Abstract
Introduction
During the early coronavirus disease (COVID-19) pandemic in 2020, researchers cautioned about the potential neuroinvasive capability of the virus and long-term neurological consequences. Although a few preliminary studies have found delayed communication, fine motor, and problem-solving skills in infants after COVID-19 infection, there continues to be a paucity of data on long-term development of neonates diagnosed with COVID-19.
Methods
We conducted a prospective study of 20 neonates who acquired severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection during the first wave of the pandemic (April–July 2020). At 18–24 months corrected age, we assessed neurodevelopment by Bayley Scales of Infant and Toddler Development, the third edition (BSID-III), along with growth, hearing, and vision evaluation.
Results
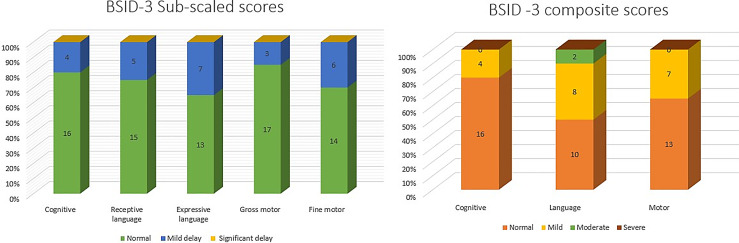
The mean corrected age at assessment was 21 months 11 days ± 1 month 28 days. We found developmental delay in nearly half of the children with scores below one standard deviation in either of the BSID-III domains. Mild delay in either motor, cognitive, or language domains was found in 9 (45%) children and moderate delay in 2 (10%). Expressive language, fine motor, and receptive language were predominantly affected. None of the children had hearing impairment, blindness, or significant growth faltering including clinically severe microcephaly. The mean composite cognitive, language, and motor scores were significantly lower in those with neurodevelopmental delay (p value – 0.02, 0.000, and 0.03, respectively) without any differences in their disease characteristics.
Conclusion
Neonates infected with SARS-CoV-2 have an increased risk of developmental delays in expressive language, fine motor, and receptive language skills at 18–24 months of age. The severity of delays is predominantly mild.
Keywords: COVID-19, Infant, Neurodevelopmental impairment, Coronavirus infection
Introduction
Coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) started as an outbreak of atypical pneumonia in December 2019 and has since become a global pandemic [1]. As we continue into the third year of the pandemic, the scientific focus has shifted to the short- and long-term consequences of the infection. Early in the pandemic in 2020, researchers described a wide range of neurological manifestations of SARS-CoV-2 infection in young children [2]. The recent discovery of angiotensin-converting enzyme 2 receptors in neural tissues which can act as a viral receptor has added to ongoing concerns [3]. In the past, viral outbreaks of HINI, Rubella, and Zika were eventually found to have long-term neurological consequences [4–7].
Newborn infants are particularly vulnerable as infections during this time disrupt important stages of neurodevelopment. Furthermore, for children, even mild deficits can be challenging and significantly impact their learning, social interactions, and quality of life [8, 9]. Early diagnosis and intervention are crucial in order to take advantage of neuroplasticity. Hence, studying the development of neonates affected by SAR-CoV-2 is crucial to prevent having a population with unmet needs in the future.
There are a few preliminary follow-up studies published recently that evaluated the neurodevelopmental outcomes at various stages of infant development using different structured examinations [8–11]. Of these, one study found transient early fine motor impairments in children born to mothers with COVID-19 by the Denver Developmental Screening Test in the first year of life [8]. Another study used Neonatal Behavioral Assessment Scale at 6 weeks of life in 21 neonates born to mothers with COVID-19 and compared to 21 unexposed neonates and found suboptimal scores in infant interactive behavior and motor skills [10]. A cohort of full-term neonates born to mothers with SARS-CoV-2 was assessed at 16–18 months with Ages and Stages Questionnaires and an increased risk of neurodevelopmental delays in communication (29%), fine motor (31%), and problem-solving (24%) skills was found [12]. Additionally, in COVID-19 pandemic, the contribution of social isolation hindering a healthy environment for growth and development with increased morbidity following a severe infection could have additional impacts on the overall neurodevelopmental outcomes. This possibility was explored by a study which compared neurodevelopment outcomes at 6 months of age in children born during and before the pandemic and found pandemic-born children to have lower scores on gross motor, fine motor, and social skills regardless of in utero exposure to COVID-19 [13].
