Abstract
Corals are imminently threatened by climate change–amplified marine heatwaves. However, how to conserve coral reefs remains unclear, since those without local anthropogenic disturbances often seem equally or more susceptible to thermal stress as impacted ones. We disentangle this apparent paradox, revealing that the relationship between reef disturbance and heatwave impacts depends upon the scale of biological organization. We show that a tropical heatwave of globally unprecedented duration (~1 year) culminated in an 89% loss of hard coral cover. At the community level, losses depended on pre-heatwave community structure, with undisturbed sites, which were dominated by competitive corals, undergoing the greatest losses. In contrast, at the species level, survivorship of individual corals typically declined as local disturbance intensified. Our study reveals both that prolonged heatwaves projected under climate change will still have winners and losers and that local disturbance can impair survival of coral species even under such extreme conditions.
Staggering coral mortality from a prolonged marine heatwave can be exacerbated by local human disturbance.
INTRODUCTION
Marine heatwaves threaten the persistence of tropical scleractinian corals (1–3) and, with them, the biologically diverse ecosystems that these foundational reef-building species support. Corals are particularly vulnerable to temperature anomalies, with increases of only 1°C capable of disrupting their obligate symbiosis with the photosynthetic dinoflagellate microalgae (family Symbiodiniaceae) that normally fuel them, causing the coral animal to expel its symbionts and bleach (4, 5). Prolonged bleaching typically leads to coral starvation and mortality (5). Although climate change has long been recognized as a serious threat to tropical corals (6–8), the recent preponderance of marine heatwaves—persistent anomalously warm ocean temperatures (9)—has shifted focus from the threats posed by gradually rising temperatures and ocean acidification (8) to these punctuated disturbances (1, 3). Already, three global coral bleaching events triggered by El Niño–fueled marine heatwaves (1997–1998, 2010, and 2014–2017) have caused devastating coral losses (10, 11). Climate change models project that both the intensity and frequency of marine heatwaves will increase in the coming decades (1, 3), such that many of the world’s coral reefs are predicted to undergo annual bleaching events by midcentury (12). These events will, however, not occur in isolation. On almost all reefs, climate change is superimposed on a suite of chronic local anthropogenic disturbances (13)—ranging from coastal development and associated pollution and reef sedimentation to overexploitation, destructive fishing practices, and disease—that have already substantially altered coral communities through reductions in coral cover and changes to community composition, with largely unknown consequences for species and ecosystem resilience to thermal stress.
Given the intensification of marine heatwaves and the ubiquity of local anthropogenic disturbances on coral reefs, there is an urgent need to understand how these stressors interact (14). However, to date, few coral bleaching studies have explicitly examined multiple stressors (15). Chronic local anthropogenic disturbance might mediate coral reef responses to thermal stress, either increasing susceptibility—as documented for massive corals on the Mesoamerican Reef following the 1998 El Niño (16, 17)—or conversely enhancing resilience—if disturbances have already eliminated the most vulnerable coral species, leaving behind only the hardiest ones (18)—as documented on Kenyan reefs (19). Alternatively, exposure to chronic local disturbance may have no effect, with thermal stress affecting corals irrespective of underlying protection, as found recently on the Great Barrier Reef (20). The degraded state of most modern reefs is widely acknowledged (8, 13, 21, 22), and as managers seek to understand how to manage coral reefs under climate change, one might expect that examination of multiple stressors would be common practice in modern coral reef research. However, when we systematically reviewed studies reporting on the effects of recent marine heatwaves (2014–2021)—a period that included six of the seven hottest years on record at the time of study—we found that only 10% (n = 20 of 194) had explicitly tested if local anthropogenic disturbance influenced heat stress effects on corals (fig. S1 and data file S1). The most common comparisons in these studies were between sites exposed to local disturbance and ones actively managed inside a marine protected area, although we note that coral reefs may also be de facto protected from local disturbance by the absence of impacts in remote locations. Almost half (n = 9) of the 20 studies reported a positive effect of protection (i.e., decreased coral bleaching and/or mortality) on corals during heat stress events, 40% (n = 8 of 20) reported no effect, and 15% (n = 3 of 20) concluded that protection was detrimental to corals during heat stress. Conflicting evidence among the few bleaching studies that have tested for the effects of local anthropogenic disturbance and the overall lack of attention to this fundamental aspect of modern coral reefs impedes understanding of how best to manage these ecosystems in a warming world.
How coral reefs are transformed by climate change this century will depend not only on their exposure to thermal stress and local anthropogenic disturbance but also on the sensitivity and response capacity of individual coral species to these stressors (23–25). Coral sensitivity to thermal stress is determined by biological traits, such as tissue thickness (26), and physiological tolerance, which is influenced by factors including the type and abundance of the coral colony’s obligate algal endosymbionts (27–30). Response capacity, in turn, may reflect species-specific propensity for acclimatization (e.g., the flexibility to switch or shuffle symbionts or to up-regulate host thermal stress responses) and adaptation (e.g., selection for traits of either the host or symbionts that confer a fitness advantage under stressful conditions) (28, 31, 32). Interspecific differences in sensitivity to thermal stress have long been recognized (23, 26, 33, 34), with “winners” generally able to either avoid bleaching during thermal stress or recover from it after warming subsides, and “losers” tending to bleach and die quickly in response to warming (34). Because environmental filtering is stronger under stressful conditions (35–37), reefs increasingly stressed by marine heatwaves may lose diversity and converge toward simpler assemblages, as losers are eliminated from the species pool. However, because repeated heatwaves may turn some winners into losers and vice versa (38), questions remain about how corals with different sensitivities will respond to heatwaves of increasing frequency, duration, and intensity. Which corals will endure in communities will also depend on whether species exhibit positive or negative cotolerance to thermal stress and local anthropogenic disturbance (35, 39). Predicting future reef states thus requires not only accounting for underlying anthropogenic disturbances but also understanding interspecific variability in survivorship through heatwaves. To date, however, most heatwave studies have focused on quantifying coral bleaching, a symptom of thermal stress, rather than coral mortality, a fundamental parameter required to assess the demographic effects of such events. This disconnect reflects the challenge of quantifying coral mortality, which, under the strictest standards, requires following individual colonies over time and, at minimum, requires documenting coral cover before and after a heatwave, as opposed to bleaching assessments that require only a single site visit. Although bleaching may be an accurate proxy for mortality in short heatwaves, during prolonged events that are becoming the norm, for corals that either bleach quickly and die (and hence are unlikely to be recorded in the bleached state) or those that can persist in a bleached state for prolonged periods, it is not (40).
Here, we took advantage of the ecosystem-scale natural experiment that occurred at the epicenter of the 2015–2016 El Niño, the central equatorial Pacific Ocean, where prolonged heat stress blanketed a spatial gradient of chronic local anthropogenic disturbance on the world’s largest atoll, Kiritimati. We quantified thermal stress around the atoll using high-precision in situ temperature loggers and satellite data, and human disturbance using a combined metric of local human population and fishing pressure. Our primary objective was to evaluate how exposure to local disturbance modulates the impacts of heat stress on corals at both the community (i.e., among coral species) and species (i.e., for individual coral species) levels. In addition, we sought to assess whether coral bleaching, the most commonly recorded reef metric during heatwaves, accurately predicts coral mortality. Thus, over the course of nine expeditions before (2013–2015), during (2015–2016), and after (2016–2017) the heatwave, at sites exposed to consistent heat stress but varying levels of local disturbance (Fig. 1, A and D, and figs. S2 and S3), we quantified coral community composition and bleaching (n > 250,000 points from 94 photo surveys) and, in one of the largest longitudinal studies of individual corals to date (41), tracked the fate of >850 individual coral colonies.
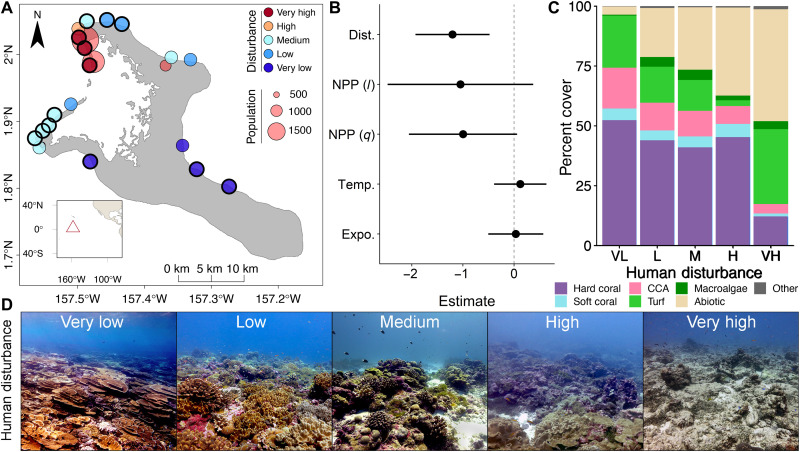
Fig. 1. Reef communities across a gradient of chronic human disturbance before the 2015-2016 El Niño.
(A) Reef sites on Kiritimati (central equatorial Pacific Ocean) at which coral community structure and individually tagged coral colonies (sites encircled in black) were tracked over the course of the 2015–2016 El Niño. (B) Parameter estimates and 95% confidence intervals for factors examined [Dist., local human disturbance; NPP, net primary productivity (l, linear; q, quadratic); Temp., maximum monthly mean (MMM) temperature; Expo., wave exposure] for their influence on hard coral cover before thermal stress (table S4). (C) Mean community composition (CCA, crustose coralline algae; turf, turf algae; abiotic, sediment, sand, and rubble) of the forereef benthos among sites, classified by their exposure to chronic local human disturbance. VL, very low; L, low; M, medium; H, high; VH, very high. (D) Photos of the coral reef communities before the El Niño at sites representing each of the atoll’s levels of local human disturbance. Photo credits: D. Claar (very low and very high), University of Victoria (UVic); M. Watson (medium), UVic; and The Baum Lab (low and high), UVic.
RESULTS
Prolonged heat stress superimposed on reefs spanning a local disturbance gradient
Before the El Niño, benthic communities varied markedly across the atoll’s forereefs, with sites ranging from a high of 62.7% hard coral cover to a low of only 1.6% (Fig. 1). Chronic local human disturbance (figs. S2 to S4 and table S1; detailed in the Supplementary Materials), including dredging, water pollution, and fishing, was the primary determinant of these differences, with coral cover declining significantly as local disturbance increased (z = −3.257, P = 0.001; Fig. 1B). Abiotic factors, including oceanographic productivity, site exposure (windward versus sheltered), and sea surface temperature (SST), did not significantly influence coral cover among sites (Fig. 1B and table S4). Almost three quarters of the benthos of reefs far from villages, with very low exposure to chronic local human disturbance, was composed of hard corals (mean = 52.4 ± 13.2%; SD), soft corals (mean = 4.8 ± 9.0%), and beneficial crustose coralline algae (mean = 17.1 ± 10.9%; Fig. 1C). In contrast, reefs exposed to the highest levels of local human disturbance had little hard coral cover (12.2 ± 17.3%), with most of the benthic community composed of turf algae (31.3 ± 18.6%), sediment (28.4 ± 15.5%), sand (11.9 ± 3.2%), and rubble (5.1 ± 1.7%; Fig. 1C). The effects of different intensities of chronic human disturbance on reef states were notably evident visually before the El Niño-induced heatwave (Fig. 1D).
