Abstract
Background: The ARROW study demonstrated that once-weekly carfilzomib and dexamethasone (wKd) therapy significantly prolonged progression-free survival compared with twice-weekly carfilzomib and dexamethasone therapy in relapsed or refractory multiple myeloma patients. Aim: To describe the treatment patterns, effectiveness and safety of wKd therapy in real-world settings in Japan. Methods: We investigated data from the medical records of 126 Japanese patients with relapsed or refractory multiple myeloma. Results: The overall response rate was 66.3%. The median progression-free survival was 9.5 months. The incidence of treatment-emergent adverse events of any grade and grade ≥3 were 45.8 and 20.8%, respectively. Conclusion: There were no new or unexpected safety signals in this study. This study demonstrated the effectiveness and safety profiles of wKd therapy in Japan.
Keywords: : ARROW study, carfilzomib, Japanese, real-world, relapsed or refractory multiple myeloma
Plain language summary
Carfilzomib became available for daily clinical practice as a drug for cancer of bone marrow (multiple myeloma) that comes back or does not respond to previous drug (relapsed or refractory). This drug was approved in the USA in 2012, and in Japan in 2016. In this study, we looked at how once-weekly carfilzomib works and how safe it is in real-life situations in Japan. We screened 126 patients with relapsed or refractory multiple myeloma in Japan. The median age of the patients was 70 years, with 25% being over 75 years. This study also included some patients who were not in the best overall health, had a history of many treatments or had heart complications. In 66.3% of patients, the cancer had disappeared or the extent of the cancer had reduced after treatment. Side effects and serious side effects occurred in 45.8 and 14.2% of patients, respectively. The most common side effects were low levels of blood platelets (9.2%), high blood pressure (5.8%), loose or watery stools (5.0%), fever (5.0%), and low levels of red blood cells (4.2%). Heart disorders occurred in five patients. But all patients recovered or improved with treatment such as blood pressure lowering drugs and diuretics. These results showed that once-weekly carfilzomib works well and is safe in real-world settings in Japan. This information can help us think about how to pick the right patients and handle heart disease risks when using carfilzomib treatment.
Plain language summary
Summary points.
Once-weekly carfilzomib in combination with dexamethasone (wKd) therapy is approved for the treatment of relapsed or refractory multiple myeloma (RRMM).
Although the ARROW study demonstrated that wKd therapy significantly prolonged progression-free survival compared with twice-weekly carfilzomib and dexamethasone (20/27 mg/m2) therapy in RRMM patients, there are few reports based on real-world data for wKd therapy in Japanese patients.
This Weekly-CAR study is an observational study that evaluated real-world clinical data on wKd treatment in Japanese patients with RRMM.
In this study, the overall response rate and median progression-free survival were 66.3% and 9.5 months, respectively.
There were no new or unexpected safety signals in this study.
Additionally, this study demonstrated the effectiveness and safety in patients who were not recruitable to the clinical trial of wKd therapy, for example those with an Eastern Cooperative Oncology Group performance status ≥2, or those with 1 or ≥4 prior regimens.
These data demonstrated the effectiveness and safety profiles of wKd therapy in real-world settings in Japan.
Multiple myeloma (MM) is a hematologic malignancy caused by the abnormal differentiation and proliferation of plasma cells. MM is a disease that is characterized by various clinical symptoms such as elevated serum calcium, renal and hematopoietic disorders.
Although therapy with melphalan and prednisone has been the standard treatment for patients with MM, new therapies such as the immunomodulatory agent lenalidomide and the proteasome inhibitor bortezomib were shown to improve survival in the 2000s [1]. Bortezomib is the first proteasome inhibitor approved for treatment of patients with MM and works by inhibiting chymotrypsin-like and caspase-like enzymes [2]. Conversely, bortezomib has occasionally been reported to cause peripheral neuropathy [3]. Carfilzomib is a second-generation proteasome inhibitor, which irreversibly and highly selectively inhibits chymotrypsin-like activity [2,4]. Carfilzomib can induce tumor cell death through accumulation of ubiquitinated proteins [4].
In July 2012, the US FDA approved carfilzomib for the treatment of patients with MM who have received at least two prior therapies including bortezomib and an immunomodulatory agent, and who had shown disease progression on or within 60 days of completion of their last therapy [5].
In Japan, carfilzomib in combination with lenalidomide and dexamethasone (KRd) therapy was approved in July 2016 for the treatment of patients with relapsed or refractory multiple myeloma (RRMM), based on results of the ASPIRE study and a phase I study in Japan [6,7].
In recent years, triplet regimens including an immunomodulator such as lenalidomide or pomalidomide have become mainstream in the treatment of MM. Lenalidomide is commonly used in triplet regimens, but it is well known that long-term use of lenalidomide results in resistance to this agent. In addition, there are reports that extension of the immunomodulator-free period before restarting regimens containing an immunomodulator can be expected to prolong progression-free survival (PFS) [8]. In such cases, the use of regimens that do not contain lenalidomide is a possible solution.
Carfilzomib in combination with dexamethasone therapy was approved for treatment of patients with RRMM as a twice-weekly carfilzomib 20/56 mg/m2 (20 mg/m2 initial dose; 56 mg/m2 thereafter) dosing regimen (twKd [20/56 mg/m2]) in May 2017 based on the ENDEAVOR study [9]. However, as carfilzomib in combination with dexamethasone therapy is a twice-weekly dosing regimen, it can be burdensome for patients because carfilzomib is generally administered as an intravenous infusion at an outpatient institution on 2 consecutive days for 3 weeks out of a 4-week treatment cycle.
The CHAMPION-1 study was a phase I/II study to establish the maximum tolerated dose of once-weekly carfilzomib therapy in RRMM and it determined that the maximum tolerated dose of carfilzomib was 70 mg/m2 [10]. The ARROW study was a multicenter, open-label phase III trial. The study demonstrated that once-weekly carfilzomib 20/70 mg/m2 (20 mg/m2 initial dose; 70 mg/m2 thereafter) and dexamethasone (wKd) therapy significantly prolonged PFS compared with twice-weekly carfilzomib 20/27 mg/m2 (twKd [20/27 mg/m2]) therapy [11]. In Japan, wKd therapy was approved for the treatment of patients with RRMM in November 2019, based on the results of the ARROW study.