Between April and July 2020, during the initial wave of the COVID-19 pandemic in India, we published a retrospective cohort study involving 198 neonates who were suspected of SARS-CoV-2 infection. The infants were suspected if they were born to mothers who were diagnosed with COVID-19 or manifested symptoms which raised clinical suspicion of COVID-19 [14]. We found a SARS-CoV-2 infection rate of 10.6%, with 21 neonates testing positive. The majority of the infected neonates were asymptomatic, while one-third (33.3%) showed symptoms, predominantly of respiratory (33.3%) and gastrointestinal (4.8%) involvement. Intensive care was required for six neonates (28.6%), of whom four were preterm and two were term. All neonates improved and were successfully discharged home. At 6–8 weeks of telephonic follow-up, one neonate had passed away due to cyanotic congenital heart disease and the rest remained healthy. In this study, we report neurodevelopment and growth outcomes of the COVID-19-infected neonates at 18–24 months corrected age.
Materials and Methods
We conducted a prospective follow-up study of a previously published cohort of COVID-19-positive neonates [14]. These neonates were seen in high-risk infant follow-up clinic between 18 and 24 months corrected age in a level III b neonatal intensive care unit in one of the major public hospitals in western India. Key maternal and neonatal demographic details including maternal age, parity, education, socio-economic status, mode of delivery, need of resuscitation, antenatal steroid coverage, birth weight, and gestational age were obtained from patient record sheets. Clinical details of maternal and neonatal COVID-19 infection, outcomes, and duration of hospital stay were noted from the records. History of breastfeeding, complementary feeding, immunization, subsequent hospitalization, growth, vision, hearing, and development was obtained from parents.
Assessment of Chronological and Corrected Age
For infants who were born before the completion of 37 weeks, the corrected age was used to adjust scores up to 24 months of age to compare the development to age-related peers, as per Bayley Scales of Infant and Toddler Development, third edition (BSID-III).
Assessment of Neurodevelopment Outcomes
The neurodevelopmental outcomes were assessed at 18–24 months of corrected age by either of the two trained examiners (MG and PRR) using the BSID‐III (cognitive, language, motor). We calculated subscaled scores and composite scores for cognitive, language, and motor domains. The composite score was calculated with a mean of 100 and a standard deviation (SD) of 15. A composite score between −1 and −2 SD below the mean (70–84), between −2 and −3 SD (55–69), and below −3 SD (<55) was classified as mild, moderate, and severe delay, respectively. Subscaled scores were calculated for five subscales including cognition, receptive language, expressive language, gross motor, and fine motor. These were classified as mild delay if the subscaled score was less than 7 and as significant delay if it was less than 4.
Assessment of Growth
Anthropometric variables including head circumference, length, weight, mid-upper arm circumference (MUAC), and body mass index (BMI) were measured. The weight was obtained without clothes/diapers using an electronic weighing scale with a sensitivity of 10 grams. An infantometer with a sensitivity of 5 millimeters was used for recumbent length. Head circumference was measured by a nonstretchable plastic tape with an accuracy of 1 millimeter. MUAC was recorded at the mid-point between the tip of the acromion process and the olecranon process using a nonstretchable plastic tape with an accuracy of 1 millimeter. Each measurement was collected independently by two observers and averaged. BMI was calculated from weight and recumbent length. All measurements were plotted on the sex-specific World Health Organization (WHO) growth charts 2006. The child was classified as underweight, stunted, and wasted, if weight-for-age, length-for-age, and weight-for-length were below 2 SD based on sex-specific charts. Severe microcephaly was defined as head circumference-for-age below 3 SD. MUAC was categorized as > 12.5 cm (no acute malnutrition), 11.5–12.5 cm (moderate acute malnutrition), and <11.5 cm (severe acute malnutrition).
Assessment of Hearing and Vision
Parental concerns about hearing and vision were recorded. Hearing assessment was carried out by auditory brainstem evoked potential (ABR) testing and profound hearing loss was defined as hearing impairment requiring unilateral or bilateral hearing aid or cochlear implants. The vision was assessed by pediatric ophthalmologists and any loss of useful vision in unilateral or bilateral eyes was considered blindness.
Statistical Analysis
Data were entered in MS Excel and analyzed using SPSS software version 23. Categorical variables were represented by percentages. Continuous variables were represented by mean with standard deviations and median with interquartile range. Independent t-test was used for continuous data and χ2 and Fisher’s exact test were used for categorical data. A p value <0.05 was taken as significant.