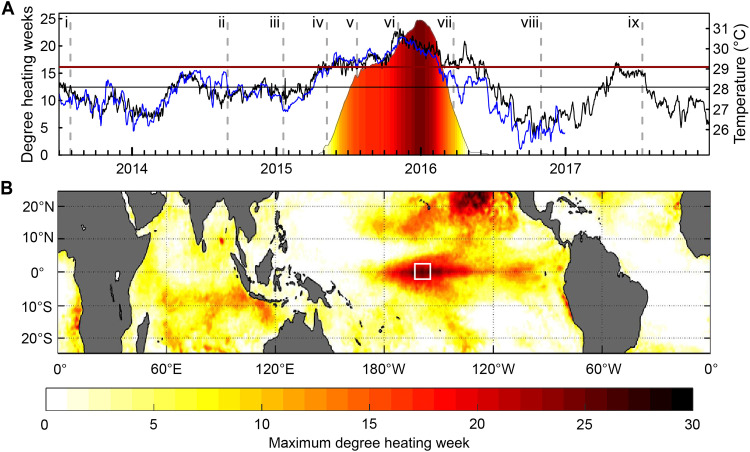
As the epicenter of the 2015–2016 El Niño, Kiritimati’s coral reefs experienced a sustained heatwave [degree heating weeks (DHW; °C-weeks) > 0] for approximately 1 year (Fig. 2). Heat stress started accumulating on 17 April 2015 and exceeded 4°C-weeks [National Oceanic and Atmospheric Administration’s (NOAA’s) Coral Reef Watch (CRW) Bleaching Alert Level 1] from 28 May 2015 to 13 April 2016; DHWs >0 persisted until 17 July 2016 (Fig. 2A). Accumulated heat stress rapidly exceeded both NOAA’s CRW Bleaching Alert Level 2 threshold (8°C-weeks) and its 12°C-weeks threshold, reaching an unprecedented level (>24.7°C-weeks; Fig. 2A and table S3) by January 2016. Heat stress was remarkably consistent around the atoll, varying by a maximum of only 4.3% (1.08°C-weeks) across sites over the course of the event (fig. S5 and table S3). Maximum temperature anomalies ranged across sites from 2.83° to 3.05°C above the reef’s normal maximum monthly mean (MMM) temperature during the event (table S3). The heat stress sustained by Kiritimati’s reefs during this El Niño far exceeds that at any other time point from the recent past for which there are records (fig. S6).
Fig. 2. Thermal stress at the epicenter of the 2015–2016 El Niño.
(A) In situ (blue line) and satellite [black line; from National Oceanic and Atmospheric Administration (NOAA) (90)] temperature on Kiritimati’s reefs, with MMM temperature and bleaching threshold for reference [black and red horizontal lines, respectively; from NOAA Coral Reef Watch (CRW) (95)]; all right axis. Color shows cumulative heat stress on Kiritimati as degree heating weeks (DHW; °C-weeks; left axis) from NOAA CRW (95). Dashed vertical lines denote the timing of expeditions before (i to iv), during (v to vii), and after (vii to ix) the event. (B) Global heat stress on coral reefs during the 2015–2016 El Niño (May 2015 to June 2016) from NOAA CRW, with white box denoting Kiritimati’s location at the epicenter of the heat stress during this event. Color (scale at bottom) indicates maximum thermal stress (°C-weeks).
Prolonged heatwave impacts on coral cover primarily reflect differences in community composition
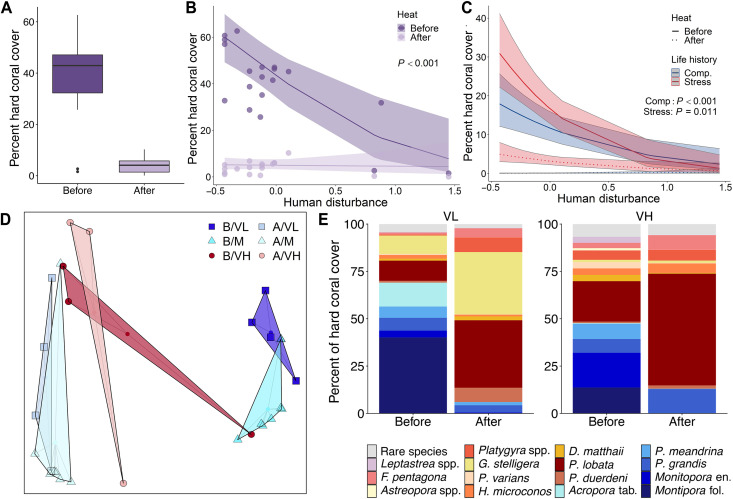
The exceptional heat stress unleashed on Kiritimati during the 2015–2016 El Niño caused staggering coral bleaching (fig. S7) and mortality, culminating in an overall estimated loss of 89.3 ± 7.1% of the forereef’s hard coral cover (Figs. 3A and 4). Consistency in thermal stress around the atoll meant that DHW was not a significant factor explaining variability in the overall hard coral cover among sites (z = −0.683, P = 0.495; table S4). Instead, we found that heat stress period (i.e., before versus after the event; z = −22.538, P < 0.001) and local disturbance (z = −3.643, P < 0.001) were both highly significant predictors of coral cover; NPP was also statistically significant (quadratic relationship; linear term: z = −1.669, P = 0.095 and quadratic term: z = −2.240, P = 0.025). Moreover, the interaction between local disturbance and heat stress period was significant, indicative of the absolute loss of corals being much greater at minimally disturbed sites than at those exposed to very high disturbance (Fig. 3B), which resulted in the strong inverse relationship between coral cover and local disturbance being completely eroded by the end of the heatwave (z = 5.290, P < 0.001; slope = −0.09, 95% confidence interval: −0.94 to 0.75; Fig. 3B and table S4). Relative coral cover losses also tended to be greater with lower local disturbance: On average, minimally disturbed sites underwent an estimated 92.8 ± 1.4% decline in coral cover, ending the heatwave with only 3.9 ± 1.3% coral cover, while sites exposed to very high levels of local disturbance—which already had depressed levels of coral cover—declined by a further 63.6 ± 16.8%, ending the heatwave with only 3.7 ± 4.7% coral cover (Figs. 3B and 4). However, this difference in relative coral cover losses was not statistically significant (t = −3.0014, P = 0.09), likely due to variable losses at the high local disturbance sites. Reef-building corals were replaced primarily by turf algae, which rapidly overgrew the dead coral, and at some sites also by macroalgae (Fig. 4 and fig. S8).
Fig. 3. Impacts of a prolonged heatwave on coral cover and community composition.
(A) Overall change in hard coral cover across all sites from before to after the 2015–2016 El Niño on Kiritimati (sites as in Fig. 1A). Model predictions of the effect of local human disturbance on percent coral cover before versus after the heatwave for (B) the overall coral community and (C) stress-tolerant (red) and competitive (blue) corals. (D) Principal coordinates analysis plots of coral assemblage structure before (B) and after (A) the event at VL, M, and VH levels of local human disturbance. (E) Comparison of average coral community composition across sites exposed to VL disturbance versus those exposed to VH levels of disturbance before and after the heatwave (stress-tolerant species in shades of red-yellow and competitive species in blue; see table S6 for full species names).
Fig. 4. Transformation of a minimally disturbed coral reef by a heatwave of unprecedented duration.
(A) Before (July 2015) and (B) after (July 2017) the 2015–2016 El Niño-induced mass coral mortality event at one site (VL1) with very low exposure to chronic local stressors on Kiritimati. Virtually, all coral in (B) is dead and overgrown by turf algae. Photo credits: (A) K. Cox and (B) K. Tietjen, UVic.
We hypothesized that differences in coral cover loss across the local disturbance gradient reflected distinct coral communities, composed of species with variable thermal stress tolerances, found at different disturbance levels. Examining these coral communities revealed that community composition not only varied with disturbance independent of the heatwave [permutational multivariate analysis of variance (PERMANOVA), pseudo-F = 4.4, P = 0.001] but also changed significantly following the heat stress (PERMANOVA, pseudo-F = 15.3, P = 0.002), with sites exposed to very low or medium local disturbance experiencing greater turnover than those exposed to very high disturbance (PERMANOVA, pseudo-F = 3.9, P = 0.022; Fig. 3D). Underlying this change was the loss of corals with a competitive life history strategy, namely, all large tabulate and corymbose Acropora at very low local disturbance sites (100% loss) and all foliose Montipora at very low and medium local disturbance sites (100% loss; Figs. 3E and 4). Models testing the relationship between pre-heatwave community composition and coral cover showed that sites dominated by “competitive” corals were more strongly affected by the heatwave than those dominated by “stress-tolerant” corals (z = −2.428, P = 0.015; fig. S9). Moreover, separate models of competitive and stress-tolerant coral cover revealed that, while the cover of both life histories decreased significantly due to the heatwave (competitive: z = −21.665, P < 0.001 and stress-tolerant: z = −14.464, P < 0.001; Fig. 3C and table S4), the degree of change was substantially greater for competitive corals (98.6 ± 2.3%) than for stress-tolerant ones (78.2 ± 10.2%; t = −8.3014, P < 0.001). Although we also found that net primary productivity (NPP) was significantly related to the cover of competitive corals and the magnitude of thermal stress at each site (°C-weeks) was significantly related to the cover of stress-tolerant corals, the effect sizes were smaller than those of local disturbance or heat stress period (table S4).