Since wKd therapy reduces the burden of having to make hospital visits, it becomes a convenient treatment option for RRMM patients, and more patients are expected to receive wKd therapy than twKd (20/56 mg/m2) therapy in clinical practice.
The ARROW study was conducted as a global phase III study and 478 patients were registered. However, the number of Japanese patients in this study was just 26 [12]. There are also few reports containing real-world data on carfilzomib, and just a small number of cases included wKd therapy [13,14]. In clinical practice, the drug is often used in patients with various backgrounds that are not included in clinical trials, such as patients with poor Eastern Cooperative Oncology Group performance status (ECOG-PS) or those with many prior treatment regimens. Therefore, dissemination of information on the treatment pattern, and on the effectiveness and safety of wKd therapy in clinical practice would be expected to contribute to more appropriate use in the treatment of RRMM. Consequently, in this Weekly-CAR study, we investigated the real-world effectiveness, safety and treatment of wKd therapy at 18 sites in Japan.
Materials & methods
Study design, patients
The Weekly-CAR study is a multicenter, observational study in patients with RRMM in a real-world setting in Japan. Data related to patients receiving wKd therapy were obtained from clinical records at 18 sites.
The inclusion criteria were as follows: age ≥18 years at the time of registration, patients with RRMM, wKd therapy started between November 2019 and December 2020. The exclusion criteria were as follows: hypersensitivity to carfilzomib or dexamethasone, pregnant patients, initial dose of carfilzomib other than 20 mg/m2, prior carfilzomib treatment within 6 months before starting wKd therapy.
This study was registered in University Hospital Medical Information Network, registry No. UMIN000043049.
Treatment patterns
Treatment pattern outcomes included patient characteristics, dosage of carfilzomib and dexamethasone, number of cycles of wKd therapy, and reasons for discontinuation of wKd therapy. According to the approved dosage and administration regimen, patients received a 30 min intravenous infusion of carfilzomib 20/70 mg/m2 on days 1, 8 and 15 of each 28-day cycle and dexamethasone (40 mg oral or intravenous infusion) on days 1, 8, 15 and 22 of the cycle. This study did not collect data on supportive therapy, such as anticoagulants or infection prevention drugs.
Effectiveness evaluations
Effectiveness included overall response rate (ORR) – defined as the proportion of patients who achieved stringent complete response (sCR), complete response (CR), very good partial response (VGPR) or partial response (PR), according to the International Myeloma Working Group Uniform Response Criteria [15,16] – PFS and overall survival (OS). PFS was defined as the period up to the date of exacerbation or the date of death from any cause, whichever was earlier. Patients who survived and were not judged to have progressed were censored on the last day on which no progression was confirmed clinically (final day of confirmation of PFS) or the start date of the next treatment, whichever came first. OS was defined as the time from start of wKd therapy to the date of death from any cause. Other outcomes included PFS and OS rate at 12 months of treatment; best response rate of sCR, CR, VGPR, PR, minimal response according to the European Group for Blood and Bone Marrow Transplant, stable disease and progressive disease; ≥CR; ≥VGPR; disease control rate (defined as the proportion of patients who achieved sCR, CR, VGPR, PR or stable disease lasting for over 8 weeks); time to response (defined as the time from start of wKd therapy to achievement of PR or better); time to next treatment (TTNT; defined as the time from start of wKd therapy to the start of subsequent treatments). This study collected data from medical records, in which the data assessed by the old International Myeloma Working Group response criteria and European Group for Blood and Bone Marrow Transplant criteria were included.
Safety evaluations
Adverse events (AEs) and treatment-emergent adverse events (TEAEs) were extracted and analyzed from medical records from each center. AEs were coded in accordance with MedDRA version 25.0. TEAEs related to cardiac disorders were also analyzed. All AEs and TEAEs were classified and graded according to the Common Terminology Criteria for Adverse Events version 4.0. Serious adverse events (SAEs) were also extracted and analyzed from medical records. SAEs encompassed events leading to death, posing a threat to life, requiring hospitalization for treatment or an extension of hospitalization or care, resulting in persistent or serious impairment or dysfunction, causing congenital abnormalities, or involving other significant medical events.
Statistical methods
The target number for enrollment was 150 patients. Since this is an observational study, sample size was not statistically determined. It would be possible to evaluate the similarity of ORR between this study and the ARROW study within 10% of the error rate (confidence level of 95%) with data from approximately 150 patients.
Patients who did not meet any of the exclusion criteria were included in the safety analysis set. The effectiveness analysis set excluded patients who were administered treatment twice a week during the first cycle, violated the wKd dosing schedule or did not meet the inclusion criteria. Patient characteristics, treatment pattern, effectiveness and safety outcomes were assessed using descriptive statistics. Subgroup analysis was performed on the ORR and TEAEs, and Fisher's exact test (two groups with unpaired nominal data) and the Cochran–Armitage test were used to compare between subgroups. Significance was fixed at a two-sided level of 5%. Summary statistics of relative dose intensity (RDI) were calculated based on carfilzomib and dexamethasone dosage. For PFS and OS, the median time to event was calculated using the Kaplan–Meier method. The hazard ratio (HR) and corresponding 95% CI were estimated using a Cox proportional hazard model stratified by the subgroup categories.
All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., NC, USA) for Windows.
Results
Treatment patterns
We screened 126 patients with RRMM who started new wKd treatment between November 2019 and December 2020 at 18 sites in Japan. After excluding patients who were ineligible for this study, 120 and 114 patients were included in the safety and effectiveness analysis sets, respectively (Figure 1).
Figure 1.
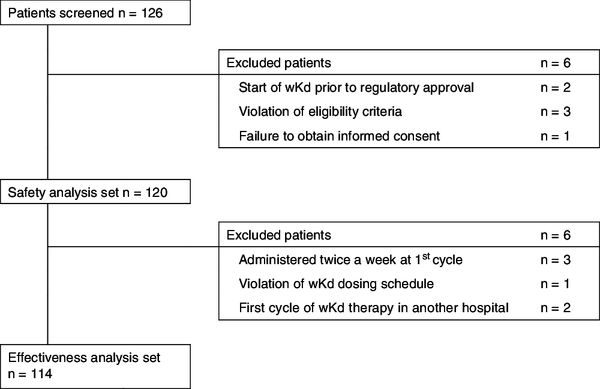
Patients disposition in the Weekly-CAR study.
wKd: Once-weekly carfilzomib and dexamethasone.
