Abstract
Introduction
Neonatal presentation of coarctation of the aorta (CoA) is a potentially life-threatening condition that is difficult to diagnose in fetal life. We therefore sought to validate and compare novel metrics that may add diagnostic value for fetal CoA, including the diastolic to systolic aortic isthmus VTI ratio (VTId:VTIs), ascending aorta to descending aorta angle (AAo-DAo), transverse aorta to descending aorta angle (TAo-DAo), and LV longitudinal strain (LVS), then to evaluate whether these novel metrics improve specificity to identify fetuses at the highest risk for postnatal CoA without compromising sensitivity.
Methods
Retrospective cohort study of fetuses followed a prospective clinical pathway and previously classified as mild, moderate, or high-risk for CoA based on standard fetal echo metrics. Novel metrics were retrospectively measured in a blinded manner.
Results
Among fetuses with prenatal concern for CoA, VTId:VTIs, AAo-DAo angle, TAo-DAo angle, and LVS were significantly different between surgical and non-surgical cases (p < 0.01 for all variables). In the subgroup of moderate- and high-risk fetuses, the standard high-risk criteria (flow reversal at the foramen ovale or aortic arch) did not discriminate effectively between surgical and non-surgical cases. VTId:VTIs, AAo-Dao angle, Tao-DAo angle, and LVS all demonstrated greater discrimination than standard high-risk criteria, with specificity of 100% and PPV (positive predictive value) of 78–100%.
Conclusions
The incorporation of novel metrics added diagnostic value to our clinical pathway for fetal CoA with higher specificity than the previous high-risk criteria. The incorporation of these metrics into the evaluation of fetuses at moderate- or high-risk for surgical CoA may improve prenatal counseling, allow for more consistent surgical planning, and ultimately optimize hospital resource allocation.
Keywords: Fetal echocardiography, Coarctation, Congenital heart disease, Cardiac surgery, Myocardial strain
Plain Language Summary
Coarctation of the aorta is challenging to diagnosis in fetal life. New fetal echocardiographic techniques have been developed to help predict the development of surgical coarctation of the aorta after birth but have not been externally validated or compared to each other. We sought to validate these metrics and compare their utility in predicting surgical coarctation of the aorta in a retrospective cohort of previously identified fetuses at risk for coarctation of the aorta. We found that among fetuses with prenatal concern for coarctation of the aorta, novel metrics were significantly different between surgical and non-surgical cases. Our results indicate that the incorporation of novel metrics can add diagnostic value for fetal coarctation of the aorta with higher specificity than previous high-risk criteria, without compromising the sensitivity of the low-risk group. The ability to identify fetuses at the highest risk for surgical coarctation may improve prenatal counseling, allow for more consistent surgical planning, and ultimately optimize hospital resource allocation.
Introduction
Neonatal presentation of coarctation of the aorta (CoA) is a potentially life-threatening condition that remains challenging to diagnose in fetal life [1, 2]. When accurately identified and appropriately treated, it carries a low risk of immediate mortality and lasting morbidity [3]. Fetal diagnosis remains difficult because of the patency of the ductus arteriosus and the primarily parallel circulation, in which only 20% of fetal cardiac output crosses the aortic arch [4]. Current strategies optimize sensitivity at the cost of specificity, leading to frequent, unnecessary medical interventions for these patients and undue excessive burden of care for their families [5–8].
Recent work by a multidisciplinary group at our institution demonstrated that the implementation of a fetal stratification pathway effectively discriminates between infants at different levels of risk for surgical coarctation and reduces unnecessary medicalization [8]. The criteria used a combination of anatomic measures (standard 2D measurements, derived z-scores, and/or ratios of left-to-right-sided structures) to identify moderate-risk fetuses, and physiologic features (reversed foramen ovale or aortic arch flow) to identify those at the highest risk of CoA. Although the risk categorization schema and postnatal treatment pathway are novel, the actual measurements used to derive risk categories are well-established and used clinically at major fetal heart centers [8–10]. The results of the study demonstrated high sensitivity but less than ideal specificity in identifying surgical CoA, with only 63% of those stratified as high-risk with true surgical CoA in the neonatal period [8].
To improve specificity in detecting surgical CoA without compromising sensitivity, novel fetal echocardiography metrics have been proposed by various groups, including aortic arch angulation measurements, diastolic to systolic aortic isthmus VTI ratio (VTId:VTIs), and LV longitudinal strain (LVS) [11–13]. Differences in arch angulation are thought to represent conformational changes due to differences in flow characteristics of fetuses who develop surgical CoA [11]. With significant vascular narrowing, the complete LV stroke volume cannot completely pass through the isthmus during systole, resulting in flow accumulation in the proximal aorta and increased diastolic flow through the isthmus, resulting in elevated VTId:VTIs ratio [12]. While not fully understood, reduction in LVS in fetuses with surgical CoA are thought to be related to impaired contraction related to increased afterload and the compensatory ventricular changes [13]. These metrics were selected due to their relatively simple implementation, use of commonly obtained fetal echocardiographic images, and reported association with surgical coarctation. Prior studies of these novel metrics were limited to retrospective, single-center studies, applied in idealized clinical settings, and did not compare the diagnostic power of novel metrics. Fetuses across the studies were also limited to second-trimester gestational ages [11–13].
We aimed to improve the ability of our institutional clinical pathway to appropriately risk stratify fetuses at increased risk for postnatal surgical CoA. To this end, our objectives were (1) to validate novel metrics (VTId:VTIs ratio, AAo-DAo angle, TAo–DAo angle, and LVS), (2) to compare novel metrics between each other and with previously studied criteria which represent the standard of care at our institution, and (3) to apply these metrics specifically to the moderate- or high-risk group. We hypothesized that implementation of these metrics would improve the specificity of high-risk criteria without compromising the sensitivity of the low-risk group.
Methods
We queried our aortic arch watch database for fetuses who met criteria for inclusion in the pathway from 2016 to 2021. Fetuses met criteria for the pathway, and for inclusion in the current study, if they met criteria for at least mild risk for postnatal surgical CoA [4]. These criteria were as described in the previous report of our clinical pathway and include: transverse or arch isthmus z-score < −2, or isthmus ductus ratio <0.8 [14]. Mild-risk fetuses met the above criteria without meeting the criteria for moderate risk. Moderate-risk criteria included more severe aortic arch narrowing (Z < −3) or ≥1 other specific anatomic finding consistent with CoA (increased carotid-subclavian artery index, mitral/aortic valve hypoplasia, significant RV/LV discrepancy, posterior shelf, and structural cardiac defects known to be associated with CoA) [8]. High-risk criteria included moderate-risk anatomical features in addition to any aortic arch flow reversal or any foramen ovale left-to-right flow, including bidirectional flow at these levels [15]. Fetal echocardiograms were then reviewed to ensure fetuses were correctly classified by CoA risk and that adequate images were present to measure the required metrics. Exclusion criteria included: significant prematurity (GA <28 weeks), other significant non-cardiac diseases, CHD that otherwise required PGE infusion, fetal cardiac intervention, fetal demise, or insufficient images to perform the measurements necessary to derive novel metrics. All subjects in the study cohort received at least one postnatal echocardiogram. The primary outcome of all the analyses was neonatal CoA surgery [8]. Criteria for surgery included: clear surgical CoA on postnatal imaging (echocardiogram, CT angiogram, and/or cardiac MRI), diminished or absent lower extremity pulses, signs of otherwise unexplained acute renal dysfunction or mounting lactic acidosis, and/or systolic blood pressure gradient ≥20 mm Hg. Fetuses known to have structural cardiac anomalies that require surgical repair, but do not confer dependency on PGE or neonatal intervention, were included.