Chronic local disturbance can exacerbate heatwave impacts on individual coral species
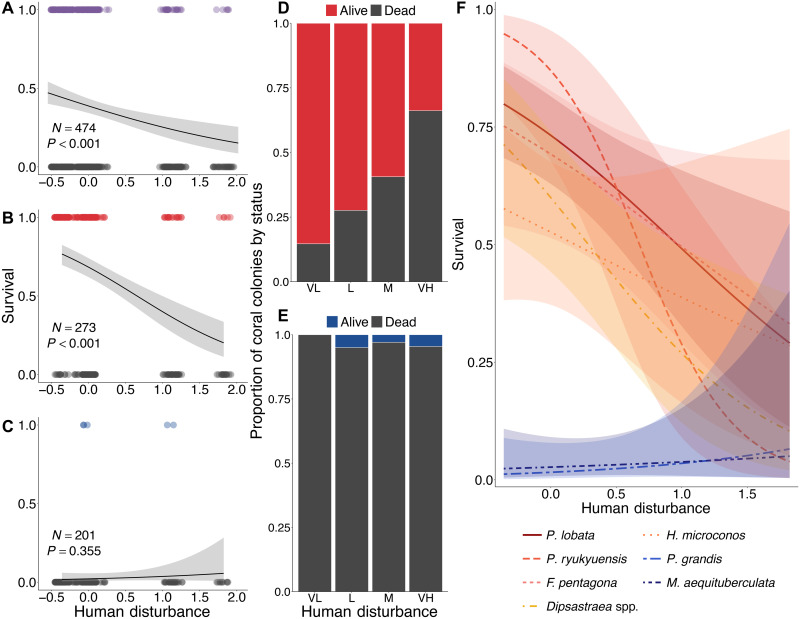
In contrast to the observed changes at the community level, at the species level, we found a clear signal of the negative influence of chronic local disturbance on coral survival through prolonged heat stress (Fig. 5 and figs. S10 and S11). Examining a representative sample of tagged coral colonies of the same seven species across sites revealed a significant negative relationship between local disturbance and coral survival (z = −3.79, P < 0.001; Fig. 5A and table S5). This signal became clearer when distinguishing between life history strategies, with survival of the stress-tolerant coral species strongly negatively related to local disturbance (Fig. 5, B, D, and F), while that of the competitive coral species showed no relationship with disturbance, namely because these colonies were so sensitive to the heat stress that few of them survived anywhere (2.0% survival for Pocillopora grandis and 2.97% survival for Montipora aequituberculata; Fig. 5, C, E, and F). Individually, each stress-tolerant coral species exhibited an inverse relationship between survival and local disturbance, with the massive corals Platygyra ryukyuensis and Porites lobata exhibiting the steepest relationships (an estimated 95 and 80% survival at very low sites and 4 and 29% survival at very high sites for the two species, respectively) and Hydnophora microconos exhibiting the shallowest (58% survival at very low sites to 28% survival at very high sites; Fig. 5F, figs. S10 and S11, and table S5). Survivorship of the two competitive species each showed weakly positive, but nonsignificant, relationships with local disturbance, with mortality at all sites exceeding 97.5% (Fig. 5F and figs. S10 and S11). Bleaching prevalence early in the heatwave was not related to the ultimate survival of any coral species (P > 0.78; figs. S12 and S13).
Fig. 5. Survival of individually tracked coral colonies throughout the prolonged heatwave.
Logistic regressions of coral survival versus human disturbance for (A) all coral colonies, (B) stress-tolerant (red) species, (C) competitive (blue) species, and (F) all coral colonies, with species modeled as a fixed effect in a two-way interaction with local human disturbance. Bar plots of coral colony survival by local human disturbance level for (D) stress-tolerant and (E) competitive coral species.
Emergent winners and losers
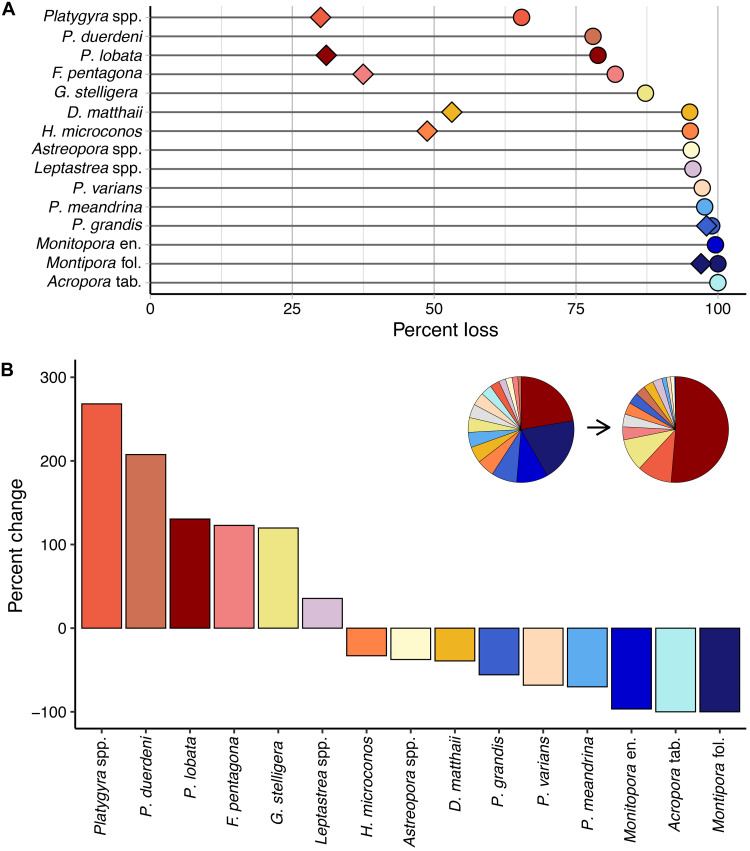
Given strong interspecific differences in survival, certain coral species emerged from this unprecedented heatwave as winners, while others were clear losers (Fig. 6). All of the competitive coral species were losers, having each lost more than 97.5% of their cover (Fig. 6A). In contrast, the winners were all stress-tolerant coral species that underwent smaller losses in cover (65.4 to 87.2% for the five biggest winners) and thus increased considerably in their relative proportion of coral cover (Fig. 6). Notably, the massive coral Porites spp., which was already the most common coral before the heatwave, underwent more than a twofold relative increase, such that more than half of the atoll’s remaining coral community is now composed of this one slow-growing, stress-tolerant species (Fig. 6B). Although two other corals—Platygyra spp. and Pavona duerdeni—had even greater proportional gains, because they were initially relatively rare, they still comprised only a small proportion of the coral community at the end of the heatwave (10.6 and 3.1%, respectively; Fig. 6B). Finally, substantially lower rates of colony mortality than coral cover loss for the five individually tracked stress-tolerant species reflect the fact that while many colonies sustained partial mortality during the heatwave, a portion of the colony was still alive at the end of the event (Fig. 6A).
Fig. 6. Interspecific variation in prolonged heat stress impacts on corals.
(A) Overall change in percent cover of individual coral taxa (circles) for the 15 taxa comprising the greatest proportion of benthic cover before the heatwave, ordered from least to greatest cover loss; overall percent colony mortality for the seven individually tracked coral species is overlaid (diamonds). (B) Percent change in proportion of overall coral cover, from before to after the heatwave, ordered left to right from species that underwent the greatest proportional gains (winners) to the greatest proportional loss (losers). Inset pie charts show species composition of the overall coral assemblage before (left) and after (right) the heatwave (gray, rare species). See table S6 for taxonomic and other details.
DISCUSSION
Despite the urgent need to leverage all available solutions for enhancing coral reef resilience to thermal stress in the face of escalating climate change, it has been unclear whether managing local anthropogenic disturbances on reefs helps or hinders in this regard. Our study sheds new light on this debate. Tracking whole coral communities and individual species exposed to consistent thermal stress, but different levels of underlying local anthropogenic disturbance, clarified that the relationship between local anthropogenic disturbance and climate resilience varies qualitatively across biological scales. At the community level, we found that reefs exposed to high levels of chronic local anthropogenic disturbance fared better through a prolonged heatwave than those shielded from local disturbance, an outcome driven by differences in the coral community composition among sites. In contrast, when comparing survival rates within individual coral species, the predominant relationship for those species that were not eradicated by the heatwave was one of declining survivorship as local disturbance increased. These findings have implications for managing and restoring coral reefs, as climate change–driven marine heatwaves continue to intensify in frequency and duration.
Winners and losers in prolonged heatwaves
Overall, we documented mass coral mortality (89% hard coral cover loss) arising from a heatwave that persisted for a remarkable 10 months unabated. At its peak, heat stress accumulated to 25°C-weeks (DHWs), a level that had not previously been anticipated to occur on any reef until midcentury (26). Its occurrence 35 years earlier than predicted underscores how rapidly climate change is advancing (2). Although corals typically exhibit high interspecific variability in their sensitivity to thermal stress (33), a heatwave this extreme might have been expected to overwhelm the tolerance of even the most resilient species, resulting in high mortality rates across the board. Instead, we found that interspecific coral cover losses still varied widely, from the complete loss of tabular Acropora and foliose Montipora to a loss of only 65% in the stress-tolerant mounding coral Platygyra spp. Mortality rates for the individual colonies that we tracked were also highly variable across species, but with lower mortality rates for the stress-tolerant species, because many of the colonies of these species had surviving corallites, thus potentially paving the way for their recovery. Only on nearby tiny Jarvis Island was thermal stress more extreme (maximum of 31.58°C-weeks) during this heatwave (42, 43). There, reefs underwent an estimated >98% decline in hard coral cover, with severe losses of Montipora spp. (100%), Pocillopora spp. (>90%), and Pavona spp. (~85%) recorded in the surveyed areas (43, 44). Together, these results illustrate that extremely prolonged heatwaves still have winners and losers, such that these events will cause not only enormous coral losses but also substantial changes in community composition.
Influence of local anthropogenic disturbance on coral survival
In species-specific models testing the influence of exposure to chronic local anthropogenic disturbance on coral resilience to thermal stress, we detected an inverse relationship for those species with stress-tolerant life histories. Survivorship of all stress-tolerant species was at least twice as high at sites absent local disturbance compared to sites with the highest exposure and was more than 10 times as high for the species with the strongest relationship, P. ryukyuensis. Increased coral sensitivity to thermal stress with greater human disturbance is, we expect, most likely attributable to the diminished water quality at the most highly disturbed sites. On Kiritimati, raw sewage and pollution inputs have resulted in increased turbidity and sedimentation (fig. S3) as well as greater concentrations of bacteria, virus-like particles, and potential pathogens in the water column at these sites (45, 46). Previous studies have shown that low water quality can change the coral microbiome (47–49), and microbiome analyses of subsets of our tracked corals before the heatwave showed increased bacterial diversity at highly disturbed sites (50). Such changes can have knock-on effects for coral physiology and survivorship even in the absence of warming (51), which may then be exacerbated during heatwave events. Poor water quality, as with other environmental stressors, can also lead to changes in coral-algal symbioses (52–54), which may then influence coral resilience to subsequent thermal stress. Analyses of our tracked P. ryukyuensis colonies, and two of the three cryptic coral lineages in tracked P. lobata colonies, revealed symbioses with distinct Symbiodiniaceae across the disturbance gradient that were linked to coral survival during heat stress (55, 56). Although the mechanism underlying the relationship between coral survival and local disturbance remains unclear for the other stress-tolerant corals that we tracked—and may differ across species—we suggest that it likely results from distinct coral microbiomes and their associated physiological traits found across the disturbance gradient. We attribute the failure to detect an effect of local disturbance on coral resilience to thermal stress in our two competitive coral species to their extremely high mortality. A recent study of a less severe heatwave on Moorea, French Polynesia showed that bleaching severity was significantly increased by local nitrogen pollution in the competitive coral genera Acropora and Pocillopora (57). Overall, these results suggest that impairment of coral resilience to thermal stress by local stressors may be a general phenomenon and at least deserves increased research and management attention.
