Abstract
Machu Picchu originally functioned as a palace within the estate of the Inca emperor Pachacuti between ~1420 and 1532 CE. Before this study, little was known about the people who lived and died there, where they came from or how they were related to the inhabitants of the Inca capital of Cusco. We generated genome-wide data for 34 individuals buried at Machu Picchu who are believed to have been retainers or attendants assigned to serve the Inca royal family, as well as 34 individuals from Cusco for comparative purposes. When the ancient DNA results are contextualized using historical and archaeological data, we conclude that the retainer population at Machu Picchu was highly heterogeneous with individuals exhibiting genetic ancestries associated with groups from throughout the Inca Empire and Amazonia. The results suggest a diverse retainer community at Machu Picchu in which people of different genetic backgrounds lived, reproduced, and were interred together.
Individuals buried at Machu Picchu are highly genetically diverse exhibiting ancestries from throughout the Inca empire.
INTRODUCTION
Machu Picchu is arguably the best-known archaeological site in the Western Hemisphere, and, before the 2020 pandemic, it attracted over a million travelers from throughout the world. In recent decades, its image has been used as an icon of the Peruvian nation and a symbol of the historic accomplishments of Latin America’s indigenous peoples (1). Despite its fame, little was known of Machu Picchu’s function and the daily life of its inhabitants until recently. These lacunae were due to the absence of references to Machu Picchu in 16th and 17th century Spanish accounts and the failure of modern investigators to decipher the knotted string records (quipus) that the Incas used to record their history (2, 3). However, over the past two decades, scholars have begun to understand the site as a result of archaeological fieldwork and the application of new scientific techniques to laboratory research. The latter has yielded important results related to the diet and health of Machu Picchu’s ancient population and insights into the daily activities carried out there (4).
There is a now a consensus among archaeologists and historians that Machu Picchu was part of a royal estate belonging to the lineage (or panaca) of the emperor Pachacuti (2, 3, 5), the ruler credited with establishing the Inca Empire (or Tahuantinsuyu) (6). The monumental architecture at the core of Machu Picchu is actually the remains of a country palace located within the royal estate of Pachacuti (2, 7, 8).
Royal estates were lands claimed by an Inca emperor for his noble lineage that was maintained in perpetuity for the ostensible purpose of caring for and making offerings to the ruler and, after his death, the ruler’s mummy (9). Often, these royal estates were established to commemorate conquests, and Machu Picchu may have been built to celebrate Pachacuti’s conquest of the lower Urubamba Valley (7). The emperor and members of his lineage only resided seasonally in the elaborate palaces built within these country estates, but a retinue of retainers was left behind to maintain the facilities there. The Urubamba Valley was a favored location for royal estates and Machu Picchu, Pisac, Ollantaytambo, and over a dozen others have been identified in the drainage (2, 10). Some royal estates such as Cheqoq lacked palaces but fulfilled economic roles for the panacas such as maize cultivation, pottery production, and salt mining (5, 9). The royal lineages were served by individuals known as yanacona who were ethnically non-Inca and were permanently resettled to attend to the daily needs of the Inca, his mummy, and his guests. The yanacona were believed to be privileged compared to the general population, and this was expressed in their proximity to royalty and material benefits, such as luxury goods (9, 11, 12). They were taken from conquered lands by the emperor or presented as gifts by other panacas, even after the death of the founder of the royal estate (13, 14). The yanacona, who were male, appear to have received wives from the class of females known as aclla, “chosen women” who were severed from their ethnic group and educated in special facilities (15–17).
Machu Picchu would have been occupied by several hundred permanent retainers (yanacona and former aclla) throughout the year and, at peak season, a still larger population of attendants, members of the Inca royalty, and their guests. Judging from the intact architecture, it is unlikely that more than 750 people ever resided in Machu Picchu at one time (4). While the Inca elite individuals who visited the palace were buried in the capital of Cusco, the yanacona and former aclla were usually interred in cemetery areas outside the palace walls. Isotopic (12, 18, 19), osteological (20), and artifact studies (21) indicate that there was substantial diversity in this attendant population but these studies have had limited success in answering questions about where the residents came from, how they were related to each other, and how their regional and ethnic background affected the way in which they lived and were buried. This article presents an ancient DNA (aDNA) analysis of a large sample of individuals buried around the palace at Machu Picchu to begin answering these questions. In addition, we provide aDNA results for individuals excavated in the Inca capital of Cusco and nearby sites coeval with Machu Picchu for comparative purposes. These samples highlight the remarkable genomic composition of the Machu Picchu yanacona population and the considerable genomic variability of the Cusco inhabitants.
Machu Picchu’s occupants
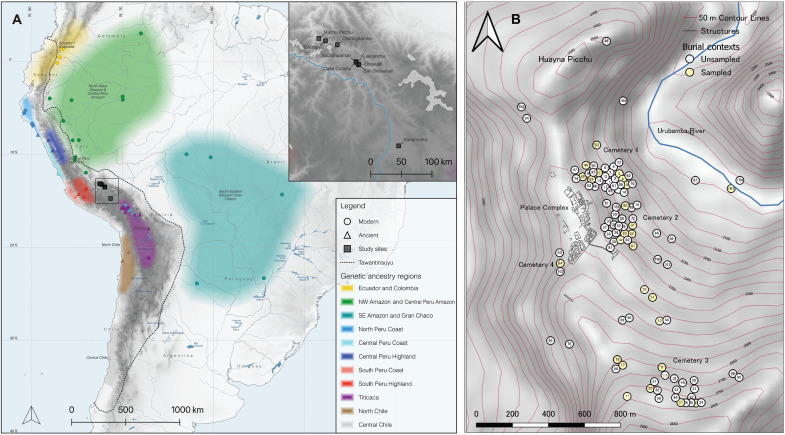
Machu Picchu is located in southern Peru on the eastern slopes of the Andes (Fig. 1A) at 2430 m above sea level on a ridge overlooking the Urubamba River. The Urubamba/Vilcanota drainage was a favored location for country palaces by many Inca rulers because of its rich lands, good climate, and proximity to the capital. Located 75 km from Cusco, Machu Picchu would have been especially attractive during the dry season (May to October). The ruler, his family, and his guests would have appreciated Machu Picchu’s tropical climate and vegetation and the absence of nightly frosts that occur in Cusco during the winter months. According to historic accounts, the Inca royalty carried out a range of activities at royal estates including feasting, singing, and dancing in the plazas and hunting in the forested lands surrounding the palaces of royal estates (13, 14, 22), The Spanish chronicles also describe numerous religious ceremonies practiced by the Inca priests that were linked to celestial events, sacred geography, and agricultural fertility. It is likely that the emperor and/or his representatives would also have participated in these ceremonies when he visited Machu Picchu (4, 23, 24).
Fig. 1. Maps of the provenience of the sites, groups, and individuals analyzed in this study.
(A) Map of South America showing the geographic distribution of genetic ancestry groups described (33, 34), the location of published reference genomes from modern-day (circles) and ancient (triangles) individuals that constitute these groups and of the archaeological sites from which individuals in this study derived (squares, also see inset). (B) Map showing the location of burial caves at Machu Picchu and highlighting the caves sampled in this study [adapted from figure 2 of (25)].
A recent radiocarbon study of the osteological collection from the site based on 26 human bone and tooth samples concluded that Machu Picchu had been occupied from circa 1420 to 1532 CE (25). Subsequently, the same AMS measurements were analyzed using a Bayesian single phase model, and the probabilities produced suggest an occupation from 1400 to 1435 CE to 1470 to 1520 CE (95.4%) (see Supplementary Text; table S1, B and C; and fig. S1).
Excavations by the Yale Peruvian Scientific Expedition in 1912 documented 107 burials containing the remains of a minimum of 174 individuals (20, 26). These simple interments sometimes included multiple individuals and were in shallow chambers with protective coarse stone walls beneath large boulders or underneath natural overhangs. Many burials lacked grave goods or contained only a small number of pots or other artifacts. A large number of the ceramics were in provincial Inca or non-Inca styles from the Peruvian north coast, the Peruvian central coast, the circum-Titicaca area, and Chachapoyas. In contrast, exotic ceramics are rare or absent from nonburial contexts at the site (8, 21, 27). The dead also showed evidence of different types of cranial modification, including forms associated with Lake Titicaca and the coast rather than Cusco or Urubamba (20). The nature of the skeletons and the burial goods found with them led investigators to posit that the burial caves held the remains of retainers and attendants who served the elite at Machu Picchu (4, 12, 17, 19).
Most burials at Machu Picchu were found along the periphery of the site (Fig. 1B), grouping in four clusters referred to as cemeteries 1 to 4 (26, 28). Additional research on burial ceramics (21, 27), skeletal morphology and cranial modification (20), stable carbon (C) and nitrogen (N) isotopes (18, 19), and lead (Pb), strontium (Sr), and oxygen (O) isotopes (12, 17) demonstrated a high degree of diversity among the Machu Picchu burial population, consistent with the interpretation that the buried individuals were yanacona and former aclla. However, cultural identifiers such as burial contents may have been acquired on site rather than being indicators of an individual’s homeland. Isotopic methods on their own are limited for determining the ancestral regions from where these individuals might have originated. For example, high degrees of Sr-isotopic variability and the absence of regional and pan-regional isoscapes limit inferences (29). After reviewing all of the available evidence, Inca specialist D’Altroy (2) concluded that the people of the estate complex at Machu Picchu were largely from areas to the south of Cusco, but he hesitated to be more specific.
This study was undertaken to further elucidate the origins of the individuals buried at Machu Picchu and/or of their ancestors, as well as to shed light on their genetic histories and potential multigenerational relationships (see “Ethics statement” in Supplementary Text). For this purpose, we generated genome-wide aDNA data from 34 individuals deriving from all four cemeteries at Machu Picchu (Fig. 1B and table S1) excavated in 1912. Samples were selected to optimize comparisons with data generated by the aforementioned previous studies investigating cranial modification types and isotopes. We further generated genome-wide data for 36 individuals from the Urubamba Valley, sites in neighborhoods of modern-day urban Cusco, and Kanamarka in the southern Cusco region (Fig. 1A and table S1). We extracted DNA from teeth and enriched for a targeted set of ~1.2 million single-nucleotide polymorphisms (SNPs). The DNA data for each individual exhibit low nuclear and mitochondrial (mtDNA) contamination rates and damage rates characteristic of aDNA (table S1 and Supplementary Text). We analyzed these data jointly with published ancient and modern genomes available from South America (see Supplementary Text). In addition, we generated radiocarbon dates for 15 individuals buried at the sites in the Urubamba Valley and Cusco (table S1 and fig. S1) and for 26 individuals buried at Machu Picchu (25), all of whom were included in the genetic analysis.
RESULTS AND DISCUSSION
Ancestry and genetic population structure in the Urubamba Valley and Cusco preceding the Imperial Inca period
Studies of pre-Hispanic and modern-day genomic diversity indicate that the regional genetic substructure in the Central Andes and adjacent geographic regions has persisted for at least 2000 years (30–34). This allows for the identification of genomic ancestries that are associated with larger geographic regions during that time span [e.g., NorthPeruCoast, SouthPeruHighland, and TiticacaBasin following the nomenclature by Nakatsuka et al. (34)] (Fig. 1A). While 16th century documents indicate ethnic diversity in the Inca city of Cusco and among the remaining yanacona at Yucay, a royal estate in Urubamba belonging to Huayna Capac (35, 36), before this study, this claim could not be evaluated because only limited genome-wide aDNA had been reported for individuals from the Urubamba Valley and Cusco.
Radiocarbon dates obtained from the individuals in our sample buried at Ollantaytambo, Urubamba Valley (~1040 to 1380 CE) and San Sebastian, Cusco (~1300 to 1400 CE) indicate that these burials pre-date the Inca imperial expansion and the construction of Machu Picchu (table S1B, fig. S1A, and Supplementary Text). These individuals come from the groups inhabiting the Cusco area before the reign of Pachacuti, and, while limited in numbers, they are one possible source of information permitting us to investigate genetic changes that may have occurred because of subsequent Inca policy and occupation (table S1, fig. S1, and Supplementary Text). We combined the individuals for each site into groups named Ollantaytambo_LIP and San Sebastian_LIP, respectively, after confirming intragroup genetic homogeneity using f4 tests (table S3 and Supplementary Text). Statistical tests of the type f4 (Mbuti, X; Ollantaytambo_LIP, San Sebastian_LIP) indicate that the individuals from Ollantaytambo share significantly more alleles with ancient individuals from the ancestry group SouthPeruHighland described by Nakatsuka et al. (34) (Fig. 1) than the individuals from San Sebastian (table S2 and Supplementary Text). Furthermore, qpWave modeling (see Supplementary Text) reveals that Ollantaytambo_LIP and SouthPeruHighland are consistent with one source of ancestry (P > 0.01 for rank 0; table S2). San Sebastian, however, is best modeled (P = 0.2058) as a two-way mixture between 80 ± 8% ancient PeruSouthHighland ancestry and 20 ± 8% ancestry associated with ancient Titicaca Basin groups using qpADM (table S2 and Supplementary Text) (34). This is expected as the Cusco Valley and the Titicaca Basin had cultural and economic ties long before the Incas. Both Luis Lumbreras and Charles Stanish independently have observed that the pre-Hispanic societies of Cusco and the Lake Titicaca region maintained strong links for over two millennia before the appearance of the Inca Empire (37, 38), and, while their conclusions were based mainly on ceramic styles, they are consistent with studies of long-distance obsidian exchange in the southern highlands of Peru and northern Bolivia (39).