Due to the wide range of gestational ages present, the presence of multiple studies for each fetus, and the significant role gestational age can play on image quality, the fetal echocardiogram closest to 27 weeks was selected for this research study (with a mean age of 25.3 weeks).
All measurements were obtained by an observer blinded to CoA risk score and postnatal outcome and were applied to previously obtained echocardiograms. All 110 blinded studies were then randomized and 10% were selected to assess for interobserver reliability. The randomized blinded studies were distributed to two separate observers who performed the measurements. Intraclass correlation coefficient and coefficients of variation were then calculated between the two reviewers. The identity of the reviewers was blinded during calculations. Measurement variability was minimized through the training of blinded observers, initial selection of only studies with optimized desired images, standardization of image plane, and adherence to the specific measurement protocol as described by the original author [11–13].
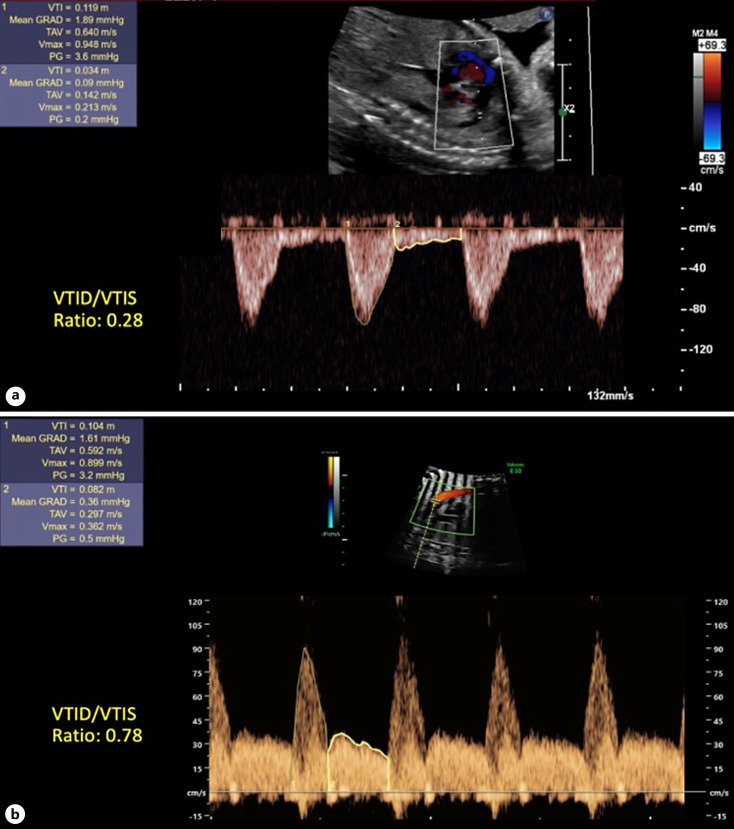
To measure the VTId:VTIs ratio, pulse-wave Doppler was performed at the narrowest part of the aortic isthmus parallel to the direction of the aortic isthmus. The systolic velocity-time integral (VTIs) and diastolic velocity-time integral (VTId) were measured, and the VTId:VTIs ratio was calculated [12, 16, 17] (Fig. 1).
Fig. 1.
VTId:VTIs ratio. a VTI ratio <0.56 in patient without CoA. b VTI ratio >0.56 in patient with surgical CoA.
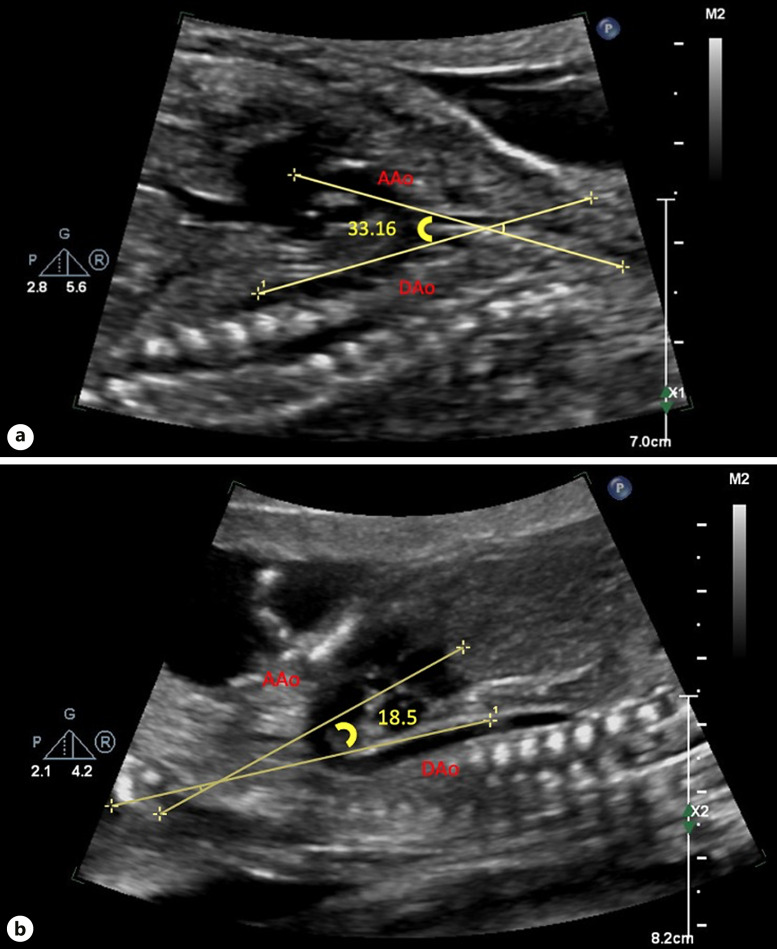
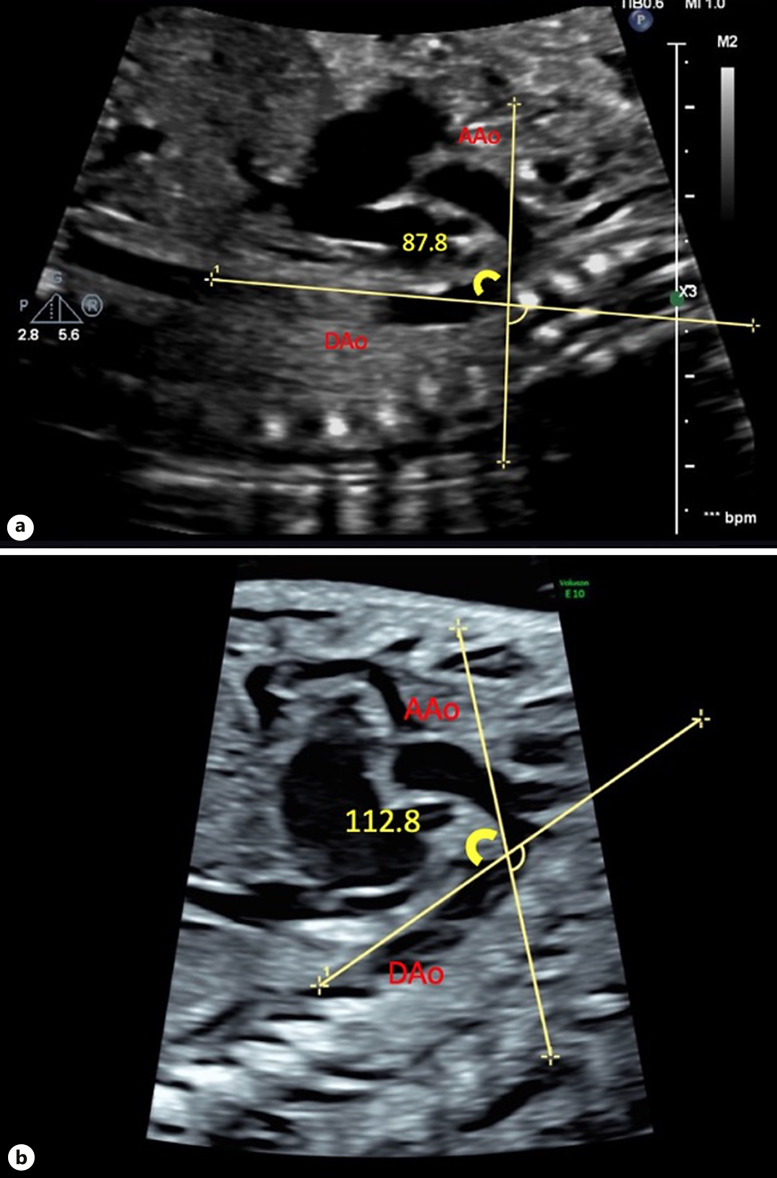
Both arch angulation measurements were obtained in the sagittal view during systole. To measure the AAo-DAo angle, a straight line was drawn from just cranial to the AoV immediately prior to the first arch vessel along the lesser curvature of the AAo. The second line was drawn from the transverse aortic arch immediately proximal to the aortic isthmus to the thoracic abdominal aorta along the lesser curvature of the aortic arch [14] (Fig. 2). To measure the TAo-DAo angle, the first line was drawn from the takeoff of the first head vessel to the takeoff of the third head vessel along the lesser curvature of the aortic arch. The second line was drawn just prior to the aortic isthmus to the thoracic abdominal aorta, along the lesser curvature of the aortic arch [14] (Fig. 3).
Fig. 2.
AAo-DAo angle. a AAo-DAo angle >20.3° in patient without CoA. b AAo-DAo angle in patient with surgical CoA <20.3°.
Fig. 3.
TAo-DAo angle. a TAo-DAo angle <96.2° in patient without CoA. b TAo-DAo angle >96.2° in patient with surgical CoA.