Notably, however, these species-level results stand in contrast to our own community-level results and previous studies, which have suggested that local disturbance enhances coral reef resilience to thermal stress. Over a decade ago, Côté and Darling (58) argued that this would be the case for coral reefs exposed to—and altered by—local stressors, because the most sensitive coral species would already have been eliminated, leaving behind a more stress-tolerant community. That is, if organisms exhibit cotolerance to stressors (or, conversely, cosensitivity) such that they respond similarly to them, then the combined effects of the stressors may be antagonistic, resulting in a response that is less than the sum of their individual effects (14, 39, 59). Although antagonistic effects due to stressor cotolerance are not a given—as multiple stressors may also exhibit synergistic or additive effects—such interactions appear to be fairly common on reefs exposed to local stressors and global climate change. Darling et al. (19) found that while the stress-tolerant corals that dominated fished reefs in Kenya before the 1998 El Niño were barely affected by the bleaching event, reefs in no-take reserves had more diverse coral assemblages, including many corals with competitive life history traits that exhibited cosensitivity to fishing and bleaching, and incurred heavy losses. More recently, Cannon et al. (60) showed that central Pacific reefs in the Gilbert Islands that were exposed to higher levels of chronic local pressure were dominated by a coral species tolerant of nutrient loading and turbidity and were subsequently less affected during a bleaching event than nearby reefs with fewer local pressures. At Kiritimati’s highest disturbance sites, we found that of the competitive coral species, Acropora were completely absent, and while some encrusting Montipora persisted, only a few colonies of the foliose form (common in less disturbed sites) were recorded. Thus, the “positive” effect of local disturbance reflects different community compositions and the variable thermal sensitivities of the coral species that dominate disturbed reef communities, rather than there being a mechanism by which local disturbance itself enhances coral resilience to thermal stress.
More difficult to reconcile with either our species- or community-level findings are studies reporting that coral responses to thermal stress occur irrespective of local protection or remoteness, influenced only by the reef’s exposure to thermal stress. In surveys of the Great Barrier Reef Marine Park during the 2016 marine heatwave, for example, Hughes et al. (20) documented severe bleaching on reefs in each of the park’s types of management zones and concluded that local management of water quality (assessed using long-term chlorophyll a concentration) and fishing pressure had little to no influence on coral resistance to extreme heat. Similarly, a study in one of Indonesia’s oldest marine parks during the same heatwave found that management zone made no difference to coral losses (61). More recently, Baumann et al.’s (62) global meta-analysis tested the relationship between human influence and coral resilience and concluded that reefs isolated from human pressures are not more resilient to climate change, noting that even the world’s most remote reefs bear the impacts of intense marine heatwaves. We concur that, at broad spatial scales, exposure to thermal stress will be highly variable across reefs, and this may well be the primary determinant of reef impacts; remote reefs are not immune to high thermal stress exposure levels. Such an emphasis on current and future thermal stress exposures has proven useful when considering future thermal refugia for coral reefs, as in the “50 Reefs” conservation prioritization (63, 64). At finer spatial scales, however, where thermal stress exposure is the same (or very similar) across reefs, a corollary of the conclusion that coral responses are the same irrespective of protection is that coral sensitivities and response capacities to thermal stress must be the same across the protection levels. Because this outcome seems unlikely, we posit that, in these studies: (i) corals were exposed to similar conditions inside and outside the protected area, such that the communities did not differ, or (ii) exposures to thermal stress across protection levels were not actually equal; or (iii) different impacts were not detected because of insufficient power, or bleaching was measured at only a single time point such that the full ecological impacts of the event were not quantified.
Considering our results together across scales suggests that, although local anthropogenic disturbance can result in the loss of sensitive coral species such that the remaining community is more tolerant to subsequent thermal stress, when comparing “apples with apples”—that is, the same species across different levels of local anthropogenic disturbance—there is clear evidence that local disturbance can impair survival. Thus, while there is compelling recent evidence that coral reef recovery following bleaching events may not be aided by minimizing human disturbances to reefs (18), our study suggests that previous conflicting results pertaining to coral community resilience to thermal stress may be resolved through consideration of biological scale.
Coral bleaching does not foretell demographic impacts of prolonged heatwaves
Our repeated reef surveys during an extended bleaching event also provide an empirical test of the relationship between coral bleaching and mortality. Given the many challenges associated with conducting in situ assessments of coral bleaching events—including the need to marshal resources quickly when heatwaves arise, the limited reef area that can be assessed by divers, and the complexities of accessing remote reefs—rapid reef surveys at a single time point during a heatwave are often used to assess ecological impacts. However, whereas bleaching incidence can lead to decreased coral growth and reproduction, the capacity of corals to recover from bleaching means that it may not accurately foretell coral mortality, and hence the overall demographic impacts of heatwaves. We found no relationship between bleaching prevalence and subsequent mortality levels in any of our tagged coral species. Instead, we found that the species with the highest bleaching incidence early in the event (P. ryukyuensis and Favites pentagona) had among the lowest mortality, while a species with very low bleaching incidence (P. grandis) suffered near complete mortality (Fig. 6A and figs. S12 and S13). Mismatches between bleaching and mortality could arise if certain coral species can resist the onset of bleaching more than others but then only persist in a bleached state for a short period (40, 65). Such mismatches will be more likely in the prolonged heatwaves that are projected to become more common under climate change (40, 66), thus highlighting the need for increased sampling during these events to accurately gauge demographic impacts. As the capacity to use satellite-derived data to accurately monitor coral bleaching increases, these sources could help to overcome this challenge.
Coral reef recovery from prolonged heatwaves unlikely under climate change
We posit that coral reef recovery from prolonged heatwaves is increasingly unlikely because of long ecosystem recovery times and the diminishing interval between successive heatwaves under climate change (67, 68). On Kiritimati, our sampling up until 3 years after the end of the heatwave (2019, before the onset of coronavirus disease 2019) revealed juvenile corals and regrowth of colonies that had experienced partial mortality, which together resulted in some increase in overall coral cover but still left the ecosystem a long way from full recovery. Long-term studies of coral reefs from the Indian and Pacific Oceans following the major 1998 El Niño found that recovery of hard coral cover typically took more than a decade and involved substantial turnover of community composition, with “recovered” reefs tending to have lower coral diversity and be dominated by fast-growing corals (69–72). Recovered reefs in Moorea, for example, are now dominated by “fields” of Pocillopora (73), while recovering reefs in the Seychelles became dominated by fast-growing, branching Acropora corals (74). Reef recovery following mass bleaching events is also not guaranteed. Following the 1998 El Niño, more than 40% of surveyed reefs in the Seychelles underwent regime shifts to fleshy macroalgae (72). Those that were on a recovery trajectory, which had high coral cover before the 1998 El Niño, still had not fully recovered by 2014, and although full recovery was projected to be complete within 17 to 29 years (74), progress was nullified by Seychelles’ 2016 bleaching event (75). Such outcomes are increasingly likely with climate change (67). Thus, as with many reefs, the probability of full recovery of Kiritimati’s reefs now seems slim.
Persistence of coral reefs throughout the 21st century will be dictated almost entirely by the extent to which greenhouse gas emissions are reduced (66). Our study shows that prolonged heatwaves under climate change will not only substantially reduce coral cover but also transform the remaining coral community composition. All of Kiritimati’s reefs suffered staggering losses during this study, including those exposed to very low disturbance, which had arguably been among the most pristine remaining on the planet before the heatwave. Diminishing intervals between recurrent heatwaves will leave most of the world’s reefs with insufficient time to recover after such events (67). Emissions reductions that only limit warming to 2°C are projected to result in the loss of virtually all coral reefs (99%), whereas if warming is limited to 1.5°C, then losses could be limited to between 70 and 90% (66, 76). Under such dire conditions, strategies additional to greenhouse gas emissions reductions that can reliably enhance coral resistance to, or recovery from, marine heatwaves should be broadly deployed. However, the efficacy and scalability of the potential options remains uncertain. Our study provides evidence that, at least for some coral species, resilience to thermal stress is enhanced as local anthropogenic disturbances are reduced. These findings imply that alleviating local disturbances—such as by improving water quality, which is likely one of the most tractable options for reef managers—could not only benefit natural coral reefs but also aid coral restoration efforts, improving the odds of success for the individual coral species that are out-planted on reefs. With much still to learn about the interactions between multiple stressors on coral reefs, we encourage researchers to explicitly incorporate local disturbances into future studies of marine heatwave impacts on reefs. In addition to urgent reductions in greenhouse gas emissions, evidence-based local management actions that are both scalable and durable are urgently needed as a means of increasing the odds of persistence for these imperiled ecosystems under climate change.
MATERIALS AND METHODS
Literature survey
We conducted a systematic review of the primary literature to quantify the extent to which field studies assessing the impacts of recent marine heatwaves (i.e., 2014 to 2021) on corals quantified underlying local anthropogenic disturbance at their study site and tested for an effect of disturbance on coral outcomes through the heat stress event. On 9 September 2021, we conducted a search for papers published between 2015 and 2021 using all databases on the Web of Science, with the following search terms: [(“coral*”) and (“mortal*” or “bleach*” or “cover*” or “health*”) and (“El Niño” or “El Nino” or “ENSO” or “heat*” or “thermal stress” or (“temperature” and “anomal*”) or “bleaching event”)]. We evaluated each of the n = 721 papers returned from this search, reviewing the titles, abstracts, and method sections, to first determine whether the paper examined corals during a heatwave between 2014 and the present day; we excluded papers describing lab-based studies or heatwaves before 2014. In addition, we added n = 10 papers that were not returned from this search but were known to quantify the effects of a heatwave between 2014 and the present day. The remaining n = 184 papers that met our criteria were classified on the basis of whether the study included an anthropogenic disturbance (searching for “anthropogenic,” “human,” “disturbance,” “stressor,” “cumulative effects,” or “protection”) and whether the study analyzed or made conclusions about the effect of anthropogenic disturbances. We noted the type of heat stress effect (e.g., bleaching and mortality), the type of disturbance (e.g., fishing and pollution), if the study included sites without disturbance as a control, the coral sampling method (e.g., randomized quadrats, etc.), as well as the frequency of sampling before, during, and after the heatwave event.
Study site
Situated in the central equatorial Pacific Ocean at the center of the Niño 3.4 region (a designation used to quantify El Niño presence and strength) (77), Kiritimati (Christmas Island) is the world’s largest atoll by landmass (388 km2, 150 km in perimeter). Coral reefs are exposed to vastly different levels of chronic local human disturbance depending on their location around the atoll (Fig. 1A). Human impacts—including pollution from sewage outflow (78, 79) and an oil company, major infrastructure (i.e., a pier), and fishing pressure on the forereef—are densely concentrated on the northwest coast, where the two main villages are located and the majority of the population resides (Fig. 1A and table S1). In contrast, reefs on the atoll’s north, east, and south coasts are minimally affected (Fig. 1 and fig. S2; detailed in the Supplementary Materials).