The baseline characteristics of the patients are shown in Table 1. The proportion of males was 48.3%. The median age was 70.0 years (range: 44–89). The proportion of patients aged ≥75 years was 25.0%. The most common MM subtype was IgG (57.5%), followed by Bence Jones type (30.8%). IgA type was observed in 15.0%. High-risk cytogenetics having at least one of del(17p), t(4;14) or t(14;16) were observed in 29.2% of patients. The proportion of patients with complications was 50.8%, among which cardiac, renal and pulmonary disorders, and hypertension were seen in 8.3, 27.5, 5.8 and 35.8% of patients, respectively. The proportion of patients who received 1, 2–3 and ≥4 prior regimens was 17.5, 34.2 and 48.3%, respectively. The proportion of patients with bortezomib, lenalidomide and autologous stem cell transplantation as prior treatments was 90.8, 88.3 and 35.8%, respectively. The proportion of patients refractory to bortezomib, lenalidomide and both was 42.5, 51.7 and 28.3%, respectively. The ECOG-PS at baseline was 0–1 in 56.7%, ≥2 in 10.8% and unknown in 32.5% of patients.
Table 1.
Patient demographic and clinical characteristics in the safety analysis set.
| Characteristics | n (%) |
|---|---|
| Total | 120 |
| Sex | |
| Male | 58 (48.3) |
| Female | 62 (51.7) |
| Age (years) | |
| Median (range) | 70.0 (44–89) |
| <65 | 33 (27.5) |
| ≥65, <75 | 57 (47.5) |
| ≥75 | 30 (25.0) |
| Multiple myeloma subtypes† | |
| IgG | 69 (57.5) |
| IgA | 18 (15.0) |
| IgD | 3 (2.5) |
| IgM | 1 (0.8) |
| Bence Jones | 37 (30.8) |
| Nonsecretory | 1 (0.8) |
| Eastern Cooperative Oncology Group performance status | |
| 0–1 | 68 (56.7) |
| ≥2 | 13 (10.8) |
| Unknown | 39 (32.5) |
| Complication, yes | 61 (50.8) |
| Cardiac complications | 10 (8.3) |
| Renal complications | 33 (27.5) |
| Pulmonary complications | 7 (5.8) |
| Hypertension | 43 (35.8) |
| Cytogenetics | |
| del(17p), yes | 14 (11.7) |
| t(4;14), yes | 19 (15.8) |
| t(11;14), yes | 21 (17.5) |
| t(14;16), yes | 8 (6.7) |
| High risk (at least one of del[17p], t[4;14], t[14;16]), yes | 35 (29.2) |
| Number of prior treatments | |
| 1 | 21 (17.5) |
| 2–3 | 41 (34.2) |
| ≥4 | 58 (48.3) |
| Prior bortezomib or lenalidomide treatment, yes | |
| Bortezomib | 109 (90.8) |
| Lenalidomide | 106 (88.3) |
| Refractory | |
| Bortezomib | 51 (42.5) |
| Lenalidomide | 62 (51.7) |
| Both lenalidomide and bortezomib | 34 (28.3) |
| Autologous stem cell transplantation, yes | 43 (35.8) |
| Best response to prior treatment | |
| Stringent complete response | 12 (10.0) |
| Complete response | 5 (4.2) |
| Very good partial response | 14 (11.7) |
| Partial response | 31 (25.8) |
| Stable disease | 30 (25.0) |
| Progressive disease | 27 (22.5) |
| Unknown | 1 (0.8) |
| Number of cycles of once-weekly carfilzomib and dexamethasone therapy | |
| Median (range) | 4.0 (1–22) |
| Reasons for starting once-weekly carfilzomib and dexamethasone therapy | |
| Relapse | 19 (15.8) |
| Refractory | 64 (53.3) |
| Other | 37 (30.8) |
| Observational period (month) | |
| Median (range) | 17.1 (1–29) |
Some patients had multiple subtypes.
The median number of cycles of wKd therapy was four (range: 1–22). The median observational period was 17.1 months (range: 1–29 months).
The mean RDI of carfilzomib and dexamethasone was 79.8 (standard deviation [SD]: 21.4) and 51.0% (SD: 30.2), respectively. The mean RDI of carfilzomib and dexamethasone in patients aged ≥75 years was 71.5 (SD: 24.0) and 37.6% (SD: 23.6), respectively, and those in patients aged <75 years were 82.6 (SD: 19.8) and 55.6% (SD: 31.0), respectively (Table 2).
Table 2.
The relative dose intensity of carfilzomib and dexamethasone.
| Relative dose intensity | Mean % (standard deviation) |
|---|---|
| Carfilzomib | |
| All | 79.8 (21.4) |
| <75 | 82.6 (19.8) |
| ≥75 | 71.5 (24.0) |
| Dexamethasone | |
| All | 51.0 (30.2) |
| <75 | 55.6 (31.0) |
| ≥75 | 37.6 (23.6) |
Discontinuation of wKd therapy was observed in 113 patients (Table 3). The reasons were disease progression (45.1%), followed by AEs or TEAEs (23.0%) and termination due to confirmation of effectiveness (17.7%).
Table 3.
Reasons for discontinuation of once-weekly carfilzomib and dexamethasone therapy.
| Reason for treatment discontinuation | n (%) |
|---|---|
| Total | 113 |
| Disease progression | 51 (45.1) |
| AEs or treatment-emergent AEs (including AEs leading to death) | 26 (23.0) |
| Termination due to confirmation of effectiveness | 20 (17.7) |
| Patient request | 3 (2.7) |
| Death | 1 (0.9) |
| Other reasons | 12 (10.6) |
AE: Adverse event.
Effectiveness
Best response is shown in Table 4. The ORR was 66.3%. The proportion of patients with sCR and CR was 12.5 and 4.8%, respectively. The proportion of ≥CR and ≥VGPR was 17.3 and 33.7%, respectively. The disease control rate was 70.2%.
Table 4.
