Abstract
Aim: To investigate changes in treatment patterns in extensive-stage small-cell lung cancer (ES-SCLC) in France, Germany, Italy, Spain and the UK (EU5) between 2018 and 2021. Methods: Cross-sectional data from an oncology database were analyzed retrospectively. Results: Of 5832 eligible patients, 88.4% had stage IV disease at diagnosis. Among patients receiving first-line treatment, 91.8% (1079 /1176) received the platinum-etoposide (PE) combination in 2018 which decreased to 42.3% (509/1203) by 2021. Usage of the PE-atezolizumab combination increased from 0 to 41.2% during the same timeframe. Topotecan monotherapy remained the most widely used second-line treatment regardless of patients' platinum sensitivity. Conclusion: The first-line standard of care for ES-SCLC has evolved in EU5 with the PE-atezolizumab/durvalumab combination gradually superseding PE usage.
Keywords: : Europe, immunotherapy, real-world, small cell lung cancer, treatment patterns
Plain language summary
Lung cancer is the leading cause of cancer-related deaths. Small-cell lung cancer (SCLC) is fast-growing type of lung cancer. New treatments for SCLC using medicines that stimulate the immune system to kill cancer cells (called immunotherapies) have recently been approved for use in Europe. The purpose of the study was to describe the type of treatments that patients received in five European countries before and after the introduction of these new treatments to determine how quickly these new treatments were adopted in a real-world setting. This study found that most patients treated between 2018 and 2020 still received a platinum-based chemotherapy as their first anticancer therapy, but immunotherapies were used more often in later years and became the most common first treatment in 2021 for patients who had never been treated for their cancer. Topotecan, a type of chemotherapy, was the most used treatment for patients whose cancer came back after treatment. There is still a clear unmet need for new, safe and effective therapies for the treatment of patients with SCLC whose cancer comes back again after treatment.
Plain language summary
Summary points.
Real-world data from 5,832 patients with extensive-stage small-cell lung cancer (ES-SCLC) who were treated in France, Germany, Italy, Spain and the UK (EU5) between 2018 and 2021 were analyzed to identify patient characteristics and changes in treatment patterns following the approval of atezolizumab and durvalumab in combination with platinum-etoposide chemotherapy for first-line treatment of ES-SCLC in Europe.
Atezolizumab with platinum-etoposide chemotherapy was rapidly adopted as first-line treatment in France, Germany and the UK while the adoption was relatively slower in Italy and Spain. The country-specific differences in the adoption of immunotherapy were potentially related to the different time points at which reimbursement for anti-PD-L1 immunotherapy was initiated in these countries.
Topotecan remained the most widely used second-line treatment for patients regardless of platinum sensitivity or the duration of the platinum-free interval after first-line therapy (3–6 months vs ≥6 months). This study highlights the presence of an unmet need for newer treatment options for ES-SCLC in the second-line and beyond.
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Small-cell lung cancer (SCLC) is an aggressive, high-grade cancer that accounts for 15% of all lung cancers [2]. In Europe, the estimated prevalence of SCLC is 1–5 per 10,000 people [3,4]. Approximately two-thirds of patients present with extensive-stage SCLC (ES-SCLC) at the time of primary diagnosis [5]. SCLC is associated with poor prognosis; the 5-year survival rate for SCLC ranges from 27% in patients with localized SCLC to 3% in patients with extensive disease [2,6]. Patients treated for ES-SCLC have a median survival of 6–12 months whereas untreated patients have a median survival of only 2–4 months following diagnosis [7].
Until recently, in Europe and the USA, the most common first-line (1L) therapy for SCLC has been chemotherapy with a platinum-etoposide (PE) combination [8]. Disease progression after 1L platinum-based chemotherapy in patients with ES-SCLC is common, with most patients relapsing within 6 months [4,9]. Patients are categorized as either platinum-sensitive, i.e., progression at ≥3 months after the last dose of platinum-based induction chemotherapy or platinum-resistant, i.e., progression within 3 months from the last dose of platinum-based induction chemotherapy per the European Society for Medical Oncology (ESMO) guidelines [4]. There is no consensus on how to define sensitivity to platinum-based therapies, as the National Comprehensive Cancer Network (NCCN) guidelines for the USA use disease progression in a 6-month window (following the end of 1L platinum-based chemotherapy) to define these two categories [10]. Responsiveness to 1L platinum-based chemotherapy correlates with prognosis and guides subsequent treatment strategies [9].
The only approved second-line (2L) treatment for ES-SCLC in Europe is topotecan. Lurbinectedin was conditionally approved for the treatment of adult patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy by the US FDA in June 2020 but is not yet approved in Europe [11,12]. In general, the response rates in patients receiving 2L chemotherapy for ES-SCLC range between 20 and 30% in platinum-sensitive patients to 15% in platinum-resistant patients [4]. No third-line (3L) regimen has received regulatory approval for ES-SCLC in Europe and the USA.
Following a 30-year period during which there were only limited therapeutic advances in the treatment landscape for ES-SCLC, two anti-programmed death-ligand 1 (anti-PD-L1) antibodies, atezolizumab and durvalumab, were approved in quick succession by the European Medicines Agency. Atezolizumab in combination with carboplatin/etoposide and durvalumab in combination with platinum (carboplatin/cisplatin)-etoposide were approved for 1L treatment of adult patients with ES-SCLC in 2019 and 2020, respectively [13,14]. Updated European treatment guidelines recommend the incorporation of atezolizumab or durvalumab into a platinum-based chemotherapy regimen and as maintenance therapy for 1L treatment of patients with ES-SCLC [4,15].
There are limited data on the extent of adoption of the newly approved immunotherapy regimens in European patients with ES-SCLC. The most recent publications describing real-world treatment patterns in SCLC have either focused on understanding treatment patterns among patients in the USA, have included a limited number of patients, analyzed data derived from a single hospital database, or analyzed data from several years before the approval of these chemoimmunotherapy combinations for ES-SCLC [8,16-18]. As a result, there is a lack of information about the current extent of adoption of these newly approved immunotherapy regimens in the first line of treatment for ES-SCLC across Europe. Mapping the therapeutic landscape is critical for understanding unmet patient needs with regard to treatment availability and identifying potential treatment gaps and issues regarding access to new treatments.
The primary objective of this repeated cross-sectional, retrospective, observational study was to describe the characteristics of patients with ES-SCLC and the real-world treatment patterns in five European countries (EU5: France, Germany, Italy, Spain and the UK) between 2018 and 2021.
