Abstract
Patient and surgical treatment An 11-year-old, castrated male cat was referred for insulin-resistant diabetes mellitus. It had a ravenous appetite, increased body weight, polyuria/polydipsia and a dull hair coat. The cat was receiving 25 IU insulin four times daily but blood glucose concentrations remained elevated. Plasma concentrations of growth hormone (GH) (51 μug/l, reference range 0.8–7.2 μg/l) and insulin-like growth factor 1 (IGF-1) (3871 μg/l, reference range 39–590 μg/l) were highly elevated, whereas those of alpha-melanocyte-stimulating hormone, adrenocorticotropic hormone and Cortisol were normal. Computed tomography revealed a thick palatum molle and an enlarged pituitary gland, indicating a pituitary neoplasm. Microsurgical transsphenoidal hypophysectomy was performed and microscopic examination of the surgical specimen revealed an acidophilic, infiltrative pituitary adenoma that showed positive immunostaining for GH.
Outcome The clinical signs resolved and 3 weeks after surgery the cat no longer required insulin administration. One year after hypophysectomy the plasma concentrations of GH and IGF-1 were 2.4 μg/l and 113 μg/l, respectively.
Practical relevance This is the first report detailing transsphenoidal hypophysectomy as a feasible and effective treatment for feline acromegaly due to a pituitary somatotroph adenoma. Moreover, in this patient, concurrent insulin-resistant diabetes mellitus resolved completely. The surgery is discussed in the context of human and other feline therapies for acromegaly.
In cats, chronic hypersecretion of growth hormone (GH) by a functional pituitary adenoma causes acromegaly. 1 The clinical signs are dominated by concurrent insulin-resistant diabetes mellitus. Although acromegaly is being recognised in cats with increasing frequency, 2 there have been few reports of experience with treatment. Usually treatment is aimed at regulation of glucose levels with insulin medication, but as long as the primary cause remains untreated, insulin requirements usually increase over time. This case report documents, for the first time, successful treatment of acromegaly in a diabetic cat by pituitary surgery.
Case history
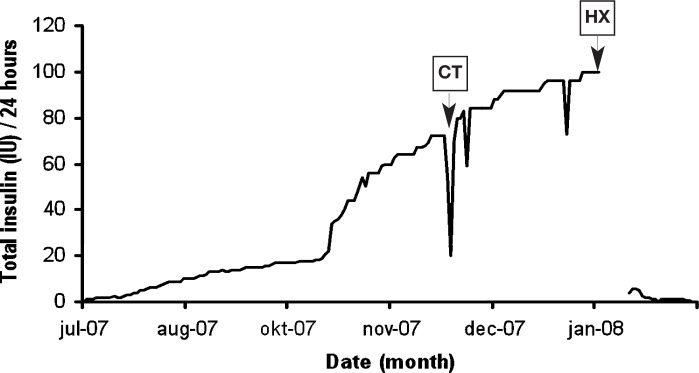
Over a period of 4 months, an 11-year-old, castrated male crossbred cat had developed a ravenous appetite which had led to an increase in body weight. At the time of referral the cat weighed 7.2 kg (Fig 1). It had a dull hair coat and was diagnosed with diabetes mellitus. On a day curve, plasma glucose concentrations varied between 17 and 22 mmol/l while the cat was treated with 9 IU of insulin twice daily (q12h), indicating insulin resistance. Initially the cat had been treated with injections q12h (Caninsulin; Intervet), but at the time of referral it was being injected four times daily (q6h) with 25 IU of insulin (Levemir; Novo Nordisk) (Fig 2).
FIG 1.

An 11-year-old, castrated male diabetic cat with acromegaly, showing increased body weight and a dull hair coat
FIG 2.
Insulin requirements over a 6-month period in a diabetic cat with acromegaly treated by transsphenoidal hypophysectomy (HX). CT indicates when computed tomography was performed. By week 3 after surgery the cat no longer required insulin treatment
Acromegaly was confirmed by elevated plasma concentrations of GH of 19 and 82 μg/l (reference range 0.8–7.2 μg/l) 3 and of insulin-like growth factor 1 (IGF-1) of 3678 and 4064 μg/l (reference range 39–590 μg/l)3,4 in two blood samples collected 7 weeks apart (Table 1). The pituitary-adrenocortical system was further investigated by measuring plasma concentrations of alpha-melanocyte-stimulating hormone (α-MSH), adrenocorti-cotropin (ACTH) and cortisol by methods described previously. 5 The basal plasma concentrations of theses hormones were within their reference ranges (Table 1). 5 The plasma total thyroxine concentration was also within the reference range (Table 1).
TABLE 1.