Best response with once-weekly carfilzomib and dexamethasone therapy.
| Best response | n† (%) |
|---|---|
| Total | 104 |
| sCR | 13 (12.5) |
| CR | 5 (4.8) |
| VGPR | 17 (16.3) |
| PR | 34 (32.7) |
| Minimal response | 4 (3.8) |
| Stable disease | 13 (12.5) |
| Progressive disease | 18 (17.3) |
| Overall response rate (sCR, CR, VGPR or PR) | 69 (66.3) |
| ≥CR (sCR or CR) | 18 (17.3) |
| ≥VGPR (sCR, CR or VGPR) | 35 (33.7) |
| Disease control rate (sCR, CR, VGPR, PR, minimal response or stable disease lasting over 8 weeks) | 73 (70.2) |
Best response was analyzed after excluding ten patients without medical record that contained relevant information.
CR: Complete response; PR: Partial response; sCR: Stringent complete response; VGPR: Very good partial response.
Baseline factors associated with ORR are shown in Supplementary Table 1. The ORR for patients aged ≥75 years was 57.7%, which was not significantly different from that (69.2%) for patients aged <75 years. No association was observed between high-risk cytogenetics, refractoriness to bortezomib, lenalidomide or both, or ECOG-PS and ORR. The ORRs for patients with 1 and ≥4 prior regimens, who would not have been enrolled in the ARROW study, were 90.5 and 57.1%, respectively. While no statistical analysis was conducted for individual comparisons between categories, the ORR was higher for patients with fewer prior treatments.
The median PFS was 9.5 months (95% CI: 6.3–12.4), and the median OS was not reached (Figure 2). The PFS rate and OS rate at 12 months were 38.9 (95% CI: 27.1–50.5) and 82.8% (95% CI: 74.3–88.7), respectively.
Figure 2.
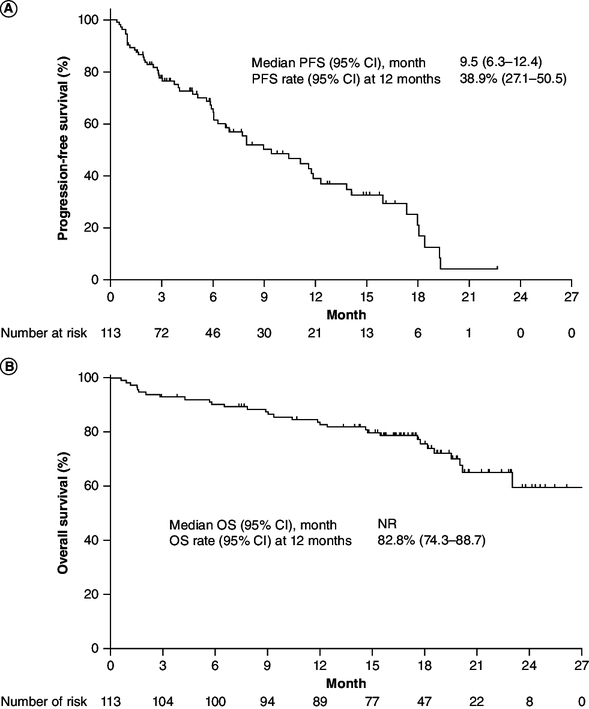
Kaplan–Meier plots.
(A) PFS and (B) OS. PFS and OS were analyzed after excluding one patient whose data were not recorded in the medical records.
NR: Not reached; OS: Overall survival; PFS: Progression-free survival.
In the subgroup analysis for PFS, the median PFS for patients aged ≥75 years and <75 years was 9.0 (95% CI: 3.8–12.0) and 9.5 months (95% CI: 6.1–14.2), respectively (Figure 3). There was no significant difference between these subgroups (HR: 1.205; 95% CI: 0.7–2.1; p = 0.517). Baseline factors including high-risk cytogenetics and refractoriness to bortezomib, lenalidomide or both were not associated with PFS (Figure 3). The PFS for patients with 1 or ≥4 prior regimens, categories not enrolled in the ARROW study, was not significantly different from that for patients with 2–3 prior regimens (Supplementary Table 2). The PFS for patients with ECOG-PS ≥2, also not enrolled in the ARROW study, was not significantly different from that for patients with ECOG-PS 0–1.
Figure 3.
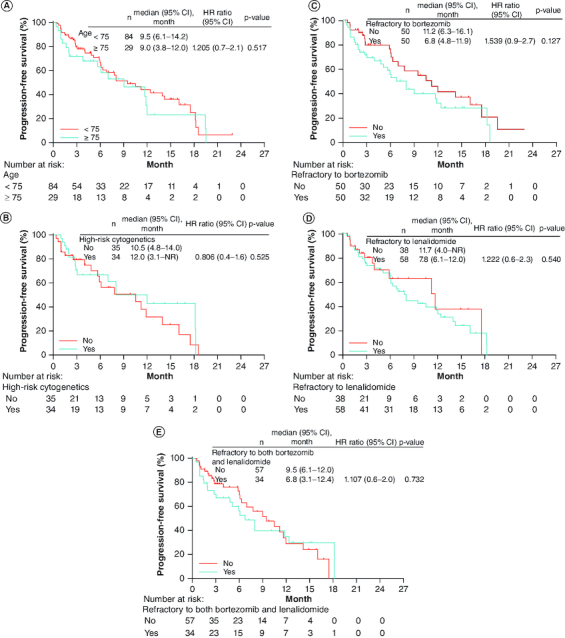
Kaplan–Meier plot for progression-free survival by subgroup.
(A) Subgroup of patients aged <75 and ≥75 years, (B) subgroup of patients with or without high-risk cytogenetics, (C) subgroup of patients refractory to bortezomib, or not, (D) subgroup of patients refractory to lenalidomide, or not and (E) subgroup of patients refractory to both bortezomib and lenalidomide, or not.
HR: Hazard ratio; NR: Not reached.
For patients with RDI ≥80 and <80%, the ORR was 71.2 and 57.9%, respectively, and the median PFS was 9.5 (95% CI: 6.1–14.0) and 8.0 months (95% CI: 4.8–14.2), respectively. There was no significant difference between these subgroups (HR: 0.904; 95% CI: 0.5–1.5; p = 0.709).