Patients & methods
Data source & study design
This cross-sectional, observational study was based on data from IQVIA's Oncology Dynamics™ (Oncology Dynamics, IQVIA Ltd, London, UK) database (ONC-D). The ONC-D database collects anonymized patient-level information from participating physicians on drug-treated cancer patients at quarterly intervals (Supplementary Figure 1) [18,19]. Participating physicians are drawn from IQVIA's OneKey database that contains the details and affiliations of approximately 11 million physicians globally. Physicians are selected from the OneKey database based on stratified random sampling; physicians are randomly sampled from a representative distribution of physician specialties that care for SCLC patients in each participating country. Selected physicians include both hospital and office-based physicians and all major cancer-treating specialties. At the site-level, the type of cancer treatment site, physician specialties within that site, and the type of cancers treated at each site are all factored into physician selection, and no more than three physicians from each site are included to avoid patient clustering effects. Physician and site participation is voluntary, and physicians are remunerated for their participation. At a country level, Italy is oversampled compared with the other four included countries to account for sub-national treatment differences in Italy, resulting in a higher proportion of patients from Italy included in the sample.
The selected physicians are asked to complete electronic case report forms (eCRFs) on the medical histories for the most recent series of consecutive patients that the physician has personally treated for a specific cancer type (i.e., with cytotoxic therapy, targeted therapy, hormonal or immunotherapy) during a quarterly reporting period. The responses to all questions are mandatory. The maximum number of included patients in each quarter is determined by the physician's specialty, the size of the hospital and country, the number of patients a physician sees within the reporting period, and the distribution of cancer types the patients are drawn from. Patients are excluded if they are solely treated with radiotherapy, surgery, supportive care or are on active surveillance.
ONC-D conducts quarterly cross-sectional surveys in oncology centers located in ten countries, five of which are in Europe (France, Germany, Italy, Spain and the UK). The included patient distributions and demographics in ONC-D have been found to be similar to study samples of published epidemiological studies [19]. The database provides retrospective information on patient characteristics and treatment history from the time the participating physician completes the case report form until diagnosis. At each quarterly data collection period, physicians record the current and previous systemic treatment(s) for each patient (i.e., if a patient was receiving 2L treatment, both 1L and 2L treatments are documented). Each quarterly data collection period collects data on a new sample set of patients and therefore the longitudinal treatment history of each patient is not recorded.
Institutional review board approval or informed consent from individual patients were not required as this study describes the secondary analysis of de-identified patient data [20].
Study participants
The study population included all patients in the ONC-D database who were ≥18 years of age, diagnosed with ES-SCLC, and had received systemic treatment between January 2018 and December 2021 in five European countries (France, Germany, Italy, Spain and the UK; EU5).
Treatment sites
Patients had received treatment at either academic hospitals (a university hospital that may or may not contain specialist cancer facilities), specialist cancer facilities (a non-university hospital which is either a cancer hospital or a hospital that has a cancer unit/pediatric cancer ward), general hospitals (a non-university hospital which is not a cancer hospital and does not have a cancer unit), or had been treated by office-based practitioners (specialized outpatient care provided by hospital specialists within office-based clinics that were introduced in Germany in 2004). These office-based clinics offer treatment for severe progressive forms of disease and for rare diseases, as well as carrying out highly specialized procedures. Details of what is permitted under these care facilities and the qualification requirements for the treating physicians are defined by Germany's Federal Joint Committee.
Statistical analyses
No formal a priori hypothesis was tested. All statistical analyses were carried out on a cross-sectional time point separately. Descriptive statistics were used to report analyzed results. For continuous variables, the number of patients (non-missing and missing), mean, median, standard deviation, 95% CI, interquartile range, minimum and maximum values are presented. For categorical/ordinal variables, the number and percentage of patients (non-missing and missing) distributed in each category are presented. All statistical analyses were carried out using SAS Software (SAS Enterprise Guide 7.1.; SAS Institute Inc., NC, USA).
Results
Patient characteristics
A total of 5832 ES-SCLC patients (1430 in 2018; 1406 in 2019; 1589 in 2020; and 1407 in 2021) were considered eligible for analysis in the period between January 2018 and December 2021. Of these, most patients were treated in Italy (31.7%, 1850/5832), followed by Germany (26.0%, 1518/5832), the UK (19.7%, 1149/5832), Spain (13.2%, 771/5832) and France (9.3%, 544/5832).
The majority of patients (84.0%, 4898/5832) were receiving 1L treatment for ES-SCLC at the time of data collection, 13.8% (804/5832) were receiving 2L treatment, 1.7% (98/5832) were receiving 3L treatment and 0.6% (32/5832) were receiving fourth-line treatment.
The study population was predominantly male (65.9%, 3843/5832), had a median age of 66 years (range, 27.0–83.5 years), and 90.4% (4000/4425) were either current smokers or had a history of smoking. The majority of patients (88.4%, 5158/5832) had stage IV disease at the time of primary diagnosis. The primary sites of metastases were the liver (55.4%, 3230/5832) and the lung (50.0%, 2917/5832; Table 1).
