ABSTRACT
We report a 38–year–old woman with a malignant catecholamine–secreting vagal paraganglioma. In the preceding year she had intermittent severe frontoparietal headaches. While she was receiving radiotherapy (35 Gy in 15 fractions) she developed palpitations, which steadily worsened over the following three years. A repeat CT scan showed no change in the size of the tumor. Urinary catecholamines were elevated and a MIBG scan showed increased uptake in the region of the vagal paraganglioma. She underwent near total resection of her tumor via a Fisch type C approach. Pathological examination showed a paraganglioma with metastasis involving a cervical lymph node. The palpitations and headaches resolved completely after surgery. This report and other published case reports suggest that catecholamine secretion from head and neck paragangliomas does not appear to respond to radiotherapy. Patients with cardiovascular symptoms from catecholamine–secreting paragangliomas are best managed surgically.
Keywords: Paraganglioma, extra–adrenal, catecholamines, radiotherapy, surgery
CASE REPORT
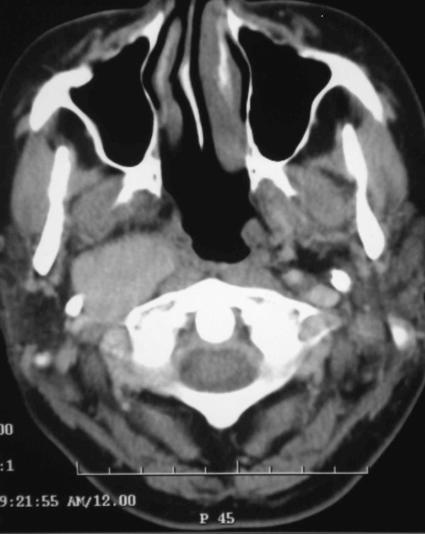
A 38–year–old woman presented with a brief history of right–sided throat discomfort. In the preceding year she had experienced intermittent severe frontoparietal headaches. A pulsatile submucosal swelling was present on the right side of her soft palate. The remainder of her examination was normal. Computed tomography (CT) (Fig. 1) showed a large right–sided parapharyngeal mass. There was no bony erosion. A vagal paraganglioma was diagnosed.
Figure 1.
Axial computed tomography showing a large right–sided parapharyngeal mass.
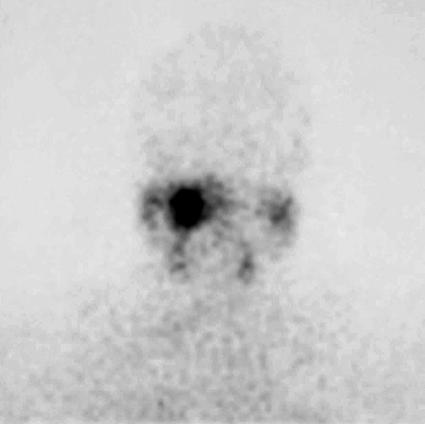
The patient had no history of palpitations before beginning radiotherapy, and urinary catecholamines were not measured. While receiving radiotherapy she developed palpitations, light–headedness, and sweating, which initially were attributed to the radiotherapy treatment itself. However, the symptoms persisted after the completion of radiotherapy (35 Gy in 15 fractions) and steadily worsened over the next three years. Eventually after two normal tests, mildly elevated urinary catecholamines were noted: normetanephrines, 3.8 micromol/ deciliter (normal, up to 3.3 micromol/ deciliter); metanephrines, 0.4 micromol/deciliter (normal, up to 1.7 micromol/deciliter); and vanillymandelic acid, 18 micromol/deciliter (normal, 6–36 micromol/ deciliter). A 131I–meta–iodobenzylguanidine (MIBG) scan (Fig. 2) showed markedly increased uptake localized to the region of the tumor. Again CT showed no change in the size of the tumor.
Figure 2.
131Iodine meta–iodobenzylguanidine (MIBG) scan showing increased uptake localized to the region of the tumor.