Genomic insights into the burial population at Machu Picchu
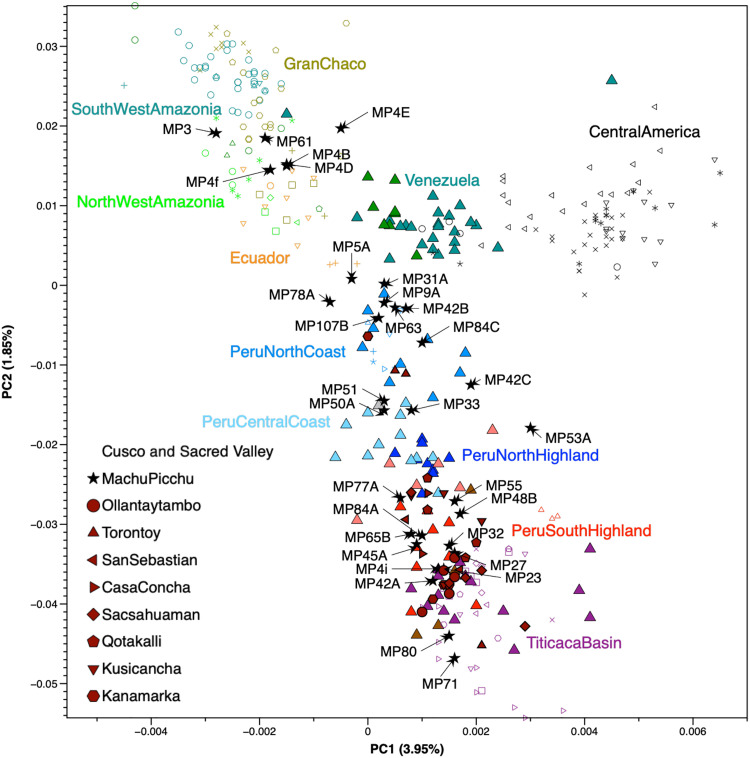
To explore the genetic diversity and affinities of the individuals from Machu Picchu and Cusco in the broader context of South and Central America, we performed a principal components analysis (PCA) using modern-day genomes from these regions as references and projecting the genomes obtained in this study and other published ancient genomes onto these axes (Fig. 2 and Supplementary Text). We find that the individuals from Machu Picchu cluster with individuals not only from throughout the Andean highlands but also from the North to South Peruvian coast. Furthermore, six individuals (MP3a, MP4b, MP4d, MP4e, MP4f, and MP61) group with modern individuals from the central and northwestern Peruvian Amazon, as well as the Ecuadorian and Colombian Amazon regions (Fig. 2). Individual profiles of shared genetic drift computed using f3 statistics (fig. S2 and Supplementary Text) reflect the diversity of genetic affinities observed in the PCA, with some individuals (e.g., MP3a, MP4b, MP4d, MP4e, MP4f, MP5a, MP61, and MP107b) sharing most genetic drift with either Northwestern or Southwestern Amazonian groups and some (MP9b, MP31a, MP63, and MP78) exhibiting distinct genetic attraction to modern-day Kichwa speakers from Ecuador and southern Colombia (33). Accordingly, most of the Machu Picchu individuals for which we obtained sufficient data (>100,000 SNPs, n = 30) share excess alleles with other regional coastal or highland Andean ancestry and adjacent tropical forest lowland ancestry groups (Fig. 1), when compared to the pre-Inca inhabitants of the Urubamba Valley (Ollantaytambo_LIP) or the preimperial inhabitants of the Inca capital Cusco (San Sebastian_LIP) using f4 statistics (Fig. 3, fig. S3, table S3, and Supplementary Text) to the limits of our statistical resolution.
Fig. 2. Genetic affinity of the individuals from Machu Picchu, the Urubamba Valley, and Cusco.
PCA plot projecting previously published individuals (32–34, 41, 46, 75–82, 84–86) and the ancient individuals studied here onto principal components calculated from present-day Native American populations. The Machu Picchu individuals are depicted by black stars.
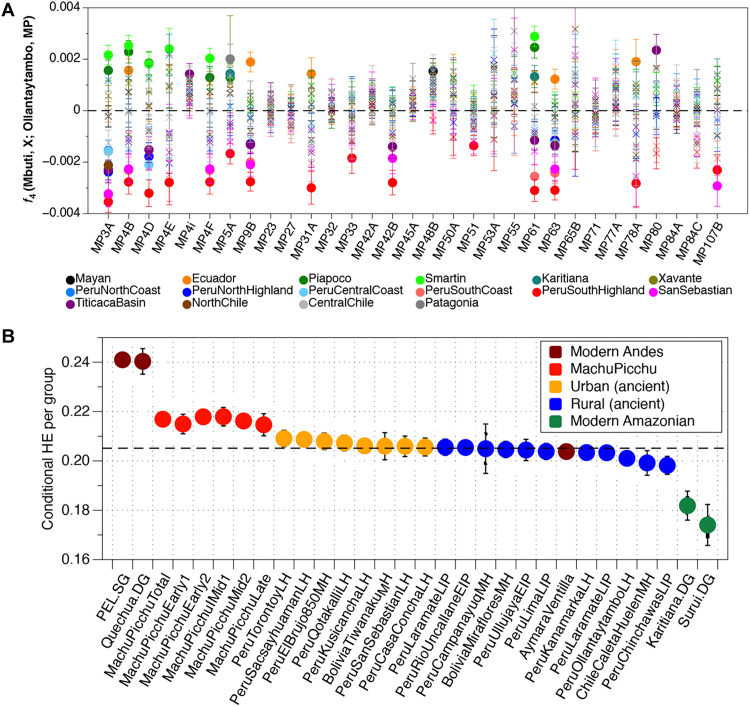
Fig. 3. Genomic structure and diversity of the individuals from Machu Picchu.
(A) f4 statistics of the type f4(Mbuti, X; Ollantaytambo/San Sebastian, MPindividual) using the HumOrg dataset (26). X represents either one of the Andean ancestry clusters or selected non-Central Andean groups from South and Central America (complete overview, see fig. S3 and table S3). (B) Conditional heterozygosity estimates (HE). Narrow and thicker black bars, ±1.96 and 3 SEs, respectively.
We used qpWave and qpADM to test whether any of the Machu Picchu retainers can be modeled as sharing the same ancestral population with any of the groups representing the regional Andean ancestry clusters (34) and any non-Andean South American ancestry cluster (33, 40, 41) or as a two-way admixture of the two (Fig. 1 and Supplementary Text). To the limits of our statistical resolution, we discerned that 17 of 30 individuals can be linked to a single ancestral source shared with one of the mentioned regional ancestry groups (P > 0.05 for rank 0; Fig. 4 and table S4). Six of these individuals (20%), all biologically female, exhibit ancestry associated with modern-day groups from the Peruvian North and Central Western Amazon. Additional tests indicate that these individuals share more genetic drift with groups living along the eastern piedmont of the Central Peruvian Andes, such as the Matsigenka, Shipibo, Piros, and the Ashaninka, than with groups inhabiting the northwestern Peruvian Amazon (figs. S2 and S4, table S5, and Supplementary Text). The Ashaninka are one of the contemporary tropical forest groups geographically closest to Machu Picchu, living in the lower Urubamba drainage and the lowlands of the Departments of Junín and Ucayali, north of Machu Picchu (Fig. 1).
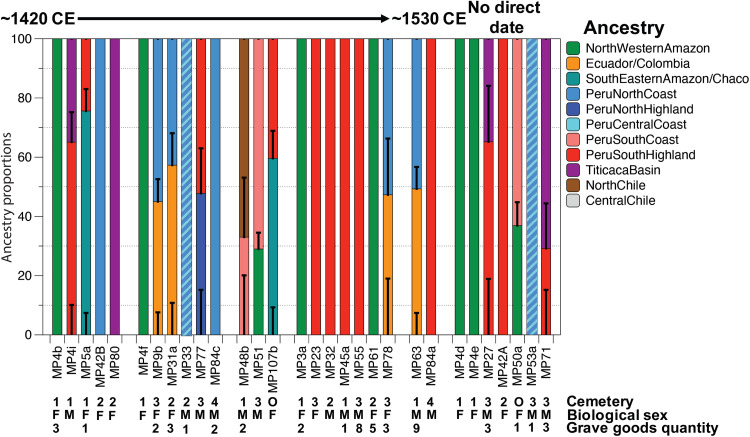
Fig. 4. Ancestry modeling for Machu Picchu individuals.
Estimated ancestries for 30 individuals buried at Machu Picchu sorted by median date (table S1), considering qpWave and qpADM results. For admixed individuals, we only display only the model with the highest P value (including SE), but, in most cases competing models (P > 0.05) were observed (table S6). The light blue/middle blue candy stripes indicate that it was not possible to differentiate between PeruNorthCoast and PeruCentralCoast ancestry.
Four individuals from Machu Picchu (three males and one female) exhibit ancestry associated with pre-Hispanic and modern-day individuals from the Peruvian Northern and Central Coast (table S4). One male individual shares the same ancestry with San Sebastian_LIP, representing the Cusco region, while only six individuals (four males and two females) exhibit ancestry associated with the ancestral inhabitants of the Urubamba Valley (Ollantaytambo_LIP) and the broader SouthPeruHighland ancestry group. This does not necessarily mean that these six individuals were locals because the regional ancestry cluster SouthPeruHighland includes individuals from Ayacucho and Huancavelica, with some individuals in this group found at archaeological sites up to 260 km west of Ollantaytambo in the upper reaches of the Nasca drainage (Fig. 1).
We fitted two-way mixture models for the 13 remaining individuals (Fig. 4). In many cases, we observed multiple competing models, with several Andean sources fitting, while non-Andean sources were less problematic (table S6). The difficulty of determining distinct Andean sources when the number of SNPs is low is best explained by the low genetic differentiation among the Andean regional groups, most of whom share very recent ancestry (34, 40). Five male individuals were modeled as mixtures between two Andean sources, such as NorthHighland-SouthHighland or SouthHighland-TiticacaBasin (Fig. 3). For all other individuals, the models with the highest support (highest P value) suggest mixtures between an Andean and a non-Andean source (Fig. 4, table S6, and Supplementary Text). For two of the individuals, the models suggest a mixture of Central Andean ancestry (~63 to 70%) with a source from the Peruvian Amazon (~30 to 37%) that is closely related to the ancestry for the previously described six individuals of Amazonian ancestry. However, there are also two individuals for which the best fitting models (table S6) were a mixture of Andean ancestry (~30 to 40%) and ancestry associated with groups from the southeastern Amazon and Gran Chaco region of Bolivia, Brazil, Argentina, and Paraguay (~60 to 70%) such as the Chané, Guarani, Karitiana, and Xavante. We cannot exclude that the observed admixture reflects an unsampled regional ancestry, such as groups from the Eastern Slopes of the Bolivian Andes, rather than an admixture event in Inca times. Archaeological and ethnohistoric sources confirm interactions between the Inca and the ancestors of the Chané in the foothills of the Bolivian Andes, making both models plausible (42, 43).
Four individuals (three females and one male) can be modeled as a mixture between a genomic source sharing ancestry with modern-day eastern Ecuadorian Kichwa speakers (33) and ancestry from the Peruvian North Coast (Fig. 4, table S6, and Supplementary Text). Additional tests indicated that these individuals are genetically homogenous (table S7); thus, we grouped them to increase our statistical power and found that a model considering a mixture of 46 ± 6% Ecuadorian-Kichwa–associated ancestry and 54 ± 6% NorthPeruCoast ancestry produces the highest P value (P = 0.41). However, alternative models considering other Andean sources (NorthHighland and CentralCoast) are supported as well (table S6). There are no published ancient or modern-day genomes from individuals living in the Ecuadorian highlands or along the Ecuadorian coast, which limits our ability to assess whether the observed admixture indicates a recent admixture event or whether it reflects the genetic ancestry of an unsampled group somewhere in Ecuador. Similar to the previous case, the intensive interactions between Ecuadorian populations and the Inca make such an alternative possible (2, 44, 45).
On the basis of our results, Machu Picchu was substantially more genetically diverse—measured as conditional heterozygosity for nuclear DNA and nucleotide diversity for mtDNA—than contemporary rural villages in the Andes (Fig. 3 and fig. S5). Among the yanacona of Machu Picchu, we observe genetic ancestries that represent all regions comprising the Inca Empire, except Central Chile/Western Argentina, which represents the southern frontier. This observation is consistent with previous analyses of the Machu Picchu burials that indicated that many individuals exhibited nonlocal isotopic signatures (Sr and O) or cultural indicators (12, 20, 21) not associated with local cultural traditions (table S1).
The rate of variation at polymorphic sites observed for Machu Picchu seems to remain consistently high throughout the site’s occupation (Fig. 2) (25). Individuals of nonregional (Ollantaytambo, Cusco) genetic ancestry and nonlocal strontium 87Sr/86Sr are found throughout the burial population across radiocarbon ages (e.g., MP63 and MP107; table S1). When combined with the genetic diversity estimates, this pattern suggests that the addition of yanacona and former aclla to the Machu Picchu community continued after the death of Pachacuti. This is consistent with historical evidence that yanacona were gifted to royal estates after the death of the founder (11, 14) because the mummies who founded their royal estates continued to be recognized as the owners of the estates and were expected to entertain mummies from other lineages and their guests.
We observe a significant difference (P = 0.0157) when we compare the genetic ancestries of biologically male and female individuals, with most male individuals exhibiting ancestries associated with highland regions, while female individuals exhibit more diverse nonhighland ancestries (Fig. 4 and table S1). The genetic diversity observed at Machu Picchu also exceeds the diversity observed for the only genetically studied communities of mitimaes (i.e., ethnic groups forcibly resettled by the Inca state) from the Chincha Valley on the central Peruvian coast (46). This pattern is consistent with the conclusion that the burial population at Machu Picchu was composed of yanacona and former aclla (17) rather than mitimaes. The presence of individuals exhibiting ancestries associated with Amazonia (e.g., MP4b), Ecuador (e.g. MP9b), and the Peruvian North Coast (MP42b) in the earliest occupation phases of Machu Picchu (Fig. 4 and table S1) may suggest that the expansion of the Inca Empire occurred earlier or differently from models derived from historical sources. This observation is consistent with the increasing view among Inca specialists that the historicist chronology dominant since 1945 is untenable in light of recent archaeological evidence from Cusco and distant Inca provinces (2, 6, 42, 47, 48) (see Supplementary Text).
The early presence of individuals exhibiting ancestry associated with, for example, Ashaninka and other Arawak speaking groups along the eastern Andean piedmont is exciting but expected. Archaeological, ethnohistorical, linguistic, and genetic sources indicate that the inhabitants of this region interacted with the adjacent highlands even before the Inca (33, 40, 49–51), and recent studies suggest a complementary relationship between the Inca and these groups, rather than one dominated by conflict and conquest (49). All individuals buried at Machu Picchu exhibiting this ancestry have been determined to be biologically female, suggesting that some might have been gifted as wives to favored individuals, a process recorded in historical sources (13, 16).