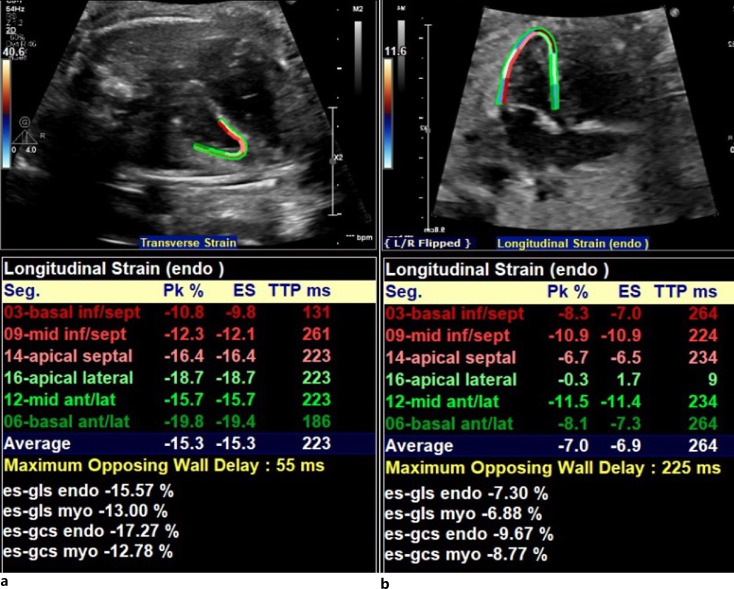
LVS was measured in the four-chamber view using vendor-independent software (TOMTEC Imaging Systems GmbH Freisinger Strasse 9 2D strain package V 41.00) in a semi-automated fashion [18–21]. Points of interest were placed at the basal LV free wall, basal septum, and apex, with a minimal frame rate of 40 frames/s. The LV endocardium was then detected using the software. Manual adjustments were made to ensure tracking of the endocardial border throughout the cardiac cycle, with data recorded as absolute values (Fig. 4).
Fig. 4.
LV longitudinal strain. a Normal LVS in patient without CoA. b Abnormal LVS in patient with surgical CoA.
Statistical analyses were performed using SPSS for Mac version 28.0 (IBM), with a significance level of <0.05. Descriptive statistics are presented as counts (percentages) or mean ± standard deviation (SD). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of fetal echocardiographic measures for surgical CoA are described. McNemar’s test was used to analyze the paired nominal data. Categorical data were compared using the χ2 or Fisher’s exact test. Receiver operating characteristic curve analyses with a paired sample design were used to compare the discriminatory power of previously published risk stratification criteria to the following novel metrics: AAo-DAo angle, Tao-DAo angle, VTId:VTIs ratio, and LVS to distinguish patients with and without surgical CoA. Optimal cutoff points were identified using the Youden index method and compared to previously described thresholds [11–13]. The Stanford University Institutional Review Board approved the ongoing study of the fetal CoA pathway.
Results
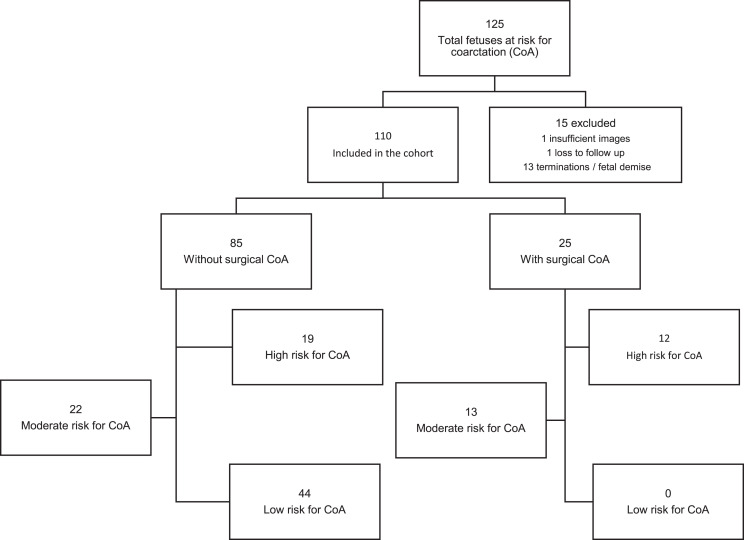
During the study period, 125 fetuses were followed through the CoA pathway. Of these, 15 (12%) fetuses were excluded from analysis: 13 terminations/fetal demise, one with insufficient images, and one loss to follow-up. Among the remaining cohort of 110, 44 (40%) were classified as mild-risk, 35 (32%) moderate-risk, and 31 (28%) high-risk. Surgical CoA was performed in 25 (23%) Figure 5 and Table 1 describe the characteristics of fetuses included in the study. None of the fetuses in the mild-risk category developed surgical CoA. There were no statistically significant differences in baseline characteristics between fetuses that underwent surgery in terms of race/ethnicity, fetal sex, presence of identifiable underlying genetic disease, estimated fetal weight, or estimated fetal cardiac output. The mean gestational age of the included fetal echocardiograms was 25.3 ± 5.9 (weeks).