We quantified the intensity of chronic local human disturbance at each forereef site (described below in the “Field methods” section), as in (55, 80), using two spatial data sources: (i) the number of people residing within 2 km of each site, as a proxy for localized impacts, based upon the Government of Kiribati’s 2015 population census data for each village on Kiritimati (81); and (ii) subsistence fishing pressure, quantified through detailed semistructured interviews conducted with heads of household in each of the atoll’s villages in 2013 (82) and represented using a kernel density function as a measure of its intensity at each site (fig. S4). We combined these data with equal weight to create a quantitative metric of chronic local human disturbance at each site (table S1). This metric correlated strongly with sedimentation, turbidity, and bacterial loads, three other indicators of disturbance (see Supplementary Methods and fig. S3), and, we believe, is the most accurate measure of disturbance for the atoll. For visualization purposes and to contrast reefs exposed to local disturbance extremes, we also classified each site as one of five distinct disturbance levels (very low, low, medium, high, and very high) based on clear breakpoints in our continuous disturbance metric (Fig. 1A and table S1) (55, 80). These terms should be regarded as being relative to other levels of disturbance around the atoll, rather than absolute levels of human disturbance.
In addition to local human disturbance, we also quantified site-specific oceanographic parameters to assess their influence on benthic community composition around the atoll (detailed in Supplementary Methods). We extracted remotely sensed data for maximum NPP and wave energy from the open-source data product Marine Socio-Environmental Covariates (83) and defined site exposure (i.e., windward versus leeward) based on the dominant wind direction (southeasterly) (84).
Field methods
To examine how heat stress interacts with chronic local human disturbance, we conducted benthic surveys at 19 forereef sites on the atoll during nine expeditions between 2013 and 2017: four before the onset of thermal stress (July 2013, August 2014, and January and May 2015), three during the El Niño–induced heat stress (July and November 2015 and March/April 2016), and two after the event (November 2016 and July 2017). The surveyed reefs at each site are all at 10 to 12 m in depth on sloping, fringing reefs, with no back reef or notable reef crest formations, and adjacent sites are all more than 1 km apart (with one exception) (85). On average, we surveyed 10.4 ± 4.9 sites per expedition, for a total of 94 surveys (tables S7 and S8); logistical and weather constraints associated with working in such a remote location prevented surveying all sites in each time point (tables S7 and S8).
To survey sites, we photographed the benthos underneath a 1-m2 gridded quadrat (mean = 28.1 ± 4.1 quadrats per site) at randomly selected positions adjacent to a 60-meter transect that had been placed along the 10- to 12-m isobath (n = 2637 photos total; tables S7 and S8). Photographs were taken with a Canon PowerShot digital camera (G15 and G16 models with an Ikelite housing and wide-angle lens dome) that was white-balanced at depth on each dive. We analyzed all benthic photos using CoralNet, an open-source online software for benthic image analysis (86), by projecting 100 random points onto each image and manually identifying the substrate beneath each point (n = 259,359 in total) from our custom label set (n = 103 identification tags), which consisted of coral (table S6) and noncoral animals, algae, bacteria, and abiotic substrates, such as sand and sediment. Recognizing that some corals cannot be definitively identified to the species level by morphology alone, we have identified some corals to the genus level only. For each coral taxon, we confirmed that the morphotype was consistent across all sites. We also included separate labels for bleaching and nonbleaching coral tissue (e.g., “bleaching Porites” and “Porites”), thus allowing us to determine the proportion of points per site in each expedition that were bleaching for each coral taxon. We quantified each site’s benthic community composition in each surveyed time point by dividing the total number of points for each substrate type by the total number of annotated points from all quadrats (detailed in Supplementary Methods).
In addition, we tagged and photographed 834 individual coral colonies from seven species at 13 of our 19 monitoring sites (Fig. 1A) and tracked their fate over the course of the El Niño event. We selected three common corals as our focal species (P. lobata, P. grandis, and M. aequituberculata), because they were found at all sites and include different life history strategies, with the first classified as being a stress-tolerant coral, a group that is defined by slow-growing, massive species and has the capacity to tolerate chronically stressful and variable environments, and the latter two considered to be competitive corals, a group typified by large, branching and plating species with fast growth and has the capacity to dominate communities (table S6) (34, 87–89). We aimed to tag 12 colonies of each of these three species per site. We also tagged up to six colonies per site of each of four less common species [F. pentagona, Dipsastraea spp. (primarily Drawida matthaii), P. ryukyuensis, and H. microconos], each of which has a stress-tolerant life history strategy (table S6) (88). Tagged coral colonies were located along the same transects as the benthic photoquadrats. Colonies were first tagged and photographed during the August 2014 expedition, before the onset of heat stress. For each coral, we photographed the entire colony parallel to the colony surface with a ruler next to it for scale; macro shots were taken of the colony surface to aid in identification where necessary. In each subsequent expedition (except November 2015), we rephotographed each colony that could be relocated and also tagged and photographed additional colonies.
We assigned each coral colony a bleaching status for each time point in which it was photographed using the following visual criteria: (i) no bleaching or paling, (ii) some light bleaching but less than 5 cm across the largest patch and less than 50% of colony pale, (iii) bleaching in patches >5 cm or more than 50% of colony pale, and (iv) severe or complete bleaching (>80%) or the entire colony pale. For binomial treatments of bleaching, we considered categories 1 and 2 to be “healthy” and categories 3 and 4 to be “bleached.” Thus, colonies were assigned to bleached if they had at least one patch of their surface that was bleached and greater than 5 cm across or if more than 50% of the surface of the coral was faded.
In total, we were able to determine the survivorship status of 474 of the tagged colonies (average of n = 36.5 colonies per site, range = 9 to 56; table S9); the remaining colonies could not be relocated after the heatwave. Corals were recorded as surviving the heatwave if they were found alive at any time point following the event and as not surviving it if they were found dead upon first inspection after heatwave. This occurred at the end of the heatwave (March/April 2016) for most colonies and in the two subsequent expeditions for corals located at sites that we had either been unable to fully sample (i.e., one dive instead of two to three to search for all corals) or sample at all (due to unfavorable weather conditions or logistical constraints) in March/April 2016.
Temperature and thermal stress
We quantified temperature on Kiritimati during the 2015–2016 El Niño event using both remotely sensed data extracted from NOAA’s CoralTemp product (90) and high-precision in situ temperature loggers (Sea-Bird Scientific SBE 56; ± 0.001°C precision). In situ loggers were deployed at sites around the atoll (minimum of one logger deployed per disturbance level; n = 17 sites, including n = 12 of the sites surveyed here) between 2011 and 2016, all at ~10 m depth (range: 8 to 12 m) on the forereef (fig. S2). For both data sources, we quantified temperature for all available sites around the atoll as in Claar et al. (91) and averaged across sites to produce a measure of island-wide temperature.
To assess the potential influence of baseline temperatures on coral communities around the atoll before the 2015–2016 El Niño, we also extracted the MMM temperature (92) for each site from NOAA CRW’s monthly mean SST climatology, which are produced at a 5-km spatial resolution (https://www.star.nesdis.noaa.gov/pub/sod/mecb/crw/data/5km/v3.1/climatology/nc/).
We quantified thermal stress on Kiritimati during the 2015–2016 El Niño as DHW (°C-weeks), the metric most commonly used to assess coral bleaching risk. Corals are sensitive to temperatures more than 1°C above their long-term MMM SST, known as the bleaching threshold. DHW is a measure of accumulated thermal stress, which is defined as the rolling sum of temperatures above the bleaching threshold during the preceding 12 weeks (93, 94). Considerable coral bleaching is expected to occur once cumulative thermal stress has exceeded 4°C-weeks (NOAA CRW Bleaching Alert Level 1), with widespread bleaching and some mortality typically expected at >8°C-weeks (NOAA CRW Bleaching Alert Level 2) (95).
DHW values for Kiritimati were extracted from the U.S. NOAA CRW’s 5-km DHW product (NOAA CRW daily global 5-km satellite coral bleaching heat stress DHW version 3.1) (94) for January 2011 to December 2016 (91), for each of the 19 study sites, and used to calculate an island-wide mean (table S3). Comparisons of these satellite-derived thermal stress values to in situ estimates in a previous study (91) yielded consistent results. Here, we present the satellite-derived DHW data and analyses using these data for comparability with other coral bleaching studies. In addition, to compare the thermal stress experienced on Kiritimati during this heatwave to earlier events, we extracted DHW values (as above) from 1985 to 2018 (fig. S5).
Statistical analyses
Analyses were conducted in R 4.0.4. interfaced with RStudio 1.4.1106. We fit a series of generalized linear models (GLMs) and generalized linear mixed-effects models (GLMMs) with the benthic community composition data, in which the overall proportion of hard coral cover (i.e., the response variable) was modeled with a beta error distribution and a logit link, using the glmmTMB package (96): (i) To examine influences on coral cover before the 2015–2016 El Niño, we modeled the overall hard coral cover as a function of chronic local disturbance (continuous) and three environmental variables: maximum NPP (mg C m−2 day−1), SST, and site exposure (windward versus sheltered; GLM). NPP was modeled as a polynomial (quadratic) relationship, as examination of the model residuals indicated a missing quadratic effect. (ii) To assess the influence of prolonged heat stress on coral communities and whether chronic local human disturbance modulates heat stress impacts, we modeled the overall hard coral cover as a function of heatwave period (before versus after), chronic local disturbance, maximum heat stress experienced during the El Niño (i.e., maximum site-level DHW), and a two-way interaction between heatwave period and disturbance (GLMM). To directly quantify the impacts of heat stress on corals with distinct life history types, we also fit separate models for the cover of stress-tolerant corals and the cover of competitive corals using the same model structure. Because at some sites all competitive corals were lost and the beta distribution is not defined at zero or one, the competitive coral models were fit with a zero-inflation parameter. (iii) We also examined whether the impact of the heatwave on coral cover was modulated by the pre–El Niño coral community composition. To do so, we defined a “dominant coral life history” covariate for each site by classifying each coral species according to its life history strategy [following (88) and table S6] and then classifying each site as “stress-tolerant dominated” (if >60% of the corals at that site had this life history strategy), “competitive dominated” (if >60% of the corals at that site had this life history strategy), or “mixed” (if there was no dominant life history type). We then modeled overall hard coral cover as a function of heatwave period (before versus after), dominant life history type, maximum site-level DHW, and maximum site-level NPP, with a two-way interaction between life history and heatwave period (GLMM). Although our environmental covariates (NPP, SST, and site exposure) did not significantly influence coral cover before the heatwave (Fig. 1B), examination of residuals for model sets (ii) and (iii) indicated missing predictors for some models such that NPP was selectively included and modeled as a quadratic relationship; similarly, because of missing predictors, DHW was modeled as a quadratic relationship in one model (see table S4 for details). Overall, “site” was included as a random effect in the GLMMs to account for the nonindependence of data collected at the same site over time, and continuous explanatory variables for all models were standardized using the “rescale” function in the arm package. All models were fit using site-level coral cover averaged across expeditions within each heatwave period. To test the robustness of the model results, we ran all models again, first using all available data points from the “before” and “after” heatwave periods, such that some sites had more than one data point per heatwave period, because they were sampled in multiple expeditions, and second using only the data from the largest expeditions conducted before (July 2013) and after (July 2017) the heatwave (table S8). These models resulted in minimal quantitative changes to the model results, with all conclusions regarding heat stress and local disturbance remaining the same (see table S4 and Supplementary Methods for additional details).