The median time to response and TTNT for wKd therapy were 32.0 (range: 8–500) and 149.0 days (range: 16–558), respectively.
Safety
The incidences of any grade, grade ≥3 and SAEs were 48.3, 24.2 and 16.7%, respectively. The incidence of AEs leading to discontinuation was 21.7%. The incidences of any grade, grade ≥3 and serious TEAEs were 45.8 and 20.8 and 14.2%, respectively (Table 5). Two deaths related to AEs were observed. In one case, relevance to carfilzomib treatment could not be ruled out, but the specific cause was unknown. In the other case, death was ruled out as being related to carfilzomib – the patient was adjudicated to have died from COVID-19.
Table 5.
Adverse events and treatment-emergent adverse events with once-weekly carfilzomib and dexamethasone therapy: the safety analysis set (n = 120).
| Adverse events | Number of patients (%) |
|---|---|
| AEs | |
| All grades | 58 (48.3) |
| Grade 3 or higher | 29 (24.2) |
| Serious AEs | 20 (16.7) |
| Leading to death | 2 (1.7) |
| Leading to discontinuation | 26 (21.7) |
| Leading to drug interruption | 12 (10.0) |
| Treatment-emergent AEs | |
| All grades | 55 (45.8) |
| Grade 3 or higher | 25 (20.8) |
| Serious treatment-emergent AEs | 17 (14.2) |
AE: Adverse events.
Table 6 shows TEAEs that occurred with a frequency greater than or equal to 2%. The most common TEAEs were thrombocytopenia (9.2%), hypertension (5.8%), diarrhea (5.0%), pyrexia (5.0%) and anemia (4.2%). No baseline factor was associated with the incidence of TEAEs (Supplementary Table 3). The incidences for any grade and grade ≥3 TEAEs were comparable between patients with RDI ≥80% (45.9 and 20.3%, respectively) and <80% (45.7 and 21.7%, respectively).
Table 6.
Treatment-emergent adverse events with once-weekly carfilzomib and dexamethasone therapy.
| Treatment-emergent adverse events | Number of patients (%) | |
|---|---|---|
| All grades† | Grade 3 or higher | |
| Thrombocytopenia | 11 (9.2) | 4 (3.3) |
| Hypertension | 7 (5.8) | 2 (1.7) |
| Diarrhea | 6 (5.0) | 0 (0.0) |
| Pyrexia | 6 (5.0) | 0 (0.0) |
| Anemia | 5 (4.2) | 1 (0.8) |
| Neutropenia | 4 (3.3) | 4 (3.3) |
| Blood creatinine increased | 4 (3.3) | 2 (1.7) |
| Alanine aminotransferase increased | 4 (3.3) | 1 (0.8) |
| Renal disorder | 4 (3.3) | 0 (0.0) |
| Decreased appetite | 3 (2.5) | 1 (0.8) |
| Nausea | 3 (2.5) | 0 (0.0) |
| Aspartate aminotransferase increased | 3 (2.5) | 0 (0.0) |
Incidence of ≥2%.
TEAEs related to cardiac disorders were reported in five patients for any grade and four patients for grade ≥3. One case each of cardiac arrest, cardiac failure, cardiac failure congestive, cardiotoxicity and cardiac disorder was reported. All patients recovered or improved with treatment such as antihypertensives and diuretics (Table 7).
Table 7.
Cardiac disorders with once-weekly carfilzomib and dexamethasone therapy.
| Age | Cardiac complication | Events | Grade | Time to adverse events (days) | Treatment | Outcome | Time to recovery or improvement day (days) |
|---|---|---|---|---|---|---|---|
| 59 | Yes | Cardiac arrest | 4 | 4 | Cardiopulmonary resuscitation, discontinuation of carfilzomib treatment | Recovered | 1 |
| 71 | Yes | Cardiac failure congestive | 4 | 103 | Angiotensin converting enzyme II inhibitor, loop diuretic, discontinuation of carfilzomib treatment | Improvement | 53 |
| 74 | No | Cardiac disorder | 2 | 8 | Vasopressin V2 receptor antagonist, discontinuation of carfilzomib treatment | Recovered | 33 |
| 76 | No | Cardiac failure | 3 | 31 | Oxygen administration, loop diuretic, aldosterone antagonist, discontinuation of carfilzomib treatment | Recovered | 744 |
| 81 | No | Cardiotoxicity | 3 | 70 | Angiotensin1 receptor antagonist, loop diuretic, angiotensin converting enzyme II inhibitor, discontinuation of carfilzomib treatment | Improvement | 331 |
Discussion
The Weekly-CAR study is a multicenter observational study of wKd therapy. Although a considerable amount of evidence has already been reported in support of KRd and twKd (20/56 mg/m2) therapy in real-world settings [13,14,17,18], there are few reports of wKd therapy [13,14]. Furthermore, even in the cited reports, patients treated with wKd therapy comprised just a small portion [13,14]. It is thus necessary to clarify the treatment pattern, effectiveness and safety of wKd therapy in real-world settings. The Weekly-CAR study is the first such study to report real-world treatment pattern, effectiveness and safety of wKd therapy using data from over 100 RRMM patients in Japan.
Regarding the baseline characteristics of this study, the median age of patients and the proportion of patients ≥75 years were similar to those in the ARROW study [11,12]. The proportion of high-risk cytogenetics was higher and the proportion of patients refractory to lenalidomide was lower than in the ARROW study [11,12]. Fewer than half the patients had previously undergone extensive MM treatment, such as receiving ≥4 regimens. On the other hand, approximately 20% of patients had received just 1 prior regimen, and wKd therapy was selected as a second-line therapy in this population. The mean RDI of carfilzomib and dexamethasone was 79.8 and 51.0%, respectively, indicating that doses are being adjusted in clinical practice. In Japan, since the 70 mg/m2 dose of carfilzomib is the first approved dose for wKd therapy and had not been used in clinical practice prior to approval of this therapy, it has been assumed that this dose was used with caution, taking into consideration the patient's condition including age and complications.
The median number of cycles of wKd therapy was four, and it was found that administration was completed relatively early in clinical practice in Japan.
