ABSTRACT
Primary intraosseous cavernous hemangiomas (PICHs) of the skull base are extremely rare tumors. These lesions are most common in the frontal and parietal bones of the calvarium. The authors describe a 40–year–old female who presented with progressive headaches. Serial imaging revealed a contrast–enhancing intraosseous lesion of the lateral body of the sphenoid bone and the greater wing associated with encroachment of the inferior cavernous sinus and mild posterior displacement of the cavernous carotid artery. Follow–up imaging 9 years later revealed slow growth of the lesion. The patient underwent complete excision of the PICH through an extradural frontopolar approach. Pathological examination revealed an intraosseous cavernous hemangioma. PICHs of the skull base can mimic other more common skull base lesions and thus can be difficult to diagnose preoperatively. Diagnosis is usually made at surgery. The authors review the literature regarding the clinical presentation, radiological characteristics, pathological features, and surgical management of PICHs.
Keywords: Cavernous hemangioma, skull base, intraosseous tumor
Primary intraosseous cavernous hemangiomas (PICHs) of the cranium are benign tumors that account for ∼0.2 % of all bone tumors and 10 % of benign skull tumors.1 They are most frequently found in the calvarium, particularly in the parietal and frontal bones. Less commonly, they arise from craniofacial bones, including the zygoma, maxilla, vomer, and mandible.2, 3, 4, 5, 6, 7 When multiple bones of the orbit are involved, they are termed primary intraosseous orbital hemangiomas.8, 9, 10 PICHs of the skull base are exceedingly rare. We present a patient with a PICH arising from the medial sphenoid wing, which was treated with a radical excision of the lesion and associated skull base.
ILLUSTRATIVE CASE
History and Examination
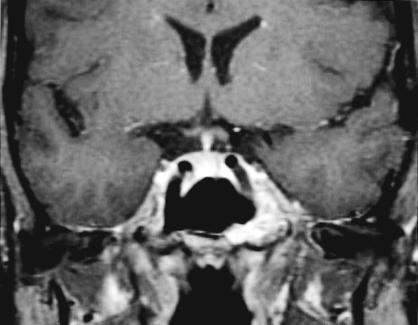
A 40–year–old, otherwise healthy woman initially had sought treatment 9 years earlier for chronic headaches. Magnetic resonance imaging (MRI) at that time revealed an intraosseous mass in the left sphenoid (Fig. 1). After consultation with the senior author (William T. Couldwell), the patient elected to have the lesion observed conservatively and she was followed by a neurologist for her headaches. She had chronic daily headaches and intermittent common migraines that increased in frequency and severity in the last several years. The patient failed multiple medical therapies, including acetaminophen, nonsteroidal anti–inflammatory drugs, high–dose narcotics, depakote, verapamil, imitrex, and diamox. She was referred to our center for repeat neuroimaging and a follow–up neurosurgical evaluation, which was within normal limits. A cranial nerve examination revealed no abnormality of facial sensation in the distribution of V2 or V3.
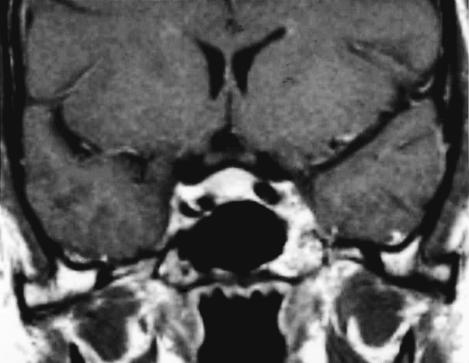
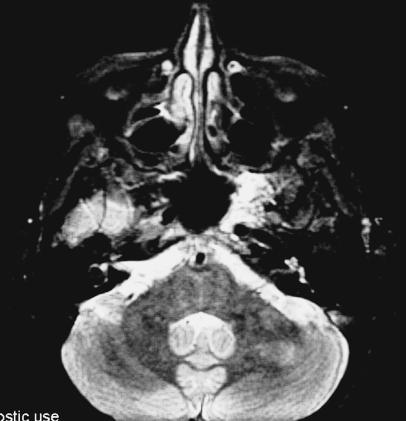
Figure 1A.
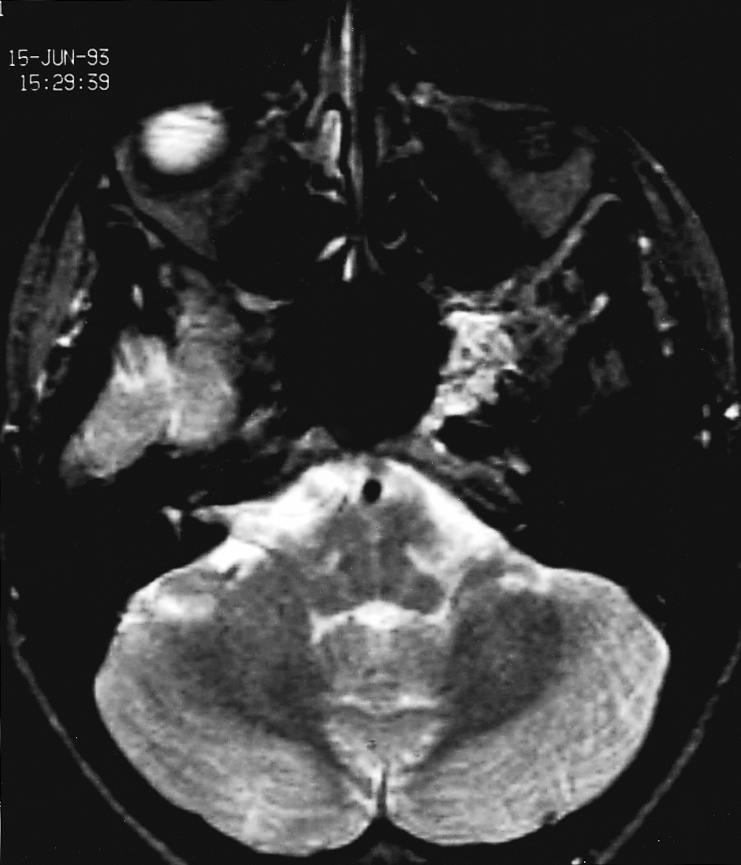
T2–weighted coronal MRI with gadolinium infusion from 1993 demonstrating intraosseous hemangioma in the left sphenoid bone with encroachment on the cavernous sinus and displacement of the cavernous carotid artery.