Fig. 5.
Flow chart of study cohort showing the total number of fetuses included and excluded number of subjects with and without surgical CoA, and number of subjects within each risk stratification tier.
Table 1.
Cohort characteristics of subjects with surgical CoA and without CoA
| Total cohort (N = 110) | No CoA (N = 85) | CoA (N = 25) | p value | |
|---|---|---|---|---|
| Maternal age | 32.0±5.4 | 32.2±5.8 | 31.7±4.3 | 0.66 |
| Race/ethnicity, n (%) | White/Caucasian: 38 (35) | White/Caucasian: 29 (34) | White/Caucasian: 9 (36) | 0.97 |
| Hispanic: 32 (29) | Hispanic: 25 (29) | Hispanic: 7 (28) | ||
| Asian: 25 (22) | Asian: 19 (22) | Asian: 6 (24) | ||
| Black/African American: 3 (3) | Black/African American: 2 (2) | Black/African American: 1 (4) | ||
| Other: 2 (2) | Other: 2 (2) | Other: 0 | ||
| Unknown: 10 (9) | Unknown: 8 (9) | Unknown: 2 (8) | ||
| Indication for referral (CHD vs. screening)*, n (%) | 68 (62) | 48 (56) | 20 (80) | 0.03 |
| Average gestational age first fetal echo, weeks | 25.3±5.9 | 25.5±6.0 | 24.8±5.8 | 0.59 |
| Average gestational age last fetal echo, weeks | 31.9±3.6 | 31.9±3.6 | 32.4±3.5 | 0.57 |
| Fetal sex (female), n (%) | 47 (43) | 36 (42) | 11 (44) | 0.88 |
| Genetic/syndromic, n (%) | 14 (13)3 | 10 (12) | 4 (16) | 0.58 |
| Associated major CHD**, n (%) | 31 (28) | 18 (21) | 13 (52) | <0.01 |
| Estimated fetal weight, g | 1,627.7±640 | 1,628.9±645.8 | 1,734.7±574.9 | 0.46 |
| Estimated fetal Co., mL/kg/min*** | 366.2±175 | 383.6±167 | 346±174 | 0.34 |
| Excluded fetuses | 1 insufficient images, 1 loss to follow-up, 13 terminations/fetal demise | |||
*CHD, cases specifically referred for fetal echocardiography due to concern for congenital heart defect on obstetric ultrasound. Screening refers to other indications of perform a fetal echocardiogram.
**Structural cardiac anomalies which require surgical repair but do not confer dependency on PGE or neonatal intervention.
***Cardiac output.
Of the 25 fetuses who underwent neonatal CoA surgery, 76% met previously described standard high-risk criteria (flow reversal at either foramen ovale or aortic arch). Combined aortic and mitral valve z-score < −4 was found in all patients who underwent CoA surgery. VTId:VTIs, AAo-DAo angle, TAo-DAo angle, and LVS were statistically different in the surgical CoA group versus the non-surgical CoA group, with optimal cut-points of 0.31, 21°, 98° and 12.8%, respectively (p < 0.01 for all, Tables 2, 3). Surgical CoA was present in 13 (37%) moderate-risk fetuses compared with 12 (38%) high-risk fetuses. The previously studied and current institutional high-risk criteria did not effectively discriminate between surgical CoA and non-surgical CoA cases in the entire cohort or in the moderate- and high-risk subgroup (Tables 4, 5, p = 0.21). Combined mitral and aortic z-score with an optimal cut point of (−4.1) demonstrated discriminatory power, with PPV of 56% and NPV of 77% (p = 0.03). Each novel metric discriminated effectively between fetuses who ultimately had surgical CoA in both the entire cohort and in the moderate- and high-risk subgroups. The novel metrics that demonstrated the highest discriminatory power were AAo-DAo Angle, TAo-DAo and LVS (Tables 6–9, Fig. 6).
Table 2.
Comparison of novel metrics with previous standards in entire cohort between surgical CoA and No CoA (N = 110)
| No CoA (N = 85) | CoA (N = 25) | p value | |
|---|---|---|---|
| Novel metrics | |||
| VTI diastolic systolic ratio | 0.31±0.09 | 0.51±0.3 | <0.01 |
| AAo-DAo angle | 26.4±3.0 | 18.0±2.7 | <0.01 |
| TAo-DAo angle | 85.9±4.7 | 103.4±9.11 | <0.01 |
| LV longitudinal strain | 16.6±2.8 | 9.04±3.05 | <0.01 |
| Standard metrics | |||
| Combined mitral/aortic Z-score | −2.66±1.89 | −4.35±2.03 | <0.01 |
| Flow reversal PFO or aortic arch | 91 | 19 | <0.01 |
VTI, velocity time interval; AAo, ascending aorta; DAo, descending aorta; TAo, thoracic aorta; LV, left ventricular; PFO, patent foramen ovale.
Table 3.
Comparison of novel metrics with previous standards in entire cohort between surgical CoA and No CoA (N = 65)
| No CoA (N = 40) | CoA (N = 25) | p value | |
|---|---|---|---|
| Novel metrics | |||
| VTI diastolic systolic ratio | 0.32±0.1 | 0.54±0.3 | <0.01 |
| AAo-DAo angle | 26.7±2.9 | 18.2±2.8 | <0.01 |
| TAo-DAo angle | 87.7±4.9 | 102.9±9.24 | <0.01 |
| LV longitudinal strain | 16.6±2.6 | 8.7±3.1 | <0.01 |
| Standard metrics | |||
| Combined mitral/aortic Z-score | −3.14±1.9 | −4.42±2.0 | <0.01 |
| Flow reversal PFO or aortic arch | 43 | 22 | 0.10 |
VTI, velocity time interval; AAo, ascending aorta; DAo, descending aorta; TAo, thoracic aorta; LV, left ventricular; PFO, patent foramen ovale.
Table 4.