Table 1.
Patient demographics and clinical characteristics.
| Country |
EU5 |
France |
Germany |
Italy |
Spain |
UK |
|---|---|---|---|---|---|---|
| N | 5832 | 544 | 1518 | 1850 | 771 | 1149 |
| Gender, n (%) | ||||||
| Female | 1989 (34.1) | 150 (27.6) | 543 (35.8) | 573 (31.0) | 210 (27.2) | 513 (44.6) |
| Male | 3843 (65.9) | 394 (72.4) | 975 (64.2) | 1277 (69.0) | 561 (72.8) | 636 (55.4) |
| Age at diagnosis (derived) | ||||||
| Mean (SD) | 64.7 (8.6) | 63.1 (8.3) | 63.8 (8.3) | 65.8 (8.7) | 64.2 (8.3) | 65 (9.2) |
| Median (Q1, Q3) | 66 (58, 72) | 63 (58, 68) | 63 (58, 68) | 67.7 (58, 72.8) | 63 (58, 71.8) | 67 (58, 72.4) |
| Min, Max | 27.0, 83.5 | 27.0, 83.5 | 31.0, 83.5 | 33.0, 83.5 | 37.4, 83.5 | 32.0, 83.5 |
| Smoking status not including 2021†, n (%) | ||||||
| n = 4425 | n = 374 | n = 1142 | n = 1454 | n = 594 | n = 861 | |
| Yes | 2316 (52.3) | 234 (62.6) | 649 (56.8) | 670 (46.1) | 303 (51.0) | 460 (53.4) |
| No, but has smoked in the past | 1684 (38.1) | 113 (30.2) | 405 (35.5) | 613 (42.2) | 226 (38.1) | 327 (38.0) |
| No, and has not smoked in the past | 364 (8.2) | 23 (6.2) | 78 (6.8) | 133 (9.2) | 65 (10.9) | 65 (7.6) |
| Not known | 61 (1.4) | 4 (1.1) | 10 (0.9) | 38 (2.6) | 0 (0.0) | 9 (1.1) |
| Disease stage at primary diagnosis, n (%) | ||||||
| ≤ II | 50 (0.9) | 11 (2.0) | 14 (0.9) | 10 (0.5) | 8 (1.0) | 7 (0.6) |
| III | 304 (5.2) | 30 (5.5) | 66 (4.4) | 78 (4.2) | 53 (6.9) | 77 (6.7) |
| IV | 5158 (88.4) | 503 (92.5) | 1438 (94.7) | 1443 (78.0) | 709 (92.0) | 1065 (92.7) |
| Unknown | 320 (5.5) | 0 (0.0) | 0 (0.0) | 319 (17.2) | 1 (0.1) | 0 (0.0) |
| ECOG performance status‡, n (%) | ||||||
| 0 | 651 (11.2) | 32 (5.9) | 94 (6.2) | 429 (23.2) | 38 (4.9) | 58 (5.0) |
| 1 | 3,699 (63.4) | 362 (66.5) | 960 (63.2) | 1138 (61.5) | 534 (69.3) | 705 (61.4) |
| 2 | 1336 (22.9) | 135 (24.8) | 432 (28.5) | 237 (12.8) | 189 (24.5) | 343 (29.9) |
| 3 | 112 (1.9) | 15 (2.8) | 30 (2.0) | 16 (0.9) | 10 (1.3) | 41 (3.6) |
| 4 | 4 (0.1) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) |
| N/A | 30 (0.5) | 0 (0.0) | 0 (0.0) | 30 (1.6) | 0 (0.0) | 0 (0.0) |
| Site of treatment, n (%) | ||||||
| Academic hospital | 2041 (35.0) | 214 (39.3) | 156 (10.3) | 439 (23.7) | 575 (74.6) | 657 (57.2) |
| Specialist cancer facility | 2419 (41.5) | 236 (43.4) | 503 (33.1) | 1090 (58.9) | 196 (25.4) | 394 (34.3) |
| General hospital | 519 (8.9) | 94 (17.3) | 10 (0.7) | 317 (17.1) | 0 (0.0) | 98 (8.5) |
| Office-based practitioner | 849 (14.6) | 0 (0.0) | 849 (55.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 4 (0.1) | 0 (0.0) | 0 (0.0) | 4 (0.2) | 0 (0.0) | 0 (0.0) |
ECOG: Eastern Cooperative Oncology Group; EU5: France, Germany, Italy, Spain and the UK; N/A: Not available; Q1: Quartile 1; Q3: Quartile 3; SD: Standard deviation.
Smoking status from 2018 to 2020; data on smoking status were not collected in 2021.
ECOG performance status scale interpretation: 0-asymptomatic; 1-symptomatic fully ambulatory; 2-symptomatic in bed less than 50% of the day; 3-symptomatic in bed greater than 50% of the day but not bedridden; 4-bedridden.
Most patients had been treated at either a specialist cancer facility (41.5%, 2419/5832) or at an academic hospital (35.0%, 2041/5832; Table 1). However, there were wide variations in the treatment site among the EU5 countries. Most patients from Spain (74.6%, 575/771) and the UK (57.2%, 657/1149) received their treatment in an academic hospital, while 58.9% of patients (1090/1850) from Italy received treatment in a specialist cancer facility. In Germany, one in two patients (55.9%, 849/1518) was treated by an office-based practitioner, while none of the patients from the other four countries were treated at a similar site.
First-line treatment patterns
Overall, 4898 of the 5832 patients (84.0%) were receiving 1L treatment for ES-SCLC between 2018 and 2021. In 2018, the majority of patients received platinum-based chemotherapy as 1L treatment. The most common treatment regimen by far in 2018 was PE (91.8%, 1079/1176 [carboplatin-etoposide: 58.6%, 632/1079; cisplatin-etoposide: 41.4%, 447/1079]) followed by platinum monotherapy (2.6%, 30/1176), etoposide monotherapy (1.5%, 18/1176) and platinum-based chemotherapy with other agents (1.5%, 18/1176). There was a progressive decline in the use of PE as 1L treatment from 91.8% in 2018 to 42.3% in 2021. In contrast, the use of atezolizumab with platinum-based chemotherapy increased from 0% in 2018 to 41.2% in 2021 and the use of durvalumab with platinum-based chemotherapy increased from 0% in 2018 to 4.6% in 2021 (Figure 1). By 2021, atezolizumab with platinum-based chemotherapy was the most commonly used 1L regimen in Germany (54.8%), France (48.5%) and the UK (43.7%).
Figure 1.
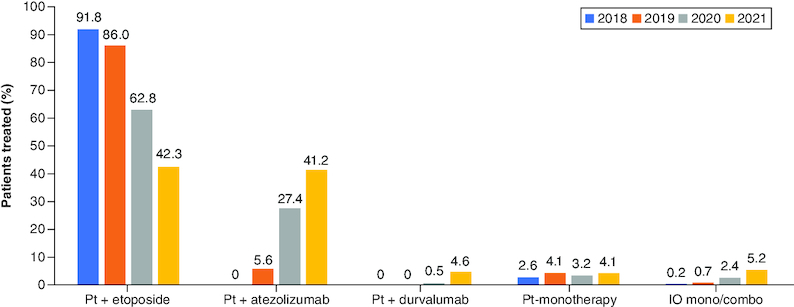
First-line treatment regimens for extensive-stage small cell lung cancer in EU5*.
*Regimens that contributed <2% individually to total percentage from 2018 to 2021 are not shown.
EU5: France, Germany, Italy, Spain and the UK; IO mono/combo: Immunotherapy monotherapy or combination, including ipilimumab/nivolumab combination regimen; Pt: Platinum; Pt + atezolizumab: Platinum-based chemotherapy with atezolizumab combination regimen; Pt + durvalumab: Platinum-based chemotherapy with durvalumab combination regimen; Pt + etoposide: Platinum-based chemotherapy with etoposide combination regimen.
The use of PD-L1–based chemoimmunotherapy regimens (atezolizumab or durvalumab in combination with platinum-based chemotherapy) varied across countries. A higher proportion of patients in Germany and France were treated with the atezolizumab and platinum-based chemotherapy combination compared with the other three countries in all years (Figure 2A). The difference was particularly pronounced in 2020, where a more than fivefold higher proportion of patients was treated with this regimen in Germany and France compared with patients in Italy. After Germany and France, the steepest increase in the use of atezolizumab with platinum-based chemotherapy combination occurred in Italy (3.4-fold increase) and the UK (2.1-fold increase) between 2020 and 2021. The use of atezolizumab combination therapy in Spain remained low, with fewer than one in five patients treated with this regimen in 2020 and 2021.
Figure 2.