The relationship between the Inca and populations of the former Kingdom of Chimor on the Peruvian North Coast and Ecuadorian groups such as the Cañaris were more bellicose, and the presence of some of the individuals at Machu Picchu with ancestries from these regions may be explained by their incorporation as yanacona who were craft specialists skilled in metal working. The identification of unfinished metal objects and production debris have allowed archaeologists to identify metal working as one of the activities present at Machu Picchu but apparently lacking at many other royal estates (8, 52).
Implications for daily life at Machu Picchu
Osteological analyses (20) indicate that the yanacona and former aclla of Machu Picchu led relatively comfortable lives. They were not involved in heavy agricultural labor or construction projects. None of them showed head wounds or other pathologies frequently produced by warfare, nor do they display growth disruptions resulting from childhood illnesses or food shortages (20). Notably, many of them (n = 67) survived to maturity (15 to 49 years), and a substantial number (n = 14) reached old age (over 50). As noted, their varied ethnic backgrounds were suggested by the frequent presence of exotic ceramics and metal tools in the graves (21). The large number of vessels that showed evidence of repairs implies that these foreign objects, perhaps from their homelands, continued to have special meaning for the yanacona and former aclla.
A previous study established that all four cemeteries of Machu Picchu were used over the duration of the site’s occupation, with multiple interments in the same caves made over several decades (25). Our analyses show that individuals of differing genetic ancestry were buried in the same cemeteries and, in some cases, in the same burial cave, as in Burial Caves 4 and 42 (Figs. 1 and 4). When testing for biological relatedness, we found only one pair of first-degree relatives, most likely a mother-daughter pair, in a single cave (MP4b and MP4f, r/k0 ratio: 2.88; table S8). Both nuclear DNA and mtDNA indicate that the other individuals investigated here—even those buried together in the same location—shared no closer familial relationship (table S8). Our observations suggest that neither genetic ancestry nor ancestral ethnicity of the retainers was a major factor in structuring mortuary patterns, a conclusion consistent with an earlier observation that a single set of shared burial practices was practiced at Machu Picchu (4, 21). Moreover, these findings further support the conclusion that people arrived at Machu Picchu as individuals rather than communities or extended families, a pattern predicted for yanacona and former aclla based on historic accounts (11, 13, 45).
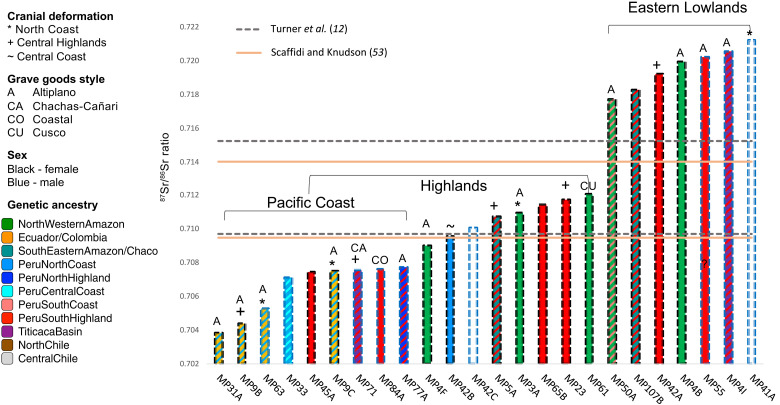
The genomic histories of the retainers at Machu Picchu further suggest that ancestry or ethnicity did not constrain their daily life and reproductive choices. Several of the retainers displayed an admixture of genetic ancestries associated with diverse geographies. While in some cases, such admixture patterns might reflect ancestries of currently unsampled regional groups (30, 33, 34), other cases seem to be the result of mating between individuals of different ancestries that comprised the community at Machu Picchu (e.g., MP48b, MP50a, MP51, and MP77; Fig. 4 and table S6). We further observe that the geographic regions associated with the cultural, genomic, and geochemical signatures do not align for some individuals (Fig. 5 and Supplementary Text). The complexity of individual life histories is, for example, suggested by the isotopic analyses and the current interpretations of their significance (Fig. 5 and table S1). In the case of the mother-daughter pair MP4b-MP4f, both of Amazonian ancestry (Fig. 4), the putative parent (MP4b) exhibits a nonlocal strontium 87Sr/86Sr signature associated with the Amazonian lowlands, which matches their genetic ancestry. However, the daughter (MP4f) exhibits a signature that matches broader highland or coastal Andean regions (12, 53). Other individuals of nonlocal, nonhighland ancestries or admixed ancestries exhibit 87Sr/86Sr ratios, indicating that those individuals may have spent their younger ages during which the investigated teeth formed and erupted (~7 to 17 years) in the highlands (e.g., MP3a, MP5b, and MP61; Fig. 4) before coming to Machu Picchu.
Fig. 5. Comparison of genetics, Sr isotopes, cranial modification, and grave goods.
The graph shows the 87Sr/86Sr for individuals studied reported by Turner et al. (12) and sorted by geographic macro region (Coast, Highland, and Lowlands) (53). The solid and the dashed horizontal lines represent the bioavailable 87Sr/86Sr range for geological features surrounding Machu Picchu as determined by Turner et al. (12) or Scaffidi and Knudson (53), respectively. The colored outline of the bars indicates biological sex, and the fill represents the genetic ancestry. Symbols above the bars indicate cranial modification styles and grave goods observed with the individuals.
Together with the burial distribution patterns and burial analysis, our genomic analyses reveal that the retainer community at Machu Picchu was very diverse and that their lives were not structured primarily by ethnic or regional backgrounds. People of different genomic backgrounds were buried together in the same cemetery areas and, on occasion, even in the same caves. Judging from the number of individuals with mixed genomic backgrounds, sometimes from regions quite distant from each other, it would appear that among the yanacona and former aclla, genomic background was not the main determinant in selecting mates and producing children. Genomic ancestry is of course not a conscious category but merely a product of regional reproductive history and other evolutionary processes and does not necessarily reflect an experienced identity.
Unfortunately, the analyses reported here tell us nothing about the genetic identity of the Inca royalty and their guests for whom the country palace was built. These elite individuals resided in Cusco and would not have lived full time at Machu Picchu or been buried there. Despite the inherent limitations, our analyses of the nonelite individuals demonstrate that genomic information, in combination with archaeological and ethnohistorical sources, can reveal a more nuanced and comprehensive view of daily life at Machu Picchu than has been available in the past.
Regional context of the Machu Picchu results
In contrast to the Machu Picchu results, most of the individuals sampled from other sites in the Urubamba Valley and Cusco area cluster with other pre-Hispanic and modern-day individuals from the Southern Peruvian Highlands and the Titicaca Basin (33, 34, 40) in the PCA (Fig. 2). A small number of individuals from Kanamarka and Torontoy appear closest to individuals from the Peruvian North Coast and some from Sacsahuaman and Torontoy closest to pre-Hispanic and modern-day individuals from the Titicaca Basin. These genetic affinities are also reflected in the f3 statistics (figs. S2 and S7). When computing f4 statistics of the type f4(Mbuti, X; Ollantaytambo/San Sebastian, Urubamba/Cusco), we observe that several individuals/groups from those sites share access alleles with other nonlocal groups from the highlands, especially the northern Peruvian highlands or the Titicaca Basin (table S2 and fig. S8). Seven individuals from three sites in Cusco (Kusicancha, Casa Concha, and Qotakalli) share the same ancestry with the early Cusco individuals from San Sebastian and, similar to the latter, can be modeled as a two-way mixture between SouthPeruHighland (~70 to 80%) and TiticacaBasin ancestry (~20 to 30%; table S6 and Supplementary Text) using qpADM. Four other individuals from the major Inca ceremonial center of Sacsahuaman in Cusco (54) (see Supplementary Text) exhibit the same two ancestry sources but show higher proportions of TiticacaBasin-associated ancestry (~60%; table S6). Another individual exhibits an unadmixed TiticacaBasin-associated ancestry (table S4 and Supplementary Text). Thirteen of the 17 individuals deriving from sites situated in or near Cusco exhibit some degree of affinity with genomic ancestries observed within groups living in the Titicaca Basin. All efforts to date the admixture event that led to the ancestry observed in San Sebastian and other Cusco sites did not produce reliable results (see Supplementary Text). However, as discussed before, archaeological records indicate that interactions between Cusco and the Titicaca Basin already existed in the Middle Formative (1500 to 500 BCE) (37, 38). Only one individual from Cusco exhibits SouthPeruHighland/Ollantaytambo ancestry, while some others show some degree of ancestry associated with the northern Peruvian highlands (table S6 and fig. S8). The individuals from the rural Inca settlement of Kanamarka to the south of Cusco (Fig. 1) all exhibit SouthPeruHighland/Ollantaytambo ancestry, except for one individual who shares the same mixture of ancestry associated with Ecuadorian Kichwa populations and the Peruvian north coast identified among several retainers in our sample from Machu Picchu (e.g., MP63).
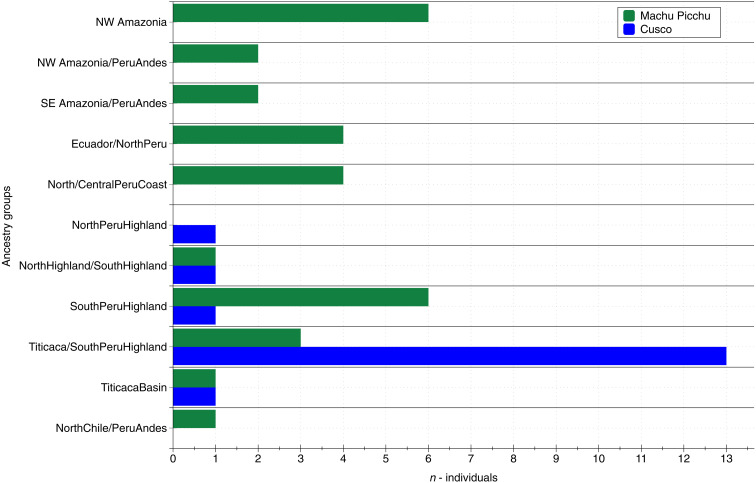
In summary, as would be expected from the historical documents, the population of the Inca Cusco was diverse (Fig. 2). However, while the retainer community at Machu Picchu exhibited ancestries from throughout the Inca Empire, ancestries observed in our sample of Cusco’s inhabitants are mostly associated with the Peruvian highlands and the Titicaca Basin (Fig. 6). This contrast may be the result of our limited sample from Cusco, a complex urban center with over 100,000 inhabitants (2, 3, 15). It is likely that a larger sample from more sectors in the city might yield evidence of greater genomic diversity. Nonetheless, it is also possible that these differences were an expression of the differing structure and functions that Cusco and Machu Picchu had within Tahuantinsuyu. The genomic diversity at both Cusco and Machu Picchu is in stark contrast to the genetic composition of groups inhabiting rural support settlements such as Paucarcancha and Patallacta in the Urubamba Valley, where individuals seem to have been of local ancestry, much like the individuals from Ollantaytambo (55).
Fig. 6. Comparison of ancestry distribution between Cusco and Machu Picchu.
Simplified overview of ancestries/two-way admixed ancestries exhibited by individuals buried in Machu Picchu and the sites from urban Cusco (excluding: Urubamba Valley sites and Kanamarka).
As the historic records imply, the demographic and genetic structure of Inca sites was determined by their function within the empire, but the aDNA analyses offer a more detailed and nuanced view of the degree of genomic diversity that existed among the retainers at Machu Picchu and the inhabitants of Cusco. The representation of a wide range of genomic backgrounds from the entire empire among Machu Picchu’s retainers and the degree to which they were intermixed in life and in death was previously unknown. The strong presence of individuals, especially females, from multiple zones of the forested eastern Andean slopes and Amazonian lowlands was an unanticipated result, and it points to the need for additional investigation of the role of tropical forest groups in the Inca Empire.
MATERIALS AND METHODS
Sampling
DNA extractions were carried out in dedicated facilities at the University of California, Santa Cruz Paleogenomics Lab (UC-PGL; USA) and the Max Planck Institute for Evolutionary Anthropology (MPI-EVA; Germany). Extractions from tooth powder were performed following a silica column–based protocol optimized for the recovery of small aDNA molecules (56) using ~60 mg of pulverized sample. Before analysis, we added a bleach treatment before digestion as described by Boessenkool et al. (57). Aliquots of 60 mg of tooth powder were first incubated in 1 ml of 0.5% sodium hypochlorite solution (Sigma-Aldrich) at room temperature for 15 min. After centrifuging, the supernatant was discarded, and the remaining bone powder pellet washed using 1 ml of molecular-grade H2O three times.
For several teeth, we followed the minimal destructive extraction method described by Harney et al. (58) that does not involve any mechanical destruction of the tooth. We wrapped tooth crowns of the cleaned teeth in ultraviolet-irradiated parafilm and emerged part of the roots in 1 ml of the lysis buffer used for the previously described method for ~3 hours at 37°C. The lysate was then purified as described for the destructive sampling (56, 58). All extraction batches were accompanied by at least two negative controls.
Sequencing library preparation
We used two sequencing library preparation methods for the data generated in this study. For most individuals from Machu Picchu, we built double-indexed, double-stranded DNA (dsDNA) libraries from 25 μl of DNA extract. All extracts were partially Uracil-DNA Glycosylase (UDG)–treated before library building to reduce, but not eliminate, the amount of deamination-induced damage toward the ends of the aDNA fragments (59).
At University of California, Santa Cruz (UCSC), for DNA extracts from all other individuals, we used a library protocol that uses directional splinted ligation of Illumina’s P5 and P7 adapters to convert natively single-stranded DNA (ssDNA) and heat-denatured dsDNA into sequencing libraries in a single enzymatic reaction (60). We followed the protocol as described by the authors using 25 μl of DNA extract for each individual. For libraries produced at MPI EVA, another ssDNA library protocol described by Gansauge et al. (61) was used, using the fully automated version of the protocol.