Results
The study included a follow-up of a cohort of 21 COVID-19-positive neonates with a median (range) birth weight and gestation age of 2,662 (996–3,714) g and 39 (30–41) weeks, respectively. Only one had neurological manifestations in the neonatal period and presented with lethargy; however, it was neurologically normal at discharge. He was born at 30 weeks with a birth weight of 1,200 g, tested positive on day three of life, and required ventilation and surfactant for respiratory distress syndrome. All four preterm neonates in the cohort underwent cranial ultrasound in the first week of life and had normal studies. Two neonates required subsequent brief hospitalization, one at the age of 3 months for acute bronchiolitis and the other at 8 months for acute gastroenteritis, and were discharged home within a week.
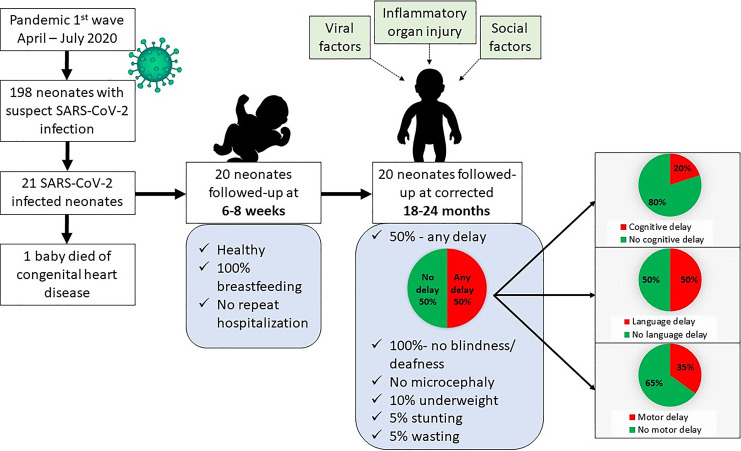
The cohort was followed up for long-term neurodevelopmental outcomes at 18–24 months of corrected age as depicted in Figure 1. The mean chronological age and corrected age at assessment were 22 months 29 days ± 2 months 18 days and 21 months 11 days ± 1 month 28 days, respectively (Table 1). The mean weight was 10.25 ± 1.37 kg with two children (10%) underweight and median weight-for-age z scores were −0.97 (−1.44, −0.24). The mean recumbent length was 81.7 ± 3.25 cm with one (5%) child stunted and median length-for-age z scores were −1.01 (−1.42, −0.43). Wasting was recorded in one child (5%) and median weight-for-length z scores were −0.79 (−1.55, 0.36). The mean head circumference was 44.62 ± 0.76 cm and the median head circumference-for-age z score was −2.22 (−2.56, −1.83). None of the children had severe microcephaly. The mean BMI was 15.35 ± 1.52 kg/m2 and the median BMI-for-age z score was −0.36 (−1.39, 0.56). None of the children had severe acute malnutrition and the mean MUAC was 12.21 ± 0.44 cm.
Fig. 1.
Study flow diagram.
Table 1.
Maternal and infant demographic characteristics
| Study characteristics | Result |
|---|---|
| Maternal age | |
| Median (range) | 26 (18–33) years |
| Maternal education, n/N (%) | |
| Primary school | 6/20 (30) |
| High school | 8/20 (40) |
| Graduate and above | 6/20 (30) |
| Socio-economic class, n/N (%) | |
| Lower middle | 11/20 (55) |
| Upper lower | 9/20 (45) |
| Maternal history of psychological illness | 0/20 |
| Maternal SARS-CoV-2 infection, n/N (%) | |
| SARS-CoV-2 positive in the antenatal period | 18/20 (90) |
| Symptomatic COVID-19 | 5/20 (25) |
| Severe COVID-19 requiring ICU care | 0/20 (0) |