Sensitivity and specificity of novel metrics with previous standards in entire cohort between surgical CoA and No CoA (N = 110)
| Metric | Sensitivity, % | Specificity, % | Confidence interval sensitivity | Confidence interval specificity | PPV, % | NPV, % | p value |
|---|---|---|---|---|---|---|---|
| Novel metrics | |||||||
| VTI diastolic systolic ratio (>0.31) | 32 | 90 | 0.21–0.46 | 0.79–0.96 | 53 | 80 | <0.01 |
| AAo-DAo angle (<21 degrees) | 100 | 98 | 0.94–1.0 | 0.72–0.98 | 92 | 100 | <0.01 |
| TAo-DAo angle (>98 degrees) | 100 | 97 | 0.94–1.0 | 0.7–0.96 | 90 | 100 | <0.01 |
| LV long strain (<12.8%) | 85 | 96 | 0.63–0.94 | 0.68–0.95 | 88 | 96 | <0.01 |
| Standard metrics | |||||||
| Combined mitral/aortic Z-score (<−4.1) | 39 | 81 | 0.27–0.44 | 0.58–0.89 | 28 | 90 | 0.08 |
| Flow reversal PFO or aortic arch | 47 | 82 | 0.37–0.52 | 0.6–0.92 | 36 | 88 | 0.32 |
VTI, velocity time interval; AAo, ascending aorta; DAo, descending aorta; TAo, thoracic aorta; LV, left ventricular; PFO, patent foramen ovale.
Table 5.
Sensitivity and specificity of novel metrics with previous standards in moderate and high risk cohort between surgical CoA and No CoA (N = 65)
| Metric | Sensitivity, % | Specificity, % | Confidence interval sensitivity | Confidence interval specificity | PPV, % | NPV, % | p value |
|---|---|---|---|---|---|---|---|
| Novel metrics | |||||||
| VTI diastolic systolic ratio (>0.31) | 45 | 100 | 0.27–0.52 | 0.89–1.0 | 100 | 78 | <0.01 |
| AAo-DAo angle (<21°) | 92 | 100 | 0.72–0.99 | 0.89–1.0 | 100 | 97 | <0.01 |
| TAo-DAo angle (>98°) | 90 | 100 | 0.67–0.97 | 0.89–1.0 | 100 | 95 | <0.01 |
| LV long strain (<12.8%) | 86 | 100 | 0.63–0.94 | 0.89–1.0 | 100 | 93 | <0.01 |
| Standard metrics | |||||||
| Combined mitral/aortic Z-score (<−4.1) | 63 | 65 | 0.43–0.74 | 0.48–0.78 | 56 | 77 | 0.03 |
| Flow reversal PFO or aortic arch | 41 | 79 | 0.22–0.68 | 0.61–0.89 | 50 | 71 | 0.21 |
VTI, velocity time interval; AAo, ascending aorta; DAo, descending aorta; TAo, thoracic aorta; LV, left ventricular; PFO, patent foramen ovale.
Table 6.
Paired-sample area difference under the ROC curves – entire cohort (N = 110)
| Test result pair(s) | AUC difference | p value |
|---|---|---|
| VTId:VTIs versus AAo-DAo angle | −0.21 | <0.01 |
| VTId:VTIs versus TAo-DAo angle | −0.17 | 0.01 |
| VTId:VTIs versus LVS | −0.21 | <0.01 |
| VTId:VTIs versus combined mitral aortic Z-score | 0.09 | 0.33 |
| VTId:VTIs versus PFO or aortic arch flow reversal | 0.14 | 0.08 |
| AAo-DAo angle versus TAo-DAo angle | 0.05 | 0.17 |
| AAo-DAo angle versus LVS | 0.01 | 0.80 |
| AAo-DAo angle versus combined mitral aortic Z-score | 0.30 | <0.01 |
| AAo-DAo angle versus PFO or aortic arch flow reversal | 0.35 | <0.01 |
| TAo-DAo angle versus LVS | −0.04 | 0.28 |
| TAo-DAo angle versus combined mitral aortic Z-score | 0.26 | <0.01 |
| TAo-DAo angle versus PFO or aortic arch flow reversal | 0.31 | <0.01 |
| LVS versus combined mitral aortic Z-score | 0.30 | <0.01 |
| LVS versus PFO or aortic arch flow reversal | 0.35 | <0.01 |
| Combined mitral aortic Z-score versus PFO or aortic arch flow reversal | 0.05 | 0.46 |
VTId:VTIs, velocity time interval diastolic to systolic ratio; AAo-DAo angle, ascending to descending aorta angle; TAo-DAo angle, transverse to descending aorta angle; LVS, left ventricular longitudinal strain; PFO, patent foramen ovale.
Table 9.
AUC novel metrics moderate/high risk cohort
| Parameter | AUC (95% CI) |
|---|---|
| VTId:VTIs | 0.76 (0.63, 0.88) |
| AAo-DAo angle | 0.97 (0.94, 1.0) |
| TAo-DAo angle | 0.93 (0.83, 1.0) |
| LVS | 0.97 (0.93, 1.0) |
| Combined mitral aortic Z-score | 0.53 (0.538, 0.81) |
| PFO or aortic flow reversal | 0.52 (0.52, 0.72) |
VTId:VTIs, velocity time interval diastolic to systolic ratio; AAo-DAo angle, ascending to descending aorta angle; TAo-DAo angle, transverse to descending aorta angle; LVS, left ventricular longitudinal strain; PFO, patent foramen ovale.
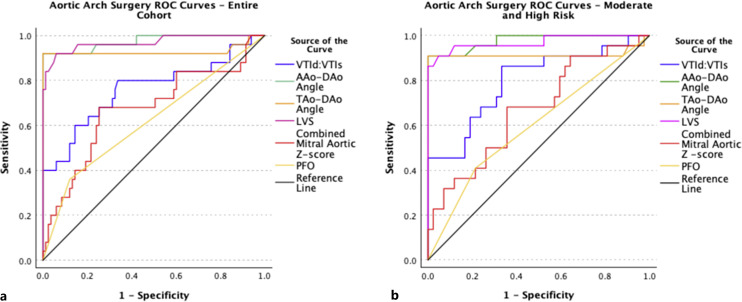
Fig. 6.
Aortic arch surgery receiver operating characteristic (ROC) curve. These ROC curves depict the ability of each fetal echocardiographic measure to discriminate between fetuses who did and did not undergo neonatal aortic arch surgery in the entire cohort (a) and fetuses in the moderate- or high-risk groups who did and did not undergo neonatal arch surgery (b).
Table 7.
AUC novel metrics entire cohort
| Parameter | AUC (95% CI) |
|---|---|
| VTId:VTIs | 0.80 (0.67, 0.92) |
| AAo-DAo angle | 0.98 (0.94, 1.0) |
| TAo-DAo angle | 0.92 (0.8, 1.0) |
| LVS | 0.97 (0.92, 1.0) |
| Combined mitral aortic Z-score | 0.67 (0.53, 0.81) |
| PFO or aortic flow reversal | 0.60 (0.48, 0.72) |
VTId:VTIs, velocity time interval diastolic to systolic ratio; AAo-DAo angle, ascending to descending aorta angle; TAo-DAo angle, transverse to descending aorta anglemargo LV Left ventricular longitudinal strain; PFO, patent foramen ovale.
Table 8.