All library batches were accompanied by at least two blanks, and libraries were also built and tested for the extraction blanks. Postindexing polymerase chain reaction libraries from both methods were quantified using the High-Sensitivity DNA Assay on an Agilent 2200 TapeStation (Agilent Technologies Inc., Santa Clara, CA, USA). For initial screening of DNA, preservation libraries from preparation batches were then pooled in equimolar amounts and sequenced on a NextSeq500 sequencer (Illumina Inc., San Diego, CA, USA) for 2 × 75 cycles at UC-PGL.
Targeted enrichment and high-throughput sequencing
Both dsDNA and ssDNA libraries that, after shotgun sequencing, showed the presence of aDNA-specific damage and a percentage of human endogenous DNA of over 0.5% were transferred to MPI-EVA for target enrichment. Libraries from 34 individuals buried at Machu Picchu showed DNA preservation sufficient for enrichment. In addition, libraries from 30 individuals (table S1) from the Sacred/Urubamba Valley (Ollantaytambo = 5 and Torontoy = 3), sites in neighborhoods of modern-day Cusco (Casa Concha = 2, Kusicancha = 4, Qotakalli = 4, Sacsahuaman = 5, and San Sebastian = 3), and Kanamarka (n = 4) in the southern Cusco Region, ~150 km from Cusco (Espinar Province), were submitted for enrichment. The data from the individuals buried at Torontoy and San Sebastian have been reported in Nakatsuka et al. (34).
At the MPI-EVA, the libraries were enriched for a set of 1,237,207 targeted SNPs across the human genome (1240K in-solution capture) as described previously (62). The enriched DNA products were sequenced on an Illumina HiSeq 4000 instrument (Illumina Inc., San Diego, CA, USA) with 75 single-end run cycles or 50 paired-end run cycles using the manufacturer’s protocol. The output was then demultiplexed using bcl2fastq version 2.17.1.14 (Illumina conversion software) and dnaclust version 3.0.0 (63).
Sequencing read processing, chromosomal sex determination, and screening and DNA authenticity
After demultiplexing, resulting sequencing reads were processed using the UCSC-PGL in-house computational pipeline developed for aDNA described in (34) and available at (https://github.com/mjobin/batpipe, v1, 10 May 2021). This pipeline clips adapters, merges paired-end reads (default parameters), maps sequencing reads against a user specified reference genome, removes duplicate reads, and estimates quality traits. Residual adaptor sequences were trimmed and merged using BC_bin_clip (https://github.com/svohr/bc_bin_clip, v1. 015b4dc, 2 October 2022) and SeqPrep 2 (64) (https://github.com/jeizenga/SeqPrep2, v2, 2 October 2022), with a minimum overlap of 11 base pairs needed to merge paired-end reads. Sequencing reads were mapped using the Burrows-Wheeler Aligner version 0.7.12 (65), disabling seeding (-l 16500, -n 0.01) against UCSC genome browser’s human genome reference GRCh37/hg19. Duplicates were removed with DeDup version 0.12.2 (66), which removes reads with identical start and end coordinates. A mapping quality filter of 30 was further applied using SAMtools version 1.3.
We estimated the contamination rate for the sequencing reads using two methods. For all individuals, we used recommended parameters in Contammix (67) to estimate mitochondrial contamination rates. In addition, we also estimated contamination on the X chromosome for all biologically male individuals with ANGSD (68), which creates an estimate based on the rate of heterozygosity observed on the X chromosome. Mitochondrial and X-chromosomal contamination rates for all individuals sequenced were low (mitochondrial: <3%; X-chromosomal: <1%), indicating authenticity for the obtained genomic data (table S1). We estimated patterns of DNA damage using MapDamage 2 (69) and observe damage rates at the read termini of >3% for all individuals (table S1), as expected for aDNA. For ssDNA libraries, we observe the expected terminal deamination frequency disbalance reported before (60).
Chromosomal sex of the studied individuals was determined by evaluating the ratio (Ry) of reads aligning to the Y chromosome (nY) compared to the total number of reads aligning to the sex chromosomes (nX + nY), i.e., Ry = (nY/nY + nX), as described in (70). In addition, we used a X-chromosomal normalization rate (Rx) approach that compares the Rx ratio to the variability observed in all autosomes (71). We were able to determine chromosomal sex for 33 of the 34 Machu Picchu individuals that were enriched using the 1240k capture. The analyzed set is composed of 19 biological females and 14 biological males. The molecular sex estimates are in agreement with the morphological sex assessment reported by Verano (20). The sex estimates for all individuals analyzed in this study can be found in table S1. We were able to determine molecular sex for a total of 67 individuals of the 68 ancient genomes newly sequenced in this study (Machu Picchu and Urubamba and Cusco groups). Of those, a total of 27 individuals exhibit male and 40 female biological sex.
Y chromosome and mtDNA analyses
For Y chromosome haplogroup calling, we used the original BAM files and performed an independent processing procedure. We filtered out reads with a mapping quality of <30 and bases with a base quality of <30 and trimmed the first and last 2 base pairs (bp) of each sequence to remove potential damage induced substitutions. We determined the haplogroup by determining the most derived mutation for each sample using the tree of the 2019–2020 International Society of Genetic Genealogy version 15.37 (accessed 15 April 2021) using the LineageTracker classify function and the hg19 reference genome build (-b 37) implemented in Y-LineageTracker (72). The mitochondrial haplotypes of the individuals were analyzed as described in Llamas et al. (73) (see Supplementary Text).
Genotyping and reference data
We trimmed 2 bp from each end of the reads from the partially UDG-treated enriched libraries to reduce potential bias introduced by DNA damage. Different sequencing runs and libraries from the same individuals were merged, and duplicates removed and sorted again using SAMtools (74). For each of the 1240k SNP positions enriched in the sequencing libraries, a read was chosen at random to represent this position using the genotype caller pileupcaller (https://github.com/stschiff/sequenceTools, v1.5.3, 15 February 2023). The data were then merged with previously published ancient genomes from the Americas (32, 34, 46, 75–82), present-day human data from the Simons Genome Diversity Project (83), which included 26 Native American individuals from 13 groups, and genomic data from further individuals genotyped for the 1,196,358 SNPs targeted by the 1240k SNP capture, compiled and provided by the Reich laboratory (84). We also included data from 224 Native American individuals from 34 different populations genotyped on the Affymetrix Human Origins (HO) array (33, 41, 85, 86) not included in the 1240k dataset. For several analyses that involved modern populations and that benefitted from the larger diversity and geographic representation of individuals in the HO dataset, we restrict the data to the intersection of 597,503 SNPs between the 1240k SNP set and the HO dataset. However, because of the overall low coverage obtained for several of the Machu Picchu (MP) individuals, the substantial loss of statistical power resulting from that was not acceptable. In most cases, we performed analyses using both the full 1240k dataset and the HO dataset (also showing the limits of resolution). For a limited number of analyses, we also merged our HO dataset with the genotypes of 229 individuals from Central and South America, including individuals from Cashibo, Shipibo, Huambisa, Ashaninka, and Yanesha communities from the Central Peruvian Amazon, genotyped with the HumanOmniExpress 1.1 BeadChip (OMNI, Illumina, San Diego, CA), to increase the geographic diversity of representative groups (40). The combination of the HO and OMNI (HO_Omni) sets resulted in an overlap of 125,320 SNPs retained.
Genomic diversity estimates
To estimate autosomal genetic diversity, we performed conditional heterozygosity analyses for the Machu Picchu and Cusco groups reported here and previously reported genomic data from several pre-Hispanic and modern-day Andean and South American groups from rural and urban contexts using POPSTATS with default parameters (85). Conditional heterozygosity is an estimate of genetic diversity in a group obtained by sampling a random allele from each of two randomly chosen individuals at a known panel of polymorphisms.
Relatedness
To analyze relatedness or biological kinship between individuals buried at Machu Picchu, we used both READ (87) and lcMLkin (88). We ran lcMLkin with population allele frequencies calculated from n = 150 pre-Hispanic and modern-day Native American individuals published in (32, 34, 46, 75, 78, 82), excluding modern-day individuals exhibiting admixture with non-Native American ancestry (see Supplementary Text).
Principal components analysis
We performed PCA using the smartpca version 16680 in EIGENSOFT (89). We used the default parameters and the lsqproject: YES and newshrink: YES options and performed PCA on the HO dataset of present-day unadmixed Central and South American. We projected the ancient individuals onto the principal components determined from the present-day individuals. Individuals with less than 20,000 SNPs were not projected.
Grouping Urubamba Valley and Cusco individuals into analysis clusters
The PCA indicates that many of the individuals from each of the Urubamba Valley and Cusco region share similar genetic ancestry; so to identify the differences on an individual basis and to identify a sensible grouping, we used qpWave (90) to test whether the individuals from the sites in the Urubamba Valley and Cusco region are for genetically homogenous within those groups, doing pairwise comparisons between each individual of one group. When individuals within a site were consistent with one wave of ancestry using multiple Andean groups as outgroups (P > 0.01 for rank 0), we assigned them to one group/population (table S2). In the case of Ollantaytambo and San Sebastian, all pairwise comparisons indicated that all individuals in the respective group are homogenous in ancestry. For all other groups, this was not the case resulting in several subgroupings (e.g., Peru_Kanamarka1_LH, Peru_Kanamarka2_LH, and Peru_Kanamarka3_LH) for each site (see table S1 for all groupings used in the subsequent analyses). Because of our interest to identify individual genetic histories, we did not group any of the individuals from Machu Picchu for most of the analyses.
f3 statistics
Using the full 1240k dataset, we used the qp3pop package (v650) in ADMIXTOOLS (90) to compute f3 statistics with SEs computed with a weighted block jackknife over 5-Mb blocks. We used the inbreed: YES parameter to compute f3 statistics to account for our random allele choice at each position. We computed “outgroup f3” statistics of the form f3(Mbuti; Pop1, Pop2), which measure the shared genetic drift between population 1 and population 2. We created a matrix of the outgroup f3 values between all pairs of populations and between the Machu Picchu individuals. We converted the original f3 values to distances by taking the inverse of the values and generating a neighbor joining tree (NJ-tree) using PHYLIP version 3.696’s (91) neighbor function and setting USA-MT_Anzick1_12800BP as the outgroup (default settings were used for the rest of the analysis). For the NJ-tree, we only included the Cusco and Urubamba individuals grouped as described before, but not the Machu Picchu individuals. We displayed the tree using FigTree (http://tree.bio.ed.ac.uk/software/) (fig. S4).
In addition, we computed f3 statistics of the form f3(Mbuti; Ancient Andean, Present-Day SouthAmerican) for each Machu Picchu individual and the Urubamba/Cusco groups using the HO dataset and for selected individuals using the Omni dataset. We plot the f statistics on a heatmap using R (https://github.com/pontussk/point_heatmap, v1, 2 May 2022) (figs. S2 and S7).
To further investigate the shared drift between each individual buried at Machu Picchu and to test whether their genetic ancestry correlates with their distribution throughout the different cemeteries and burial caves, we computed f3 statistics of the type f3(Mbuti; MP_ind1, MP_ind2) and visualized those as a similarity matrix (fig. S6) for the Machu Picchu individuals. Only individuals with >50,000 SNPs were included.
f4 statistics, qpWave, and qpADM
We used the tools qpDstats, qpWave, and qpADM packaged in ADMIXTOOLS (90) to test for admixture. To assess that the relative connections between the ancient individuals and groups share with each other and other ancient and present-day populations, we computed several f4 statistics using the qpDstat (v970) package in ADMIXTOOLS using f4mode: YES, and printse: YES parameters. SEs were computed using a jackknife block size of 0.050. For details on the specific tests, refer to the later Supplementary Text and tables S3 and S7.
We used qpWave (v1200) from ADMIXTOOLS (90) to determine the minimum number of ancestry sources for each individual and groups of individuals using ancient and modern populations. Reference groups (right populations) were chosen to represent branches that are considered basal to the populations under investigation, keeping the number of populations at minimum as suggested in the software documentation. More precisely, we tested, e.g., whether any of the Machu Picchu, Cusco, or Urubamba individuals or groups are consistent with one wave of ancestry (P > 0.05) for rank 0 when paired with either SanSebastian_combined, Ollantaytambo_LIP, or any of the regional Andean ancestry clusters determined by Nakatsuka et al. (34) or other non-Andean South Americans. For all qpWave analyses, we used the default settings except for the change that we set allsnps: YES. The compiled results using both the full 1240k and the HumOrg datasets can be found in table S4, and the extended description of all tests can be found in the Supplementary Text.
For all individuals/groups where rank = 0 was rejected in the qpWave analyses, we used qpADM (90) to test two-way and three-way admixture models using the rotating model approach suggested as implemented in qpADM_wrapper (https://github.com/pontussk/qpAdm_wrapper, v1, 12 March 2022) (92). Under this rotating approach, populations are consistently moved from the source set to the set of reference populations. We used a fixed set of sources/outgroups for the analysis consisting of all ancient Andean ancestry clusters determined by Nakatsuka et al. and other ancient and modern-day populations from the Amazon, the Southern Cone, and Central America, cumulating in 120 tested models per individual/group for the two-way models. We set the details: YES parameter, which reports a normally distributed Z score for the fit (estimated with a block jackknife). The compiled results using both the full 1240k and the HumOrg datasets can be found in table S5.
Acknowledgments
We are grateful to colleagues at the Universidad Nacional de San Antonio Abad del Cusco (UNSAAC), Museo Machu Picchu, Museo Inka, and the regional representatives of the Ministry of Culture in Cusco for granting access to the human remains studied here and for collaboration and support. We thank the Ministry of Culture for granting us research and exportation permission (see “Ethics statement” section in Supplementary Text). We also thank A. Caccone (Yale U) for providing laboratory resources and advice for the initial investigations and W. Garner, C. Thomas, R. Clasby, and C. Milan for support during the initial sampling of the Machu Picchu individuals.
Funding: This work was supported by National Science Foundation grant 1515138 (to L.F.-S., R.Bu., and B.B.), National Science Foundation grant 1842447 (to E.W. and L.F.-S.), German Scientific Foundation (DFG) grant FE1161/1-1 (to L.F.-S.), Alberts Fund (to R.Bu. and L.S.), and President’s Office at Yale (to R.Bu. and L.S.).