| Multiple gestations, n/N (%) | |
| Twin | 1/20 (5) |
| Mode of delivery, n/N (%) | |
| Vaginal | 8/20 (40) |
| Assisted vaginal | 1/20 (5) |
| Cesarean | 11/20 (55) |
| Skin-to-skin contact following birth | 0/20 (0) |
| Mother-child separation following delivery | 8/20 (40) |
| Need of resuscitation | 0/20 (0) |
| Antenatal steroid coverage of preterm births | 4/4 (100) |
| Preterm (<37 completed weeks) | 4/20 (20) |
| Male | 11/20 (55) |
| Low birth weight | 8/20 (40) |
| Small for gestational age | 6/20 (30) |
| Asian race | 20/20 (100) |
| Any breastfeeding | 20/20 (100) |
| Exclusive breastfeeding till 6 months | 14/20 (70) |
| Onset of neonatal COVID-19 infection, n/N (%) | |
| ≤3 days of life | 15/20 (75) |
| >3 days of life | 5/20 (25) |
| Symptoms of COVID-19 in neonates | 7/20 (35) |
| Respiratory | 7/7 |
| Central nervous system | 1/7 |
| Gastrointestinal | 1/7 |
| Immunized for age, n/N (%) | 20/20 (100) |
| Parental concerns, n/N (%) | |
| Delay in milestones | 1/20 (5) |
| Hearing difficulties | 0/20 |
| Visual difficulties | 0/20 |
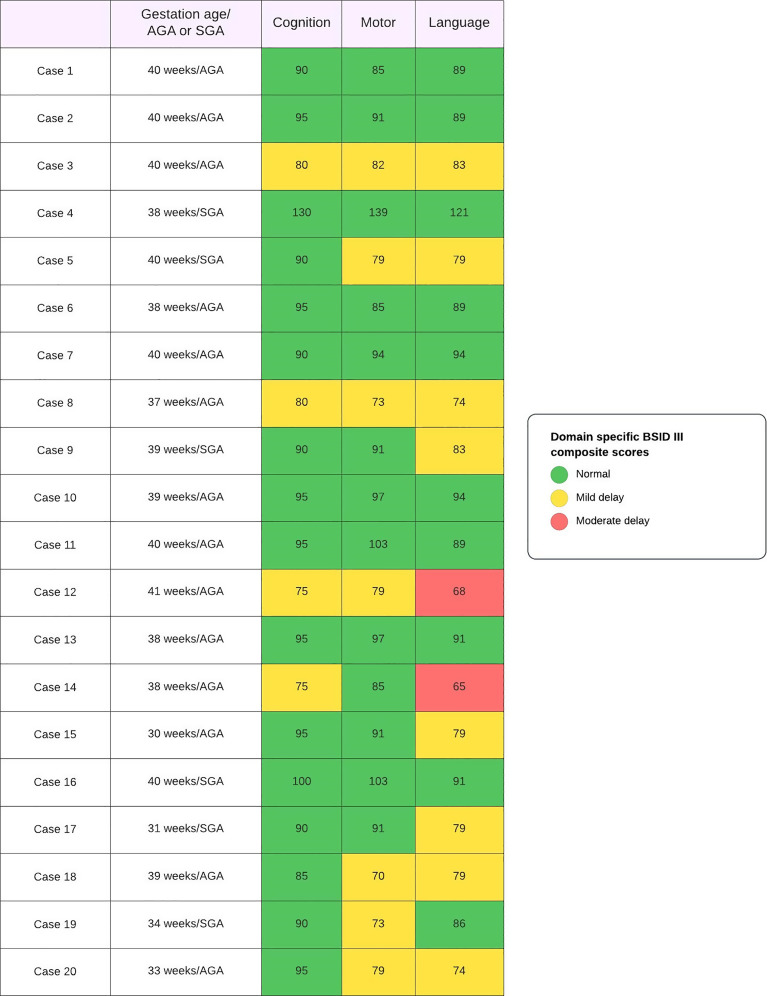
Hearing assessment with ABR and ophthalmologic assessment was normal for all children. Mean raw, subscaled, and composite scores are summarized in Table 2. The individual domain-specific composite score for all 20 children is illustrated in Figure 2 and of the complete cohort in Figure 3. Composite scores for the cognitive domain found a mild delay in 4 (20%) children. Of these four, three had motor and language delays as well. The motor delay was mild in all three children. However, language delay was mild in two of them and moderate in one child. The child who had moderate language delay with mild motor and cognitive delay was born at term gestation and had acquired SARS-CoV-2 infection on day 5 of life. However, the child remained asymptomatic and had no neurological concerns at discharge.
Table 2.
BSID-III (cognitive, language, and motor) scores on neurodevelopmental follow-up at 18–24 months corrected age
| Domain | Mean raw score | Mean scaled score | Mean composite score | Median percentile rank (Q1, Q3) |
|---|---|---|---|---|
| Cognitive | 54.05±5.19 | 8.3±2.30 | 91.5±11.48 | 25 (22.75, 37) |
| Language | 14.75±4.02 | 84.80±11.87 | 15.5 (8, 24) | |
| Expressive | 21.9±4.64 | 7.20±1.82 | ||
| Receptive | 19.7±3.66 | 7.55±2.33 | ||
| Motor | 16.45±5.03 | 89.35±15.09 | 21.5 (8, 36) | |
| Gross | 49.9±5.17 | 8.20±2.42 | ||
| Fine | 34.15±3.20 | 8.25±2.71 |
Fig. 2.