Cut points novel metrics moderate/high risk cohort
| Parameter | Cut point |
|---|---|
| VTId:VTIs | >0.31 |
| AAo-DAo angle | <21 |
| TAo-DAo angle | >98 |
| LVS | <12.8 |
VTId:VTIs, velocity time interval diastolic to systolic ratio; AAo-DAo angle, ascending to descending aorta angle. TAo-DAo angle, transverse to descending aorta angle; LVS, left ventricular longitudinal strain; PFO, patent foramen ovale.
AAo-DAo and LVS had the highest discriminatory power between fetuses with and without CoA with an area under the curve (AUC) of 0.97 and 0.97 respectively, closely followed by TAo-DAo angle (0.93). VTId:VTIs had the next highest AUC, followed by the combined mitral aortic z-score (0.76 and 0.53). Flow reversal at the foramen ovale or aortic arch had the lowest AUC and was not statistically significant in the moderate- and high-risk subgroup (p = 0.30) or in the entire cohort (p = 0.21). VTId:VTIs had the lowest sensitivity in the novel metrics group (45%), with 100% specificity, PPV of 100%, and NPV of 78%. AAo-DAo Angle and TAo-DAo angles demonstrated equally high discriminatory values with 100% specificity and PPV (p < 0.01). LVS demonstrated a sensitivity of 86%, specificity of 100%, PPV of 100%, and NPV of 93% (p < 0.01; Tables 4, 5). Each of the novel metrics (AAo-DAo, TAo-DAo, LVS, and VTId:VTIs) was able to discriminate between cases with and without surgical CoA more effectively than flow reversal at the foramen ovale or aortic arch, and more effectively than combined mitral and aortic valve z-score in both the entire cohort (Table 6) and in the moderate- and high-risk cohorts (Table 10). One hundred percent specificity was achieved for VTId:VTIs ratio, AAo-DAo Angle, and TAo-DAo Angle when each of the metrics was applied retrospectively to the surgical CoA cohort with the above described cut points; however, sensitivity was significantly lower for VTId:VTIs and false negatives were present in each of the metrics (Table 5).
Table 10.
Paired-sample area difference under the ROC curves – moderate/high risk cohort N = 65
| Test result pair(s) | AUC difference | p value |
|---|---|---|
| VTId:VTIs versus AAo-DAo angle | −0.18 | <0.01 |
| VTId:VTIs versus TAo-DAo angle | −0.12 | 0.07 |
| VTId:VTIs versus LVS | −0.17 | 0.00 |
| VTId:VTIs versus combined mitral aortic Z-score | 0.13 | 0.22 |
| VTId:VTIs versus PFO or aortic arch flow reversal | 0.20 | 0.03 |
| AAo-DAo angle versus TAo-DAo angle | 0.06 | 0.16 |
| AAo-DAo angle versus LVS | 0.01 | 0.73 |
| AAo-DAo angle versus combined mitral aortic Z-score | 0.31 | <0.01 |
| AAo-DAo angle versus PFO or aortic arch flow reversal | 0.38 | <0.01 |
| TAo-DAo angle versus LVS | −0.06 | 0.22 |
| TAo-DAo angle versus combined mitral aortic Z-score | 0.25 | 0.01 |
| TAo-DAo angle versus PFO or aortic arch flow reversal | 0.32 | <0.01 |
| LVS versus combined mitral aortic Z-score | 0.30 | <0.01 |
| LVS versus PFO or aortic arch flow reversal | 0.37 | <0.01 |
| Combined mitral aortic Z-score versus PFO or aortic arch flow reversal | 0.07 | 0.39 |
VTId:VTIs, velocity time interval diastolic to systolic ratio; AAo-DAo angle, ascending to descending aorta angle; TAo-DAo angle, transverse to descending aorta angle; LVS, left ventricular longitudinal strain; PFO, patent foramen ovale.
Intraclass correlation coefficient and mean percentage interobserver differences between two reviewers was 0.78 and 10% ± 4.9% for VTId:VTIs, 0.77 and 13% ± 9% for AAo–DAo, 0.79 and 7.6% ± 6% for TAo–DAo, and 0.56 and 17% ± 13% for LVS. When analyzed as a categorical variable (above or below cut-points defined in the current study), interobserver agreement was 100% for AAo-DAo and LVS, and 78% for TAo-DAo and VTId:VTIs. The coefficients of variation were 27% for VTId:VTIs, 15% for AAo–DAo angle, 9% for TAo–DAo angle, and 28% for LVS.
Discussion
We aimed to improve the ability of our institutional clinical pathway to appropriately risk stratify fetuses at increased risk for postnatal surgical CoA. To this end, we sought to validate and compare the novel metrics with our previously established standard of care. Once validated, we posited that the implementation of these metrics would improve the specificity of high-risk criteria without compromising the sensitivity of the low-risk group. We found that VTId:VTIs, AAo-DAo angle, Tao-DAo angle, and LVS discriminate between surgical and non-surgical CoA cases in fetuses at risk for CoA more effectively than previously established high-risk criteria [11–13]. In the moderate-to-high-risk subgroup, the current standard of care did not discriminate between surgical and nonsurgical cases. In contrast, the novel metrics demonstrated a high discriminatory power.
Arch angulation measurements, VTId:VTIs ratio, and LVS may improve the prenatal identification of neonatal CoA. Values beyond the assigned cut-off points should prompt increased suspicion of surgical CoA. The metrics have the greatest benefit in fetuses for which the risk of CoA by standard means is thought to be at least moderate. The incorporation of arch angulation measurements into existing protocols for fetuses with CoA is timely, requires minimal additional training for practitioners, and does not require additional software. VTId: The VTIs in this study lacked the specificity of other novel metrics. LVS showed high specificity for CoA but also demonstrated only modest interobserver reliability.
Clinical Impact
There is a large potential clinical impact of implementing novel metrics in the fetal CoA pathway. If fetuses by prior criteria are thought to be moderate or high risk and have findings on novel metrics that indicate low risk for postnatal surgical CoA, the mothers of otherwise uncomplicated fetuses may be able to deliver at their home institutions without the need for transferring to a higher level of care. While intuitional policies differ, fetuses at some institutions with the highest risk for CoA have umbilical lines presumptively placed, and prostaglandin infusion started prior to the initial postnatal echocardiogram. In our earlier report, fetuses who developed surgical CoA met standard-risk criteria at a higher rate than those who did not develop surgical CoA. However, the diagnostic power of these criteria was not sufficient for clinical use [8]. As our experience has grown, standard-risk criteria are proving to be even less reliable than previously thought as is evidenced by the results of the current study. With the addition of novel metrics to existing institutional workflows, unnecessary umbilical catheter placement, time without enteral feeding, and overall length of hospital stay may be reduced in infants who do not ultimately develop surgical CoA.
Decreasing maternal anxiety and overall turmoil involved in a fetal diagnosis of CHD is paramount, as maternal anxiety is a known factor that can impact fetal development [22]. Fetal echocardiography plays a significant role in maternal psychological impact, and mothers with a prenatal diagnosis of congenital heart disease have been shown to have improved relationships with their infants [23, 24].