Author contributions: L.S., R.Bu., and L.F.-S. conceived the study, supported by B.B. and designed it with J.K. and J.F. J.F., R.Ba., L.F.-S., J.N., K.K., E.W., and R.D. performed laboratory and computing analyses, supported by K.Z., J.D. and K.S. R.Bu., L.S., J.C., J.N., B.I.A., and J.V. assembled archaeological and anthropological information and provided context interpretation. J.F., E.W., B.I.A., L.F.-S., L.S., R.Bu., B.B., J.N., and J.C. obtained skeletal samples for analysis or facilitated sampling. R.Bu., L.F.-S., L.S., and J.N. wrote the manuscript with input from all coauthors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Aligned sequencing reads for all individuals reported in this study are available from European Nucleotide Archive (ENA), accession no: PRJEB62808, and their respective genotypes for 1240k SNPs are available from DRYAD: https://datadryad.org/stash/share/dmC5N5bjM3jzRjmKbNSOPvEJ-pGso5EipzPTp3ABP0o. All skeletal samples were exported and returned under materials transfer agreements with the curating institutions in Peru.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S8
Legends for tables S1 to S8
References
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S8
REFERENCES AND NOTES
- 1.J. A. Flores Ochoa, Contemporary significance of Machu Picchu, in Machu Picchu: Unveiling the Mystery of the Incas, R. L. Burger, L. Salazar, Eds. (Yale Univ. Press, 2008), pp. 109–123. [Google Scholar]
- 2.T. N. D’Altroy, The Incas (John Wiley & Sons, 2014). [Google Scholar]
- 3.C. Morris, A. von Hagen, The Incas: Lords of the Four Quarters (Thames & Hudson, 2012). [Google Scholar]
- 4.R. L. Burger, Scientific insights into daily life at Machu Picchu, in Machu Picchu: Unveiling the Mystery of the Incas, R. L. Burger, L. C. Salazar, Eds. (Yale Univ. Press, 2005), pp. 500–502. [Google Scholar]
- 5.K. Quave, Royal estates and imperial centers in the Cuzco Region, in The Oxford Handbook of the Incas, S. Alconini, R. A. Covey, Eds. (Oxford Univ. Press, 2018), pp. 101–118. [Google Scholar]
- 6.K. Quave, B. S. Bauer, Machu Picchu und die königlichen landsitze der region Cuzco, in Könige der Anden, D. Kurella, I. de Castro, Eds. (Verlag Phillip von Zabern, 2013), pp. 96–113. [Google Scholar]
- 7.J. H. Rowe, Machu Picchu a la luz de documentos del siglo XVI. Dent. Hist. 14, 139–154 (1990). [Google Scholar]
- 8.L. C. Salazar, Machu Picchu: Myterious royal estate in the cloud forest, in Machu Picchu: Unveiling the Mystery of the Incas, R. L. Burger, L. C. Salazar, Eds. (Yale Univ. Press, 2004), pp. 21–47. [Google Scholar]
- 9.D. Hu, K. E. Quave, Prosperity and prestige: Archaeological realities of unfree laborers under Inka imperialism. J. Anthropol. Archaeol. 59, 101201 (2020). [Google Scholar]
- 10.S. A. Niles, Considering Inka Royal Estates: Architecture, economy, history, in The Inka Empire: A Multidisciplinary Approach, I. Shimada, Ed. (University of Texas Press, 2015), pp. 233–246; 10.7560/760790-014. [DOI]
- 11.J. H. Rowe, Inca policies and institutions relating to the cultural unification of the empire, in George Collier, Renato Rosaldo, John Wirth (eds.) The Inca and Aztec States 1400–1800: Anthropology and History (Academic Press, 1982), pp. 93–118. [Google Scholar]
- 12.B. L. Turner, G. D. Kamenov, J. D. Kingston, G. J. Armelagos, Insights into immigration and social class at Machu Picchu, Peru based on oxygen, strontium, and lead isotopic analysis. J. Archaeol. Sci. 36, 317–332 (2009). [Google Scholar]
- 13.H. Santillán, Relación del orígen, descendencia, política, y gobierno de los incas, in Biblioteca de Autores Españoles (Ediciones Atlas, 1968), vol. 209, pp. 97–149. [Google Scholar]
- 14.S. Villar Cordova, La Institución de Yanacona en el Incanato (Universidad Nacional Mayor de San Marcos, 1966), vol. 1 of Nueva Coronica. [Google Scholar]
- 15.M. Rostworowski, History of the Inca Realm (Cambridge Univ. Press, 1999). [Google Scholar]
- 16.I. M. Silverblatt, Moon, Sun, and Witches: Gender Ideologies and Class in Inca and Colonial Peru (Princeton Univ. Press, 1987). [Google Scholar]
- 17.B. L. Turner, B. R. Hewitt, The acllacona and mitmacona, in The Oxford Handbook of the Incas, S. Alconini, R. A. Covey, Eds. (Oxford Univ. Press, 2018), pp. 263–282. [Google Scholar]
- 18.R. L. Burger, J. Lee-Thorp, N. Van der Merwe, Rite and crop revisited: An isotopic perspective from Machu Picchu and beyond, in The 1912 Yale Peruvian Scientific Expedition Collections from Machu Picchu: Human and Animal Remains, R. L. Burger, L. C. Salazar, Eds. (Department of Anthropology, Yale University Division of Anthropology, Peabody Museum of Natural History, Yale University Publications in Anthropology, 2003), pp. 119–137. [Google Scholar]
- 19.B. L. Turner, J. D. Kingston, G. J. Armelagos, Variation in dietary histories among the immigrants of Machu Picchu: Carbon and nitrogen isotope evidence. Chungará (Arica). 42, 515–534 (2010). [Google Scholar]
- 20.J. W. Verano, Human skeletal remains from Machu Picchu: A reexamination of the Yale Peabody Museums Collections, in The 1912 Yale Peruvian Scientific Expedition Collections from Machu Picchu: Human and Animal Remains, R. L. Burger, L. C. Salazar, Eds. (Department of Anthropology, Yale University Division of Anthropology, Peabody Museum of Natural History, Yale University Publications in Anthropology, 2003), pp. 65–117. [Google Scholar]
- 21.L. C. Salazar, Machu Picchu’s silent majority: A consideration of the Inca cemeteries, in Variations in the Expression of Inca Power, R. Burger, R. Matos, C. Morris, Eds. (Dumbarton Oaks Research and Library, 2007), pp. 165–184. [Google Scholar]
- 22.G. Tomlinson, The Singing of the New World. Indigenous Voice in the Era of European Conquest (Cambridge Univ. Press, 2009). [Google Scholar]
- 23.L. C. Salazar, Inca religion and mortuary ritual at Machu Picchu, in Mortuary Practices and Ritual Associations: Shamanic Elements in Prehistoric Funerary Contexts in South America, E. J. Currie, J. E. Staller, Eds. (Archaeopress, 2001), pp. 117–127. [Google Scholar]
- 24.B. Cobo, Inca Religion and Customs, edited by R. Hamilton (University of Texas Press, 1990).
- 25.R. L. Burger, L. C. Salazar, J. Nesbitt, E. Washburn, L. Fehren-Schmitz, New AMS dates for Machu Picchu: Results and implications. Antiquity 95, 1265–1279 (2021). [Google Scholar]
- 26.G. Eaton, The Collection of Osteological Material from Machu Picchu (Yale Univ. Press, 1916). [Google Scholar]
- 27.R. L. Burger, L. Salazar, M. D. Glascock, Analysis of Inca pottery from the Cuzco Region: Implications for the provisioning of ceramics for Machu Picchu and other Inca Sites, in Ceramics of the Indigenous Cultures of South America: Studies of Production and Exchange, M. D. Glascock, Ed. (University of New Mexico Press, 2019), pp. 97–111. [Google Scholar]
- 28.G. R. Miller, Food for the Dead, Tools for the afterlife: Zooarchaeology at Machu Picchu, in The 1912 Yale Peruvian Scientific Expedition Collections from Machu Picchu: Human and Animal Remains, R. Burger, L. Salazar, Eds. (Yale Univ. Press, Yale University Publications in Anthropology, 2004), pp. 1–63. [Google Scholar]
- 29.E. Washburn, J. Nesbitt, B. Ibarra, L. Fehren-Schmitz, V. M. Oelze, A strontium isoscape for the Conchucos region of highland Peru and its application to Andean archaeology. PLOS ONE 16, e0248209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D. N. Harris, W. Song, A. C. Shetty, K. S. Levano, O. Cáceres, C. Padilla, V. Borda, D. Tarazona, O. Trujillo, C. Sanchez, M. D. Kessler, M. Galarza, S. Capristano, H. Montejo, P. O. Flores-Villanueva, E. Tarazona-Santos, T. D. O’Connor, H. Guio, Evolutionary genomic dynamics of Peruvians before, during, and after the Inca Empire. Proc. Natl. Acad. Sci. U.S.A. 115, E6526–E6535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D. Popović, M. Molak, M. Ziółkowski, A. Vranich, M. Sobczyk, D. U. Vidaurre, G. Agresti, M. Skrzypczak, K. Ginalski, T. C. Lamnidis, N. Nakatsuka, S. Mallick, M. Baca, Ancient genomes reveal long-range influence of the pre-Columbian culture and site of Tiwanaku. Sci. Adv. 7, eabg7261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.J. Lindo, R. Haas, C. Hofman, M. Apata, M. Moraga, R. A. Verdugo, J. T. Watson, C. V. Llave, D. Witonsky, C. Beall, C. Warinner, J. Novembre, M. Aldenderfer, A. D. Rienzo, The genetic prehistory of the Andean highlands 7000 years BP though European contact. Sci. Adv. 4, eaau4921 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.C. Barbieri, R. Barquera, L. Arias, J. R. Sandoval, O. Acosta, C. Zurita, A. Aguilar-Campos, A. M. Tito-Álvarez, R. Serrano-Osuna, R. Gray, F. Mafessoni, P. Heggarty, K. K. Shimizu, R. Fujita, M. Stoneking, I. Pugach, L. Fehren-Schmitz, The current genomic landscape of western South America: Andes, Amazonia and Pacific Coast. Mol. Biol. Evol. 36, 2698–2713 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.N. Nakatsuka, I. Lazaridis, C. Barbieri, P. Skoglund, N. Rohland, S. Mallick, C. Posth, K. Harkins-Kinkaid, M. Ferry, É. Harney, M. Michel, K. Stewardson, J. Novak-Forst, J. M. Capriles, M. A. Durruty, K. A. Álvarez, D. Beresford-Jones, R. Burger, L. Cadwallader, R. Fujita, J. Isla, G. Lau, C. L. Aguirre, S. LeBlanc, S. C. Maldonado, F. Meddens, P. G. Messineo, B. J. Culleton, T. K. Harper, J. Quilter, G. Politis, K. Rademaker, M. Reindel, M. Rivera, L. Salazar, J. R. Sandoval, C. M. Santoro, N. Scheifler, V. Standen, M. I. Barreto, I. F. Espinoza, E. Tomasto-Cagigao, G. Valverde, D. J. Kennett, A. Cooper, J. Krause, W. Haak, B. Llamas, D. Reich, L. Fehren-Schmitz, A paleogenomic reconstruction of the deep population history of the Andes. Cell 181, 1131–1145.e21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R. A. Covey, D. Amado, The marquisate of orapesa and the preservations and study of its documents, in Imperial Transformations in Sixteenth Century Yucay, Peru, R. A. Covey, A. Amado, Eds. (University of Michigan Press, 2008), pp. 30–34. [Google Scholar]
- 36.R. A. Covey, C. M. Elson, Ethnicity, demography, and estate management in sixteenth-century Yucay. Ethnohistory 54, 303–335 (2007). [Google Scholar]
- 37.C. Stanish, Ancient Titicaca: The Evolution of Complex Society in Southern Peru and Northern Bolivia (University of California Press, 2003). [Google Scholar]
- 38.L. G. Lumbreras, Arqueología de la América Andina (Editorial Milla Batres, 1981).
- 39.R. L. Burger, K. L. M. Chávez, S. J. Chávez, Through the glass darkly: Prehispanic obsidian procurement and exchange in Southern Peru and Northern Bolivia. J. World Prehist. 14, 267–362 (2000). [Google Scholar]
- 40.G. A. Gnecchi-Ruscone, S. Sarno, S. De Fanti, L. Gianvincenzo, C. Giuliani, A. Boattini, E. Bortolini, T. Di Corcia, C. Sanchez Mellado, T. J. Dàvila Francia, D. Gentilini, A. M. Di Blasio, P. Di Cosimo, E. Cilli, A. Gonzalez-Martin, C. Franceschi, Z. A. Franceschi, O. Rickards, M. Sazzini, D. Luiselli, D. Pettener, Dissecting the pre-Columbian genomic ancestry of native Americans along the Andes–Amazonia divide. Mol. Biol. Evol. 36, 1254–1269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.M. A. Castro e Silva, T. Ferraz, M. C. Bortolini, D. Comas, T. Hünemeier, Deep genetic affinity between coastal Pacific and Amazonian natives evidenced by Australasian ancestry. Proc. Natl. Acad. Sci. U.S.A. 118, e2025739118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.S. Alconini, Southeast Inka Frontiers: Boundaries and Interactions (University Press of Florida, 2016); https://muse.jhu.edu/book/46400.
- 43.F. Santos-Granero, Vital Enemies: Slavery, Predation, and the Amerindian Political Economy of Life (University of Texas Press, 2010). [Google Scholar]
- 44.F. Salomon, A north Andean status trader complex under Inka rule. Ethnohistory 34, 63–77 (1987). [Google Scholar]
- 45.F. Salomon, Native Lords of Quito in the Age of the Incas: The Political Economy of North Andean Chiefdoms (Cambridge Univ. Press, 2007).