Radiographic Examination
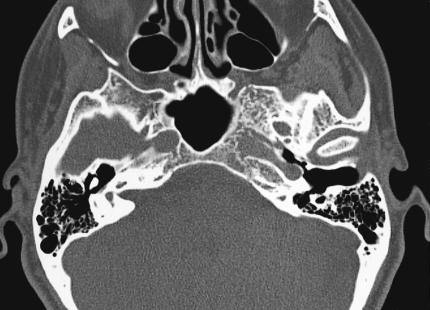
Computed tomography (CT) of the skull base revealed an abnormal mottled and trabeculated appearance of the left greater wing of the sphenoid, extending inferiorly into the pterygoid plate and superiorly into the lateral wall of the sphenoid sinus. The lesion also involved the medial and anterior wall of the horizontal carotid canal in the temporal bone (Fig. 2). MRI showed a 2.5–cm enhancing mass involving the left sphenoid bone at the skull base, encroaching on the cavernous sinus and mildly displacing the cavernous carotid artery posteriorly (Fig. 3). Compared with her initial MRI study performed 9 years earlier, the imaging on this occasion suggested a slight increase in the size of the lesion. Based on the radiographic studies, differential diagnosis included a low–grade chondrosarcoma, an intraosseous meningioma, and a hemangioma.
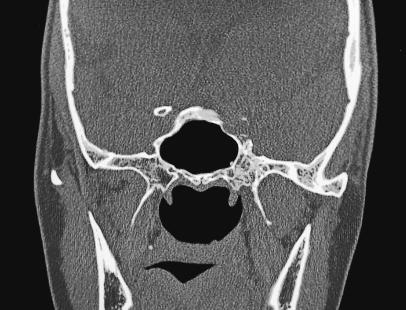
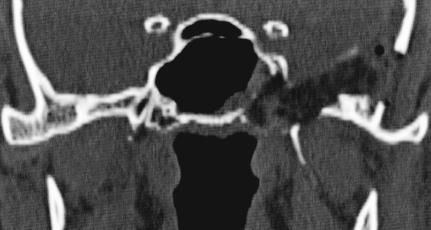
Figure 2A.
Axial CT showing characteristic trabeculated “honeycomb” appearance of the left greater wing of the sphenoid, extending inferiorly into the pterygoid plate and superiorly into the lateral wall of the sphenoid sinus.
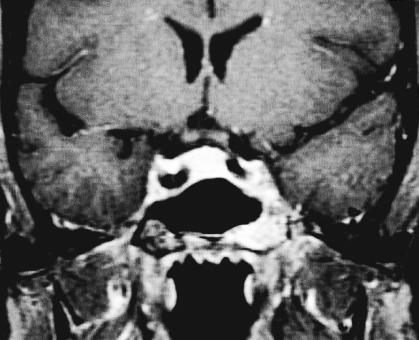
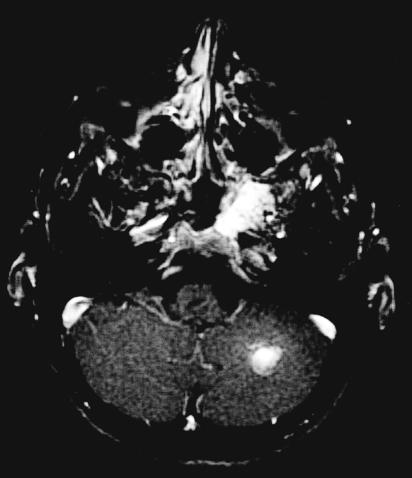
Figure 3A.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T1 coronal with gadolinium. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Surgery
Given the slight increase in the size of the sphenoid mass, the patient elected surgical removal for a definitive diagnosis and complete resection. A frontotemporal bone flap was elevated through a frontotemporal incision. The lateral aspect of the sphenoid ridge was removed with a high–speed drill. Under microscopic visualization, the middle cranial fossa surrounding the superior orbital fissure, the foramen rotundum, and the foramen ovale were exposed using a frontopolar extradural approach as described by Dolenc.11, 12, 13
Abnormal vascular soft tissue was identified in the skull base interspersed within the interstices of bone between the foramina rotundum and ovale. The mass was very vascular, with cavernous blood–filled spaces between the bony trabeculae. With a high–speed drill, the entire abnormal bony skull base of the middle fossa, including the lateral wall of the sphenoid sinus, the upper pterygoid plates, and the petrous carotid canal was removed in all directions. The second and third divisions of the trigeminal nerve as well as the petrous carotid artery were skeletonized and preserved in their entirety.