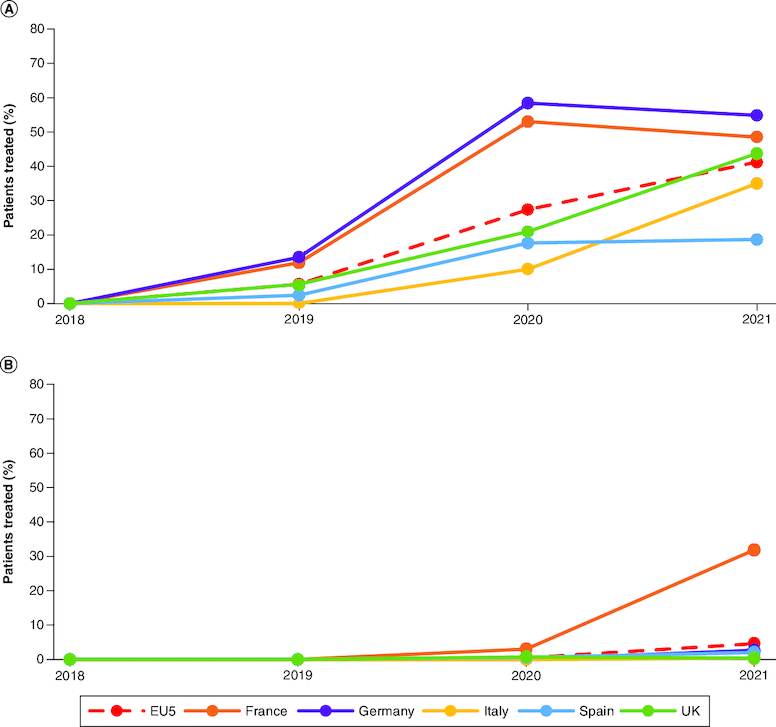
Change in the proportion of patients treated with chemoimmunotherapy between 2018 and 2021 in EU5.
Change in proportions of patients treated with (A) platinum-based chemotherapy-atezolizumab combination and (B) platinum-based chemotherapy and durvalumab combination as first-line treatment for ES-SCLC between 2018 and 2021 in EU5 (overall) and in individual EU5 countries.
ES-SCLC: Extensive-stage small-cell lung cancer; EU5: France, Germany, Italy, Spain and the UK.
Overall, the use of durvalumab and platinum-based chemotherapy combination was considerably lower compared with atezolizumab and platinum-based chemotherapy combination across all years and countries (Figure 2B). Across the EU5, 0.5 and 4.6% of patients were treated with this combination in 2020 and 2021, respectively. There was considerable variation in use by country and year, with a noticeable increase in the use of this combination in France in 2021 (31.8%) compared with its use in 2020 (3.0%). Less than 3% of patients were treated with this combination in the other EU5 countries between 2018 and 2021.
Second-line treatment patterns
Data for 804 patients who were receiving a 2L treatment for ES-SCLC between 2018 and 2021 were collected. Overall, 59.8% (2018), 57.7% (2019), 57.3% (2020) and 50.3% (2021) of patients were treated with topotecan, making it the most commonly used 2L treatment. (Figure 3). Other treatment regimens used for 2L treatment were carboplatin-etoposide combination (14.3% [2018], 9.3% [2019], 11.6% [2020] and 8.7% [2021]), cyclophosphamide, doxorubicin, and vincristine combination (CAV) (4.9% [2018], 7.7% [2019], 8.9% [2020] and 8.1% [2021]), and paclitaxel monotherapy (3.1% [2018], 3.3% [2019], 5.8% [2020] and 4.1% [2021]). These treatment trends remained stable across the study period.
Figure 3.
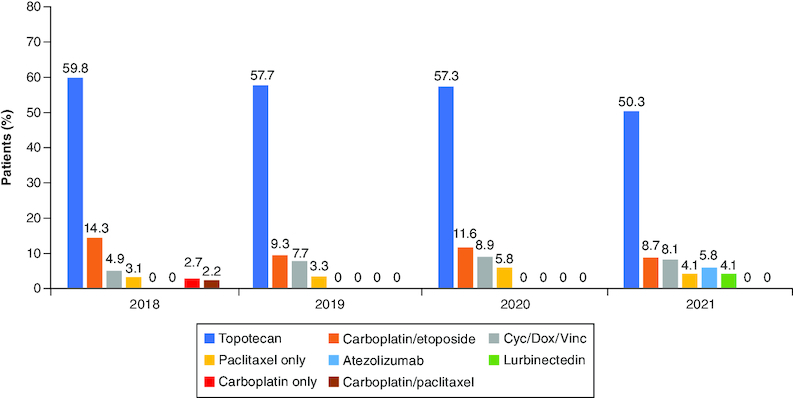
Second-line treatment regimens for extensive-stage small cell lung cancer in EU5 between 2018 and 2021*.
*Regimens that contributed <2% individually to total percentage from 2018 to 2021 are not shown. Consequently, percentages do not add up to 100% in each analyzed year.
Cyc/Dox/Vinc: Cyclophosphamide, doxorubicin and vincristine combination chemotherapy.
For this study, progressive disease was categorized as either platinum-sensitive or platinum-resistant/refractory as defined by ESMO guidelines [4]. Topotecan was the 2L treatment of choice for both platinum-sensitive and platinum-resistant patients across all years, though a higher proportion of platinum-resistant patients received topotecan (range, 55.6–73.0%) compared with the platinum-sensitive group (range, 51.9–57.4%). A higher proportion of platinum-sensitive patients were rechallenged with platinum-based chemotherapy with etoposide (range, 9.8–24.8%) compared with platinum-resistant patients (range, 0–11.1%; Figure 4A & B). Other 2L regimens that were commonly used across all years for platinum-resistant patients were CAV therapy and paclitaxel monotherapy. The use of lurbinectedin as 2L treatment was observed starting from 2020, with 4.1% of patients being treated with this drug in 2021.
Figure 4.
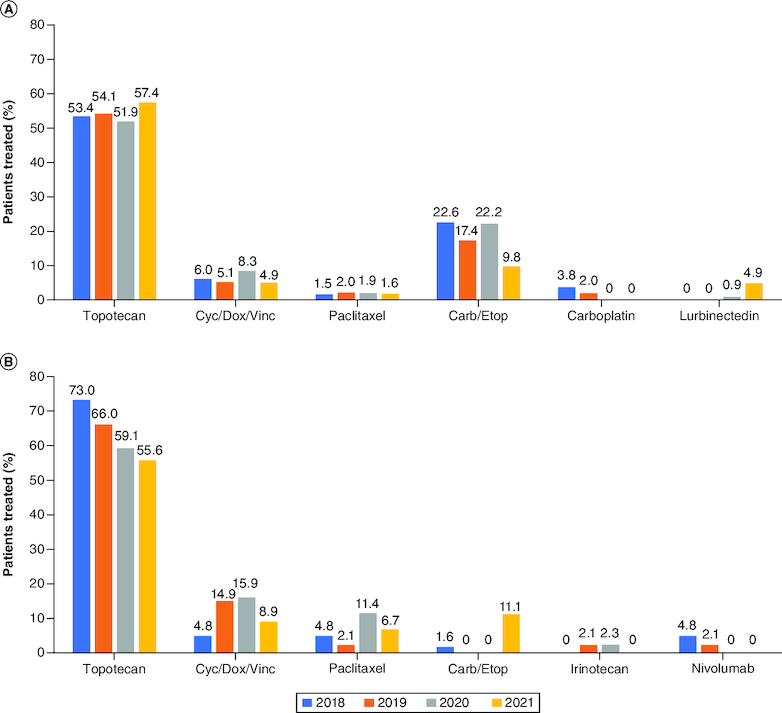
Second-line treatment patterns according to sensitivity to platinum chemotherapy*.