- 46.J. L. Bongers, N. Nakatsuka, C. O’Shea, T. K. Harper, H. Tantaleán, C. Stanish, L. Fehren-Schmitz, Integration of ancient DNA with transdisciplinary dataset finds strong support for Inca resettlement in the south Peruvian coast. Proc. Natl. Acad. Sci. U.S.A. 117, 18359–18368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.E. J. Marsh, R. Kidd, D. Ogburn, V. Durán, Dating the expansion of the Inca Empire: Bayesian models from Ecuador and Argentina. Radiocarbon. 59, 117–140 (2017). [Google Scholar]
- 48.T. N. D’Altroy, V. Williams, A. Lorandi, The Inkas in the Southlands, in Variations in the Expression of Inka Power, R. Burger, C. Morris, R. Matos, Eds. (Dumbarton Oaks Research Library and Collection, 2007), pp. 138–164. [Google Scholar]
- 49.D. Wilkinson, Incas and Arawaks: A special relationship along the Andes-Amazonian frontier. Andean Past. 13, 13 (2022). [Google Scholar]
- 50.J. R. Sandoval, D. R. Lacerda, O. Acosta, M. S. Jota, P. Robles-Ruiz, A. Salazar-Granara, P. P. R. Vieira, C. Paz-Y-Miño, R. Fujita, F. R. Santos, The genetic history of Peruvian Quechua-Lamistas and Chankas: Uniparental DNA patterns among autochthonous Amazonian and Andean populations. Ann. Hum. Genet. 80, 88–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.J. Nesbitt, R. Clasby, The Archaeology of the Upper Amazon: Complexity and Interaction in the Andean Tropical Forest (University Press of Florida, 2021); https://muse.jhu.edu/pub/227/edited_volume/book/85772.
- 52.J. W. Rutledge, R. B. Gordon, The work of metallurgical artificers at Machu Picchu, Peru. Am. Antiq. 52, 578–594 (1987). [Google Scholar]
- 53.B. K. Scaffidi, K. J. Knudson, An archaeological strontium isoscape for the prehistoric Andes: Understanding population mobility through a geostatistical meta-analysis of archaeological 87Sr/86Sr values from humans, animals, and artifacts. J. Archaeol. Sci. 117, 105121 (2020). [Google Scholar]
- 54.V. A. Andrushko, E. C. Torres Pino, V. Bellifemine, The burials at Sacsahuaman and Chokepukio: A bioarchaeological case study of imperialism from the capital of the Inca Empire. Ñawpa Pacha 28, 63–92 (2006). [Google Scholar]
- 55.K. Shinoda, N. Adachi, S. Guillén, I. Shimada, Mitochondrial DNA analysis of ancient Peruvian highlanders. Am. J. Phys. Anthropol. 131, 98–107 (2006). [DOI] [PubMed] [Google Scholar]
- 56.J. Dabney, M. Knapp, I. Glocke, M.-T. Gansauge, A. Weihmann, B. Nickel, C. Valdiosera, N. García, S. Pääbo, J.-L. Arsuaga, M. Meyer, Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. U.S.A. 110, 15758–15763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.S. Boessenkool, K. Hanghøj, H. M. Nistelberger, C. Der Sarkissian, A. Gondek, L. Orlando, J. H. Barrett, B. Star, Combining bleach and mild pre-digestion improves ancient DNA recovery from bones. Mol. Ecol. Resour. 17, 742–751 (2016). [DOI] [PubMed] [Google Scholar]
- 58.É. Harney, O. Cheronet, D. M. Fernandes, K. Sirak, M. Mah, R. Bernardos, N. Adamski, N. Broomandkhoshbacht, K. Callan, A. M. Lawson, J. Oppenheimer, K. Stewardson, F. Zalzala, A. Anders, F. Candilio, M. Constantinescu, A. Coppa, I. Ciobanu, J. Dani, Z. Gallina, F. Genchi, E. G. Nagy, T. Hajdu, M. Hellebrandt, A. Horváth, Á. Király, K. Kiss, B. Kolozsi, P. Kovács, K. Köhler, M. Lucci, I. Pap, S. Popovici, P. Raczky, A. Simalcsik, T. Szeniczey, S. Vasilyev, C. Virag, N. Rohland, D. Reich, R. Pinhasi, A minimally destructive protocol for DNA extraction from ancient teeth. Genome Res. 31, 472–483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.N. Rohland, E. Harney, S. Mallick, S. Nordenfelt, D. Reich, Partial UDG-treatment for screening of ancient DNA. Phil. Trans. R. Soc. B 370, 20130624 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.J. D. Kapp, R. E. Green, B. Shapiro, A fast and efficient single-stranded genomic library preparation method optimized for ancient DNA. J. Hered. 112, 241–249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.M. T. Gansauge, A. Aximu-Petri, S. Nagel, M. Meyer, Manual and automated preparation of single-stranded DNA libraries for the sequencing of DNA from ancient biological remains and other sources of highly degraded DNA. Nat. Protoc. 15, 2279–2300 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Q. Fu, M. Hajdinjak, O. T. Moldovan, S. Constantin, S. Mallick, P. Skoglund, N. Patterson, N. Rohland, I. Lazaridis, B. Nickel, B. Viola, K. Prüfer, M. Meyer, J. Kelso, D. Reich, S. Pääbo, An early modern human from Romania with a recent Neanderthal ancestor. Nature 524, 216–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Ghodsi, B. Liu, M. Pop, DNACLUST: Accurate and efficient clustering of phylogenetic marker genes. BMC Bioinformatics. 12, 271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.J. S. John, SeqPrep (2011); https://github.com/jstjohn/SeqPrep.
- 65.H. Li, R. Durbin, Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A. Peltzer, G. Jäger, A. Herbig, A. Seitz, C. Kniep, J. Krause, K. Nieselt, EAGER: Efficient ancient genome reconstruction. Genome Biol. 17, 60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Q. Fu, A. Mittnik, P. L. F. Johnson, K. Bos, M. Lari, R. Bollongino, C. Sun, L. Giemsch, R. Schmitz, J. Burger, A. M. Ronchitelli, F. Martini, R. G. Cremonesi, J. Svoboda, P. Bauer, D. Caramelli, S. Castellano, D. Reich, S. Pääbo, J. Krause, A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 23, 553–559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.T. S. Korneliussen, A. Albrechtsen, R. Nielsen, ANGSD: Analysis of next generation sequencing data. BMC Bioinformatics 15, 356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.H. Jónsson, A. Ginolhac, M. Schubert, P. L. F. Johnson, L. Orlando, MapDamage2.0: Fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682–1684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.P. Skoglund, J. Storå, A. Götherström, M. Jakobsson, Accurate sex identification of ancient human remains using DNA shotgun sequencing. J. Archaeol. Sci. 40, 4477–4482 (2013). [Google Scholar]
- 71.A. Mittnik, C. C. Wang, J. Svoboda, J. Krause, A molecular approach to the sexing of the triple burial at the upper paleolithic site of Dolní Věstonice. PLOS ONE 11, e0163019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.H. Chen, Y. Lu, D. Lu, S. Xu, Y-LineageTracker: A high-throughput analysis framework for Y-chromosomal next-generation sequencing data. BMC Bioinformatics. 22, 114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.B. Llamas, L. Fehren-Schmitz, G. Valverde, J. Soubrier, S. Mallick, N. Rohland, S. Nordenfelt, C. Valdiosera, S. M. Richards, A. Rohrlach, M. I. B. Romero, I. F. Espinoza, E. T. Cagigao, L. W. Jiménez, K. Makowski, I. S. L. Reyna, J. M. Lory, J. A. B. Torrez, M. A. Rivera, R. L. Burger, M. C. Ceruti, J. Reinhard, R. S. Wells, G. Politis, C. M. Santoro, V. G. Standen, C. Smith, D. Reich, S. Y. W. Ho, A. Cooper, W. Haak, Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci. Adv. 2, e1501385 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.H. Li, B. Handsaker, A. Wysoker, T. Fennell, J. Ruan, N. Homer, G. Marth, G. Abecasis, R. Durbin; 1000 Genome Project Data Processing Subgroup , The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.M. Rasmussen, S. L. Anzick, M. R. Waters, P. Skoglund, M. DeGiorgio, T. W. Stafford, S. Rasmussen, I. Moltke, A. Albrechtsen, S. M. Doyle, G. D. Poznik, V. Gudmundsdottir, R. Yadav, A.-S. Malaspinas, S. S. White, M. E. Allentoft, O. E. Cornejo, K. Tambets, A. Eriksson, P. D. Heintzman, M. Karmin, T. S. Korneliussen, D. J. Meltzer, T. L. Pierre, J. Stenderup, L. Saag, V. M. Warmuth, M. C. Lopes, R. S. Malhi, S. Brunak, T. Sicheritz-Ponten, I. Barnes, M. Collins, L. Orlando, F. Balloux, A. Manica, R. Gupta, M. Metspalu, C. D. Bustamante, M. Jakobsson, R. Nielsen, E. Willerslev, The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature 506, 225–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.M. Raghavan, M. Steinrucken, K. Harris, S. Schiffels, S. Rasmussen, M. DeGiorgio, A. Albrechtsen, C. Valdiosera, M. C. Avila-Arcos, A.-S. Malaspinas, A. Eriksson, I. Moltke, M. Metspalu, J. R. Homburger, J. Wall, O. E. Cornejo, J. V. Moreno-Mayar, T. S. Korneliussen, T. Pierre, M. Rasmussen, P. F. Campos, P. de Barros Damgaard, M. E. Allentoft, J. Lindo, E. Metspalu, R. Rodriguez-Varela, J. Mansilla, C. Henrickson, A. Seguin-Orlando, H. Malmstrom, T. Stafford, S. S. Shringarpure, A. Moreno-Estrada, M. Karmin, K. Tambets, A. Bergstrom, Y. Xue, V. Warmuth, A. D. Friend, J. Singarayer, P. Valdes, F. Balloux, I. Leboreiro, J. L. Vera, H. Rangel-Villalobos, D. Pettener, D. Luiselli, L. G. Davis, E. Heyer, C. P. E. Zollikofer, M. S. Ponce de Leon, C. I. Smith, V. Grimes, K.-A. Pike, M. Deal, B. T. Fuller, B. Arriaza, V. Standen, M. F. Luz, F. Ricaut, N. Guidon, L. Osipova, M. I. Voevoda, O. L. Posukh, O. Balanovsky, M. Lavryashina, Y. Bogunov, E. Khusnutdinova, M. Gubina, E. Balanovska, S. Fedorova, S. Litvinov, B. Malyarchuk, M. Derenko, M. J. Mosher, D. Archer, J. Cybulski, B. Petzelt, J. Mitchell, R. Worl, P. J. Norman, P. Parham, B. M. Kemp, T. Kivisild, C. Tyler-Smith, M. S. Sandhu, M. Crawford, R. Villems, D. G. Smith, M. R. Waters, T. Goebel, J. R. Johnson, R. S. Malhi, M. Jakobsson, D. J. Meltzer, A. Manica, R. Durbin, C. D. Bustamante, Y. S. Song, R. Nielsen, E. Willerslev, Genomic evidence for the Pleistocene and recent population history of Native Americans. Science 349, aab3884 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.J. V. Moreno-Mayar, B. A. Potter, L. Vinner, M. Steinrücken, S. Rasmussen, J. Terhorst, J. A. Kamm, A. Albrechtsen, A.-S. Malaspinas, M. Sikora, J. D. Reuther, J. D. Irish, R. S. Malhi, L. Orlando, Y. S. Song, R. Nielsen, D. J. Meltzer, E. Willerslev, Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature 553, 203–207 (2018). [DOI] [PubMed] [Google Scholar]
- 78.J. V. Moreno-Mayar, L. Vinner, P. de Barros Damgaard, C. de la Fuente, J. Chan, J. P. Spence, M. E. Allentoft, T. Vimala, F. Racimo, T. Pinotti, S. Rasmussen, A. Margaryan, M. I. Orbegozo, D. Mylopotamitaki, M. Wooller, C. Bataille, L. Becerra-Valdivia, D. Chivall, D. Comeskey, T. Devièse, D. K. Grayson, L. George, H. Harry, V. Alexandersen, C. Primeau, J. Erlandson, C. Rodrigues-Carvalho, S. Reis, M. Q. R. Bastos, J. Cybulski, C. Vullo, F. Morello, M. Vilar, S. Wells, K. Gregersen, K. L. Hansen, N. Lynnerup, M. M. Lahr, K. Kjær, A. Strauss, M. Alfonso-Durruty, A. Salas, H. Schroeder, T. Higham, R. S. Malhi, J. T. Rasic, L. Souza, F. R. Santos, A.-S. Malaspinas, M. Sikora, R. Nielsen, Y. S. Song, D. J. Meltzer, E. Willerslev, Early human dispersals within the Americas. Science 362, eaav2621 (2018). [DOI] [PubMed] [Google Scholar]
- 79.D. M. Fernandes, K. A. Sirak, H. Ringbauer, J. Sedig, N. Rohland, O. Cheronet, M. Mah, S. Mallick, I. Olalde, B. J. Culleton, N. Adamski, R. Bernardos, G. Bravo, N. Broomandkhoshbacht, K. Callan, F. Candilio, L. Demetz, K. S. D. Carlson, L. Eccles, S. Freilich, R. J. George, A. M. Lawson, K. Mandl, F. Marzaioli, W. C. McCool, J. Oppenheimer, K. T. Özdogan, C. Schattke, R. Schmidt, K. Stewardson, F. Terrasi, F. Zalzala, C. A. Antúnez, E. V. Canosa, R. Colten, A. Cucina, F. Genchi, C. Kraan, F. La Pastina, M. Lucci, M. V. Maggiolo, B. Marcheco-Teruel, C. T. Maria, C. Martínez, I. París, M. Pateman, T. M. Simms, C. G. Sivoli, M. Vilar, D. J. Kennett, W. F. Keegan, A. Coppa, M. Lipson, R. Pinhasi, D. Reich, A genetic history of the pre-contact Caribbean. Nature 590, 103–110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.K. Nägele, C. Posth, M. I. Orbegozo, Y. C. de Armas, S. T. H. Godoy, U. M. G. Herrera, M. A. Nieves-Colón, M. Sandoval-Velasco, D. Mylopotamitaki, R. Radzeviciute, J. Laffoon, W. J. Pestle, J. Ramos-Madrigal, T. C. Lamnidis, W. C. Schaffer, R. S. Carr, J. S. Day, C. A. Antúnez, A. R. Rivero, A. J. Martínez-Fuentes, E. Crespo-Torres, I. Roksandic, A. C. Stone, C. Lalueza-Fox, M. Hoogland, M. Roksandic, C. L. Hofman, J. Krause, H. Schroeder, Genomic insights into the early peopling of the Caribbean. Science 369, 456–460 (2020). [DOI] [PubMed] [Google Scholar]