The tumor was entirely intraosseous and extracavernous. Encroachment of, but not invasion into, the cavernous sinus was appreciated. After the tumor was removed completely the sphenoid sinus, infratemporal fossa, and pterygopalatine fossa were visualized. Autologous fat and fascia lata grafts were placed in the skull base defect. The remainder of the closure was routine. A tunneled lumbar drain was placed at the end of the operation to mitigate against the development of a cerebrospinal fluid (CSF) fistula. The estimated blood loss was ∼500 ml.
Pathological Examination and Postoperative Course
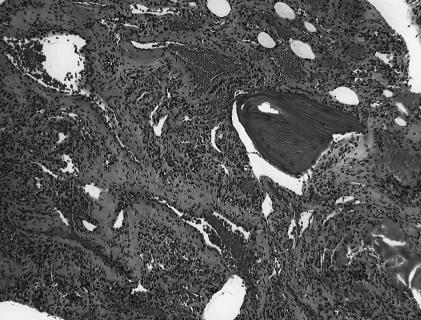
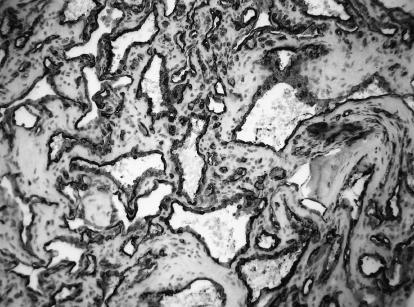
Pathological examination revealed thin–walled vascular channels lined by a single layer of flattened endothelial cells interspersed among bony trabeculae, consistent with an intraosseous cavernous hemangioma (Fig. 4A). Immunohistochemical studies showed immunopositivity for CD34 marker in the dilated vascular channels lined by endothelial cells. This finding supported the diagnosis of an intraosseous cavernous hemangioma (Fig. 4B).
Figure 4A.
Pathological examination demonstrating intraosseous cavernous hemangioma composed of thin–walled vascular channels lined by a single layer of flattened endothelial cells interspersed among bony trabeculae (hematoxylin–eosin).
Figure 4B.
Immunohistochemical studies for CD34 showing the vascular spaces lined by strongly immunoreactive endothelium.
Postoperatively, the patient was awake and alert with some residual facial numbness in the distribution of the left V2. CT obtained on postoperative Day 1 confirmed complete resection of the skull base hemangioma (Fig. 5). There was no evidence of a CSF leak, and the lumbar drain was removed on postoperative Day 2. The remainder of her hospital course was unremarkable, and she was discharged on postoperative Day 3. Her facial numbness resolved in the ensuing weeks and her headache symptoms improved dramatically. The patient has remained stable after 1 year of follow–up.
Figure 5A.
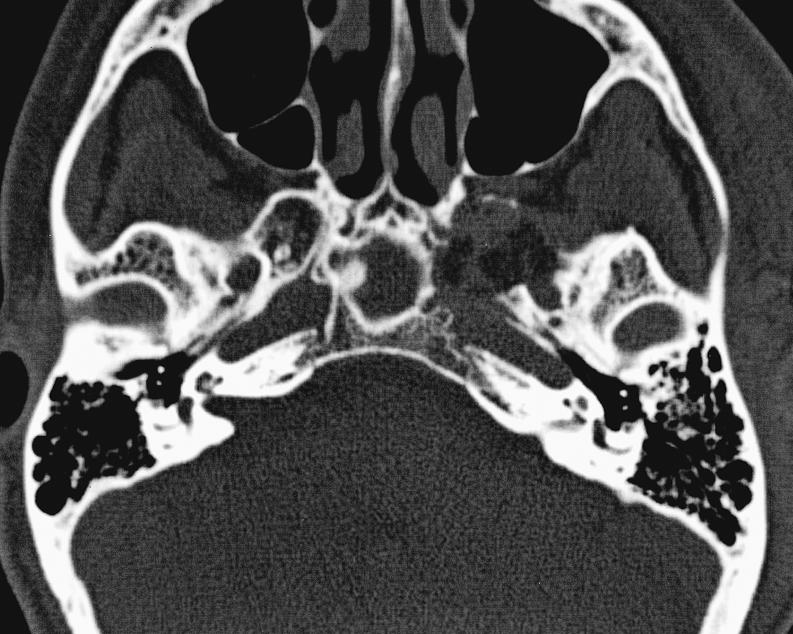
Postoperative axial CT performed one day after surgery showing complete resection of skull base cavernous hemangioma.