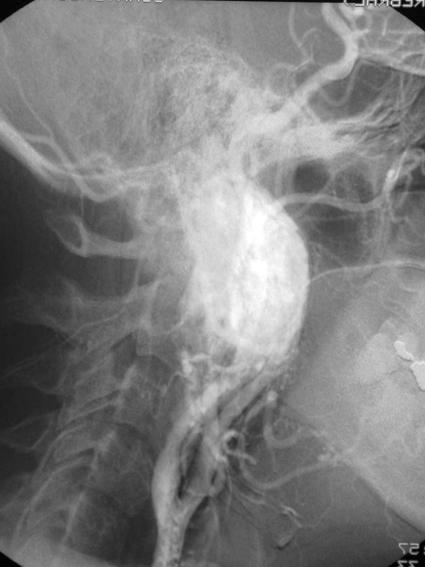
In preparation for surgery she was commenced on both α and β–blockers. An arteriogram (Fig. 3) was performed and the tumor was embolized. The tumor was resected subtotally via a Fisch type C approach. A small amount of tumor was left at the skull base attached to the internal carotid artery (ICA). Complete removal would have required resection of the ICA. A selective neck dissection of level II was performed. The superior part of level V was dissected at the same time. The patient's cardiovascular symptoms and headaches resolved completely after surgery.
Figure 3.
Carotid arteriogram showing displacement of the internal carotid artery anteriorly and marked enhancement of the tumor.
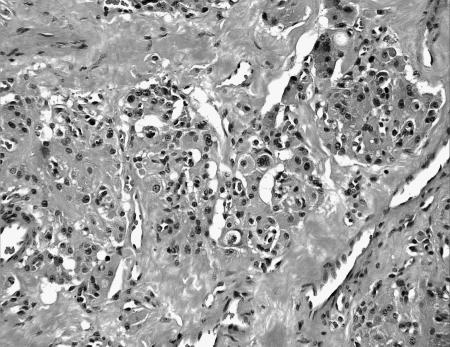
Pathology showed a tumor composed of large chief cells arranged in a typical “zellballen” pattern. There was no significant cytological atypia. Sustentacular cells were surrounded by thin fibrovascular septa (Fig. 4). Immunohistochemical staining showed strong staining of chief cells with chromogranin (Fig. 5a) and of sustentacular cells with S–100 (Fig. 5b). The histopathological diagnosis was a paraganglioma with metastasis present within the subcapsular sinus of one cervical lymph node.
Figure 4.
Photomicrograph showing chief cells, with no significant cellular atypia and typical zellballen pattern.
Figure 5A.
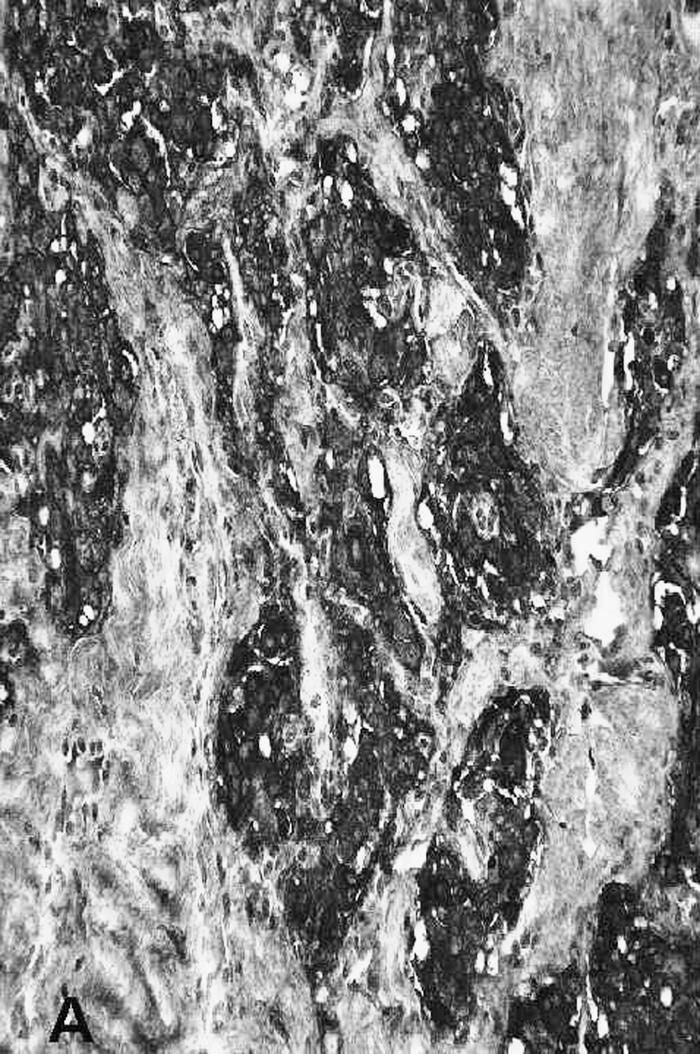
Immunohistochemical staining of the chief cells with chromogranin.
Figure 5B.