Second-line treatment regimens used for patients with (A) platinum-sensitive and (B) platinum-resistant ES-SCLC between 2018 and 2021 in EU5.
*Regimens that collectively contributed <2% to total percentage from 2018 to 2021 are not shown. Consequently, percentages may not add up to 100%.
Carb: Carboplatin; Cyc/Dox/Vinc: Cyclophosphamide/doxorubicin/vincristine; Etop: Etoposide; ES-SCLC: Extensive-stage small-cell lung cancer; EU5: France, Germany, Italy, Spain and the UK.
A total of 125 patients were rechallenged with platinum-based chemotherapy in the 2L (after having been treated with platinum-based chemotherapy in the 1L). Of these, 107 patients (85.6%) were platinum-sensitive, ten (8.0%) were platinum-resistant, and the platinum sensitivity was unknown for eight (6.4%) patients. Of the platinum-sensitive patients who were rechallenged with platinum-based chemotherapy in the 2L, 72.9% (78/107) had received their last dose of platinum-based chemotherapy ≥6 months before the rechallenge and 27.1% (29/107) had received their last dose of platinum-based chemotherapy 3–6 months before the rechallenge.
Third- & fourth-line treatment regimens
Between 2018 and 2021, a total of 98 and 32 patients received 3L and fourth-line (4L) treatment, respectively. Non–platinum/non–etoposide-based monotherapy and combination chemotherapy regimens (irinotecan with fluorouracil, docetaxel, gemcitabine, irinotecan, lurbinectedin, paclitaxel, pemetrexed and topotecan) were the most commonly used 3L and 4L treatment regimens followed by other chemotherapy-based regimens (the most used combination therapies were based on cyclophosphamide; Table 2).
Table 2.
Treatment regimens for small-cell lung cancer in the third line and beyond.
| Current regimen, n (%) | Third-line treatments | Fourth-line and subsequent treatments | ||||||
|---|---|---|---|---|---|---|---|---|
| 2018 |
2019 |
2020 |
2021 |
2018 |
2019 |
2020 |
2021 |
|
| n = 23 | n = 26 | n = 26 | n = 23 | n = 7 | n = 8 | n = 9 | n = 8 | |
| Non–platinum-based chemotherapy (excluding etoposide)† | 10 (43.5) | 14 (53.9) | 13 (50.0) | 13 (56.5) | 2 (28.6) | 3 (37.5) | 3 (33.3) | 4 (50.0) |
| Other chemotherapy combination‡ | 8 (34.8) | 2 (7.7) | 3 (11.5) | 5 (21.7) | 2 (28.6) | 2 (25.0) | 1 (11.1) | 0 (0.0) |
| Platinum-based chemotherapy + etoposide | 2 (8.7) | 1 (3.9) | 2 (7.7) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (22.2) | 3 (37.5) |
| Other non-chemotherapy§ | 2 (8.7) | 5 (19.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Platinum-based chemotherapy + other | 1 (4.4) | 3 (11.5) | 2 (7.7) | 2 (8.7) | 2 (28.6) | 0 (0.0) | 2 (22.2) | 0 (0.0) |
| Immunotherapy monotherapy or combination, including ipilimumab + nivolumab | 0 (0.0) | 1 (3.9) | 6 (23.1) | 3 (13.0) | 0 (0.0) | 3 (37.5) | 1 (11.1) | 0 (0.0) |
| Etoposide monotherapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
Non–platinum-based chemotherapies included irinotecan with fluorouracil, docetaxel, gemcitabine, irinotecan, lurbinectedin, paclitaxel, pemetrexed and topotecan.
Other chemotherapy combinations included cyclophosphamide/doxorubicin, cyclophosphamide/doxorubicin/ vincristine, cyclophosphamide/epirubicin, cyclophosphamide/epirubicin/vincristine, bevacizumab/topotecan, bevacizumab/paclitaxel, docetaxel/nintedanib. gemcitabine/irinotecan, gemcitabine/paclitaxel and irinotecan/paclitaxel.
Other non-chemotherapy options included bevacizumab, erlotinib, osimertinib, rovalpituzumab tesirine and tinostamustine.
Discussion
In this retrospective, cross-sectional analysis, we analyzed treatment patterns for ES-SCLC in five European countries between 2018 and 2021. Data from 5832 patients were evaluated and the analysis of this large dataset has provided a robust estimate of the current, real-world treatment patterns for ES-SCLC in the EU5 countries. Overall, we found a consistent increase in the use of atezolizumab with platinum-based chemotherapy combination as 1L treatment of ES-SCLC in EU5 between 2018 and 2021. The extent to which this regimen was prescribed, however, varied widely between countries with usage being noticeably higher in France and Germany. A few factors could have accelerated the adoption of these newly approved immunotherapies in these two countries relative to their adoption in the other EU5 countries. Reimbursement for atezolizumab was initiated in France as part of the early access program (Autorisation Temporaire d'Utilisation [Temporary Authorization for Use, ATU]) from May 2019 and was fully reimbursed starting from May 2020. Atezolizumab became eligible for full reimbursement in Germany from October 2019, in the UK from May 2020, in Italy from July 2020, and in Spain from June 2021. Durvalumab became eligible for reimbursement as part of the ATU in France in March 2020 and fully reimbursable in March 2021 and was fully reimbursed in Germany from October 2020. The early inclusion of atezolizumab and durvalumab in reimbursement policies in France and Germany is a likely reason for their early incorporation in 1L treatment regimens as it was observed that the number of patients receiving these chemoimmunotherapy-combinations increased almost immediately after the immunotherapy regimen was deemed eligible for reimbursement in individual countries. Similar patterns of adoption of the atezolizumab with platinum-based chemotherapy combination were observed in the UK and Italy; the percentage of patients treated with this combination increased threefold (Italy) or twofold (UK) in the year following the inclusion of atezolizumab in the respective national reimbursement programs. Relatively lower proportions of patients from Spain were treated with atezolizumab (or durvalumab) with platinum-based combination regimens during the study period. The multiple levels of approval and the decentralized regional pricing negotiations that are required before the inclusion of a therapy in the reimbursement program in Spain [21,22] may have led to the delayed coverage of atezolizumab as late as in 2021. These data support the findings from other studies which have identified inequities in patient access to oncology drugs, especially newly launched oncology medicines, across European countries [23,24]. It is important to note that despite the steady increase in number of patients treated with the atezolizumab-platinum-based chemotherapy combination in the 1L, only 41.2% of patients were treated with this regimen in 2021. Several factors such as contra-indication to immunotherapy, poor Eastern Cooperative Oncology Group performance status, frequent use of corticosteroids for symptom palliation could have restricted the number of patients who were prescribed this regimen.