Validating Prior Work
As each of the original novel metrics was described in single-center studies, we first sought to validate their findings and subsequently compare them [11–13]. Although our study is retrospective, we applied each of the metrics to a cohort that was prospectively enrolled into a clinical pathway. Since clinical information and images were obtained prospectively, we hoped to avoid the common biases present in retrospective chart reviews. Additionally, standard metrics were obtained using clinical data in a standard workflow along the clinical pathway. Although we did not obtain novel metrics prospectively, we utilized the metrics in a clinical setting.
The results of this study were largely congruent with previous descriptions of the novel metrics. With regard to arch angulation, the original cutoff points for predicting surgical CoA for AAo-DAo and TAo-DAo angles described by Arya et al. [11] (≤20.31° and ≥96.15°) were very similar to those found in our study (≤21.0° and ≥98.0°) [14]. It has been shown that the distance from the carotid artery to subclavian artery in neonatal echocardiograms can indicate CoA in presence of a large ductus arteriosus, which is thought to be secondary to stretch and change in the overall contour of the transverse to proximal DAo [6]. The novel angle measurements obtained in the sagittal view likely reflect a similar arch contour change, which may be due to early changes in aortic flow characteristics. The more acute the angle between the AAo and DAo and the more obtuse the angle between the TAo and DAo, the larger the distance between the second and third head vessels [14]. Slight differences between the cut points reported in our study and those previously established are related to the overall small size of anatomic structures and the high magnitude of change in angle with small changes in vessel location. These small differences are informative because they reflect the expected degree of error in the measurements.
The original cutoff points to predict surgical CoA for VTId:VTIs described by Wang et al. were ≥0.56 compared to ≥0.31 in our study. Among all the novel metrics, VTId:VTIs had the largest difference in reported cut-off points. When we applied prior cut-off points established by Wang et al. [12] of 0.56), sensitivity and specificity reduced to 45% and 3%. Due to the significant difference in cut-off points, it remains unclear which cut-off point should be used for future clinical studies, bringing into question the overall validity of this metric (although internally reproducible), which requires further investigation. Based on our results, in most clinical settings, it would not provide benefits beyond standard metrics, but in certain clinical cases (poor 2D windows), a low number may be reassuring. It is thought that the elevated VTId:VTIs ratio in CoA is caused by narrowing of the aortic arch, resulting in hemodynamic changes. With significant narrowing, the complete LV stroke volume cannot completely pass through the isthmus during systole, resulting in flow accumulation in the ascending aorta and increased diastolic flow through the isthmus [12]. The differences between our studies may be related to the consistency in obtaining PW Dopplers at the narrowest position of the aortic isthmus parallel to the direction of the isthmus. Although we optimized the angle of interrogation of each spectral Doppler interrogation, we did not employ angle correction in our study, as it is not our clinical standard to do so. It has also been shown that ductus arteriosus flow affects the aortic isthmus flow in both diastole and systole [25]. Differences in fetal ductal physiology between our patient populations could have caused significant differences in diastolic flow, which would not have been accounted for in this study.
The original cut-off point to predict surgical CoA for LVS described by Devore et al. [13] was <10% compared to <12.8% in our study. Although LVS demonstrated only modest interobserver reliability as a continuous variable, there was 100% agreement when treated as a binary variable. This suggests that in a real-world setting, two different observers may obtain slightly different LVS% values but would agree on whether a particular patient would be at increased risk for CoA. Normal values for fetal LVS can vary widely with strain values changing across gestational age, normal distribution reported from some sources as 95th percentile confidence intervals ranging from 10 to 32% or 15–25% [13, 18]. There are multiple challenges to obtaining an accurate fetal LVS, including small cardiac dimensions, high heart rate, fetal movement, and differences in fetal positioning. These factors led to the relatively low interobserver reliability in our study. Given these challenges and the small numbers in both studies, the difference in identified cutoff point of only 2.8% between the studies is quite consistent. Finally, a major benefit of the LVS over other novel metrics is that it is obtained in a 4 chamber view. In comparison, the sagittal aortic arch view can be particularly difficult to obtain, especially in cases with aberrant anatomy.
Limitations
The limitations of our study include the fact that it is a single-center retrospective study. Although clinical data continue to be prospectively obtained, novel metrics have been retrospectively applied. While the study measurements were collected blindly, they were drawn from a pool of fetuses known to be at risk for surgical CoA. Even though the initial sample size was 125 fetuses, 15 subjects were excluded due to loss of follow-up, inadequate imaging, or fetal demise/termination, with the number of surgical cases remaining relatively small. Novel metrics were applied to fetal studies that took place at the beginning of the third trimester, consistent with our institutional pathway. A large prospective multicenter validation study with fetuses measured at varying gestational ages would help to demonstrate the clinical utility of these measurements. This study used vendor‐independent software when measuring LVS. In transthoracic echocardiography, vendor-independent software generally provides consistent agreement with vendor‐specific software for global longitudinal strain [26]. However, there may be variability between platforms not fully explored for fetal echocardiography. While this study used a single imaging platform and vendor independent software for analysis, generalizability may be affected by choice of imaging and processing platform.
Future Studies
The potential for each of these measurements to predict surgical coarctation merits a prospective, multi-center, study to assess the diagnostic power of a clinical pathway incorporating these novel metrics. Additional studies exploring alternative end points for these metrics such as prediction of surgical approach (sternotomy vs. thoracotomy) and postoperative outcomes should be considered. Finally, the insights garnered in this study may influence future study of potential in utero therapies to advance the growth of left-sided structures such as maternal hyperoxygenation.
Conclusions
In this study, novel metrics – VTId:VTIs, AAo-DAo angle, TAo-DAo angle, and LVS-discriminate effectively between surgical and non-surgical CoA. The incorporation of novel metrics added diagnostic value for fetal CoA with higher specificity than previously established criteria, without compromising the sensitivity of the low-risk group. Metrics of aortic arch angulation require no additional software and a lower amount of training relative to LVS and represent the most practical addition to existing fetal CoA pathways. Although aortic arch angulation has minimal interobserver variability, optimized aortic arch imaging is required for accurate angle measurements. In comparison, LVS requires only an optimized 4-chamber view which is often easier to obtain than arch imaging. In the moderate-to-high-risk group, VTId:VTIs demonstrated low sensitivity, whereas the other novel metrics were particularly powerful. Standard metrics demonstrated high sensitivity, and adding these novel metrics to existing clinical CoA metrics and pathways will improve PPV diagnostic accuracy and may ultimately result in reduced unnecessary medicalization and improved care of neonates who do not have surgical CoA.
Acknowledgment
We thank all the valuable members of the Fetal Cardiology Program at Stanford Children’s Healthcare for their hard work and dedication.
Statement of Ethics
This study protocol was reviewed and approved by the chair of Panel on Medical Human Subjects, Stanford University, approval number 51341. The need for informed consent was waived by the chair of Panel on Medical Human Subjects, Stanford University, approval number 51341.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Study conception and design: Aaron Anthony Phillips, Shiraz A. Maskatia, and Rajesh Punn. Data collection: Aaron Anthony Phillips, Shiraz A. Maskatia, Kelly Thorson, and Amy Quirin. Author analysis and interpretation of results: Shiraz A. Maskatia, Claudia Algaze, and Yair J. Blumenfeld. Author draft manuscript preparation: Aaron Anthony Phillips, Valerie Y. Chock, David M. Kwiatokowski, and Theresa A. Tacy. All authors have reviewed the results and approved the final version of the manuscript.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.