The most common reason for discontinuing carfilzomib in this study was disease progression (45.1%), which was similar to the ARROW study (97/238 patients, 40.8%) [11]. Additionally, in Table 3, termination due to confirmation of effectiveness indicates that the treatment was terminated at the discretion of the physician upon confirmation of its effectiveness. This is distinct from a patient request, as the request for termination came from the physician, not the patient.
In this study, the ORR and median PFS were 66.3% and 9.5 months, respectively, and in the ARROW study the corresponding values were 62.9% and 11.2 months. These results are comparable with respect to ORR, but PFS in this study was shorter than in the ARROW study. This could be explained by the median PFS of 7.0 months for patients with ≥4 prior treatments in this study.
In patients who had received 2–3 prior regimens and received wKd therapy, the median PFS was 11.7 months (95% CI: 6.3–17.5) in this study, 11.2 months (95% CI: 8.6–13.0) in the ARROW study and 14.8 months (95% CI: 7.5–not reached) in the Japanese subgroup of the ARROW study. The ORRs were 64.7, 62.9 and 73.1% in each population, respectively [11,12]. These results demonstrated that wKd therapy was also an effective treatment for Japanese patients. Moreau et al. reported the results of a post hoc analysis comparing twKd (20/56 mg/m2) with wKd therapies using the results from the ENDEAVOR, ARROW and CHAMPION-1 studies. The HR for PFS and the odds ratio for ORR were respectively 0.91 (95% CI: 0.69–1.19; p = 0.47) and 1.12 (95% CI: 0.74–1.69; p = 0.61) [19]. Dimopoulos et al. also reported that carfilzomib had a favorable benefit–risk profile across both twKd (20/56 mg/m2) and wKd therapies in Asian patients with RRMM [20]. Although there are a few reports on twKd (20/56 mg/m2) and wKd therapies in Japanese patients with RRMM in a clinical setting, there are few reports on real-world studies that compared these treatments. Onda et al. reported that the ORR, median PFS and OS rate at 12 months were 62.0%, 7.1 months and 70.9%, respectively, in all Japanese patients who received twKd (20/56 mg/m2) and wKd therapies [13]. They also reported that the ORR of wKd therapy was not inferior to that of twKd (20/56 mg/m2) therapy (77.8 and 58.5%, respectively), and that the incidences of AEs were not significantly different between twKd (20/56 mg/m2) and wKd therapies (cardiovascular AEs were 11.1 and 7.3%, respectively; noncardiovascular AEs were 44.4 and 56.1%, respectively). These results are comparable to those from the current study. Kawaji-Kanayama et al. reported the results from a prospective and observational study of twKd (20/56 mg/m2) therapy in Japan, in which the ORR, median PFS and OS were 73.7%, 9.3 months and 28.1 months, respectively [18]. The median PFS in our study was 9.5 months, and comparable to that of the study by Kawaji-Kanayama et al. The median PFS was shorter than that of twKd (20/56 mg/m2) therapy (17.0 months) in the ENDEAVOR study. These differences are likely attributable to the high percentage of patients with ≥4 prior regimens.
In this study, the TTNT was 140 days whereas PFS was over 9 months. This study collected real-world medical data, and as a result, includes cases where patients were switched to the next treatment before disease progression was observed during carfilzomib treatment. In the PFS analysis, these cases are treated as censored. Therefore, in this study, TTNT was shorter than PFS.
Post-marketing surveillance (PMS) research in Japan demonstrated the effectiveness and safety of KRd and twKd (20/56 mg/m2) therapies in 991 patients with RRMM in real-world settings [17]. The ORRs in the PMS were 46.5% for the total patient cohort, 42.3% for patients with KRd therapy and 52.9% for those with twKd (20/56 mg/m2) therapy. The incidences of any grade and grade ≥3 TEAEs were 66.0 and 46.2%, respectively. The ORR in our study was higher than that in the PMS. Safety outcomes were comparable to each other, and there were no new safety signals in this study.
The proportion of patients aged ≥75 years was 25.0% in this study. The ORR, PFS and safety outcomes did not show significant differences between subgroups. In subgroup analyses of the ARROW study, the ORRs for wKd therapy were 65.2 (≥75 years), 60.0 (65–74 years) and 64.4% (<65 years), and comparable across all age subgroups [21]. The incidences of grade ≥3 TEAEs in the ARROW study were 84.4 (≥75 years), 68.9 (65–74 years) and 59.2% (<65 years), and tended to be higher in the older age subgroup. On the other hand, there was no significant difference in the incidence of any grade TEAEs and grade ≥3 TEAEs between subgroups in this study. This difference may be partially explained by the lower RDI in the older subgroup. The mean RDI in this study for patients aged ≥75 and <75 was 71.5 and 82.6%, respectively. These results suggest that the dose of carfilzomib was adjusted for the older patients by their physicians, because the approved dose of 70 mg/m2 for wKd therapy was considered high. However, in this study, the incidences of TEAEs were comparable between patients with RDI ≥80 and <80%. Therefore, this finding may not be explained simply by RDI alone, and the decisions made by the physicians may have been informed by careful observation. The ORR and PFS of patients with RDI ≥80% tended to be slightly higher than those with RDI <80%, suggesting that dose reductions may affect effectiveness. Recommendations on appropriate use should be made taking into account effectiveness and safety.
In actual clinical practice, wKd therapy would commonly be administered to patients with a history of prior regimens including immunomodulatory drugs. The ORR and PFS in this study were similar between patients who were and were not refractory to lenalidomide, demonstrating that wKd therapy would also be effective in patients who are refractory to lenalidomide.
This study enrolled patients who were not recruitable to the ARROW study, for example those with an ECOG-PS ≥2, or those with 1 or ≥4 prior regimens. The proportion of patients with ECOG-PS ≥2 was 10.8% in this study. The effectiveness and safety in patients with ECOG-PS ≥2 were not significantly different from those in patients with ECOG-PS 0–1. In clinical practice, wKd therapy has been used in a wide range of RRMM patients, and evaluating the effectiveness and safety in patients who were not included in a clinical trial was thought to serve as a reference for future clinical practice. While the ORR tended to be generally higher with fewer prior treatments, in this study, the ORR for patients with ≥4 prior regimens was comparable to that for patients with 2–3 prior regimens. In this study, there were no significant differences in PFS or safety among subgroups defined by the number of prior regimens. These results suggest that wKd therapy would be effective in patients with history of treatment using multiple modalities.