In addition to inclusion in reimbursement plans, other factors that may have promoted early adoption in some countries include early access programs which provides early access to innovative and life-saving medicines to patients even before a market authorization is granted [25]. Atezolizumab was included in the ATU program in 2019 and its inclusion was followed by the steep rise in its usage in the subsequent year. In Germany, therapies are immediately covered by reimbursement plans following their approval, which could explain the early adoption [26,27]. In our analysis, we observed that 55.9% of patients in Germany received their treatment from an office-based practitioner in contrast to the other EU5 countries where none of the patients were treated at a similar facility. A systematic literature review that analyzed the characteristics of early prescribers of newly approved drugs found that office-based clinical investigators were more likely to prescribe newly marketed drugs compared with other physicians [28].
A substantially lower proportion of patients received durvalumab with platinum-based chemotherapy combination as 1L treatment in the EU5 countries. Currently, durvalumab is reimbursed only for 1L ES-SCLC treatment in France, Germany and Italy with complete reimbursement starting from October 2020 in Germany, from March 2021 in France, and from November 2022 in Italy. Durvalumab was, however, covered under the early access reimbursement program in France from March 2020. Durvalumab is currently not covered under reimbursement policies for ES-SCLC in Spain and the UK. Interestingly, the use of durvalumab with platinum-based chemotherapy combination remained low in Germany despite being eligible for full reimbursement since 2020.
Topotecan was the most commonly prescribed 2L treatment in EU5, regardless of platinum sensitivity, even though topotecan is currently indicated in Europe only for the treatment of SCLC in patients for whom re-treatment with the 1L regimen is not considered appropriate [29]. In addition, topotecan treatment has yielded a modest objective response rate of only 8–27% in patients with relapsed SCLC [30]. The widespread use of topotecan as 2L treatment observed in this analysis is in keeping with data from another study assessing treatment patterns in Europe which showed that topotecan was used as 2L treatment in 59.5% of platinum-resistant patients, 56.1% of platinum-sensitive (>3 months) patients, in 61.6% of platinum-sensitive patients who had received platinum chemotherapy 3–6 months ago, and in 50.0% of platinum-sensitive patients who had not been treated with platinum for >6 months [8]. In clinical studies, topotecan has a response rate (complete response and partial response) ranging from 5 to 17% and a 1-year overall survival rate of 9–27% depending on whether the disease progression was platinum-sensitive or platinum-resistant [31]. Additionally, topotecan use is associated with a high percentage of severe hematological adverse events, such as grade III/IV neutropenia for which a pooled incidence of 69% has been reported in patients [31]. Thus, there is a need for safer and more effective alternatives to topotecan in the 2L and beyond.
Several treatments for relapsed SCLC are currently in development or are yet to receive regulatory approval in the European Union. Lurbinectedin, a synthetic alkaloid that binds double-stranded DNA to disrupt DNA-protein interactions and RNA transcription received accelerated approval in the USA for the treatment of patients with disease progression on or after platinum-based chemotherapy [11]. The approval was based on a phase II trial of 105 patients with metastatic SCLC where an objective response rate of 35.2% was noted with the median duration of response of 5.3 months [32]. However, in a subsequent phase III trial, a lurbinectedin-doxorubicin combination regimen did not improve overall survival in patients with relapsed ES-SCLC when compared with the physician's choice of standard-of-care chemotherapy [33]. Other trials of lurbinectedin in combination with atezolizumab (as maintenance therapy for ES-SCLC; phase III, NCT05091567), and topotecan/irinotecan for relapsed SCLC (phase III, NCT05153239) are currently recruiting patients. The efficacy of lurbinectedin in these trials remains to be seen.
Several delta-like ligand 3 (DLL3)-targeting therapies for relapsed SCLC (2L) are currently in various stages of clinical development, including the bi-and tri-specific T-cell engagers, tarlatamab, BI 764532, HPN328, and QLS31904 [34-36]. The DLL3-targeting T-cell engager tarlatamab has shown a tolerable safety profile and clinical activity as monotherapy in phase I studies and is now in phase II of clinical development for relapsed ES-SCLC [37]. Tarlatamab is also being evaluated in phase I studies in combination with an anti-PD-1 antibody [38,39].
ESMO and NCCN treatment guidelines recommend that patients with platinum-sensitive disease be rechallenged with platinum-based chemotherapy and that patients who are platinum-resistant be treated with a non–platinum-based alternative in the 2L setting [40,41]. In this study, we observed that 8.0% of platinum-resistant (progression <3 months from last platinum dose) patients were rechallenged with platinum-based regimens in the 2L. This suggests that real-world clinical practice is not always in concordance with the ESMO treatment guidelines and is likely a reflection of the limited treatment options available for 2L treatment though it has to be also noted that there is currently no consensus on how to define sensitivity to platinum-based therapies. In clinical trials, the addition of atezolizumab or durvalumab to 1L platinum-based chemotherapy has been shown to provide a modest extension of progression-free survival periods or reduction in the risk of progression/death in patients with ES-SCLC compared with those receiving platinum chemotherapy alone [42,43]. The long-term effect of the addition of atezolizumab/durvalumab to platinum-based chemotherapy on the duration of treatment-free intervals after 1L therapy remains to be determined as the pivotal trials involved the use of maintenance immunotherapy regimens that may not be feasible in different countries in a real-world setting.
This study had a few limitations. First, this study had a cross-sectional design. Data on only the current and previous therapies were collected for each patient; data on the total number of therapy lines or all the treatments a patient had received were not collected. Second, the data for this study were drawn from an oncology database that collects data from treatment facilities in five European countries with distinctive drug access programs and reimbursement policies for oncology therapies. Therefore, treatment patterns observed in this study may not be generalizable to other European countries. Third, physician participation in the ONC-D database is voluntary and limited to those physicians who are able to collect and report data according to the database-specified criteria and are able to obtain the necessary research approvals from the participating physician's hospital or treatment center. It may have been easier to gain approvals from some participating centers, e.g., from office-based practitioners than from other facilities such as large general hospitals. Hence, a selection bias may have been introduced both at the physician- and patient-level and patients in the ONC-D database may not have been drawn from a fully representative group of the general SCLC population. Fourthly, to ensure a representative patient population, the documenting physicians were selected based on stratified random sampling of the distribution of physician specialties that is representative of the specialties that care for SCLC patients in the country; however, documenting physicians who voluntarily participate in ONC-D may differ from physicians who do not participate. It is, therefore, possible that patients who were treated and managed by oncologists are overrepresented in ONC-D with consequent underrepresentation of patients managed by physicians from other specialties, e.g., pulmonologists.