Distribution of composite scores (cognition, language, and motor) of 20 SAR-CoV-2-infected neonates followed up at 18–24 months corrected age.
Fig. 3.
Distribution of BSID-III subscaled and composite scores of the follow-up cohort at 18–24 months corrected age.
The composite motor scores of 7 (35%) children suggested mild delay; however, none had moderate/severe delay. The isolated motor delay was seen in one case, where the child was born at 34 weeks with fetal growth restriction, had acquired SARS-CoV-2 on day 3 of life, and had no neurological abnormality at the time of discharge. Under the language domain, we found mild delay in 8 (40%) children and moderate delay in 2 (10%) children. One of the cases of moderate delay has been described previously. The other was a neonate born at term gestation and acquired asymptomatic SARS-CoV-2 infection on day 2 of life and remained otherwise healthy on follow-up. In both cases, language delay was associated with mild cognitive delay.
We compared the subgroups of those with neurodevelopment delays with those without for key confounding factors that could alter neurodevelopmental outcomes and found no difference. The parameters for growth assessment did not show significant differences; however, the cognitive, language, and motor composite scores were significantly lower in the group with neurodevelopmental delay as summarized in Table 3.
Table 3.
Comparison of subgroups with neurodevelopmental delay and no neurodevelopmental delay
| Variable | No neurodevelopmental delay (n = 10) | Neurodevelopmental delay present (n = 10) | p value | 95% confidence interval of the difference |
|---|---|---|---|---|
| Chronological age at assessment | 22 months 15 days ± 1 month 25 days | 24 months 14 days ± 3 months 6 days | 0.44 | −3.38–1.52 |
| Corrected age at assessment | 21 months 28 days ± 1 month 28 days | 20 months and 25 days ± 1 month 28 days | 0.23 | −0.75–2.89 |
| Baseline maternal and neonatal characteristics | ||||
| Maternal age, years | 25.40±4.03 | 27.20±3.71 | 0.31 | −5.44–1.84 |
| Low maternal education level (below graduate), n/N (%) | 7/10 (70) | 7/10 (70) | 1.00 | |
| Low socio-economic background (lower-middle class), n/N (%) | 5/10 (50) | 6/10 (60) | 1.00 | |
| History of maternal SARS-CoV-2 in the antenatal period, n/N (%) | 10/10 (100) | 8/10 (80) | 0.47 | |
| History of symptomatic maternal SAR-CoV-2 infection in the antenatal period, n/N (%) | 3/10 (30) | 2/10 (20) | 1.00 | |
| Need of resuscitation, n/N (%) | 0/10 (0) | 0/10 (0) | – | |
| Preterm (<37 completed weeks), n/N (%) | 1/10 (10) | 3/10 (30) | 0.58 | |
| Antenatal steroids, n/N (%) | 1/10 (10) | 3/10 (30) | 0.58 | |
| Male, n/N (%) | 5/10 (50) | 6/10 (60) | 1.00 | |
| Very low birth weight, n/N (%) | 1/10 (10) | 2/10 (20) | 1.00 | |
| Small for gestational age, n/N (%) | 3/10 (30) | 3/10 (30) | 1.00 | |
| Breastfeed after birth, n/N (%) | 10/10 (100) | 10/10 (100) | – | |
| Exclusive breastfeeding till 6 months, n/N (%) | 8/10 (80) | 6/10 (60) | 0.63 | |
| Early onset neonatal COVID-19, n/N (%) | 7/10 (70) | 8/10 (80) | 1.00 | |
| Symptomatic neonatal COVID-19 | 2/10 (20) | 5/10 (50) | 0.35 | |
| Evidence of intraventricular hemorrhage in preterm cranial ultrasound, n/N (%) | 0/1 (0) | 0/3 (0) | – | |
| Need for neonatal intensive care, n/N (%) | 0/10 (0) | 3/10 (30) | 0.21 | |
| Need for rehospitalization, n/N (%) | 1/10 (10) | 1/10 (10) | 1.00 | |
| Growth and neurodevelopmental follow-up | ||||
| Underweight, n/N (%) | 1/10 (10) | 1/10 (10) | 1.00 | |
| Wasting, n/N (%) | 0/10 (0) | 1/10 (10) | 1.00 | |
| Stunting, n/N (%) | 1/10 (10) | 0/10 (0) | 1.00 | |
| Severe microcephaly, n/N (%) | 0/10 (0) | 0/10 (0) | – | |
| Weight-for-age z score | −0.68±1.04 | −1.18±1.91 | 0.27 | −0.42–1.42 |
| Length-for-age z score | −1.13±1.20 | −0.99±0.79 | 0.77 | −1.09–0.82 |
| Head circumference-for-age z scores | −2.18±0.64 | −2.10±0.38 | 0.74 | −0.57–0.41 |
| BMI-for-age z scores | 0.05±1.05 | −0.83±1.18 | 0.10 | −0.17–1.93 |
| Weight-for-length z scores | −0.15±1.03 | −0.93±1.12 | 0.12 | −0.23–1.80 |