DISCUSSION
PICHs are rare, benign skeletal tumors most commonly found in the spinal vertebral column. Overall, they represent 0.7 % of all osseous neoplasms.14 Less commonly, PICHs can involve the bones of the cranium. The earliest description in the English literature was in 1845 by Toynbee,15 who reported a vascular tumor arising in the confines of the parietal bone. Although the origin of PICHs remains obscure, some authors believe that they represent congenital lesions that manifest in adulthood.1
Within the skull, calvarial hemangiomas are the most common. In a review by Wyke,1 70 % of cranial PICHs were localized to the calvarium, particularly the parietal and frontal bones.16, 17, 18, 19, 20, 21, 22 PICHs are slow–growing lesions and typically occur in women in the fourth and fifth decades of life. As they enlarge, they present as immobile lumps on the head associated with periodic, dull throbbing headaches that occasionally develop into severe headaches. The severity of the headaches may increase as the hemangioma expands.1, 16 Neurological deficits are unusual because these tumors tend to expand externally, but intracranial expansion has been reported.10
Intraosseous hemangiomas can also arise from craniomaxillofacial bones. The mandible, zygoma, maxilla, and frontal bones are most frequently involved (∼100 cases), and lesions in this area usually manifest with progressive painless facial swelling.3, 4, 5, 6, 7, 23 In one case, a PICH arose from the vomer, which was resected using a Le Fort I osteotomy.2
In a rare hereditary form, primary intraosseous hemangiomas are confined to the craniofacial bones. These patients present with severe dysmorphic facial features in the second decade of life.24
About 33 cases of intraosseous hemangiomas arising from the bones of the orbit, such as the frontal, ethmoid, sphenoid, and zygomatic bones have been reported.8, 9, 19, 25, 26, 27, 28, 29, 30 Some authors classify these as primary intraosseous orbital hemangiomas, which represent less than 5 % of cranial PICHs.8, 9 Initially, patients may present with supraorbital swelling, supraorbital neuralgia, an immobile nontender mass, or nasolacrimal obstruction. In severe cases, patients can experience progressive proptosis, blepharoptosis, diplopia, and visual loss.8, 9, 10, 19, 20, 29, 30, 31 In most cases, these tumors expand downward into the orbits, but intracranial expansion has been reported.10
PICHs in other regions of the skull base are distinctly unusual tumors, and most arise from the petrous temporal bone.32, 33, 34, 35 In 1921 Brandt reported the first case of a hemangioma arising from the left petrous bone, as documented by Bucy and Capp.36 In some cases, an intra–aural mass is detected incidentally and subsequent biopsy can lead to profuse hemorrhage.35 Only one case of a spontaneous hemorrhage from a petrous cavernous hemangioma resulting in a fatal epidural hematoma has been reported in an 8–year–old girl.37
Temporal bone hemangiomas that involve the facial nerve typically are referred to as facial nerve hemangiomas.38 We believe that these lesions are a distinct group of skull base hemangiomas because of their origin and unique location and because of the surgical management of the facial nerve. These tumors arise from extensive vascular plexuses distributed along the course of the facial nerve, mostly at the geniculate ganglion and the fundus of the internal auditory canal. Clinically, they tend to manifest with facial nerve paralysis, hemifacial spasm, and auditory or vestibular symptoms.
When bone is involved resulting in reactive osteoclastic and osteoblastic remodeling, the characteristic trabeculated, honeycomb appearance is visible on CT. In these cases, the lesions are often further classified as ossifying or osseous hemangiomas of the temporal bone.33, 34, 38, 39 In general, they do not invade the nerve sheath. In some cases, they can be dissected from the facial nerve while its anatomical integrity and function are preserved.38
Few reports have documented microscopic neural integrity at the site of a hemangioma.40 More commonly, the hemangioma produces an intense perineural reaction that precludes the establishment of clear surgical planes between the nerve and tumor. In these cases, some have advocated aggressive resection of the hemangioma and the involved facial nerve with subsequent primary anastomosis or cable nerve grafting.34, 38, 40
Skull base hemangiomas have been reported to arise from the occipital condyle, causing neck pain and torticollis, from the foramen magnum and clivus causing basilar impression, and from the orbitosphenoidal ridge and orbital roof causing progressive proptosis and visual loss.1, 10, 41, 42, 43, 44, 45 Although PICHs of the skull base are slow–growing lesions, they can be expansile and involve neighboring structures such as the cavernous sinus and carotid artery. On some occasions, the radiographic findings are nonspecific and can mimic more common lesions of the skull base. The diagnosis is rarely made preoperatively and is most often determined pathologically during exploratory surgery.
Radiographic Characteristics
In 1930 Bucy and Capp36 definitively described the radiographic characteristics of a series of intraosseous hemangiomas arising from various anatomical locations, including the cranium. Classically, these lesions appear as an expansive, well–circumscribed area of rarefaction with a sunburst pattern of trabeculations radiating from a common center. When viewed en face or on axial views, a honeycomb or soap–bubble configuration is characteristic (Fig. 2).8
These characteristics are better defined on CT, especially for smaller lesions that are not obvious on plain radiographs.3, 33, 46 The cortex can be greatly expanded leaving a thin bony shell, but the periosteum remains intact. Intraosseous hemangiomas are to be differentiated from osteogenic sarcomas, which destroy the bone cortex and invade the periosteum and surrounding soft tissues. There is usually no reactive sclerosis at the margins.