Conclusion
In conclusion, our analysis of a large, real-world dataset revealed that the recently approved anti-PD-L1 immunotherapies, atezolizumab and durvalumab, have changed the treatment patterns for ES-SCLC in the EU5, though the extent of adoption of these therapies varied widely among countries. Large-scale, long-term, real-world studies are needed to better understand whether these newer therapies have had a beneficial impact on clinical outcomes in ES-SCLC. This study shows that topotecan continues to be the 2L treatment of choice for ES-SCLC in EU5 regardless of platinum sensitivity. There still exists an unmet need for new, safe and effective therapies for the treatment of relapsed ES-SCLC that can further improve treatment outcomes.
Supplementary Material
Funding Statement
Medical writing support was provided by S Raghuraman of Cactus Life Sciences (part of Cactus Communications) funded by Amgen Inc. and J Sayyah of Amgen Inc.
Author contributions
N Reguart contributed to data interpretation and manuscript revision. M Pérol contributed to data interpretation and manuscript revision. D Cortinovis contributed to data interpretation and manuscript revision. S Puntis contributed to the conception and design of the study, data analysis, data interpretation, drafting and revision of the manuscript. K Öhrling contributed to the conception and design of the work, data interpretation, drafting and revision of the paper. O Archangelidi contributed to the conception and design of the study, data acquisition, data analysis and revision of the paper. KS Louie contributed to the conception and design of the study, data analysis, data interpretation, drafting and revision of the manuscript. F Blackhall contributed to data interpretation and revision of the manuscript. M Sebastian contributed to data interpretation and revision of the manuscript. All authors have provided their approval of the final version to be submitted for publication and agree to be accountable for all aspects of the work.
Financial disclosure
This study was funded by Amgen Inc. N Reguart reports advisory board activities for Amgen Inc., AstraZeneca, Bayer, Janssen, MSD, Roche, Novartis, Sanofi and Takeda, research funding from MSD, IIT, Pfizer, and Novartis, speaker's bureau activities for Amgen Inc., Sanofi, Roche, AstraZeneca, Novartis, Bristol Myers Squibb, and MSD and membership on a board or advisory committee in MSD. M Pérol reports consultancy for AstraZeneca, Roche, Bristol-Myers Squibb and Merck Sharp & Dohme, research funding from Roche and AstraZeneca, honoraria from AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharpe & Dohme, Amgen Inc. participation in speaker's bureau activities for AstraZeneca, Amgen Inc., Bristol-Myers Squibb, Merck Sharp & Dohme, and membership on a board or Advisory committee in Astra Zeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, and Amgen Inc., D Cortinovis reports participation in speaker bureau activities for Roche, MSD, BMS, Takeda, Sanofi Genzyme, Boehringer Ingelheim, AstraZeneca, and Amgen Inc., S Puntis, K Öhrling, and O Archangelidi are employed by and hold shares in Amgen Inc., KS Louie is a former employee of and holds stocks in Amgen Inc. and is an employee of and holds stock options / shares in BioMarin Pharmaceutical Inc., F Blackhall reports consultancy for Amgen Inc. and AstraZeneca, research funding from Amgen Inc., AstraZeneca, Roche, and Boehringer Ingelheim, participation in speaker's bureau activities for Amgen Inc., and membership on a board or advisory committee in Amgen Inc. and AstraZeneca. M Sebastian has received grants from AstraZeneca, personal fees from Amgen, AstraZeneca, BioNTech, Bristol Myers Squibb, Boehringer Ingelheim, CureVac, Janssen-Cilag, Lilly, Merck-Serono, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis and Takeda, and non-financial support from Bristol Myers Squibb, Pfizer and Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
Medical writing support was provided by S Raghuraman of Cactus Life Sciences (part of Cactus Communications) funded by Amgen Inc. and J Sayyah of Amgen Inc.
Ethical conduct of research
Institutional review board approval and informed consent from individual patients were not required due to the retrospective nature of the study and the use of de-identified patient data.
Data sharing statement
The data that support the findings of this study are available from IQVIA, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are however available from the authors upon reasonable request and with the permission of IQVIA.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sung H, Ferlay J, Siegel RLet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 7(1), 3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency . Public summary of opinion on orphan designation: lurbinectedin for the treatment of small cell lung cancer (2019). Available at: www.ema.europa.eu/en/documents/orphan-designation/eu/3/19/2143-public-summary-opinion-orphan-designation-lurbinectedin-treatment-small-cell-lung-cancer_en.pdf (Accessed November 2022).
- 4.Dingemans AC, Fruh M, Ardizzoni Aet al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 32(7), 839–853 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Most recent clinical practice guidelines for small cell lung cancer published by the ESMO.
- 5.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 121(5), 664–672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer. Net . Lung Cancer-Small Cell: statistics. Updated February 2022. www.cancer.net/cancer-types/lung-cancer-small-cell/statistics (Accessed November 2022).
- 7.Huber RM, Tufman A. Update on small cell lung cancer management. Breathe. 8(4), 314–330 (2012). [Google Scholar]
- 8.DiBonaventura MD, Shah-Manek B, Higginbottom K, Penrod JR, Yuan Y. Adherence to recommended clinical guidelines in extensive disease small-cell lung cancer across the US, Europe, and Japan. Ther Clin Risk Manag. 15, 355–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comparison of real-world treament patterns among patients diagnosed with extensive-stage small-cell lung cancer in the USA, France, Germany, Italy, Spain and the UK and Japan between 2014 and 2016.
- 9.Oronsky B, Reid TR, Oronsky A, Carter CA. What's new in SCLC? A review. Neoplasia. 19(10), 842–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Small Cell Lung Cancer (Version 2.2023). www.nccn.org/professionals/physician_gls/pdf/sclc.pdf (Accessed November 15, 2022).
- 11.U.S Food & Drug Administration . FDA grants accelerated approval to lurbinectedin for metastatic small cell lung cancer (2020). www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-lurbinectedin-metastatic-small-cell-lung-cancer
- 12.PharmaMar . PharmaMar and Jazz Pharmaceuticals announce initiation of confirmatory phase III clinical trial of Zepzelca® (lurbinectedin) for the treatment of patients with relapsed Small Cell Lung Cancer (2021). https://pharmamar.com/en/pharmamar-and-jazz-pharmaceuticals-announce-initiation-of-confirmatory-phase-iii-clinical-trial-of-zepzelca-lurbinectedin-for-the-treatment-of-patients-with-relapsed-small-cell-lung-cancer
- 13.Roche . European Commission approves Roche's Tecentriq in combination with chemotherapy for the initial treatment of people with extensive-stage small cell lung cancer. Published September 06, 2019. https://www.roche.com/media/releases/med-cor-2019-09-06b (Accessed November 2022).