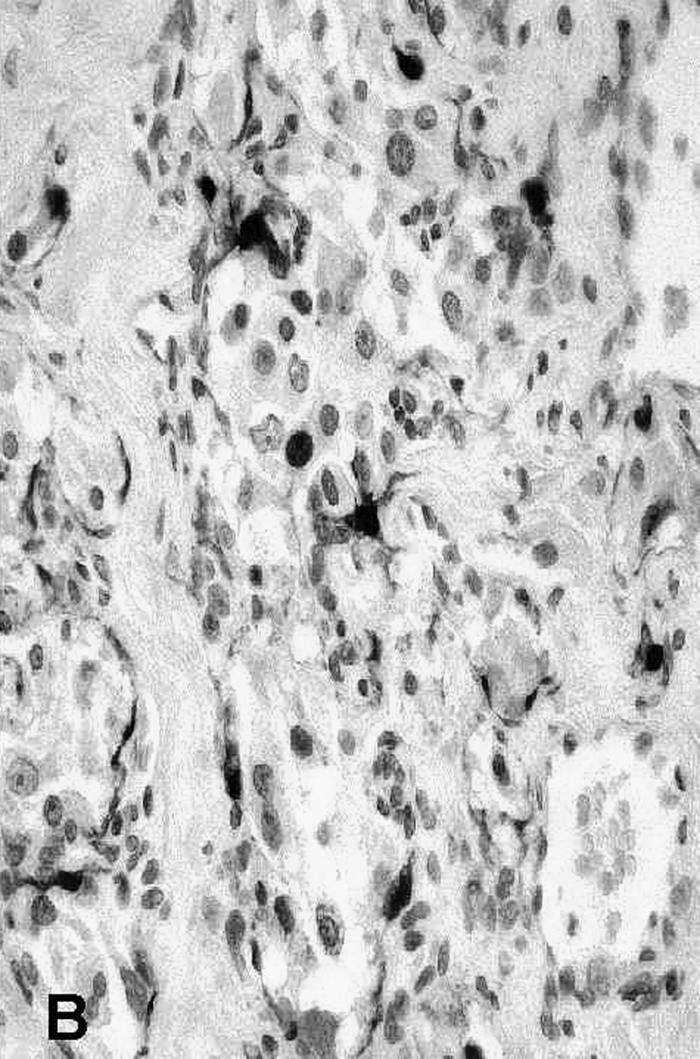
Immunohistochemical staining of the sustentacular cells with S-100.
Subsequently the patient underwent a thyroplasty for right vagal palsy. After two years of follow up, her cardiovascular symptoms and headaches have not recurred and serial CT scans have shown no change in the residual tumor.
DISCUSSION
We have presented a 38–year–old woman with a malignant, catecholamine–secreting vagal paraganglioma who was symptomatic with headaches and palpitations. Catecholamine secretion was confirmed by elevated urinary catecholamines and increased uptake in the region of the vagal paraganglioma on MIBG scanning. Pathologic examination confirmed the diagnosis of paraganglioma and revealed the presence of metastatic tumor in a lymph node, thereby fulfilling the criteria for malignancy.
Vagal paragangliomas are rare tumors. Approximately 200 cases have been reported in the medical literature.1 This figure is almost certainly an underestimate and many cases are probably not reported. Ten to 19 % of vagal paragangliomas are malignant,2, 3 and 1 to 3 % of head and neck paragangliomas secrete catecholamines and are functionally active.4
The rates of local control of vagal paragangliomas and jugular paragangliomas with radiotherapy and surgery are similar, although radiotherapy is thought to be associated with a lower rate of complications.1, 5, 6, 7, 8 The outcomes of surgery and radiotherapy are not exactly comparable. In the context of head and neck paragangliomas, the term “local control” has two different meanings-one for surgery and another for radiotherapy. Surgery aims to remove the entire tumor completely and local control refers to no evidence of a local recurrence after surgery. Radiotherapy does not usually result in complete disappearance of the tumor, and local control refers to no evidence of tumor progression after radiotherapy. Furthermore, surgery and radiotherapy are usually compared on the basis of retrospective series and sometimes modern radiotherapy techniques have been compared to outdated surgical techniques or vice versa. Our current approach is to treat most carotid body tumors and tympanic paragangliomas surgically. Vagal and jugular paragangliomas are treated with radiotherapy to minimize morbidity. Surgery is reserved for salvage.
