpartum.5 The clinical evidence of the effect of pregnancy on intracranial meningioma was best documented by Hagedoorn in 1937.6 He used sequential visual acuity and visual field studies in several pregnancies.6 Since then a few case reports and mini–series have related the aggravation of signs and symptoms to one of the following mechanisms: (a) fluid retention, vascular engorgement, and increased edema; (b) the presence of hormone receptors on tumor cells, leading to dramatic growth of the meningioma during pregnancy.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
The clinical picture of headache, vomiting, or seizure can be confused with hyperemesis gravidarium early in pregnancy or with eclampsia late in pregnancy. However, the presence of an abnormal fundoscopic examination, visual impairment, focal seizures, and lateralizing neurological deficits should alert physicians to the possibility of an intracranial lesion and prompt further investigations with MRI to establish the diagnosis. The cautious use of CT with shielding of the uterus has been superseded by MRI because of its high–sensitivity diagnostic index and no radiation effect.
The management strategy for brain tumors during pregnancy should be tailored to the individual case. Advances in noninvasive fetal monitoring, such as fetal biophysical profit scoring system (Tables 1 and 2) and umbilical artery Doppler velocimetry, and in neuroanesthesia and microsurgical techniques permit safe neurosurgical management of brain tumors during pregnancy. Urgent intervention should be reserved for patients with a (a) malignancy, (b) active hydrocephalus requiring shunting, or (c) a benign brain tumor such as a meningioma associated with signs of impending herniation, progressive neurological deficit, or both. In our patients, visual impairment was the primary indication for urgent intervention.
Table 2.
Suggested Clinical Intervention in Response to Biophysical Profile Scores
| BPP Score | Significance | Recommendation |
| 10/10 | no evidence of FA | no active intervention on fetal basis, serial testing |
| 8/10 (AFV normal) | ||
| 8/10 (AFV oligo) | chronic fetal compromise likely | exclude asymptomatic rupture of membrane, deliver at viable gestation > 26 weeks |
| 6/10 (AFV normal) | equivocal test FA not excluded | repeat test immediately, if score remains 6/10, deliver mature fetus; if fetus immature repeat test in 24 hour and deliver if score 6/10 |
| 4/10 | acute FA likely | deliver + continuous monitoring |
| 2/10 | acute FA most likely | deliver by Cæsarean section |
| 0/10 | severe/acute FA | deliver by Cæsarean section |
BPP, biophysical profile; AFV, amniotic fluid volume; FA, fetal asphyxia.The table shows the importance of the BPP scoring system in fetal management and the significance of oligohydramnios.
When surgery is indicated, attention should be paid to intraoperative blood loss, hypotension, hypovolemia, and hypoxia to avoid their potentially hazardous effects on fetal perfusion. Corticosteroids may be very helpful in treating severe edema perioperatively or peripartum. The usual regimen is a divided dose of 2–4 mg dexamethasone every 6 hours, tapered slowly over a few days. Administration of mannitol should be cautious and reserved only for acute emergency because it crosses the placenta and affects the fetus. Seizures should be controlled, preferably with monotherapy. Physicians should realize that the risk or complications of epilepsy are more serious than the side effects of anticonvulsants.18 Patients with seizures should be maintained on folic acid from early pregnancy and should receive supplemental vitamin–K1 three weeks before and during confinement to minimize the risk of drug–induced neural tube defects or blood dyscrasias.19, 20
In contemporary practice, it is feasible in most cases of meningioma and other benign, slowly progressive tumors to continue to full–term delivery without endangering the mother or fetus.21 It is anticipated that neurosurgical management and outcome will eventually equate with that of nonpregnant women with a meningioma. Caesarean section may be considered in cases of prima gravida with protracted second–stage labor associated with clinical and radiological features of refractory, increased intracranial pressure or fetal distress.
Although this study has the inherent deficits of a retrospective review, referral bias, and lack of well–defined selection criteria, it demonstrates that urgent surgical intervention has no positive impact on visual outcome in patients who suffer from advanced optic nerve atrophy and severe visual impairment.
CONCLUSION
Advances in neuroanesthesia and the neurosurgical armamentarium allow safe resection of brain tumors during pregnancy. Nevertheless, in contemporary practice it is feasible in most patients with meningiomas and other benign brain tumors for the pregnancy to continue to term and delivery without endangering the mother of the fetus. Guidelines for urgent intervention are reserved for the management of malignancy, acute hydrocephalus, and increased intracranial pressure with signs of impending herniation and/or progressive neurological deficit. This approach will not profoundly affect visual outcome in patients with advanced optic nerve atrophy and functional visual loss.