- 81.C. L. Scheib, H. Li, T. Desai, V. Link, C. Kendall, G. Dewar, P. W. Griffith, A. Mörseburg, J. R. Johnson, A. Potter, S. L. Kerr, P. Endicott, J. Lindo, M. Haber, Y. Xue, C. Tyler-Smith, M. S. Sandhu, J. G. Lorenz, T. D. Randall, Z. Faltyskova, L. Pagani, P. Danecek, T. C. O’Connell, P. Martz, A. S. Boraas, B. F. Byrd, A. Leventhal, R. Cambra, R. Williamson, L. Lesage, B. Holguin, E. Y.-D. Soto, J. Rosas, M. Metspalu, J. T. Stock, A. Manica, A. Scally, D. Wegmann, R. S. Malhi, T. Kivisild, Ancient human parallel lineages within North America contributed to a coastal expansion. Science 360, 1024–1027 (2018). [DOI] [PubMed] [Google Scholar]
- 82.C. Posth, N. Nakatsuka, I. Lazaridis, P. Skoglund, S. Mallick, T. C. Lamnidis, N. Rohland, K. Nägele, N. Adamski, E. Bertolini, N. Broomandkhoshbacht, A. Cooper, B. J. Culleton, T. Ferraz, M. Ferry, A. Furtwängler, W. Haak, K. Harkins, T. K. Harper, T. Hünemeier, A. M. Lawson, B. Llamas, M. Michel, E. Nelson, J. Oppenheimer, N. Patterson, S. Schiffels, J. Sedig, K. Stewardson, S. Talamo, C.-C. Wang, J.-J. Hublin, M. Hubbe, K. Harvati, A. Nuevo Delaunay, J. Beier, M. Francken, P. Kaulicke, H. Reyes-Centeno, K. Rademaker, W. R. Trask, M. Robinson, S. M. Gutierrez, K. M. Prufer, D. C. Salazar-García, E. N. Chim, L. Müller Plumm Gomes, M. L. Alves, A. Liryo, M. Inglez, R. E. Oliveira, D. V. Bernardo, A. Barioni, V. Wesolowski, N. A. Scheifler, M. A. Rivera, C. R. Plens, P. G. Messineo, L. Figuti, D. Corach, C. Scabuzzo, S. Eggers, P. DeBlasis, M. Reindel, C. Méndez, G. Politis, E. Tomasto-Cagigao, D. J. Kennett, A. Strauss, L. Fehren-Schmitz, J. Krause, D. Reich, Reconstructing the deep population history of central and South America. Cell 175, 1185–1197.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.S. Mallick, H. Li, M. Lipson, I. Mathieson, M. Gymrek, F. Racimo, M. Zhao, N. Chennagiri, S. Nordenfelt, A. Tandon, P. Skoglund, I. Lazaridis, S. Sankararaman, Q. Fu, N. Rohland, G. Renaud, Y. Erlich, T. Willems, C. Gallo, J. P. Spence, Y. S. Song, G. Poletti, F. Balloux, G. van Driem, P. de Knijff, I. G. Romero, A. R. Jha, D. M. Behar, C. M. Bravi, C. Capelli, T. Hervig, A. Moreno-Estrada, O. L. Posukh, E. Balanovska, O. Balanovsky, S. Karachanak-Yankova, H. Sahakyan, D. Toncheva, L. Yepiskoposyan, C. Tyler-Smith, Y. Xue, M. S. Abdullah, A. Ruiz-Linares, C. M. Beall, A. Di Rienzo, C. Jeong, E. B. Starikovskaya, E. Metspalu, J. Parik, R. Villems, B. M. Henn, U. Hodoglugil, R. Mahley, A. Sajantila, G. Stamatoyannopoulos, J. T. S. Wee, R. Khusainova, E. Khusnutdinova, S. Litvinov, G. Ayodo, D. Comas, M. F. Hammer, T. Kivisild, W. Klitz, C. A. Winkler, D. Labuda, M. Bamshad, L. B. Jorde, S. A. Tishkoff, W. S. Watkins, M. Metspalu, S. Dryomov, R. Sukernik, L. Singh, K. Thangaraj, S. Pääbo, J. Kelso, N. Patterson, D. Reich, The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature 538, 201–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.I. Lazaridis, D. Nadel, G. Rollefson, D. C. Merrett, N. Rohland, S. Mallick, D. Fernandes, M. Novak, B. Gamarra, K. Sirak, S. Connell, K. Stewardson, E. Harney, Q. Fu, G. Gonzalez-Fortes, E. R. Jones, S. A. Roodenberg, G. Lengyel, F. Bocquentin, B. Gasparian, J. M. Monge, M. Gregg, V. Eshed, A.-S. Mizrahi, C. Meiklejohn, F. Gerritsen, L. Bejenaru, M. Blüher, A. Campbell, G. Cavalleri, D. Comas, P. Froguel, E. Gilbert, S. M. Kerr, P. Kovacs, J. Krause, D. McGettigan, M. Merrigan, D. A. Merriwether, S. O’Reilly, M. B. Richards, O. Semino, M. Shamoon-Pour, G. Stefanescu, M. Stumvoll, A. Tönjes, A. Torroni, J. F. Wilson, L. Yengo, N. A. Hovhannisyan, N. Patterson, R. Pinhasi, D. Reich, Genomic insights into the origin of farming in the ancient near east. Nature 536, 419–424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.P. Skoglund, S. Mallick, M. C. Bortolini, N. Chennagiri, T. Hünemeier, M. L. Petzl-Erler, F. M. Salzano, N. Patterson, D. Reich, Genetic evidence for two founding populations of the Americas. Nature 525, 104–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.I. Lazaridis, N. Patterson, A. Mittnik, G. Renaud, S. Mallick, K. Kirsanow, P. H. Sudmant, J. G. Schraiber, S. Castellano, M. Lipson, B. Berger, C. Economou, R. Bollongino, Q. Fu, K. I. Bos, S. Nordenfelt, H. Li, C. de Filippo, K. Prüfer, S. Sawyer, C. Posth, W. Haak, F. Hallgren, E. Fornander, N. Rohland, D. Delsate, M. Francken, J.-M. Guinet, J. Wahl, G. Ayodo, H. A. Babiker, G. Bailliet, E. Balanovska, O. Balanovsky, R. Barrantes, G. Bedoya, H. Ben-Ami, J. Bene, F. Berrada, C. M. Bravi, F. Brisighelli, G. B. J. Busby, F. Cali, M. Churnosov, D. E. C. Cole, D. Corach, L. Damba, G. van Driem, S. Dryomov, J.-M. Dugoujon, S. A. Fedorova, I. Gallego Romero, M. Gubina, M. Hammer, B. M. Henn, T. Hervig, U. Hodoglugil, A. R. Jha, S. Karachanak-Yankova, R. Khusainova, E. Khusnutdinova, R. Kittles, T. Kivisild, W. Klitz, V. Kǔinskas, A. Kushniarevich, L. Laredj, S. Litvinov, T. Loukidis, R. W. Mahley, B. Melegh, E. Metspalu, J. Molina, J. Mountain, K. Näkkäläjärvi, D. Nesheva, T. Nyambo, L. Osipova, J. Parik, F. Platonov, O. Posukh, V. Romano, F. Rothhammer, I. Rudan, R. Ruizbakiev, H. Sahakyan, A. Sajantila, A. Salas, E. B. Starikovskaya, A. Tarekegn, D. Toncheva, S. Turdikulova, I. Uktveryte, O. Utevska, R. Vasquez, M. Villena, M. Voevoda, C. A. Winkler, L. Yepiskoposyan, P. Zalloua, T. Zemunik, A. Cooper, C. Capelli, M. G. Thomas, A. Ruiz-Linares, S. A. Tishkoff, L. Singh, K. Thangaraj, R. Villems, D. Comas, R. Sukernik, M. Metspalu, M. Meyer, E. E. Eichler, J. Burger, M. Slatkin, S. Pääbo, J. Kelso, D. Reich, J. Krause, Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.J. M. M. Kuhn, M. Jakobsson, T. Günther, Estimating genetic kin relationships in prehistoric populations. PLOS ONE 13, e0195491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.M. Lipatov, K. Sanjeev, R. Patro, K. R. Veeramah, Maximum likelihood estimation of biological relatedness from low coverage sequencing data. bioRxiv, 023374 (2015). [Google Scholar]
- 89.N. Patterson, A. L. Price, D. Reich, Population structure and eigenanalysis. PLOS Genet. 2, e190 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.N. Patterson, P. Moorjani, Y. Luo, S. Mallick, N. Rohland, Y. Zhan, T. Genschoreck, T. Webster, D. Reich, Ancient admixture in human history. Genetics 192, 1065–1093 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.B. R. Baum, PHYLIP: Phylogeny Inference Package. Version 3.2. Joel Felsenstein. Q. Rev. Biol. 64, 539–541 (1989). [Google Scholar]
- 92.P. Skoglund, J. C. Thompson, M. E. Prendergast, A. Mittnik, K. Sirak, M. Hajdinjak, T. Salie, N. Rohland, S. Mallick, A. Peltzer, A. Heinze, I. Olalde, M. Ferry, E. Harney, M. Michel, K. Stewardson, J. I. Cerezo-Román, C. Chiumia, A. Crowther, E. Gomani-Chindebvu, A. O. Gidna, K. M. Grillo, I. T. Helenius, G. Hellenthal, R. Helm, M. Horton, S. López, A. Z. P. Mabulla, J. Parkington, C. Shipton, M. G. Thomas, R. Tibesasa, M. Welling, V. M. Hayes, D. J. Kennett, R. Ramesar, M. Meyer, S. Pääbo, N. Patterson, A. G. Morris, N. Boivin, R. Pinhasi, J. Krause, D. Reich, Reconstructing prehistoric African population structure. Cell 171, 59–71.e21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.L. Salazar, R. L. Burger, The Machu Picchu solution: A new approach to cultural patrimony disputes, in Finding Solutions for Protecting and Sharing Archaeological Heritage Resources, A. P. Underhill, L. C. Salazar, Eds. (Springer, 2016), pp. 87–102. [Google Scholar]
- 94.R. Burger, Nuevos alcanzes científicos sobre la vida diaria en Machu Picchu, in Machu Picchu: Investigaciones Interdisciplinarias, F. Astete, J. Bastante, Eds. (Ministerio de Cultura Peru, 2020), pp. 77–106. [Google Scholar]
- 95.R. L. Burger, L. Salazar, The 1912 Yale Peruvian Scientific Expedition Collections from Machu Picchu: Metal Artifacts (Yale Univ. Press, Yale University Publications in Anthropology, 2012). [Google Scholar]
- 96.R. L. Burger, L. Salazar, Hiram Bingham III and Machu Picchu: Contextualizing the “Scientific Discovery” and its protagonist, in Los Reinos del Sol y de la Luna, V. Pimentel, Ed. (Museum of Fine Art, Montreal, 2013), pp. 28–33. [Google Scholar]
- 97.L. Salazar, R. L. Burger, Reinventing the Incas in Contemporary Cuzco: The Cases of Inti Raymi and Machu Picchu, in The Oxford Handbook of the Incas, S. Alconini, R. A. Covey, Eds. (Oxford Univ. Press, 2018). [Google Scholar]
- 98.L. Salazar, La Mayoría Silenciosa de Machu Pichu: Una consideración de los cemeteries incas, in Machu Picchu: Investigaciones Interdisciplinarias, F. Astete, J. Bastante, Eds. (Ministerio de Cultura Peru, 2020), pp. 11–24. [Google Scholar]
- 99.R. A. Covey, Archaeology and Inka origins. J. Archaeol. Res. 26, 253–304 (2018). [Google Scholar]
- 100.J. H. Rowe, Absolute chronology in the Andean area. Am. Antiq. 10, 265–284 (1945). [Google Scholar]
- 101.R. A. Covey, Multiregional perspectives on the archaeology of the Andes during the late intermediate period (c. A. D. 1000–1400). J. Archaeol. Res. 16, 287–338 (2008). [Google Scholar]
- 102.J. Hiltunen, G. F. McEwan, Knowing the Inca Past, in Andean Archaeology H. Silverman Ed. (Blackwell Publishing, 2004), pp. 237–254. [Google Scholar]
- 103.C. Julien, On the beginning of the late horizon. Ñawpa Pacha 29, 163–177 (2008). [Google Scholar]
- 104.A. Adamska, A. Michczyński, Towards a radiocarbon chronology of the Inca state. ANDES: Boletín de la Misión Arqueológica Andina 1, 35–60 (1996). [Google Scholar]
- 105.D. E. Ogburn, Reconceiving the chronology of Inca imperial expansion. Radiocarbon 54, 219–237 (2012). [Google Scholar]
- 106.P. J. Reimer, W. E. N. Austin, E. Bard, A. Bayliss, P. G. Blackwell, C. B. Ramsey, M. Butzin, H. Cheng, R. L. Edwards, M. Friedrich, P. M. Grootes, T. P. Guilderson, I. Hajdas, T. J. Heaton, A. G. Hogg, K. A. Hughen, B. Kromer, S. W. Manning, R. Muscheler, J. G. Palmer, C. Pearson, J. van der Plicht, R. W. Reimer, D. A. Richards, E. M. Scott, J. R. Southon, C. S. M. Turney, L. Wacker, F. Adolphi, U. Büntgen, M. Capano, S. M. Fahrni, A. Fogtmann-Schulz, R. Friedrich, P. Köhler, S. Kudsk, F. Miyake, J. Olsen, F. Reinig, M. Sakamoto, A. Sookdeo, S. Talamo, The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020). [Google Scholar]
- 107.A. G. Hogg, T. J. Heaton, Q. Hua, J. G. Palmer, C. S. Turney, J. Southon, A. Bayliss, P. G. Blackwell, G. Boswijk, C. B. Ramsey, C. Pearson, F. Petchey, P. Reimer, R. Reimer, L. Wacker, SHCal20 southern hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62, 759–778 (2020). [Google Scholar]
- 108.S. Ancapichún, J. Pawlyta, A. Z. Rakowski, D. Sieczkowska, Influence of air parcels from northern and southern hemispheres on radiocarbon-based Inca chronology. Radiocarbon 64, 1431–1446 (2022). [Google Scholar]
- 109.E. J. Marsh, M. C. Bruno, S. C. Fritz, P. Baker, J. M. Capriles, C. A. Hastorf, IntCal, SHCal, or a mixed curve? Choosing 14C calibration curve for archaeological and paleoenvironmental records from tropical South America. Radiocarbon 60, 925–940 (2018). [Google Scholar]
- 110.M. Ziółkowski, J. B. Abuhadba, A. Hogg, D. Sieczkowska, A. Rakowski, J. Pawlyta, S. W. Manning, When did the Incas build Machu Picchu and its satellite sites? New approches based on radiocarbon dating. Radiocarbon 63, 1133–1148 (2021). [Google Scholar]
- 111.J. L. Swift, N. F. Bahamodes, R. J. Schulting, A radiocarbon chronology for Estadio de Quillota in the Aconcagua Valley, Central Chile, with reference to the adoption of intensive maize agriculture and revisiting the timing of Inka influence on the southern frontier. Radiocarbon 64, 1209–1237 (2022). [Google Scholar]
- 112.B. S. Bauer, J. Fonseca Santa Cruz, M. Aráoz Silva, Vilcabamba and the Archaeology of Inca Resistance (Cotsen Institute of Archaeology Press, 2015). [Google Scholar]
- 113.A. Flammang, Inca funerary practices (ca. 1400–1532): A first assessment on the basis of archaeological data. Ñawpa Pacha 42, 261–286 (2022). [Google Scholar]
- 114.H. Bingham, Machu Picchu, a Citadel of the Incas (Yale Univ. Press, 1930). [Google Scholar]
- 115.J.-P. Protzen, Inca Architecture and Construction at Ollantaytambo (Oxford Univ. Press, 1993). [Google Scholar]
- 116.B. S. Bauer, C. Stanish, Killke and Killke-related pottery from Cuzco, Peru, in the Field Museum of natural history. Fieldiana. Anthropology, 1–17 (1990). [Google Scholar]
- 117.J. Calero, M. Fernandez, La Casa del Patrón: Documentos para la Historia de la Iglesia de San Sebastian (Municipalidad Distrital de San Sebastián, 2020).