The presence of metastatic paraganglioma tissue is often accepted as proof of malignancy. Brewis et al9 performed an extensive review of metastatic jugular paragangliomas and found a “death rate” of 68 %. Of six patients with cervical lymph node metastases, three were treated with radiotherapy alone and three with radiotherapy and surgery. All six patients died of their disease. Two patients with cervical lymph node metastases were treated with surgery alone; one died perioperatively and one was still alive at 5 years. Distant recurrences were treated with radiotherapy alone in 7 patients (2 alive with follow–up of 3 and 17 years, respectively), surgery alone in 11 patients (4 alive with follow–up of 4 months to 13 years), and surgery and radiotherapy combined in 16 patients (6 patients alive with follow–up of 9 months to 21 years). The majority (97 %) had persistent or recurrent local disease, and 75 % developed distant metastases (lung, bone, liver, brain). Although the mechanism of death is stated, presumably patients who died of their disease died from involvement of vital structures or general deterioration, including aspiration pneumonia related to cranial nerve palsies.
A detailed history and examination looking for symptoms or signs of catecholamine secretion should be performed in all patients with paragangliomas of the head and neck. Symptoms of catecholamine secretion include palpitations, chest pain, headaches, perspiration, facial flushing, nausea, anxiety, tremor, and weight loss. Hypertension may be episodic and disappear upon standing. All patients with a history suggestive of catecholamine secretion should be screened with measurement of 24–hour urinary catecholamines. Localization studies (MIBG scan, selective venous sampling) confirm the site of catecholamine secretion. Although the significance of the headaches was not appreciated at the time, in retrospect, catecholamine secretion probably caused the intermittent severe frontoparietal headaches noted before our patient began radiotherapy. They resolved completely after surgery. Therefore, radiotherapy did not cause the secretion of catecholamine from the tumor.
We were unable to find any reports of control of catecholamine secretion from a paraganglioma with radiotherapy of the head and neck. There are two reports of catecholamine secretion after radiotherapy. Schwaber et al10 reported a patient with a catecholamine–secreting jugular paraganglioma and hypertension. After radiotherapy (4750 rads over a 2–month period), no changes were observed in tumor size or in the elevated catecholamine level despite follow up for 20 months. The patient's hypertension was controlled with phenoxybenzamine (an α–blocker). Duke et al11 reported a patient with a catecholamine–secreting jugular paraganglioma. The patient was eventually treated with partial resection of the tumor and postoperative radiotherapy (3000 rads over three weeks). Although symptomatically the patient improved, hypertension and elevated urinary catecholamines persisted. Catecholamine secretion from the vagal paraganglioma in our patient did not respond to radiotherapy (35 Gy in 3 weeks). Radiotherapy did appear to result in local control because there was no growth of the tumor in the 3 years between radiotherapy and surgery.
The possibility exists that a higher dose of radiotherapy may have been effective in controlling the catecholamine secretion although this response seems unlikely given what is known about the effect of radiotherapy on cellular viability in paragangliomas. Paragangliomas are composed of chief cells and sustentacular cells. Within the cytoplasm of the chief cells are secretory granules containing catecholamines. Persistent chief cells have been found after radiotherapy,12 and this may explain why catecholamine secretion persists after radiotherapy.
Surgical excision, following α and β blockage, results in cessation of cardiovascular symptoms and normalization of catecholamine levels. Schwaber et al4 report three cases of catecholamine–secreting head and neck paragangliomas, all of which were treated surgically. Symptoms resolved in two patients, and the other patient died postoperatively of a pulmonary embolus. Reuland et al13 reported one case of a catecholamine–secreting jugular paraganglioma associated with resolution of hypertension and return of urinary catecholamine levels to normal after surgical resection. Matishak et al14 reported two cases of catecholamine–secreting paragangliomas of the skull base; both were treated surgically and hypertension resolved. Tannir et al15 reported one case of a symptomatic, markedly hypertensive man with a catecholamine–secreting vagal paraganglioma that was treated surgically. After surgery the symptoms resolved, and both blood pressure and the levels of urinary catecholamines returned to normal. Our patient became asymptomatic after near total resection of her catecholamine–secreting vagal paraganglioma and has remained asymptomatic after 2 years of follow–up. In symptomatic patients with widespread metastases from paragangliomas, treatment with 131I MIBG should be considered.16
Schwaber et al4 also detailed the perioperative management of a patient with a catecholamine–secreting paraganglioma of the head and neck. The strategy is similar to the perioperative management of a pheochromocytoma with control of tachycardia and hypertension with α and β–blockers several weeks before the operation. Intraoperative measures include the avoidance of certain medications that may precipitate a hypertensive crisis (atropine, halothane, and curare) and aggressive volume replacement. Alpha and β blockade is stopped in the perioperative period once the patient is considered stable.