It has been reported that proteasome inhibitors including carfilzomib were effective in patients with high-risk cytogenetics [6,9,11]. In this study, ORR and PFS in high-risk patients did not differ from those in other patients, demonstrating that carfilzomib is an effective treatment option for high-risk patients.
Proteasome inhibitor therapy including carfilzomib is associated with clinically significant cardiovascular AEs [22]. Carfilzomib had a more potent cardiotoxic profile compared with the other proteasome inhibitors, such as ixazomib and bortezomib [22]. The incidence of grade ≥3 cardiac disorders was 3% in the ARROW study [11] and 2% in the CHAMPION-1 study [10]. In a real-world setting, the incidence of grade ≥3 cardiac disorders was reported to be 0–6.5% [13,14,17,18,23]. In this study, the incidence of grade ≥3 cardiac AEs was 3.3%, which is within the range of previous studies. While no criteria were set for the initiation of administration that took cardiac disorders into account, all patients who had cardiac AEs in this study recovered or improved after treatment with antihypertensives and diuretics, suggesting that early treatment contributes to effective management of these AEs.
Kawaji-Kanayama et al. stated that careful and appropriate patient selection, intensive monitoring of symptoms and early intervention may reduce the risk of occurrence of cardiovascular AEs [24]. Further research will have to be conducted on how cardiovascular AEs can be managed. Recently, baseline cardiovascular risk stratification proformas were presented for cancer therapies including proteasome inhibitors for MM [25]. These proformas would be useful to stratify and mitigate cardiovascular risk related to carfilzomib treatment.
Limitations
Our study has several limitations. First, this was an observational study without a control arm, intended to compare effectiveness and safety. Second, although this study comprised over 100 patients, the sample size is still relatively small. Therefore, further research with a large sample size would be useful. Third, a limited number of investigational sites participated in this study. Therefore, there is a possibility that the present data were affected by site selection bias. Fourth, median OS was not reached in this study due to the short follow-up period, so comparison with other studies was not possible. Furthermore, treatment strategies were not standardized, instead being chosen by attending physicians at each institution.
Conclusion
The Weekly-CAR study demonstrated the effectiveness and safety of wKd therapy for patients with RRMM in real-world settings in Japan. Although the median PFS in this study was shorter than that in the ARROW study, the ORR in this study was comparable to that in the ARROW study. This study demonstrated the effectiveness and safety in patients who were not recruitable to the clinical trial of wKd therapy, and there were no new or unexpected safety signals in this study.
Supplementary Material
Acknowledgments
The authors wish to thank all study participants and their families and all study sites and investigators.
Funding Statement
The medical writing support was provided by K Wakimoto (CMIC Co., Ltd), and was funded by Ono Pharmaceuticals Co. Ltd.
Author contributions
Study conception and design: T Katayama. Patient enrollment and data collection: Y Abe, S Kubonishi, M Ri, M Iino, K Sunami, T Ito, M Fukaya, T Kitano, S Ikeda, S Ota, T Kuroi, N Iriyama, T Jo, M Adachi, D Akahane, T Kai, Y Kohara, N Kadowaki. Interpretation of results: Y Abe, T Katayama. All authors have read and approved the final manuscript.
Financial disclosure
K Sunami reports research fundings from Ono, Celgene, AbbVie, Takeda, Sanofi, Bristol Myers Squibb, GSK, Chugai, Otsuka and Janssen, and honoraria from Ono, Bristol Myers Squibb, Takeda and Sanofi. S Ikeda reports lecture fees from Janssen. S Ota reports lecture fees from Novartis, Bristol Myers Squibb, Takeda, AstraZeneca, Janssen and AbbVie. N Iriyama reports speakers fees from Ono. T Jo reports research grants from Bristol Myers Squibb and Handai Biken, consulting fees from Novartis, and lecture fees from Eisai, Sanofi, Bristol Myers Squibb, AbbVie, Nippon Shinyaku and Meiji Seika Pharmacia. T Katayama is an employee of Ono. The medical writing support was provided by K Wakimoto (CMIC Co., Ltd), and was funded by Ono Pharmaceuticals Co. Ltd. This research was funded by Ono Pharmaceutical Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
The medical writing support was provided by K Wakimoto (CMIC Co., Ltd), and was funded by Ono Pharmaceuticals Co. Ltd.
Ethical conduct of research
The study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki (World Medical Association, 2013), the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, and the Act on the Protection of Personal Information. This study was approved by relevant institutional review boards of each hospital or company.* Written informed consent to enroll in this study was obtained from patients. Opportunities for opt-out were provided for patients who had difficulty providing direct written consent, such as in instances of death or cessation of hospital visits. Both of these methods have received approval from the ethics committee at each facility.
*Japanese Red Cross Medical Center Institutional Review Board (No. 1300); Japanese Red Cross Society Himeji Hospital Institutional Review Board (No. CTM20-013); Nagoya City University Hospital Clinical Research Review Board (No. 60-21-0058); Yamanashi Prefectural Central Hospital Ethical Committee (No. 2020-53); National Hospital Organization Okayama Medical Center Institution Review Board (No. 2020-292); Ethics Review Committee of Kansai Medical University Hospital (No. 2020338); Shizuoka Cancer Center Certified Review Board (No. T2021-28-2021-1-2); Institutional Review Board of Kitano Hospital, Tazuke Kofukai Medical Research Institute (No. P210301100); Certified Clinical Research Review Board, Akita University (No. 2683); Institutional Review Board of Sapporo Hokuyu Hospital (No. 210518.01); Institutional Review Board of Japan Mutual Aid Association of Public School Teachers Chugoku Central Hospital (No. 2103-03); Nihon University Itabashi Hospital Review Board (No. RK-210427-2); Japanese Red Cross Nagasaki Genbaku Hospital Institutional Review Board (No. R3-671); Ethics Committee of JCHO Sapporo Hokushin Hospital (No. 2021-5); Tokyo Medical University Hospital Institutional Review Board (No. T2021-0050); Ethics Review Committee of Kitafukushima Medical Center (No. 96); Showa Inan General Hospital Ethics Committee (No. 2021-01); The Ethics Committee, Faculty of Medicine, Kagawa University (No. 2021-135); Ono Pharmaceutical Co., Ltd ‘Medical and Health Research Involving Human Subjects’ Ethics Committee (No. A1-20-002).