References
- 1. Vigneswaran TV, Bellsham-Revell HR, Chubb H, Simpson JM. Early postnatal echocardiography in neonates with a prenatal suspicion of coarctation of the aorta. Pediatr Cardiol. 2020;41(4):772–80. [DOI] [PubMed] [Google Scholar]
- 2. Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, et al. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics. 2015;136(2):e378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toro-Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89(5):541–7. [DOI] [PubMed] [Google Scholar]
- 4. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American heart association. Circulation. 2014;129(21):2183–242. [DOI] [PubMed] [Google Scholar]
- 5. Lytzen R, Vejlstrup N, Bjerre J, Petersen OB, Leenskjold S, Dodd JK, et al. Live-born major congenital heart disease in Denmark: incidence, detection rate, and termination of pregnancy rate from 1996 to 2013. JAMA Cardiol. 2018;3(9):829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soslow JH, Kavanaugh-McHugh A, Wang L, Saurers DL, Kaushik N, Killen SAS, et al. A clinical prediction model to estimate the risk for coarctation of the aorta in the presence of a patent ductus arteriosus. J Am Soc Echocardiogr. 2013;26(12):1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hede SV, DeVore G, Satou G, Sklansky M. Neonatal management of prenatally suspected coarctation of the aorta. Prenat Diagn. 2020;40(8):942–8. [DOI] [PubMed] [Google Scholar]
- 8. Maskatia SA, Kwiatkowski D, Bhombal S, Davis AS, McElhinney DB, Tacy TA, et al. A fetal risk stratification pathway for neonatal aortic coarctation reduces medical exposure. J Pediatr. 2021;237:102–8.e3. [DOI] [PubMed] [Google Scholar]
- 9. Vena F, Donarini G, Scala C, Tuo G, Paladini D. Redundancy of foramen ovale flap may mimic fetal aortic coarctation. Ultrasound Obstet Gynecol. 2020;56(6):857–63. [DOI] [PubMed] [Google Scholar]
- 10. Beattie M, Peyvandi S, Ganesan S, Moon-Grady A. Toward improving the fetal diagnosis of coarctation of the aorta. Pediatr Cardiol. 2017;38(2):344–52. [DOI] [PubMed] [Google Scholar]
- 11. Arya B, Bhat A, Vernon M, Conwell J, Lewin M. Utility of novel fetal echocardiographic morphometric measures of the aortic arch in the diagnosis of neonatal coarctation of the aorta. Prenat Diagn. 2016;36(2):127–34. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Lei W, Liu J, Yang B, Li H, Huang D. The diastolic and systolic velocity-time integral ratio of the aortic isthmus is a sensitive indicator of aortic coarctation in fetuses. J Am Soc Echocardiogr. 2019;32(11):1470–6. [DOI] [PubMed] [Google Scholar]
- 13. DeVore GR, Jone PN, Satou G, Sklansky M, Cuneo BF. Aortic coarctation: a comprehensive analysis of shape, size, and contractility of the fetal heart. Fetal Diagn Ther. 2020;47(5):429–39. [DOI] [PubMed] [Google Scholar]
- 14. Familiari A, Morlando M, Khalil A, Sonesson SE, Scala C, Rizzo G, et al. Risk factors for coarctation of the aorta on prenatal ultrasound: a systematic review and meta-analysis. Circulation. 2017;135(8):772–85. [DOI] [PubMed] [Google Scholar]
- 15. Berning RA, Silverman NH, Villegas M, Sahn DJ, Martin GR, Rice MJ. Reversed shunting across the ductus arteriosus or atrial septum in utero heralds severe congenital heart disease. J Am Coll Cardiol. 1996;27(2):481–6. [DOI] [PubMed] [Google Scholar]
- 16. Kennelly MM, Farah N, Hogan J, Reilly A, Turner MJ, Stuart B. Longitudinal study of aortic isthmus Doppler in appropriately grown and small-for-gestational-age fetuses with normal and abnormal umbilical artery Doppler. Ultrasound Obstet Gynecol. 2012;39(4):414–20. [DOI] [PubMed] [Google Scholar]
- 17. Garcia-Canadilla P, Crispi F, Cruz-Lemini M, Valenzuela-Alcaraz B, Rudenick PA, Gratacos E, et al. Understanding the aortic isthmus Doppler profile and its changes with gestational age using a lumped model of the fetal circulation. Fetal Diagn Ther. 2017;41(1):41–50. [DOI] [PubMed] [Google Scholar]
- 18. Maskatia SA, Pignatelli RH, Ayres NA, Altman CA, Sangi-Haghpeykar H, Lee W. Longitudinal changes and interobserver variability of systolic myocardial deformation values in a prospective cohort of healthy fetuses across gestation and after delivery. J Am Soc Echocardiogr. 2016;29(4):341–9. [DOI] [PubMed] [Google Scholar]
- 19. Kapusta L, Mainzer G, Weiner Z, Deutsch L, Khoury A, Haddad S, et al. Second trimester ultrasound: reference values for two-dimensional speckle tracking–derived longitudinal strain, strain rate and time to peak deformation of the fetal heart. J Am Soc Echocardiogr. 2012;25(12):1333–41. [DOI] [PubMed] [Google Scholar]
- 20. Matsui H, Germanakis I, Kulinskaya E, Gardiner HM. Temporal and spatial performance of vector velocity imaging in the human fetal heart. Ultrasound Obstet Gynecol. 2011;37(2):150–7. [DOI] [PubMed] [Google Scholar]
- 21. Köster HA, Hammer K, Braun J, Oelmeier de Murcia K, Möllers M, Klockenbusch W, et al. Comparison of strain and dyssynchrony measurements in fetal two-dimensional speckle tracking echocardiography using Philips and TomTec. J Perinat Med. 2020;48(3):266–73. [DOI] [PubMed] [Google Scholar]
- 22. Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. 2009;52(3):425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruschel P, Zielinsky P, Grings C, Pimentel J, Azevedo L, Paniagua R, et al. Maternal-fetal attachment and prenatal diagnosis of heart disease. Eur J Obstet Gynecol Reprod Biol. 2014;174:70–5. [DOI] [PubMed] [Google Scholar]
- 24. Sklansky M, Tang A, Levy D, Grossfeld P, Kashani I, Shaughnessy R, et al. Maternal psychological impact of fetal echocardiography. J Am Soc Echocardiogr. 2002;15(2):159–66. [DOI] [PubMed] [Google Scholar]
- 25. Toole BJ, Schlosser B, McCracken CE, Stauffer N, Border WL, Sachdeva R. Importance of relationship between ductus and isthmus in fetal diagnosis of coarctation of aorta. Echocardiography. 2016;33(5):771–7. [DOI] [PubMed] [Google Scholar]
- 26. Chamberlain R, Shiino K, Scalia GM, Sabapathy S, Chan J. Advantage and validation of vendor-independent software for myocardial strain analysis compared to vendor-specific software. Australas J Ultrasound Med. 2021;24(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.