REFERENCES
- Bernard MH. Sarcome cerebral à evolution rapide au cours de la grossesse et pendant les suites des couches. Bull Soc d'Obst de Paris. 1898;1:296–298. [Google Scholar]
- Carmel PW. Neurologic surgery in pregnancy. Philadelphia, PA: WB Saunders. 1974:203–224. In: Barber HR, Grabner EA, eds. Surgical Disease in Pregnancy. [Google Scholar]
- Isla A, Alvarez A, Gonzalez A, Garcia–Grande A, Perez–Alvarez M, Garcia–Blazquez M. Brain tumor and pregnancy. Obstet Gynecol. 1997;89:19–23. doi: 10.1016/s0029-7844(96)00381-x. [DOI] [PubMed] [Google Scholar]
- Rand CW, Andler M. Tumors of the brain complicating pregnancy. Arch Neurol Psychiatry. 1950;63:1–41. doi: 10.1001/archneurpsyc.1950.02310190007001. [DOI] [PubMed] [Google Scholar]
- Cushing H, Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas. 1938 [Google Scholar]
- Hagedoorn A. The chiasmal syndrome and retrobulbar neuritis in pregnancy. Am J Ophthalmol. 1937;20:690–699. [Google Scholar]
- Bickerstaff ER, Small MJ, Guest IA. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89–91. doi: 10.1136/jnnp.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DW, Bashirelahi N, Solomon LWE, Dalton T, Saloman M, Tucker TB. Estrogen and progesterone receptors in meningiomas in relation to clinical and pathologic features. J Neurosurg. 1984;60:985–993. doi: 10.3171/jns.1984.60.5.0985. [DOI] [PubMed] [Google Scholar]
- Donnell MS, Meyer GA, Donegan WL. Estrogen–receptor protein in intracranial meningiomas. J Neurosurg. 1979;50:499–502. doi: 10.3171/jns.1979.50.4.0499. [DOI] [PubMed] [Google Scholar]
- Glick RP, Molteni A, Fors EM. Hormone binding in brain tumors. Neurosurgery. 1983;13:513–519. doi: 10.1227/00006123-198311000-00005. [DOI] [PubMed] [Google Scholar]
- Kanaan I. Hormone receptors in meningioma. Symposium on Skull Base Meningioma, Biology and Therapy; Bamberg, Germany. 1992 [Google Scholar]
- King AB. Neurological conditions occurring as complications of pregnancy. II. Arch Neurol Psychiatry. 1950;63:611–614. [Google Scholar]
- Markwalder TM, Zava DT, Goldhirsch A, Markwalder RV. Estrogen and progesterone receptors in meningiomas in relation to clinical and pathological features. Surg Neurol. 1983;20:42–47. doi: 10.1016/0090-3019(83)90104-0. [DOI] [PubMed] [Google Scholar]
- Weyand RD, MacCarty CS, Wilson RB. The effect of pregnancy on intracranial meningiomas occurring about the optic chiasm. Surg Clin N Am. 1951;31:1225–1233. doi: 10.1016/s0039-6109(16)33403-x. [DOI] [PubMed] [Google Scholar]
- Schnegg JF, Gomez F, LeMarchand–Beraud T, de Tribolet N. Presence of sex steroid hormone receptors in meningioma tissue. Surg Neurol. 1981;15:415–418. doi: 10.1016/s0090-3019(81)80024-9. [DOI] [PubMed] [Google Scholar]
- Schrell UMH, Adams EF, Fahlbusch R, et al. Hormonal dependency of cerebral meningiomas. Part I. Female sex steroid receptors and their significance as specific markers for adjuvant medical therapy. J Neurosurg. 1990;73:743–749. doi: 10.3171/jns.1990.73.5.0743. [DOI] [PubMed] [Google Scholar]
- Tilzer LL, Plapp FV, Evans JP, Stone D, Alward K. Steroid receptor proteins in human meningiomas. Cancer. 1982;49:633–636. doi: 10.1002/1097-0142(19820215)49:4<633::aid-cncr2820490404>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tomson T, Gram L, Sillanpää M, Johannessen S. Recommendations for the management and care of pregnant women with epilepsy. Petersfield, UK: Wrighton Biomedical Publishing. 1997 In: Tomson T., Gram M, Sillanpää M, Johannessen S, eds. Epilepsy and Pregnancy. [Google Scholar]
- Douglas H. Haemorrhage in the new–born. Lancet. 1966;1:816–817. [Google Scholar]
- Mountain KR, Hirsch J, Gallus AS. Neonatal coagulation defect due to anticonvulsant drug treatment in pregnancy. Lancet. 1970;1:265–268. doi: 10.1016/s0140-6736(70)90636-7. [DOI] [PubMed] [Google Scholar]
- Keating RF. Brain tumors. Philadelphia, PA: Lippincott Williams & Wilkins. 2000:547–533. In: Cohen WR, ed. Cherry & Merkatz's Complications of Pregnancy 5th Edition. [Google Scholar]
- James DK, Steer PG, Weiner CP, Gonik B. Assessing fetal health. London, Edinburgh, New York, Philadelphia, Sydney, Toronto: WB Saunders. 2000 In: James DK, Steer PG, Weiner CP, Gonik B, eds. High Risk Pregnancy. [Google Scholar]