We also used a multivariate approach to examine differences in hard coral community composition across the disturbance gradient, both before and after the heatwave, by conducting multivariate ordinations and statistical analyses using the vegan package (97). A site-by-species matrix was created for the entire hard coral community using measures of percent cover that averaged across expeditions within each heat stress period. We performed multivariate ordinations (principal coordinates analysis) using the “betadisper” function to visualize differences in the coral communities among the three most disparate (very low, medium, and very high) levels of local human disturbance and across heat stress periods. We then tested for significant differences in coral community structure using PERMANOVA tests (“adonis” function) with 999 permutations and Bray-Curtis distances. Heat stress, human disturbance, and their interaction were included as fixed effects, while site was incorporated as a blocking factor using the strata term in adonis.
We used our longitudinal tagged coral dataset to directly examine the impact of prolonged heat stress on the survival of individual coral colonies. In all cases, coral survival was modeled using GLMs, with a binomial distribution and logit link, in the stats package. To assess the overall pattern, we first modeled all corals together without life history or species in the model. We then modeled the survival of stress-tolerant and competitive corals separately to evaluate relationships at the life history level and lastly, we modeled all data with a species-by-disturbance interaction to assess the relationships between survival and disturbance for all seven coral species individually. All models initially included chronic local disturbance, maximum site-level DHW, maximum NPP, and site exposure (windward versus sheltered) as fixed effects. All continuous explanatory variables were standardized using the rescale function in the arm package. For each model type (e.g., overall model, life history models, and species models), we ran models with all possible combinations of variables. We then used the Akaike information criterion (AIC) to determine the top model for each model type. In all cases, the model with disturbance (and the two-way life history or species interactions, where included) but without any of the environmental variables had the lowest AIC. We initially also included site as a random effect, but in all cases, this worsened the model fit. We present the results and visualizations of the top models for each model type (Fig. 5). Diagnostic graphs plotting residuals using the DHARMa package (98) were analyzed for each model presented here.
Last, to assess whether bleaching is an accurate metric of heatwave outcomes for corals, we used the tagged coral colony data to test whether corals that exhibited bleaching early in the heatwave had lower survival through the event. Here, we modeled the relationship between coral survival and bleaching as a binary state (as detailed above), using GLMs with a binomial distribution and a logit link. Bleaching status, coral species, disturbance, maximum NPP, and exposure were included as fixed effects, along with a two-way interaction between coral species and bleaching status. We ran models with all possible variable combinations and then used AIC to determine the top model, which included all variables but maximum NPP. We conducted all of these models both for the overall tagged coral dataset and for the stress-tolerant and competitive coral species separately. Bleaching results did not differ across any of these different model forms. Our results were also robust to bleaching being modeled using the four different bleaching categories (detailed above). Diagnostic plots were analyzed using the DHARMa package (98).
Acknowledgments
We acknowledge our collaborators and friends on Kiritimati who facilitated this research, R. Bebe, P. Tofinga, T. Kirata, T. Alefaio, V. Hnanguie, F. Tiata, J. Teem, and L. Teem. We also acknowledge L. Szostek, M. Watson, S. McNally, K. Cox, T. Stovel, J. Mortimer, J. Burns, and K. Bruce for help collecting the field data and N. O’Brien, L. Szostek, R. Hansen, E. Giannantonio, and A. Kozachuk for assistance with benthic image processing.
Funding: This work was supported by the National Science Foundation (NSF) RAPID grant OCE-1446402 (J.K.B. and K.M.C.); Rufford Maurice Laing Foundation (J.K.B.); Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (J.K.B.); NSERC E.W.R. Steacie Memorial Fellowship (J.K.B.); Canada Foundation for Innovation (CFI) Leaders Opportunity Fund (J.K.B.); British Columbia Knowledge Development Fund (J.K.B.); University of Victoria (J.K.B. and D.C.C.); University of Victoria Centre for Asia-Pacific Initiatives (J.K.B. and D.C.C.); David and Lucile Packard Foundation (J.K.B.); The Pew Charitable Trusts, Pew Fellowship in Marine Conservation (J.K.B.); National Geographic Society, Committee for Research and Exploration Grants (NGS-146R-18 and NGS-63112R-19) (J.K.B.); NSERC Vanier Canada Graduate Scholarship (D.C.C.); NSERC Graduate Scholarships (J.M.T.M. and D.G.M.); National Oceanic and Atmospheric Administration (NOAA) Climate and Global Change Postdoctoral Fellowship Program, administered by UCAR’s Cooperative Programs for the Advancement of Earth System Science (CPAESS) award NA18NWS4620043B (D.C.C.); American Academy of Underwater Sciences (D.C.C.); International Society for Reef Studies (D.C.C.); National Geographic Young Explorers Grant (D.C.C.); Women Divers Hall of Fame (D.C.C.); Sea-Bird Electronics equipment grant (D.C.C.); and Divers Alert Network (D.C.C.).
Author contributions: Conceptualization: J.K.B. Data collection: J.K.B., D.C.C., K.L.T., J.M.M.-I., J.M.T.M., K.M.C., and D.G.M. Data processing: D.G.M. Data analysis: K.L.T., J.M.T.M., D.C.C., and J.K.B. Writing (original draft): J.K.B. Writing (review and editing): J.K.B., D.C.C., K.L.T., J.M.T.M., D.G.M., K.M.C., and J.M.M.-I.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Data reported in this paper and code to reproduce the analyses and figures are provided on Zenodo (https://zenodo.org/record/7697331#.ZATWd-zMLlw); code is also provided on GitHub (https://github.com/baumlab/Baum_etal_2023_ScienceAdvances).
Supplementary Materials
This PDF file includes:
Supplementary Methods
Figs. S1 to S13
Tables S1 to S9
Legend for data S1
References
Other Supplementary Material for this manuscript includes the following:
Data file S1
REFERENCES AND NOTES
- 1.T. L. Frölicher, E. M. Fischer, N. Gruber, Marine heatwaves under global warming. Nature 560, 360–364 (2018). [DOI] [PubMed] [Google Scholar]
- 2.S. Cooley, D. Schoeman, L. Bopp, P. Boyd, S. Donner, D. Y. Ghebrehiwet, S.-I. Ito, W. Kiessling, P. Martinetto, E. Ojea, M.-F. Racault, B. Rost, and M. Skern-Mauritzen, 2022 Oceans and Coastal Ecosystems and Their Services. In: Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, H.-O. Pörtner, D. C. Roberts, M. Tignor, E. S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama, Eds. (Cambridge Uni. Press 2022) pp. 379–550, doi:10.1017/9781009325844.005.
- 3.E. C. J. Oliver, M. T. Burrows, M. G. Donat, A. Sen Gupta, L. V. Alexander, S. E. Perkins-Kirkpatrick, J. A. Benthuysen, A. J. Hobday, N. J. Holbrook, P. J. Moore, M. S. Thomsen, T. Wernberg, D. A. Smale, Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Mar. Sci. 6, 734 (2019). [Google Scholar]
- 4.B. E. Brown, Coral bleaching: Causes and consequences. Coral Reefs. 16, S129–S138 (1997). [Google Scholar]
- 5.A. C. Baker, P. W. Glynn, B. Riegl, Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008). [Google Scholar]
- 6.T. P. Hughes, Climate change, human Impacts, and the resilience of coral reefs. Science 301, 929–933 (2003). [DOI] [PubMed] [Google Scholar]
- 7.S. D. Donner, W. J. Skirving, C. M. Little, M. Oppenheimer, O. Hoegh-Guldberg, Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Chang. Biol. 11, 2251–2265 (2005). [DOI] [PubMed] [Google Scholar]
- 8.O. Hoegh-Guldberg, P. J. Mumby, A. J. Hooten, R. S. Steneck, P. Greenfield, E. Gomez, C. D. Harvell, P. F. Sale, A. J. Edwards, K. Caldeira, N. Knowlton, C. M. Eakin, R. Iglesias-Prieto, N. Muthiga, R. H. Bradbury, A. Dubi, M. E. Hatziolos, Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- 9.E. C. J. Oliver, M. G. Donat, M. T. Burrows, P. J. Moore, D. A. Smale, L. V. Alexander, J. A. Benthuysen, M. Feng, A. Sen Gupta, A. J. Hobday, N. J. Holbrook, S. E. Perkins-Kirkpatrick, H. A. Scannell, S. C. Straub, T. Wernberg, Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9, 1324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.S. F. Heron, J. A. Maynard, R. van Hooidonk, C. M. Eakin, Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 6, 38402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.C. M. Eakin, H. P. A. Sweatman, R. E. Brainard, The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs. 38, 539–545 (2019). [Google Scholar]
- 12.R. van Hooidonk, J. Maynard, J. Tamelander, J. Gove, G. Ahmadia, L. Raymundo, G. Williams, S. F. Heron, S. Planes, Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. 6, 39666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.L. Burke, K. Reytar, M. Spalding, A. Perry, “Reefs at risk revisited” (World Resources Institute, 2011), pp. 1–130.