- 14.AstraZeneca . Imfinzi approved in the EU for the treatment of extensive-stage small cell lung cancer. Published September 1, 2020. www.astrazeneca.com/media-centre/press-releases/2020/imfinzi-approved-in-EU-for-small-cell-lung-cancer.html# (Accessed 1 October 2022).
- 15.Domine M, Moran T, Isla Det al. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol. 22(2), 245–255 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Cramer-van der Welle CM, Schramel F, van Leeuwen AS, Groen HJM, van de Garde EMW, Santeon SSG. Real-world treatment patterns and outcomes of patients with extensive disease small cell lung cancer. Eur J Cancer Care (Engl). 29(5), e13250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser SS, Bar J, Kan Iet al. Real world analysis of small cell lung cancer patients: prognostic factors and treatment outcomes. Curr Oncol. 28(1), 317–331 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganti AK, Katranji K, Seal BS, Brannman L. Treatment patterns in extensive-stage small cell lung cancer: a retrospective real-world data study. J. Clin. Oncol. 38(Suppl. 29), 288–288 (2020).31940444 [Google Scholar]
- 19.Alymova S, Kostev K, Casey Vet al. Evaluation of the representativeness of the German Oncology Dynamics dataset. Int. J. Clin. Pharmacol. Ther. 60(5), 207–216 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Committee on Strategies for Responsible Sharing of Clinical Trial Data; Board on Health Sciences Policy; Institute of Medicine . Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. National Academies Press (US); Appendix B, Concepts and Methods for De-identifying Clinical Trial Data, Washington (DC) (Published 20 April 2015).Available from: www.ncbi.nlm.nih.gov/books/NBK285994/ (Accessed 28 November 2022). [PubMed] [Google Scholar]
- 21.Rodriguez-Lescure A, de la Pena FA, Aranda Eet al. Study of the Spanish Society of Medical Oncology (SEOM) on the access to oncology drugs and predictive biomarkers in Spain. Clin Transl Oncol. 22(12), 2253–2263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliva-Moreno J, Puig-Junoy J, Trapero-Bertran M, Epstein D, Pinyol C, Sacristan JA. Economic evaluation for pricing and reimbursement of new drugs in Spain: fable or desideratum? Value Health. 23(1), 25–31 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Uyl-de Groot CA, Heine R, Krol M, Verweij J. Unequal access to newly registered cancer drugs leads to potential loss of life-years in Europe. Cancers (Basel). 12(8), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organisation for Economic Co-operation and Development . Addressing challenges in access to oncology medicines. analytical Report Published: (2020). https://www.oecd.org/health/health-systems/Addressing-Challenges-in-Access-to-Oncology-Medicines-Analytical-Report.pdf (Accessed 29 November 2022). [Google Scholar]
- 25.Pham FY, Jacquet E, Taleb Aet al. Survival, cost and added therapeutic benefit of drugs granted early access through the French temporary authorization for use program in solid tumors from 2009 to 2019. Int. J. Cancer 151(8), 1345–1354 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency . Tecentriq (atezolizumab).Summary of Opinion (Post Authorization) (2019). www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-tecentriq-ii/0019_en.pdf [Google Scholar]
- 27.Lawlor R, Wilsdon T, Darquennes Eet al. Accelerating patient access to oncology medicines with multiple indications in Europe. J Mark Access Health Policy. 9(1), 1964791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubloy A. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res. 14, 469 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Medicines Agency . Hycamtin: product information (2010). Available at: www.ema.europa.eu/en/documents/product-information/hycamtin-epar-product-information_en.pdf (Accessed 19 November 2022).
- 30.Das M, Padda SK, Weiss J, Owonikoko TK. Advances in treatment of recurrent small cell lung cancer (SCLC): insights for optimizing patient outcomes from an expert roundtable discussion. Adv Ther. 38(11), 5431–5451 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horita N, Yamamoto M, Sato Tet al. Topotecan for relapsed small-cell lung cancer: systematic review and meta-analysis of 1347 patients. Sci Rep. 5, 15437 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trigo J, Subbiah V, Besse Bet al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 21(5), 645–654 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Aix SP, Ciuleanu TE, Navarro Aet al. Combination lurbinectedin and doxorubicin versus physician's choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): a multicentre, randomised, open-label, phase 3 trial. Lancet Respir Med. 11(1), 74–86 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Wermke M, Felip E, Gambardella Vet al. Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Future Oncol. 18(24), 2639–2649 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Johnson ML, Dy GK, Mamdani Het al. Interim results of an ongoing phase 1/2 study of HPN328, a tri-specific half-life extended DLL3-targeting T-cell engager, in patients with small cell lung cancer and other neuroendocrine cancers. Poster presented at: American Society of Clinical Oncology Annual Meeting. Chicago, USA: (June 3–7, 2022). [Google Scholar]
- 36.Safety, tolerability and pharmacokinetics study of QLS31904 in patients with advanced solid tumors. ClinicalTrials.gov Identifier: NCT05461287. Updated November 10, 2022. https://clinicaltrials.gov/ct2/show/NCT05461287 (Accessed 15 November 2022).
- 37.Borghaei HP, Paz-Ares L, Johnson Met al. Phase 1 updated exploration and first expansion data for DLL3-targeted T-cell engager tarlatamab in small cell lung cancer. J Thorac Oncol. 17(9), S33 (2022). Conference Abstract. [Google Scholar]
- 38.AMG 757 and AMG 404 in subjects with small cell lung cancer (SCLC). ClinicalTrials.gov Identifier: NCT04885998 Updated September 29, 2022. https://clinicaltrials.gov/ct2/show/NCT04885998 (Accessed 15 November 2022).
- 39.First-line tarlatamab in combination with carboplatin, etoposide, and PD-L1 inhibitor in subjects with extensive stage small cell lung cancer (ES-SCLC). ClinicalTrials.gov Identifier: NCT05361395 Updated November 15, 2022. https://clinicaltrials.gov/ct2/show/NCT05361395 (Accessed 15 November 2022).
- 40.Kalemkerian GP, Loo BW, Akerley Wet al. NCCN guidelines insights: small cell lung cancer, Version 2.2018. J Natl Compr Canc Netw. 16(10), 1171–1182 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Fruh M, De Ruysscher D, Popat Set al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24(Suppl. 6), vi99–105 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Horn L, Mansfield AS, Szczesna Aet al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 379(23), 2220–2229 (2018). [DOI] [PubMed] [Google Scholar]; • Describes findings from the pivotal IMpower133 trial that formed the basis for approval of atezolizumab for small-cell lung cancer by the US FDA and the EMA.
- 43.Paz-Ares L, Dvorkin M, Chen Yet al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212), 1929–1939 (2019). [DOI] [PubMed] [Google Scholar]; • Describes findings from the pivotal CASPIAN trial that formed for the basis for approval of durvalumab for small-cell lung cancer by the FDA and the EMA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