The signal characteristics on MRI are variable. The lesion usually appears mottled and heterogeneous with both increased and decreased signal intensities on both T1– and T2–weighted images (Fig. 3). The signal depends on the quantity of slow–moving venous blood and on the ratio of red marrow to converted fatty marrow present within the lesion. On T1–weighted imaging, smaller lesions tend to have increased intensity whereas larger lesions typically show low signal intensity within the trabeculae. Lesions with more fatty marrow show increased signal intensity on T1–weighted images. PICHs typically enhance after gadolinium is administered. On T2–weighted imaging, areas of increased signal intensity correspond to slow flow or pooling of blood.3, 8, 46 These slow–flow venous lakes interspersed between bone have been demonstrated by power Doppler ultrasonography.10
Angiography of larger hemangiomas typically demonstrates a hypervascular lesion and a delayed blush with feeding arteries but no draining veins. Preoperative embolization may be helpful in the management of larger vascular lesions.3, 6, 47, 48, 49
The pathognomonic radiographic findings of sunburst trabeculations, honeycomb configurations, and hypervascularity on angiography are most common in larger calvarial PICHs. In such instances the diagnosis can be made preoperatively based on the imaging characteristics. However, these features are not as apparent in smaller lesions, especially in the skull base. Thus, small hemangiomas are often diagnosed only after pathological examination.34
Pathological Characteristics
PICHs are benign nonencapsulated lesions that grow slowly. Invasion through the dura is extremely rare and can present as a chronic subdural hematoma (unpublished case). Cushing described one such lesion as a purplish–black mass and mistakenly suggested that it was a melanotic sarcoma.50 Grossly, PICHs appear as soft, rubbery, vascular tissue separated by thin bony trabeculae. Intraoperatively, the lesion appears as “moth–eaten” bony interstices or white coral filled with vascular soft tissue. These trabeculations are the result of reactive osteoclastic and osteoblastic bone remodeling and appear continuous with normal surrounding bone.25, 34
Microscopically, intraosseous cavernous hemangiomas are composed of thin–walled vascular channels lined by a single layer of flattened endothelial cells interspersed among bony trabeculae (Fig. 4).45 Capillary hemangiomas, which comprise the other less common form of hemangioma, are composed of radially directed capillary loops lined by a single layer of cuboidal endothelial cells. Some hemangiomas are mixed types that contain elements of both cavernous and capillary patterns. Capillary hemangiomas may progress to cavernous hemangiomas.51 Most intraosseous hemangiomas arising from the skull base are cavernous.
Surgical Management of Skull Base Lesions
PICHs do not undergo spontaneous involution. As noted, in only one case has a spontaneous hemorrhage resulted in a fatal epidural hematoma.37 Most authors have recommended gross total surgical excision to treat mass effect and neurological compromise, to improve a cosmetic deformity, and to obtain a definitive diagnosis.3, 7, 8, 10, 16, 34, 52 The surgical treatment of choice is en bloc excision and establishment of normal bony margins by drill curettage. This technique minimizes intraoperative blood loss but may not be possible to achieve when the skull base is involved. Prognosis after complete excision is excellent, and recurrence is usually rare.9
The surgical approach becomes more difficult for extensive skull base PICHs. Successful excision requires the appropriate microsurgical skull base approach and technique. Our case was successfully removed through an extradural frontopolar approach as described by Dolenc.11, 13 This approach provided wide exposure for drilling of the floor of the middle fossa while preserving the branches of the trigeminal nerve.
Radiation therapy remains a viable option for unresectable skull base lesions or residual tumors from subtotal resections.9, 46 However, some have opposed radiation therapy because of scar formation, impairment of regional bone growth in children, and the risk of development of malignant transformation. Fredrickson et al53 reported a case of radiation–induced carcinoma in a hemangioma. Breast cancer, thyroid cancer, brain tumors, and bone tumors have been reported after radiation of skin hemangiomas.54 If the lesion is near the cavernous sinus, as was the present case, the risk of radiation–induced cranial nerve deficit or hypopituitarism may be greater. Because these tumors usually grow slowly, it may be more preferable to follow such lesions conservatively rather than subject a patient to radiation. If the tumor progresses despite conservative management, fractionated stereotactic radiation therapy or radiosurgery may be considered.51, 55
CONCLUSION
PICHs of the skull base are rare, slow–growing benign tumors that may mimic other more common cranial base tumors. If critical neurovascular structures are involved, they can cause progressive neurologic deficits. The preferred treatment is complete tumor removal with normal bony margins. Sometimes the classic radiographic appearances are not evident. Consequently, the diagnosis is most often made during surgical resection.
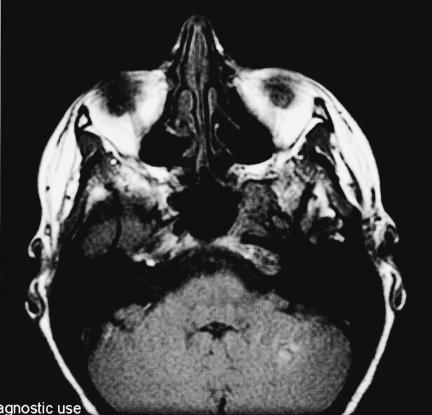
Figure 1B.
T1–weighted coronal MRI with gadolinium infusion from 1993 demonstrating intraosseous hemangioma in the left sphenoid bone with encroachment on the cavernous sinus and displacement of the cavernous carotid artery.
Figure 2B.
Coronal CT showing characteristic trabeculated “honeycomb” appearance of the left greater wing of the sphenoid, extending inferiorly into the pterygoid plate and superiorly into the lateral wall of the sphenoid sinus.