- 14.C. M. Crain, K. Kroeker, B. S. Halpern, Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 (2008). [DOI] [PubMed] [Google Scholar]
- 15.M. Ateweberhan, D. A. Feary, S. Keshavmurthy, A. Chen, M. H. Schleyer, C. R. C. Sheppard, Climate change impacts on coral reefs: Synergies with local effects, possibilities for acclimation, and management implications. Mar. Pollut. Bull. 74, 526–539 (2013). [DOI] [PubMed] [Google Scholar]
- 16.J. E. Carilli, R. D. Norris, B. A. Black, S. M. Walsh, M. McField, Local stressors reduce coral resilience to bleaching. PLOS ONE 4, e6324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.J. E. Carilli, R. D. Norris, B. Black, S. M. Walsh, M. McField, Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob Change Biol. 16, 1247–1257 (2010). [Google Scholar]
- 18.J. F. Bruno, I. M. Côté, L. T. Toth, Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann. Rev. Mar. Sci. 11, 307–334 (2019). [DOI] [PubMed] [Google Scholar]
- 19.E. S. Darling, T. R. McClanahan, I. M. Côté, Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 19, 1930–1940 (2012). [DOI] [PubMed] [Google Scholar]
- 20.T. P. Hughes, J. T. Kerry, M. Álvarez-Noriega, J. G. Álvarez-Romero, K. D. Anderson, A. H. Baird, R. C. Babcock, M. Beger, D. R. Bellwood, R. Berkelmans, T. C. Bridge, I. R. Butler, M. Byrne, N. E. Cantin, S. Comeau, S. R. Connolly, G. S. Cumming, S. J. Dalton, G. Diaz-Pulido, C. M. Eakin, W. F. Figueira, J. P. Gilmour, H. B. Harrison, S. F. Heron, A. S. Hoey, J.-P. A. Hobbs, M. O. Hoogenboom, E. V. Kennedy, C. Kuo, J. M. Lough, R. J. Lowe, G. Liu, M. T. McCulloch, H. A. Malcolm, M. J. McWilliam, J. M. Pandolfi, R. J. Pears, M. S. Pratchett, V. Schoepf, T. Simpson, W. J. Skirving, B. Sommer, G. Torda, D. R. Wachenfeld, B. L. Willis, S. K. Wilson, Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017). [DOI] [PubMed] [Google Scholar]
- 21.J. M. Pandolfi, Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 (2003). [DOI] [PubMed] [Google Scholar]
- 22.T. P. Hughes, M. L. Barnes, D. R. Bellwood, J. E. Cinner, G. S. Cumming, J. B. C. Jackson, J. Kleypas, I. A. van de Leemput, J. M. Lough, T. H. Morrison, S. R. Palumbi, E. H. van Nes, M. Scheffer, Coral reefs in the Anthropocene. Nature 546, 82–90 (2017). [DOI] [PubMed] [Google Scholar]
- 23.K. E. Carpenter, M. Abrar, G. Aeby, R. B. Aronson, S. Banks, A. Bruckner, A. Chiriboga, J. Cortes, J. C. Delbeek, L. DeVantier, G. J. Edgar, A. J. Edwards, D. Fenner, H. M. Guzman, B. W. Hoeksema, G. Hodgson, O. Johan, W. Y. Licuanan, S. R. Livingstone, E. R. Lovell, J. A. Moore, D. O. Obura, D. Ochavillo, B. A. Polidoro, W. F. Precht, M. C. Quibilan, C. Reboton, Z. T. Richards, A. D. Rogers, J. Sanciangco, A. Sheppard, C. Sheppard, J. Smith, S. Stuart, E. Turak, J. E. N. Veron, C. Wallace, E. Weil, E. Wood, One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563 (2008). [DOI] [PubMed] [Google Scholar]
- 24.C. P. Nadeau, M. C. Urban, J. R. Bridle, Climates past, present, and yet-to-come shape climate change vulnerabilities. Trends Ecol. Evol. 32, 786–800 (2017). [DOI] [PubMed] [Google Scholar]
- 25.D. J. Suggett, D. J. Smith, Coral bleaching patterns are the outcome of complex biological and environmental networking. Glob. Change Biol. 26, 68–79 (2019). [DOI] [PubMed] [Google Scholar]
- 26.O. Hoegh-Guldberg, Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 50, 839–866 (1999). [Google Scholar]
- 27.H. M. Putnam, K. L. Barott, T. D. Ainsworth, R. D. Gates, The vulnerability and resilience of reef-building corals. Curr. Biol. 27, R528–R540 (2017). [DOI] [PubMed] [Google Scholar]
- 28.H. M. Putnam, M. Stat, X. Pochon, R. D. Gates, Endosymbiotic flexibility associates with environmental sensitivity in scleractinian corals. Proc. R. Soc. B 279, 4352–4361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R. Cunning, A. C. Baker, Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 259–262 (2013). [Google Scholar]
- 30.D. J. Suggett, M. E. Warner, W. Leggat, Symbiotic dinoflagellate functional diversity mediates coral survival under ecological crisis. Trends Ecol. Evol. 32, 735–745 (2017). [DOI] [PubMed] [Google Scholar]
- 31.G. B. Dixon, S. W. Davies, G. A. Aglyamova, E. Meyer, L. K. Bay, M. V. Matz, Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460–1462 (2015). [DOI] [PubMed] [Google Scholar]
- 32.S. R. Palumbi, D. J. Barshis, N. Traylor-Knowles, R. A. Bay, Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Y. Loya, K. Sakai, K. Yamazato, Y. Nakano, H. Sambali, R. van Woesik, Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001). [Google Scholar]
- 34.E.S. Darling, L. Alvarez-Filip, T.A. Oliver, T.R. McClanahan, I.M. Côté, Evaluating life-history strategies of reef corals from species traits. Ecology Letters 15, 1378–1386 (2012). [DOI] [PubMed] [Google Scholar]
- 35.R. D. Vinebrooke, K. L. Cottingham, J. Norberg, M. Scheffer, S. I. Dodson, S. C. Maberly, U. Sommer, Impacts of multiple stressors on biodiversity and ecosystem functioning: The role of species co-tolerance. Oikos 104, 451–457 (2004). [Google Scholar]
- 36.P. A. Keddy, Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 3, 157–164 (1992). [Google Scholar]
- 37.S. I. Passy, M. Bottin, J. Soininen, H. Hillebrand, Environmental filtering and taxonomic relatedness underlie the species richness–Evenness relationship. Hydrobiologia 787, 243–253 (2017). [Google Scholar]
- 38.A. G. Grottoli, M. E. Warner, S. J. Levas, M. D. Aschaffenburg, V. Schoepf, M. McGinley, J. Baumann, Y. Matsui, The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Change Biol. 20, 3823–3833 (2014). [DOI] [PubMed] [Google Scholar]
- 39.I. M. Côté, E. S. Darling, C. J. Brown, Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 283, 20152592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D. C. Claar, J. K. Baum, Timing matters: Survey timing during extended heat stress can influence perceptions of coral susceptibility to bleaching. Coral Reefs. 38, 559–565 (2019). [Google Scholar]
- 41.M. G. Gleason, Effects of disturbance on coral communities: Bleaching in Moorea, French Polynesia. Coral Reefs. 12, 193–201 (1993). [Google Scholar]
- 42.M. D. Fox, A. L. Carter, C. B. Edwards, Y. Takeshita, M. D. Johnson, V. Petrovic, C. G. Amir, E. Sala, S. A. Sandin, J. E. Smith, Limited coral mortality following acute thermal stress and widespread bleaching on Palmyra Atoll, central Pacific. Coral Reefs. 38, 701–712 (2019). [Google Scholar]
- 43.B. Vargas-Ángel, B. Huntington, R. E. Brainard, R. Venegas, T. Oliver, H. Barkley, A. Cohen, El Niño-associated catastrophic coral mortality at Jarvis Island, central Equatorial Pacific. Coral Reefs. 38, 731–741 (2019). [Google Scholar]
- 44.H. C. Barkley, A. L. Cohen, N. R. Mollica, R. E. Brainard, H. E. Rivera, T. M. DeCarlo, G. P. Lohmann, E. J. Drenkard, A. E. Alpert, C. W. Young, B. Vargas-Ángel, K. C. Lino, T. A. Oliver, K. R. Pietro, V. H. Luu, Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016). Commun Biol. 1, 177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.E. A. Dinsdale, O. Pantos, S. Smriga, R. A. Edwards, F. Angly, L. Wegley, M. Hatay, D. Hall, E. Brown, M. Haynes, L. Krause, E. Sala, S. A. Sandin, R. V. Thurber, B. L. Willis, F. Azam, N. Knowlton, F. Rohwer, Microbial ecology of four coral atolls in the Northern Line Islands. PLOS ONE 3, e1584 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.J. M. McDevitt-Irwin, M. Garren, R. McMinds, R. Vega Thurber, J. K. Baum, Variable interaction outcomes of local disturbance and El Niño-induced heat stress on coral microbiome alpha and beta diversity. Coral Reefs 38, 331–345 (2019). [Google Scholar]
- 47.M. Garren, L. Raymundo, J. Guest, C. D. Harvell, F. Azam, Resilience of coral-associated bacterial communities exposed to fish farm effluent. PLOS ONE 4, e7319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.C. Roder, T. Bayer, M. Aranda, M. Kruse, C. R. Voolstra, Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Mol. Ecol. 24, 3501–3511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.J. R. Zaneveld, D. E. Burkepile, A. A. Shantz, C. E. Pritchard, R. McMinds, J. P. Payet, R. Welsh, A. M. S. Correa, N. P. Lemoine, S. Rosales, C. Fuchs, J. A. Maynard, R. V. Thurber, Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 7, 11833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D. C. Claar, J. M. McDevitt-Irwin, M. Garren, R. Vega Thurber, R. D. Gates, J. K. Baum, Increased diversity and concordant shifts in community structure of coral-associated Symbiodiniaceae and bacteria subjected to chronic human disturbance. Mol. Ecol. 29, 2477–2491 (2020). [DOI] [PubMed] [Google Scholar]
- 51.S. Roitman, T. López-Londoño, F. Joseph Pollock, K. B. Ritchie, C. T. Galindo-Martínez, K. Gómez-Campo, L. A. González-Guerrero, V. Pizarro, M. López-Victoria, R. Iglesias-Prieto, M. Medina, Surviving marginalized reefs: Assessing the implications of the microbiome on coral physiology and survivorship. Coral Reefs 39, 795–807 (2020). [Google Scholar]
- 52.M. Stat, R. D. Gates, Clade D Symbiodinium in Scleractinian corals: A “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011, 1–9 (2011). [Google Scholar]
- 53.T. F. Cooper, R. Berkelmans, K. E. Ulstrup, S. Weeks, B. Radford, A. M. Jones, J. Doyle, M. Canto, R. A. O’Leary, M. J. H. van Oppen, Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLOS ONE 6, e25536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.E. V. Kennedy, L. Tonk, N. L. Foster, I. Chollett, J.-C. Ortiz, S. Dove, O. Hoegh-Guldberg, P. J. Mumby, J. R. Stevens, Symbiodinium biogeography tracks environmental patterns rather than host genetics in a key Caribbean reef-builder, Orbicella annularis. Proc. R. Soc. B 283, 20161938 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D. C. Claar, S. Starko, K. L. Tietjen, H. E. Epstein, R. Cunning, K. M. Cobb, A. C. Baker, R. D. Gates, J. K. Baum, Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nat. Commun. 11, 6097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.S. Starko, J. Fifer, D. Claar, S. W. Davies, R. Cunning, A. C. Baker, J. K. Baum, Rapid selection during marine heatwaves threatens cryptic coral diversity and erodes associations amongst coevolving partners. bioRxiv 10.1101/2023.01.07.5229 (2023); 10.1101/2023.01.07.522953v1 [DOI] [PMC free article] [PubMed]
- 57.M. K. Donovan, T. C. Adam, A. A. Shantz, K. E. Speare, K. S. Munsterman, M. M. Rice, R. J. Schmitt, S. J. Holbrook, D. E. Burkepile, Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Proc. Natl. Acad. Sci. U.S.A. 117, 5351–5357 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.I. M. Côté, E. S. Darling, Rethinking ecosystem resilience in the face of climate change. PLOS Biol. 8, e1000438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A. E. Bates, R. S. C. Cooke, M. I. Duncan, G. J. Edgar, J. F. Bruno, L. Benedetti-Cecchi, I. M. Côté, J. S. Lefcheck, M. J. Costello, N. Barrett, T. J. Bird, P. B. Fenberg, R. D. Stuart-Smith, Climate resilience in marine protected areas and the “Protection Paradox”. Biol. Conserv. 236, 305–314 (2019). [Google Scholar]
- 60.S. E. Cannon, E. Aram, T. Beiateuea, A. Kiareti, M. Peter, S. D. Donner, Coral reefs in the Gilbert Islands of Kiribati: Resistance, resilience, and recovery after more than a decade of multiple stressors. PLOS ONE 16, e0255304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.E. V. Kennedy, J. Vercelloni, B. P. Neal, Ambariyanto, D. E. P. Bryant, A. Ganase, P. Gartrell, K. Brown, C. J. S. Kim, M. Hudatwi, A. Hadi, A. Prabowo, P. Prihatinningsih, S. Haryanta, K. Markey, S. Green, P. Dalton, S. Lopez-Marcano, A. Rodriguez-Ramirez, M. Gonzalez-Rivero, O. Hoegh-Guldberg, Coral reef community changes in Karimunjawa National Park, Indonesia: Assessing the efficacy of management in the face of local and global stressors. JMSE 8, 760 (2020). [Google Scholar]
- 62.J. H. Baumann, L. Z. Zhao, A. C. Stier, J. F. Bruno, Remoteness does not enhance coral reef resilience. Glob. Change Biol. 28, 417–428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O. Hoegh-Guldberg, E. V. Kennedy, H. L. Beyer, C. McClennen, H. P. Possingham, Securing a long-term future for coral reefs. Trends Ecol. Evol. 33, 936–944 (2018). [DOI] [PubMed] [Google Scholar]
- 64.H. L. Beyer, E. V. Kennedy, M. Beger, C. A. Chen, J. E. Cinner, E. S. Darling, C. M. Eakin, R. D. Gates, S. F. Heron, N. Knowlton, D. O. Obura, S. R. Palumbi, H. P. Possingham, M. Puotinen, R. K. Runting, W. J. Skirving, M. Spalding, K. A. Wilson, S. Wood, J. E. Veron, O. Hoegh-Guldberg, Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 11, e12587 (2018). [Google Scholar]
- 65.A. Baird, P. Marshall, Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237, 133–141 (2002). [Google Scholar]
- 66.V. Masson-Delmotte, “IPCC, Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, B. Zhou, Eds. (Cambridge Univ. Press. 2021).