- 118.E. Vargas Paliza, Kusikancha: Morada de las momias reales de los Inkas (Instituto Nacional de Cultura, Dirección Regional INC-C, Sub Dirección de Investigación, ed. 1, 2007).
- 119.A. Pérez, N. Olazabal, Investigaciones arqueológicas en la zona arqueológica de Qotakalli-Cusco. Patrimonio 7, 94–99 (2015). [Google Scholar]
- 120.C. J. Julien, Las tumbas de Sacsahuaman y el estilo Cuzco-Inca. Ñawpa Pacha 25, 1–125 (2004). [Google Scholar]
- 121.L. E. Valcárcel, Cuzco: Archaeological Capital of South America, 1534–1934 (Imprenta Torres Aguirre, 1934).
- 122.B. S. Bauer, Ancient Cuzco Heartland of the Inca (University of Texas Press, 2004)https://utpress.utexas.edu/books/bauanc.
- 123.M. Paredes, Prácticas funerarias incaicas en Sacsayhuamán: Enterramientos ceremoniales y complejo funerario. Boletín de Arqueología PUCP, 79–111 (2003). [Google Scholar]
- 124.J. W. Verano, V. A. Andrushko, Cranioplasty in ancient Peru: A critical review of the evidence, and a unique case from the Cuzco area. Int. J. Osteoarchaeol. 20, 269–279 (2010). [Google Scholar]
- 125.B. Aparicio Laucata, Informe de investigación arqueológica 2009: Conjunto arqueológico de Kanamarka-Espinar (Cusco, 2009).
- 126.R. Bisso-Machado, M. S. Jota, V. Ramallo, V. R. Paixao-Cortes, D. R. Lacerda, F. M. Salzano, S. L. Bonatto, F. R. Santos, M. C. Bortolini, Distribution of Y-chromosome Q lineages in Native Americans. Am. J. Hum. Biol. 23, 563–566 (2011). [DOI] [PubMed] [Google Scholar]
- 127.M. van Oven, PhyloTree build 17: Growing the human mitochondrial DNA tree. Forensic Sci. Int. Genet. Suppl. Ser. 5, e392–e394 (2015). [Google Scholar]
- 128.M. van Oven, M. Kayser, Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 30, E386–E394 (2009). [DOI] [PubMed] [Google Scholar]
- 129.H. Weissensteiner, D. Pacher, A. Kloss-Brandstätter, L. Forer, G. Specht, H.-J. Bandelt, F. Kronenberg, A. Salas, S. Schönherr, HaploGrep 2: Mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 44, W58–W63 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.L. Arias, C. Barbieri, G. Barreto, M. Stoneking, B. Pakendorf, High-resolution mitochondrial DNA analysis sheds light on human diversity, cultural interactions, and population mobility in Northwestern Amazonia. Am. J. Phys. Anthropol. 165, 238–255 (2018). [DOI] [PubMed] [Google Scholar]
- 131.S. Brandini, P. Bergamaschi, M. F. Cerna, F. Gandini, F. Bastaroli, E. Bertolini, C. Cereda, L. Ferretti, A. Gómez-Carballa, V. Battaglia, A. Salas, O. Semino, A. Achilli, A. Olivieri, A. Torroni, The Paleo-Indian entry into South America according to mitogenomes. Mol. Biol. Evol. 35, 299–311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.A. Gómez-Carballa, J. Pardo-Seco, S. Brandini, A. Achilli, U. A. Perego, M. D. Coble, T. M. Diegoli, V. Álvarez-Iglesias, F. Martinón-Torres, A. Olivieri, A. Torroni, A. Salas, The peopling of South America and the trans-Andean gene flow of the first settlers. Genome Res. 28, 767–779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.R. Davidson, L. Fehren-Schmitz, B. Llamas, A multidisciplinary review of the Inka imperial resettlement policy and implications for future investigations. Genes 12, 215 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.L. M. Valdez, K. J. Bettcher, The founding of the Inca provincial center of Tambo Viejo, Acarí, Peru. Ñawpa Pacha, 1–30 (2022). [Google Scholar]
- 135.L. Fehren-Schmitz, M. Reindel, E. Tomasto-Cagigao, S. Hummel, B. Herrmann, Pre-columbian population dynamics in Coastal Southern Peru: A diachronic investigation of mtDNA patterns in the palpa region by ancient DNA analysis. Am. J. Phys. Anthropol. 141, 208–221 (2010). [DOI] [PubMed] [Google Scholar]
- 136.M. Nei, Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89, 583–590 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.J. R. Sandoval, D. R. Lacerda, M. S. A. Jota, A. Salazar-Granara, P. P. R. Vieira, O. Acosta, C. Cuellar, S. Revollo, R. Fujita, F. R. Santos; Genographic Project Consortium , The genetic history of indigenous populations of the Peruvian and Bolivian altiplano: The legacy of the Uros. PLOS ONE 8, e73006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.J. R. Sandoval, D. R. Lacerda, M. S. Jota, R. Elward, O. Acosta, D. Pinedo, P. Danos, C. Cuellar, S. Revollo, F. R. Santos, R. Fujita, Genetic ancestry of families of putative Inka descent. Mol. Genet. Genomics 293, 873–881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.G. S. Cabana, D. A. Merriwether, K. Hunley, D. A. Demarchi, Is the genetic structure of Gran Chaco populations unique? Interregional perspectives on native south American mitochondrial DNA variation. Am. J. Phys. Anthropol. 131, 108–119 (2006). [DOI] [PubMed] [Google Scholar]
- 140.G. S. Cabana, C. M. Lewis Jr., R. Y. Tito, R. A. Covey, A. M. Cáceres, A. F. D. L. Cruz, D. Durand, G. Housman, B. I. Hulsey, G. C. Iannacone, P. W. López, R. Martínez, Á. Medina, O. O. Dávila, K. P. O. Pinto, S. I. P. Santillán, P. R. Domínguez, M. Rubel, H. F. Smith, S. E. Smith, V. R. de Celis Massa, B. Lizárraga, A. C. Stone, Population genetic structure of traditional populations in the Peruvian Central Andes and implications for south American population history. Hum. Biol. 86, 147–165 (2014). [DOI] [PubMed] [Google Scholar]
- 141.V. Ramallo, R. Bisso-Machado, C. Bravi, M. D. Coble, F. M. Salzano, T. Hünemeier, M. C. Bortolini, Demographic expansions in South America: Enlightening a complex scenario with genetic and linguistic data. Am. J. Phys. Anthropol. 150, 453–463 (2013). [DOI] [PubMed] [Google Scholar]
- 142.S. Fuselli, E. Tarazona-Santos, I. Dupanloup, A. Soto, D. Luiselli, D. Pettener, Mitochondrial DNA diversity in South America and the genetic history of Andean highlanders. Mol. Biol. Evol. 20, 1682–1691 (2003). [DOI] [PubMed] [Google Scholar]
- 143.B. M. Kemp, T. A. Tung, M. L. Summar, Genetic continuity after the collapse of the Wari empire: Mitochondrial DNA profiles from Wari and post-Wari populations in the ancient Andes. Am. J. Phys. Anthropol. 140, 80–91 (2009). [DOI] [PubMed] [Google Scholar]
- 144.C. M. Lewis, R. Y. Tito, B. Lizárraga, A. C. Stone, Land, language, and loci: mtDNA in Native Americans and the genetic history of Peru. Am. J. Phys. Anthropol. 127, 351–360 (2005). [DOI] [PubMed] [Google Scholar]
- 145.M. de Saint Pierre, F. Gandini, U. A. Perego, M. Bodner, A. Gómez-Carballa, D. Corach, N. Angerhofer, S. R. Woodward, O. Semino, A. Salas, W. Parson, M. Moraga, A. Achilli, A. Torroni, A. Olivieri, Arrival of Paleo-Indians to the southern cone of South America: New clues from mitogenomes. PLOS ONE 7, e51311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.L. Fehren-Schmitz, W. Haak, B. Mächtle, F. Masch, B. Llamas, E. Tomasto Cagigao, V. Sossna, K. Schittek, J. Isla Cuadrado, B. Eitel, M. Reindel, Climate change underlies global demographic, genetic, and cultural transitions in pre-Columbian southern Peru. Proc. Natl. Acad. Sci. U.S.A. 111, 9443–9448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.L. Excoffier, H. E. Lischer, Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (2010). [DOI] [PubMed] [Google Scholar]
- 148.B. S. Bauer, The Early Ceramics of the Inca Heartland (Field Museum of Natural History, 1999).
- 149.S. Alconini, R. A. Covey, Inca imperial identities - Colonization, resistance, and hybridity, in The Oxford Handbook of the Incas, S. Alconini, R. A. Covey, Eds. (Oxford Univ. Press, 2018), pp. 471–480. [Google Scholar]
- 150.É. Harney, N. Patterson, D. Reich, J. Wakeley, Assessing the performance of qpAdm: A statistical tool for studying population admixture. Genetics 217, iyaa045 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.V. M. Narasimhan, N. Patterson, P. Moorjani, N. Rohland, R. Bernardos, S. Mallick, I. Lazaridis, N. Nakatsuka, I. Olalde, M. Lipson, A. M. Kim, L. M. Olivieri, A. Coppa, M. Vidale, J. Mallory, V. Moiseyev, E. Kitov, J. Monge, N. Adamski, N. Alex, N. Broomandkhoshbacht, F. Candilio, K. Callan, O. Cheronet, B. J. Culleton, M. Ferry, D. Fernandes, S. Freilich, B. Gamarra, D. Gaudio, M. Hajdinjak, É. Harney, T. K. Harper, D. Keating, A. M. Lawson, M. Mah, K. Mandl, M. Michel, M. Novak, J. Oppenheimer, N. Rai, K. Sirak, V. Slon, K. Stewardson, F. Zalzala, Z. Zhang, G. Akhatov, A. N. Bagashev, A. Bagnera, B. Baitanayev, J. Bendezu-Sarmiento, A. A. Bissembaev, G. L. Bonora, T. T. Chargynov, T. Chikisheva, P. K. Dashkovskiy, A. Derevianko, M. Dobeš, K. Douka, N. Dubova, M. N. Duisengali, D. Enshin, A. Epimakhov, A. V. Fribus, D. Fuller, A. Goryachev, A. Gromov, S. P. Grushin, B. Hanks, M. Judd, E. Kazizov, A. Khokhlov, A. P. Krygin, E. Kupriyanova, P. Kuznetsov, D. Luiselli, F. Maksudov, A. M. Mamedov, T. B. Mamirov, C. Meiklejohn, D. C. Merrett, R. Micheli, O. Mochalov, S. Mustafokulov, A. Nayak, D. Pettener, R. Potts, D. Razhev, M. Rykun, S. Sarno, T. M. Savenkova, K. Sikhymbaeva, S. M. Slepchenko, O. A. Soltobaev, N. Stepanova, S. Svyatko, K. Tabaldiev, M. Teschler-Nicola, A. A. Tishkin, V. V. Tkachev, S. Vasilyev, P. Velemínský, D. Voyakin, A. Yermolayeva, M. Zahir, V. S. Zubkov, A. Zubova, V. S. Shinde, C. Lalueza-Fox, M. Meyer, D. Anthony, N. Boivin, K. Thangaraj, D. J. Kennett, M. Frachetti, R. Pinhasi, D. Reich, The formation of human populations in south and central Asia. Science 365, aat7487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.R. A. Covey, Inca Apocalypse: The Spanish Conquest and the Transformation of the Andean World (Oxford Univ. Press, 2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S8
Legends for tables S1 to S8
References
Tables S1 to S8