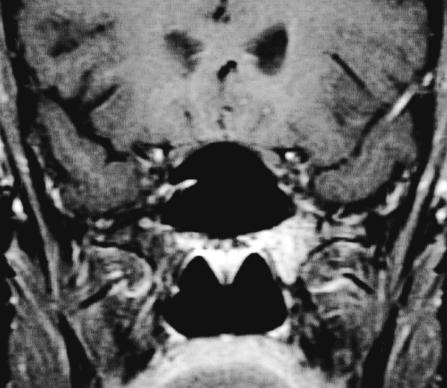
Figure 3B.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T1 coronal with gadolinium. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 3C.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T1 coronal with gadolinium. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 3D.
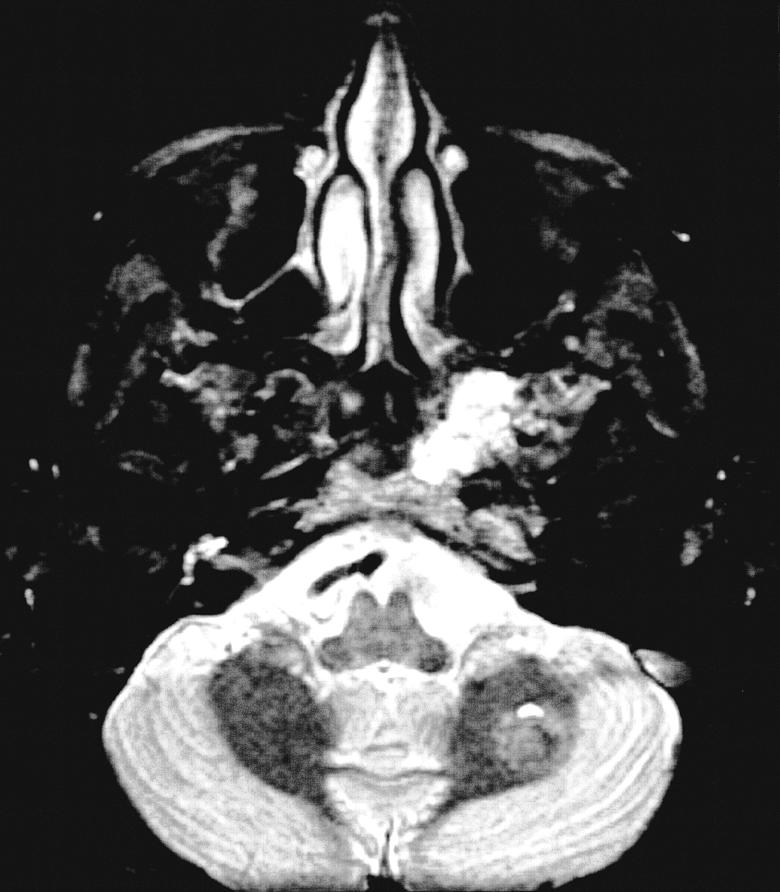
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T2 axial. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 3E.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T2 axial. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 3F.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T1 axial. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 3G.
MRI from 2002 demonstrating intraosseous hemangioma of the left sphenoid with slight enlargement. T1 axial with gadolinium reveals enhancing mass in the left sphenoid. An additional left cerebellar hemispheric vascular lesion is also appreciated.
Figure 5B.
Postoperative coronal CT performed one day after surgery showing complete resection of skull base cavernous hemangioma.
REFERENCES
- Wyke BD. Primary hemangioma of skull. A rare cranial tumour. AJR Am J Roentgenol. 1946;61:302–316. [PubMed] [Google Scholar]
- Nakahira M, Kishimoto S, Miura T, Saito H. Intraosseous hemangioma of the vomer: a case report. Am J Rhinol. 1997;11:473–477. doi: 10.2500/105065897780914956. [DOI] [PubMed] [Google Scholar]
- Moore SL, Chun JK, Mitre SA, Som PM. Intraosseous hemangioma of the zygoma: CT and MR findings. AJNR Am J Neuroradiol. 2001;22:1383–1385. [PMC free article] [PubMed] [Google Scholar]
- Cuesta G, Navarro–Vila C. Intraosseous hemangioma of the zygomatic bone. A case report. Int J Oral Maxillofac Surg. 1992;21:287–291. doi: 10.1016/s0901-5027(05)80739-8. [DOI] [PubMed] [Google Scholar]
- Ozdemir R, Alagoz S, Uysal AC, Unlu RE, Ortak T, Sensoz O. Intraosseous hemangioma of the mandible: a case report and review of the literature. J Craniofac Surg. 2002;13:38–43. doi: 10.1097/00001665-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Guibert–Trainer F, Piton J, Riche MC, Merland JJ, Caille JM. Vascular malformations of the mandible (intraosseous haemangiomas). The importance of preoperative embolization. A study of 9 cases. Eur J Radiol. 1982;2:257–272. [PubMed] [Google Scholar]
- Stassi J, Rao VM, Lowry L. Hemangioma of bone arising in the maxilla. Skeletal Radiol. 1984;12:187–191. doi: 10.1007/BF00361085. [DOI] [PubMed] [Google Scholar]
- Banerji D, Inao S, Sugita K, et al. Primary intraosseous orbital hemangioma: a case report and review of the literature. Neurosurgery. 1994;35:1131–1134. doi: 10.1227/00006123-199412000-00017. [DOI] [PubMed] [Google Scholar]
- Relf SJ, Bartley GB, Unni KK. Primary orbital intraosseous hemangioma. Ophthalmology. 1991;98:541–547. [PubMed] [Google Scholar]
- Slaba SG, Karam RH, Nehme JI, Nohra GK, Hachem KS, Salloum JW. Intraosseous orbitosphenoidal cavernous angioma. Case report. J Neurosurg. 1999;91:1034–1036. doi: 10.3171/jns.1999.91.6.1034. [DOI] [PubMed] [Google Scholar]
- van Loveren HR, Keller JT, el–Kalliny M, Scodary DJ, Tew JM Jr. The Dolenc technique for cavernous sinus exploration (cadaveric prosection). Technical note. J Neurosurg. 1991;74:837–844. doi: 10.3171/jns.1991.74.5.0837. [DOI] [PubMed] [Google Scholar]
- Dolenc VV. Extradural approach to intracavernous ICA aneurysms. Acta Neurochir Suppl (Wien) 1999;72:99–106. doi: 10.1007/978-3-7091-6377-1_9. [DOI] [PubMed] [Google Scholar]
- Dolenc VV. Frontotemporal epidural approach to trigeminal neurinomas. Acta Neurochir (Wien) 1994;130:55–65. doi: 10.1007/BF01405503. [DOI] [PubMed] [Google Scholar]
- Peterson DL, Murk SE, Story JL. Multifocal cavernous hemangioma of the skull: report of a case and review of the literature. Neurosurgery. 1992;30:778–781. [PubMed] [Google Scholar]