| Cognitive composite scores | 97.50±11.84 | 85.50±7.62 | 0.02 | 2.64–21.36 |
| Language composite scores | 93.30±10.03 | 76.30±6.02 | 0.000 | 9.23–24.77 |
| Motor composite scores | 96.70±17.46 | 82.00±7.48 | 0.03 | 2.08–27.32 |
Discussion
We describe the development and neurological outcomes of a group of 20 neonates who contracted SARS-CoV-2 during the neonatal period. Almost 50% of the children exhibited developmental delays at 18–24 months, as determined by scores below one SD in any one developmental domain in the BSID-III assessment. Mild delay in either motor, cognitive, or language domains was found in 9 (45%) children, and moderate delay was in 2 (10%). The areas of development most impacted were expressive language, fine motor skills, and receptive language. None had hearing impairment or blindness. The majority of children experienced normal growth, but two (10%) were underweight and one was also stunted. None of them had severe microcephaly.
These findings parallel epidemiologic studies carried out after influenza A virus subtype H1N1, Rubella, and Zika virus epidemics, which demonstrated an increase in long-term neurodevelopmental sequela [4–7]. H1N1 virus was associated with autism spectrum disorders, while Zika virus epidemic in 2015 resulted in distinctive symptoms such as severe microcephaly, language, and motor delays [15]. Rubella pandemic of 1964 led to an increased risk of autism spectrum disorders and schizophrenia following fetal exposure [16]. In viral infections, two major mechanisms impacting the development of a growing brain are direct viremia-mediated neurotoxicity and immune dysregulation [17]. The affinity of the SARS-CoV-2 virus for angiotensin-converting enzyme 2 receptor and its expression in multiple brain regions serve as a possible explanation for the neurological manifestations in acute infection with short- and long-term effects. This virus-mediated neurotoxicity could also possibly begin in utero following its crossing of the placental barrier. Maternal immune activation and subsequent increase in proinflammatory cytokines such as IL-6, TNF-a, and IL-1b after infection can disrupt the placental serotonin signaling, which mitigates the crucial steps of synaptogenesis, neuronal migration, and axonal targeting [18]. Another postulation suggests an alteration in fetal brain neurotransmitter signaling following this immune dysregulation [19].
Language was the predominantly affected domain followed by motor and cognition in our cohort. Expressive language was more affected than receptive language. An isolated language delay was seen in 3 children with the remaining being either mild motor delay (three children) or mild motor and cognitive delay (two children). This corroborates with the results of a large retrospective study in which 222 neonates born to SARS-CoV-2-infected mothers were compared with 7,550 neonates born to uninfected mothers, who showed developmental delays of speech-language and motor function at 12 months of age. These delays were observed to be significantly higher in the case of maternal third-trimester infection [11]. These findings could have a multifactorial attribution, apart from virus-mediated neural injury. Other crucial contributors to language-speech delay could possibly include the lack of opportunities for interaction with peers, play-based learning, and group childcare environment due to regulations imposed during a pandemic. Although we did not calculate the screen time, studies reported an increase in screen time exposure during the pandemic with an adverse impact on language development in young infants between 8 and 36 months [20, 21]. Studies have also explored the possibility of obscuring facial expressions with the use of opaque facial masks that can serve as pivotal cues in developing language and speech skills in children. Pereda et al. described associations between increases in family violence against children during COVID-19 and expressive language delays [22, 23].