- 67.T. P. Hughes, K. D. Anderson, S. R. Connolly, S. F. Heron, J. T. Kerry, J. M. Lough, A. H. Baird, J. K. Baum, M. L. Berumen, T. C. Bridge, D. C. Claar, C. M. Eakin, J. P. Gilmour, N. A. J. Graham, H. Harrison, J.-P. A. Hobbs, A. S. Hoey, M. Hoogenboom, R. J. Lowe, M. T. McCulloch, J. M. Pandolfi, M. Pratchett, V. Schoepf, G. Torda, S. K. Wilson, Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 68.N. A. J. Graham, K. L. Nash, J. T. Kool, Coral reef recovery dynamics in a changing world. Coral Reefs 30, 283–294 (2011). [Google Scholar]
- 69.J. P. Gilmour, L. D. Smith, A. J. Heyward, A. H. Baird, M. S. Pratchett, Recovery of an isolated coral reef system following severe disturbance. Science 340, 69–71 (2013). [DOI] [PubMed] [Google Scholar]
- 70.C. Sheppard, A. Harris, A. Sheppard, Archipelago-wide coral recovery patterns since 1998 in the Chagos Archipelago, central Indian Ocean. Mar. Ecol. Prog. Ser. 362, 109–117 (2008). [Google Scholar]
- 71.C. Pisapia, D. Burn, R. Yoosuf, A. Najeeb, K. D. Anderson, M. S. Pratchett, Coral recovery in the central Maldives archipelago since the last major mass-bleaching, in 1998. Sci. Rep. 6, 34720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.N. A. J. Graham, S. Jennings, M. A. MacNeil, D. Mouillot, S. K. Wilson, Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97 (2015). [DOI] [PubMed] [Google Scholar]
- 73.P. J. Edmunds, Implications of high rates of sexual recruitment in driving rapid reef recovery in Mo’orea, French Polynesia. Sci. Rep. 8, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.J. P. W. Robinson, S. K. Wilson, N. A. J. Graham, Abiotic and biotic controls on coral recovery 16 years after mass bleaching. Coral Reefs 38, 1255–1265 (2019). [Google Scholar]
- 75.S. K. Wilson, J. P. W. Robinson, K. Chong-Seng, J. Robinson, N. A. J. Graham, Boom and bust of keystone structure on coral reefs. Coral Reefs 38, 625–635 (2019). [Google Scholar]
- 76.IPCC, “Summary for Policymakers” in Global Warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, Masson-Delmotte, V., P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, and T. Waterfield, Eds. (World Meteorological Organization, Geneva, Switzerland, 2018), pp. 32.
- 77.K. E. Trenberth, The definition of El Niño. Bull. Am. Meteorol. Soc. 78, 2771–2778 (1997). [Google Scholar]
- 78.S. L. Wear, R. V. Thurber, Sewage pollution: Mitigation is key for coral reef stewardship. Ann. N.Y. Acad. Sci. 1355, 15–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.K. E. Fabricius, Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 50, 125–146 (2005). [DOI] [PubMed] [Google Scholar]
- 80.J. M. T. Magel, S. A. Dimoff, J. K. Baum, Direct and indirect effects of climate change-amplified pulse heat stress events on coral reef fish communities. Ecol. Appl. 30, e02124 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Kiribati National Statistics Office, “2015 Population and Housing Census” (Ministry of Finance, Bairiki, Tarawa, 2016), p. 197.
- 82.M. S. Watson, D. C. Claar, J. K. Baum, Subsistence in isolation: Fishing dependence and perceptions of change on Kiritimati, the world’s largest atoll. Ocean Coast. Manag. 123, 1–8 (2016). [Google Scholar]
- 83.L. A. Yeager, P. Marchand, D. A. Gill, J. K. Baum, J. M. McPherson, Marine socio-environmental covariates: Queryable global layers of environmental and anthropogenic variables for marine ecosystem studies. Ecology 98, 1976–1976 (2017). [DOI] [PubMed] [Google Scholar]
- 84.C. Bosserelle, S. Reddy, D. Lai, “WACOP wave climate reports. Kiribati, Kiritimati” (Secretariat of the Pacific Community, 2015), pp. 1–15.
- 85.S. M. Walsh, Ecosystem-scale effects of nutrients and fishing on coral reefs. J. Mar. Biol. 2011, 1–13 (2011). [Google Scholar]
- 86.O. Beijbom, P. J. Edmunds, C. Roelfsema, J. Smith, D. I. Kline, B. P. Neal, M. J. Dunlap, V. Moriarty, T.-Y. Fan, C.-J. Tan, S. Chan, T. Treibitz, A. Gamst, B. G. Mitchell, D. Kriegman, Towards automated annotation of benthic survey images: Variability of human experts and operational modes of automation. PLOS ONE 10, e0130312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.P. G. Rachello-Dolmen, D. F. R. Cleary, Relating coral species traits to environmental conditions in the Jakarta Bay/Pulau Seribu reef system, Indonesia. Estuar. Coast. Shelf Sci. 73, 816–826 (2007). [Google Scholar]
- 88.J. S. Madin, M. O. Hoogenboom, S. R. Connolly, E. S. Darling, D. S. Falster, D. Huang, S. A. Keith, T. Mizerek, J. M. Pandolfi, H. M. Putnam, A. H. Baird, A trait-based approach to advance coral reef science. Trends Ecol. Evol. 31, 419–428 (2016). [DOI] [PubMed] [Google Scholar]
- 89.J. B. C. Jackson, T. P. Hughes, Adaptive strategies of coral-reef invertebrates: Coral-reef environments that are regularly disturbed by storms and by predation often favor the very organisms most susceptible to damage by these processes. Am. Sci. 73, 265–274 (1985). [Google Scholar]
- 90.E. Maturi, A. Harris, J. Mittaz, J. Sapper, G. Wick, X. Zhu, P. Dash, P. Koner, A new high-resolution sea surface temperature blended analysis. Bull. Am. Meteorol. Soc. 98, 1015–1026 (2017). [Google Scholar]
- 91.D. C. Claar, K. M. Cobb, J. K. Baum, In situ and remotely sensed temperature comparisons on a Central Pacific atoll. Coral Reefs 38, 1343–1349 (2019). [Google Scholar]
- 92.A. E. Strong, C. S. Barrientos, C. Duda, J. Sapper, Improved satellite techniques for monitoring coral reef bleaching, in Proceedings of the 8th International Coral Reef Symposium (ICRS, 1997), vol. 2, pp. 1495–1498 [Google Scholar]
- 93.G. Liu, A. E. Strong, W. Skirving, L. F. Arzayus, Overview of NOAA Coral Reef Watch Program’s near-real-time satellite global coral bleaching monitoring activities, in Proceedings of the 10th International Coral Reef Symposium (2006) vol. 12, pp. 1783–1793
- 94.NOAA, NOAA Coral Reef Watch Daily Global 5-km Satellite Coral Bleaching Sea Surface Temperature Product v3.1 (2013); https://coralreefwatch.noaa.gov/product/5km/.
- 95.G. Liu, S. Heron, C. Eakin, F. Muller-Karger, M. Vega-Rodriguez, L. Guild, J. De La Cour, E. Geiger, W. Skirving, T. Burgess, A. Strong, A. Harris, E. Maturi, A. Ignatov, J. Sapper, J. Li, S. Lynds, Reef-scale thermal stress monitoring of coral ecosystems: New 5-km global products from NOAA Coral Reef Watch. Remote Sens. (Basel) 6, 11579–11606 (2014). [Google Scholar]
- 96.M. E. Brooks, K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Mächler, B. M. Bolker, glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017). [Google Scholar]
- 97.J. Oksanen, Vegan: An introduction to ordination (2019); http://cran.r-project.org/web/packages/vegan/index.html
- 98.F. Hartig, DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models (2022); https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html.
- 99.S. J. Dollar, Wave stress and coral community structure in Hawaii. Coral Reefs 1, 71–81 (1982). [Google Scholar]
- 100.I. D. Williams, J. K. Baum, A. Heenan, K. M. Hanson, M. O. Nadon, R. E. Brainard, Human, oceanographic and habitat drivers of central and western Pacific coral reef fish assemblages. PLOS ONE 10, e0120516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.G. J. Williams, J. E. Smith, E. J. Conklin, J. M. Gove, E. Sala, S. A. Sandin, Benthic communities at two remote Pacific coral reefs: Effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ. 1, e81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.J. P. W. Robinson, I. D. Williams, L. A. Yeager, J. M. McPherson, J. Clark, T. A. Oliver, J. K. Baum, Environmental conditions and herbivore biomass determine coral reef benthic community composition: Implications for quantitative baselines. Coral Reefs 37, 1157–1168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.D. Lüdecke, M. S. Ben-Shachar, I. Patil, P. Waggoner, D. Makowski, Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6, 3139 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Figs. S1 to S13
Tables S1 to S9
Legend for data S1
References
Data file S1