- Toynbee J. An account of two vascular tumours developed in the substance of bone. Lancet. 1845;2:676. [Google Scholar]
- McIntyre NG, Brebner DM, Gluckman J. The cavernous haemangioma of the frontal bone. A case report. S Afr Med J. 1977;52:537–538. [PubMed] [Google Scholar]
- Gupta SD, Tiwari IN, Pasupathy NK. Cavernous haemangioma of the frontal bone: case report. Br J Surg. 1975;62:330–332. doi: 10.1002/bjs.1800620421. [DOI] [PubMed] [Google Scholar]
- Sinnreich Z, Kremer S, Sade J, Bernheim J. Cavernous hemangioma of the frontal bone. ORL J Otorhinolaryngol Relat Spec. 1990;52:269–272. doi: 10.1159/000276149. [DOI] [PubMed] [Google Scholar]
- Sharma RR, Pawar SJ, Lad SD, Netalkar AS, Musa MM. Frontal intraosseous cryptic hemangioma presenting with supraorbital neuralgia. Clin Neurol Neurosurg. 1999;101:215–219. doi: 10.1016/s0303-8467(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Hook SR, Font RL, McCrary JA, et al. Intraosseous capillary hemangioma of the frontal bone. Am J Ophthalmol. 1987;103:824–827. doi: 10.1016/s0002-9394(14)74401-0. [DOI] [PubMed] [Google Scholar]
- Hoffman DF, Israel J. Intraosseous frontal hemangioma. Head Neck. 1990;12:160–163. doi: 10.1002/hed.2880120212. [DOI] [PubMed] [Google Scholar]
- Pastore FS, De Caro GM, Faiola A, Mauriello A, Giuffre R. Cavernous hemangioma of the parietal bone. Case report and review of the literature. Neurochirurgie. 1999;45:312–315. [PubMed] [Google Scholar]
- de Ponte FS, Bottini DJ, Valentini V. Surgical treatment of hemangioma of bones of the orbito–zygomatic region. Minerva Stomatol. 1994;43:365–372. [PubMed] [Google Scholar]
- Vargel I, Cil BE, Er N, Ruacan S, Akarsu AN, Erk Y. Hereditary intraosseous vascular malformation of the craniofacial region: an apparently novel disorder. Am J Med Genet. 2002;109:22–35. doi: 10.1002/ajmg.10282. [DOI] [PubMed] [Google Scholar]
- Gross HJ, Roth AM. Intraosseous hemangioma of the orbital roof. Am J Ophthalmol. 1978;86:565–569. doi: 10.1016/0002-9394(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Hornblass A, Zaidman GW. Intraosseous orbital cavernous hemangioma. Ophthalmology. 1981;88:1351–1355. doi: 10.1016/s0161-6420(81)34853-2. [DOI] [PubMed] [Google Scholar]
- Kolasa P, Januszalwasiak , Kordek R, Boronski K. Intraosseous orbital haemangioma. Folia Neuropathol. 2001;39:37–39. [PubMed] [Google Scholar]
- Mortada A. Exopththalmos and rare tumors of the orbital bones. Am J Ophthalmol. 1964;57:270–275. doi: 10.1016/0002-9394(64)91834-3. [DOI] [PubMed] [Google Scholar]
- Kiratli H, Orhan M. Multiple orbital intraosseous hemangiomas. Ophthal Plast Reconstr Surg. 1998;14:345–348. doi: 10.1097/00002341-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Okada AA, Shore JW, Rubin PA. Periorbital intraosseous hemangiomas. Int Ophthalmol Clin. 1992;32:111–121. [PubMed] [Google Scholar]
- Hwang K. Intraosseous hemangioma of the orbit. J Craniofac Surg. 2000;11:386–387. doi: 10.1097/00001665-200011040-00020. [DOI] [PubMed] [Google Scholar]
- Bottrill I, Poe DS. Imaging quiz case 2. Intraosseous cavernous–type hemangioma of the petrous temporal bone. Arch Otolaryngol Head Neck Surg. 1995;121:348. [PubMed] [Google Scholar]
- Curtin HD, Jensen JE, Barnes L Jr, May M. Ossifying” hemangiomas of the temporal bone: evaluation with CT. Radiology. 1987;164:831–835. doi: 10.1148/radiology.164.3.3112865. [DOI] [PubMed] [Google Scholar]
- Glasscock ME, Smith PG, Schwaber MK, Wissen AJ. Clinical aspects of osseous haemangiomas of the skull base. Laryngoscope. 1984;94:869–873. doi: 10.1288/00005537-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Irgens ER. Haemangioma of skull involving right petrous and occipital bone. Arch Otolaryngol. 1939;29:709–712. [Google Scholar]