Mild motor delays were seen in seven (35%) children and mild cognitive delays were found in four (20%) children. This parallels the affection of fine motor (31%) and problem-solving (24%) domains assessed by Ages and Stages Questionnaires -3 at 16–18 months of age in full-term infants, who were exposed to SARS-CoV-2 virus in utero [12]. Motor delays could also be due to the lack of play opportunities. The cognitive delays could be contributed apart from virus-mediated injury, by pandemic-related parental stress aggravated by quarantine, adverse health in family members, and socio-economic losses [24]. Studies have associated this increase in stress with anxiety and depression in caregivers, leading to a possibility of increased parent-child conflicts [25]. Wang et al. [26] in their multicenter study in Wuhan in children of ages 6–11 years found parent-child conflict, alteration in sleep pattern, decrease in physical activity, and increase in screen time to be associated with psychological problems. Importantly, another study that evaluated teleconsults during the pandemic found delay of language development and behavioral difficulties in children with a mean age of 40 ± 13 months to be the primary reason for consults [27].
We did not find any hearing or visual deficits in children. A concern for hearing abnormalities was raised with early data suggesting SARS-CoV-2 to have neurotropic properties causing direct damage to hair cells [28]. Similar to the findings in the present study, a retrospective study found COVID-19 exposure in a prenatal period not to serve as a risk factor for hearing loss [29].
Our study is strengthened by a close follow-up without attrition and a thorough neurodevelopmental evaluation using BSID-III, a standardized tool administered by a physician. This enhances the significance of our study compared to previous ones that relied on parent-administered tools. Assessment at 18–24 months corrected age remains beneficial for predicting development and implementing early intervention strategies to support neurodevelopment.
Importantly, our statistical analyses were univariate due to the small sample size from a single center which limited our ability to determine impact of each characteristic independently. As we did not have a control group, delineating the impact of pandemic per se and COVID-19 infection independently on these neurodevelopmental outcomes was limited. Hence, future studies are needed to determine whether these changes are due to the actual infection or due to the isolation of the pandemic. Larger longitudinal cohorts to continuously follow these neonates to explore the neurological sequelae that are yet to be discovered in early childhood and adolescence are imperative. Despite the nature of insults being mild, considering the number of neonates that were infected globally during COVID-19 pandemic, the future implications of such delays are of potential concern. In our opinion, all children infected with SARS-CoV-2 should be followed at least longitudinally in the first 2–3 years of life, such that delays can be recognized and early intervention services can be initiated to improve the neurodevelopment trajectory.
Conclusion
Our study highlights an increased risk of developmental delays in expressive language, fine motor, and receptive language skills in neonates infected with SARS-CoV-2 at 18–24 months of age. The severity of delays is predominantly mild.
Statement of Ethics
The study was conducted after receiving ethical permission from the Institutional Ethics Committee at Seth GS Medical College and Kind Edward Memorial Hospital, Mumbai, India (EC-OA-182/2021). Written informed consent for participation was obtained from parents/guardians of the children included in the study.
Conflict of Interest Statement
The authors have no conflicts of interest relevant to this article to disclose.
Funding Sources
No external funding was received for this manuscript.
Author Contributions
M.G., D.M., and P.R.R. conceptualized and designed the study, conducted the literature search, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. R.N. conceptualized and designed the study, coordinated and supervised data collection, critically reviewed and revised the manuscript for important intellectual content, and finalized the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding Statement
No external funding was received for this manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.WHO. Pneumonia of unknown cause – China 2020 [Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229.