Data sharing statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kumar SK, Dispenzieri A, Lacy MQet al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28(5), 1122–1128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demo SD, Kirk CJ, Aujay MAet al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 67(13), 6383–6391 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Moreau P, Pylypenko H, Grosicki Set al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 12(5), 431–440 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Parlati F, Lee SJ, Aujay Met al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood 114(16), 3439–3447 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Herndon TM, Deisseroth A, Kaminskas Eet al. US Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 19(17), 4559–4563 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Stewart AK, Rajkumar SV, Dimopoulos MAet al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 372(2), 142–152 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Ri M, Chou Tet al. Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: a phase 1 study in Japan. Cancer Sci. 108(3), 461–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kastritis E, Roussou M, Gavriatopoulou Met al. Impact of last lenalidomide dose, duration, and IMiD-free interval in patients with myeloma treated with pomalidomide/dexamethasone. Blood Adv. 3(23), 4095–4103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Moreau P, Palumbo Aet al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 17(1), 27–38 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Berenson JR, Cartmell A, Bessudo Aet al. CHAMPION-1: a phase 1/2 study of once weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood 127(26), 3360–3368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau P, Mateos MV, Berenson JRet al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (ARROW): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 19(7), 953–964 (2018). [DOI] [PubMed] [Google Scholar]; •• The phase III trial (ARROW study) comparing the efficacy and safety of once-weekly carfilzomib and dexamethasone therapy with twice-weekly carfilzomib and dexamethasone therapy in patients with relapsed and refractory multiple myeloma.
- 12.Takezako N, Shibayama H, Handa Het al. Once-weekly vs. twice-weekly carfilzomib dosing in a subgroup of Japanese relapsed and refractory multiple myeloma patients from a randomized phase 3 trial (ARROW) and comparison with ENDEAVOR. Int. J. Hematol. 113(2), 219–230 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A subgroup analysis focused on the Japanese patients with multiple myeloma enrolled in the ARROW study comparing with ENDEAVOR study.
- 13.Onda Y, Kanda J, Kaneko Het al. Real-world effectiveness and safety analysis of carfilzomib–lenalidomide–dexamethasone and carfilzomib–dexamethasone in relapsed/refractory multiple myeloma: a multicenter retrospective analysis. Ther. Adv. Hematol. 13, 1–16 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; • A retrospective analysis demonstrating the real-world effectiveness and safety of carfilzomib in combination with lenalidomide and dexamethasone and twice-weekly carfilzomib with dexamethasone therapies in Japanese patients.
- 14.Terpos E, Zambello R, Leleu Xet al. Real-world use and effectiveness of carfilzomib plus dexamethasone in relapsed/refractory multiple myeloma in Europe. Cancers 14(21), 5311 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durie BGM, Harousseau J-L, Miguel JSet al. International uniform response criteria for multiple myeloma. Leukemia 20(9), 1467–1473 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Harousseau J-L, Durie Bet al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117(18), 4691–4695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki A, Murakami H, Chou T, Matsushita M, Kizaki M. Post-marketing surveillance of carfilzomib in Japanese patients with relapsed or refractory multiple myeloma. Future Oncol. 18(24), 2661–2674 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Kawaji-Kanayama Y, Kobayashi T, Muramatsu Aet al. Prognostic impact of resistance to bortezomib and/or lenalidomide in carfilzomib-based therapies for relapsed/refractory multiple myeloma: the Kyoto Clinical Hematology Study Group, multicenter, pilot, prospective, observational study in Asian patients. Cancer Rep. 5(2), e1476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreau P, Stewart KA, Dimopoulos M, Siegel D, Facon T, Berenson J. Once-weekly (70 mg/m2) vs twice-weekly (56 mg/m2) dosing of carfilzomib in patients with relapsed or refractory multiple myeloma: a post hoc analysis of the ENDEAVOR, ARROW, and CHAMPION-1 trials. Cancer Med. 9(9), 2989–2996 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Post hoc crosstrial comparisons demonstrating the efficacy and safety profiles of once-weekly versus twice-weekly dosing schedules using data from three trials of patients with relapsed or refractory multiple myeloma.
- 20.Dimopoulos MA, Moreau P, Iida Set al. Outcomes for Asian patients with multiple myeloma receiving once- or twice-weekly carfilzomib-based therapy: a subgroup analysis of the randomized phase 3 ENDEAVOR and ARROW trials. Int. J. Hematol. 110(4), 466–473 (2019). [DOI] [PubMed] [Google Scholar]; • A subgroup analysis focused on the outcomes of Asian patients with multiple myeloma enrolled in the randomized phase III ENDEAVOR and ARROW trials.
- 21.Dimopoulos MA, Niesvizky R, Weisel Ket al. Once- versus twice-weekly carfilzomib in relapsed and refractory multiple myeloma by select patient characteristics: phase 3 ARROW study subgroup analysis. Blood Cancer J. 10(3), 35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das A, Dasgupta S, Gong Yet al. Cardiotoxicity as an adverse effect of immunomodulatory drugs and proteasome inhibitors in multiple myeloma: a network meta-analysis of randomized clinical trials. Hematol. Oncol. 40(2), 233–242 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giudice MLD, Gozzetti A, Antonioli Eet al. Carfilzomib plus dexamethasone in patients with relapsed and refractory multiple myeloma: a retro-prospective observational study. Eur. J. Haematol. 109(4), 373–380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaji-Kanayama Y, Muramatsu A, Sasaki Net al. Clinical impacts of frailty, poor performance status, and advanced age in carfilzomib-containing treatment for relapsed/refractory multiple myeloma: post hoc investigation of the KOTOSG multicenter pilot prospective observational study. Int. J. Hematol. 115(3), 350–362 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Lyon AR, Dent S, Stanway Set al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 22(11), 1945–1960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Baseline cardiovascular risk assessment and baseline risk stratification proformas in cancer patients prior to receiving the following cancer therapies, such as anthracycline chemotherapy, multiple myeloma therapies (proteasome inhibitors and immunomodulatory drugs).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