We conclude that malignancy and catecholamine secretion can occur together in the same vagal paraganglioma. This report and two other case reports suggest that catecholamine secretion from paragangliomas of the head and neck does not appear to respond to radiotherapy. Patients with cardiovascular symptoms from catecholamine–secreting paragangliomas of the head and neck are best managed surgically.
PAPER PRESENTED
Poster presentation at North American Skull Base Society annual meeting, Memphis, Tennessee, February 2003.
REFERENCES
- Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma. A review of 46 patients treated during a 20 year period. Arch Otolaryngol Head Neck Surg. 1998;124:1133–1140. doi: 10.1001/archotol.124.10.1133. [DOI] [PubMed] [Google Scholar]
- Druck NS, Spector GJ, Ciralsky RH, Ogura JH. Malignant glomus vagale. Arch Otolaryngol. 1976;102:534–536. [PubMed] [Google Scholar]
- Henrich MC, Harris AE, Bell WR. Metastatic intravagal paraganglioma. Am J Med. 1985;78:1017–1024. doi: 10.1016/0002-9343(85)90226-8. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Glasscock ME, Jackson CG, Nissen AJ, Smith PG. Diagnosis and management of catecholamine secreting glomus tumors. Laryngoscope. 1984;94:1008–1015. doi: 10.1288/00005537-198408000-00002. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Beale FA, Garrett PG, et al. The treatment of glomus tumors in the temporal bone by megavoltage radiation. Cancer. 1984;53:2635–2640. doi: 10.1002/1097-0142(19840615)53:12<2635::aid-cncr2820531211>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Powell S, Peters N, Harmer C. Chemodectoma of the head and neck: results of treatment in 84 patients. Int J Radiat Oncol Biol Physiol. 1992;22:919–924. doi: 10.1016/0360-3016(92)90788-j. [DOI] [PubMed] [Google Scholar]
- Elshaikh MA, Mahmoud–Ahmed AS, Kinney SE, et al. Recurrent head and neck chemodectomas: a comparison of surgical and radiotherapeutic results. Int J Radiat Oncol Physio. 2002;52:953–956. doi: 10.1016/s0360-3016(01)02751-1. [DOI] [PubMed] [Google Scholar]
- Hinerman RW, Mendenhall WM, Amdur RJ, Stringer SP, Antonelli PJ, Cassisi NJ. Definitive radiotherapy in the management of chemodectomas arising in the temporal bone, carotid body, and glomus vagale. Head Neck. 2001;23:363–371. doi: 10.1002/hed.1045. [DOI] [PubMed] [Google Scholar]
- Brewis C, Bottrill ID, Wharton SB, Moffat DA. Metastases from glomus jugulare tumors. J Laryngol Otol. 2000;114:17–23. doi: 10.1258/0022215001903825. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Gussack GS, Kirkpatrick W. The role of radiation therapy in the management of catecholamine–secreting glomus tumors. Otolaryngol Head Neck Surg. 1988;98:150–154. doi: 10.1177/019459988809800209. [DOI] [PubMed] [Google Scholar]
- Duke WW, Boshell BR, Soteres P, Carr JH. A norepinephrine–secreting glomus jugulare tumor presenting as a pheochromocytoma. Ann Intern Med. 1964;60:1040–1047. [Google Scholar]
- Spector GJ, Compagno J, Perez CA, Maisel RH, Ogura JH. Glomus jugulare tumors: effects of radiotherapy. Cancer. 1975;35:1316–1321. doi: 10.1002/1097-0142(197505)35:5<1316::aid-cncr2820350511>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Reuland P, Overkamp D, Aicher KP, Bien S, Muller–Schauenburg W, Feine U. Catecholamine secreting glomus tumor detected by iodine–123–MIBG scintigraphy. J Nucl Med. 1996;37:463–465. [PubMed] [Google Scholar]
- Matishak MZ, Symon L, Cheeseman A, Pamphlett R. Catecholamine–secreting paragangliomas of the base of the skull. J Neurosurg. 1987;66:604–608. doi: 10.3171/jns.1987.66.4.0604. [DOI] [PubMed] [Google Scholar]
- Tannir NM, Cortas N, Allam C. A functioning catecholamine–secreting vagal body tumor. Cancer. 1983;52:932–935. doi: 10.1002/1097-0142(19830901)52:5<932::aid-cncr2820520532>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mukherjee JJ, Kaltsas GA, Islam N, et al. Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with (131)I–meta–iodobenzylguanidine. Clin Endocrinol (Oxf) 2001;55:47–60. doi: 10.1046/j.1365-2265.2001.01309.x. [DOI] [PubMed] [Google Scholar]