- 2. Stafstrom CE, Jantzie LL. COVID-19: neurological considerations in neonates and children. Children. 2020;7(9):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: why children fare better than adults? Indian J Pediatr. 2020;87(7):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilking AN, Elliott E, Garcia MN, Murray KO, Munoz FM. Central nervous system manifestations in pediatric patients with influenza A H1N1 infection during the 2009 pandemic. Pediatr Neurol. 2014;51(3):370–6. [DOI] [PubMed] [Google Scholar]
- 5. Chess S. The influence of defect on development in children with congenital rubella. Merrill-Palmer Q Behav Dev. 1974;20(4):255–74. [Google Scholar]
- 6. Mawson AR, Croft AM. Rubella virus infection, the congenital Rubella syndrome, and the link to autism. Int J Environ Res Public Health. 2019;16(19):3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lebov JF, Brown LM, MacDonald PDM, Robertson K, Bowman NM, Hooper SR, et al. Review: evidence of neurological sequelae in children with acquired Zika virus infection. Pediatr Neurol. 2018;85:16–20. [DOI] [PubMed] [Google Scholar]
- 8. Liu HY, Guo J, Zeng C, Cao Y, Ran R, Wu T, et al. Transient early fine motor abnormalities in infants born to COVID-19 mothers are associated with placental hypoxia and ischemia. Front Pediatr. 2021;9:793561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buonsenso D, Costa S, Giordano L, Priolo F, Colonna AT, Morini S, et al. Short- and mid-term multidisciplinary outcomes of newborns exposed to SARS-CoV-2 in utero or during the perinatal period: preliminary findings. Eur J Pediatr. 2022;181(4):1507–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayesa-Arriola R, Castro Quintas Á, Ortiz-García De La Foz V, Miguel Corredera M, San Martín González N, Murillo-García N, et al. Exploring the impact of COVID-19 on newborn neurodevelopment: a pilot study. Sci Rep. 2023;13(1):2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edlow AG, Castro VM, Shook LL, Kaimal AJ, Perlis RH. Neurodevelopmental outcomes at 1 Year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw Open. 2022;5(6):e2215787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah AV, Howell HB, Kazmi SH, Zaccario M, Sklamberg FE, Groth T, et al. Developmental screening of full-term infants at 16 to 18 months of age after in-utero exposure to maternal SARS-CoV-2 infection. J Perinatol. 2023;43(5):659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shuffrey LC, Firestein MR, Kyle MH, Fields A, Alcántara C, Amso D, et al. Association of birth during the COVID-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr. 2022;176:e215563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nanavati R, Mascarenhas D, Goyal M, Haribalakrishna A, Nataraj G. A single-center observational study on clinical features and outcomes of 21 SARS-CoV-2-infected neonates from India. Eur J Pediatr. 2021;180(6):1895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat Med. 2019;25(8):1213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21. [DOI] [PubMed] [Google Scholar]
- 17. Wenner Moyer M. The COVID generation: how is the pandemic affecting kids' brains? Nature. 2022;601(7892):180–3. [DOI] [PubMed] [Google Scholar]
- 18. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shook LL, Sullivan EL, Lo JO, Perlis RH, Edlow AG. COVID-19 in pregnancy: implications for fetal brain development. Trends Mol Med. 2022;28(4):319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergmann C, Dimitrova N, Alaslani K, Almohammadi A, Alroqi H, Aussems S, et al. Young children’s screen time during the first COVID-19 lockdown in 12 countries. Sci Rep. 2022;12(1):2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McHarg G, Ribner AD, Devine RT, Hughes C. Screen time and executive function in toddlerhood: a longitudinal study. Front Psychol. 2020;11:570392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pereda N, Díaz-Faes DA. Family violence against children in the wake of COVID-19 pandemic: a review of current perspectives and risk factors. Child Adolesc Psychiatry Ment Health. 2020;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perkins S, Graham-Bermann S. Violence exposure and the development of school-related functioning: mental health, neurocognition, and learning. Aggress Violent Behav. 2012;17(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hendry A, Gibson SP, Davies C, McGillion M, Gonzalez-Gomez N. Toward a dimensional model of risk and protective factors influencing children’s early cognitive, social, and emotional development during the COVID-19 pandemic. Infancy. 2023;28(1):158–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong LP, Alias H, Farid NDN, Yusop SM, Musa Z, Hu Z, et al. Parent-child relationships and psychological distress: survey of parents from low-income families after the COVID-19 pandemic. Front Public Health. 2023;11:1158698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Chen L, Jia F, Shi X, Zhang Y, Li F, et al. Risk factors and prediction nomogram model for psychosocial and behavioural problems among children and adolescents during the COVID-19 pandemic: a national multicentre study: risk Factors of Childhood Psychosocial Problems. J Affect Disord. 2021;294:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedernera Bradichansky P, Selvatici L, Napoli S, Lejarraga C, Mato A, Urinovsky MG, et al. Teleconsultation during a pandemic. Experience of developmental pediatricians at Hospital de Pediatría “Prof. Dr. Juan P. Garrahan. Arch Argent Pediatr. 2021;119(6):419–23. [DOI] [PubMed] [Google Scholar]
- 28. Uranaka T, Kashio A, Ueha R, Sato T, Bing H, Ying G, et al. Expression of ACE2, TMPRSS2, and furin in mouse ear tissue, and the implications for SARS-CoV-2 infection. Laryngoscope. 2021;131(6):E2013–7. [DOI] [PubMed] [Google Scholar]
- 29. Goulioumis A, Angelopoulou M, Kourelis K, Mourtzouchos K, Tsiakou M, Asimakopoulos A. Hearing screening test in neonates born to COVID-19-positive mothers. Eur J Pediatr. 2023;182(3):1077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.