- Bucy PC, Capp CS. Primary hemangioma of bone with special reference to roentgenologic diagnosis. AJR Am J Roentgenol. 1930;23:1–33. [Google Scholar]
- Kessler LA, Lubic LG, Koskoff YD. Epidural haemorrhage secondary to cavernous haemangioma of the petrous portion of the temporal bone. J Neurosurg. 1957;14:329–331. doi: 10.3171/jns.1957.14.3.0329. [DOI] [PubMed] [Google Scholar]
- Friedman O, Neff BA, Wilcox TO, Kenyon LC, Sataloff RT. Temporal bone hemangiomas involving the facial nerve. Otol Neurotol. 2002;23:760–766. doi: 10.1097/00129492-200209000-00025. [DOI] [PubMed] [Google Scholar]
- Gavilan J, Nistal M, Gavilan C, Calvo M. Ossifying hemangioma of the temporal bone. Arch Otolaryngol Head Neck Surg. 1990;116:965–967. doi: 10.1001/archotol.1990.01870080087022. [DOI] [PubMed] [Google Scholar]
- Eby TL, Fisch U, Makek MS. Facial nerve management in temporal bone hemangiomas. Am J Otol. 1992;13:223–232. [PubMed] [Google Scholar]
- Garcia–Martin V, Ravina J, Trujillo E, Gonzalez–Feria L. Symptomatic cavernous hemangioma of the occipital condyle treated with methacrylate embolization. Surg Neurol. 2001;56:301–303. doi: 10.1016/s0090-3019(01)00613-9. [DOI] [PubMed] [Google Scholar]
- Rajshekhar V, Chandy MJ. Haemangioma of the skull base producing basilar impression. Br J Neurosurg. 1989;3:229–233. doi: 10.3109/02688698909002800. [DOI] [PubMed] [Google Scholar]
- Tashiro T, Inoue Y, Nemoto Y, et al. Cavernous hemangioma of the clivus: case report and review of the literature. AJNR Am J Neuroradiol. 1991;12:1193–1194. [PMC free article] [PubMed] [Google Scholar]
- Dickins JR. Cavernous hemangioma of the sphenoid wing. Arch Otolaryngol. 1978;104:58–60. [PubMed] [Google Scholar]
- Suss RA, Kumar AJ, Dorfman HD, et al. Capillary hemangioma of the sphenoid bone. Skeletal Radiol. 1984;11:102–107. doi: 10.1007/BF00348797. [DOI] [PubMed] [Google Scholar]
- Sweet C, Silbergeit R, Mehta B. Primary intraosseous hemangioma of the orbit: CT and MR appearance. AJNR Am J Neuroradiol. 1997;18:379–381. [PMC free article] [PubMed] [Google Scholar]
- Lobato RD, Lamas E, Amor T, Rivas JJ. Primary calvarial hemangioma: angiographic study. Surg Neurol. 1978;10:389–394. [PubMed] [Google Scholar]
- Simmons JB, Wolpert SM. Angiography of calvarial hemangioma. Report of a case. Radiology. 1969;93:328–330. doi: 10.1148/93.2.328. [DOI] [PubMed] [Google Scholar]
- Pompili A, Guiot G, Moret J. Sphenoid ridge haemangioma operated on after feeding artery embolization. Case report. Acta Neurochir (Wien) 1982;64:125–132. doi: 10.1007/BF01405625. [DOI] [PubMed] [Google Scholar]
- Cushing H. Surgical end–results in general, with a case of cavernous haemangioma of the skull in particular. Surg Gynecol Obstet. 1923;36:303–308. [Google Scholar]
- Tsao MN, Schwartz ML, Bernstein M, et al. Capillary hemangioma of the cavernous sinus. Report of two cases. J Neurosurg. 2003;98:169–174. doi: 10.3171/jns.2003.98.1.0169. [DOI] [PubMed] [Google Scholar]
- Zucker JJ, Levine MR, Chu A. Primary intraosseous hemangioma of the orbit. Report of a case and review of the literature. Ophthal Plast Reconstr Surg. 1989;5:247–255. doi: 10.1097/00002341-198912000-00004. [DOI] [PubMed] [Google Scholar]
- Fredrickson JM, Haight JSH, Noyek AM. Radiation induced carcinoma in a haemangioma. Otolaryngol Head Neck Surg. 1979;87:584–586. doi: 10.1177/019459987908700507. [DOI] [PubMed] [Google Scholar]
- Furst CJ, Lundell M, Holm LE. Radiation therapy for hemangiomas, 1909–1959. A cohort of 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33–36. doi: 10.3109/02841868709092974. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Shin M, Tago M, et al. Gamma knife radiosurgery for cavernous hemangiomas in the cavernous sinus. Report of three cases. J Neurosurg. 2002;97(suppl 5):S477–S480. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]