Basal plasma hormone concentrations in a diabetic cat with acromegaly pre- and post-hypophysectomy (HX)
| Hormones | Basal values pre-HX | 1 year post-HX | Reference values |
| Thyroxine nmol/l (μg/dl) | 22 (1.7) | - | 15–45 (1.2–3.5) |
| Cortisol nmol/l (μg/dl) | 211 (7.3) | - | 12–263 (0.4–9.5) |
| ACTH ng/l | 26 | - | 15–358 |
| α-MSH ng/l | 257 | - | 29–503 |
| GH μg/l | 51 | 2.4 | 0.8–7.2 |
| IGF-1 μg/l | 3871 | 113 | 39–590 |
Basal plasma concentrations of cortisol, ACTH and α-MSH are means calculated from two values in two blood samples collected the day before surgery. Before surgery basal plasma concentrations of GH and IGF-1 are means calculated from two blood samples collected 7 weeks apart. The data are presented in SI units (traditional units given in brackets)
Contrast-enhanced computed tomography (CT) 6 revealed a pituitary gland measuring 4.5 mm in height, 4.2 mm in width and 4.7 mm in length. On dynamic CT, the pituitary flush was slightly displaced to the right and dorsal side of the gland, indicative of a pituitary adenoma on the left and ventral side (Fig 3a). The palatum molle was thickened, as evident on a sagittal reconstruction of the CT images (Fig 3b). Transsphenoidal hypophysectomy was performed, as described previously.6,7 Following electrosurgical incision of the soft palate it was noted that the mucosa was thicker than normal and that the muco-periosteum covered the sphenoid bone in thick folds.
FIG 3.
(a) Dynamic contrast-enhanced CT in an 11-year-old, castrated male cat with acromegaly. The pituitary is enlarged, measuring 4.5 mm in height, 4.2 mm in width and 4.7 mm in length. The pituitary flush is displaced to the right and dorsal side of the gland (arrow), indicative of a pituitary adenoma on the left and ventral side. (b) Sagittal reconstruction showing the thickened palatum molle (arrowhead; also visible in [a]) between the tracheal tube and sphenoid bone
Usually treatment of acromegaly is aimed at regulation of glucose levels with insulin medication; but as long as the primary cause remains untreated, insulin requirements usually increase over time.
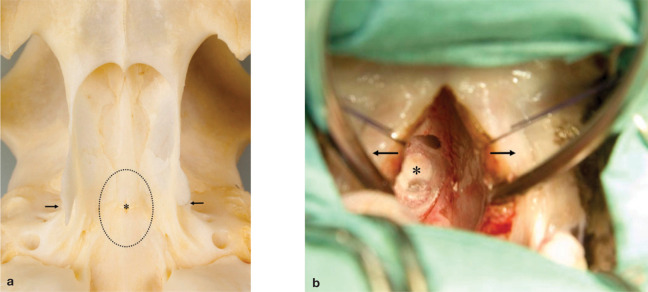
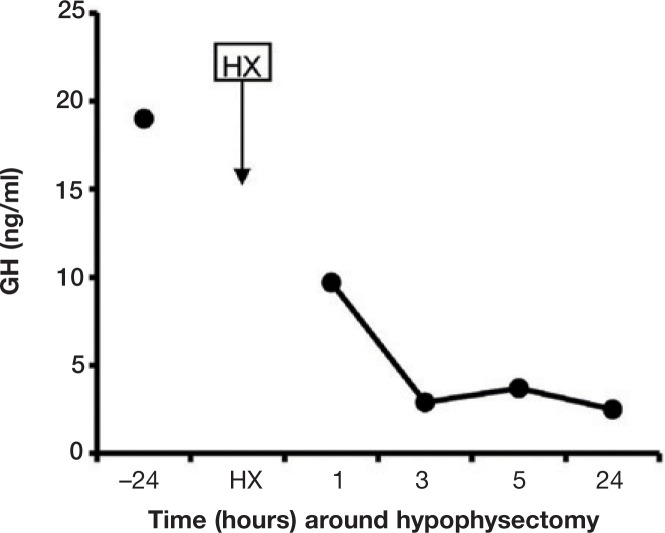
Access to the pituitary fossa (Fig 4a) was obtained with an electrically powered burr and bone punches were used to enlarge the sphenoid bone burr slot. The dura mater was coagulated and incised and the pituitary (Fig 4b) was detached from the fossa using a small ball-tipped probe and suction. The ventral and left part of the pituitary was judged to be adenomatous and was collected for routine histopathology as the tumour specimen (specimen 1). The dorsal and right part of the pituitary was judged to be normal and collected as the unaffected specimen (specimen 2). The fossa was filled with absorbable gelatine sponge, the burr slot in the sphenoid bone was filled with bone wax and the soft palate was sutured in two separate layers. Within 5 h of hypophysectomy the plasma GH concentration had declined markedly (Fig 5).
FIG 4.
(a) Ventral view of the sphenoid bone in a feline skull showing the location of the transsphenoidal slot (……)to the pituitary gland (∗). The pterygoid hamular processes are indicated with arrows. (b) Intraoperative view during transsphenoidal hypophysectomy of an 11-year-old, castrated male cat with acromegaly. The soft palate is retracted (arrows) and the pituitary tumour (∗) is visible through the transsphenoidal slot
FIG 5.
Plasma GH concentrations 24 h before and 1, 3, 5 and 24 h after hypophysectomy (HX) in a diabetic cat with acromegaly
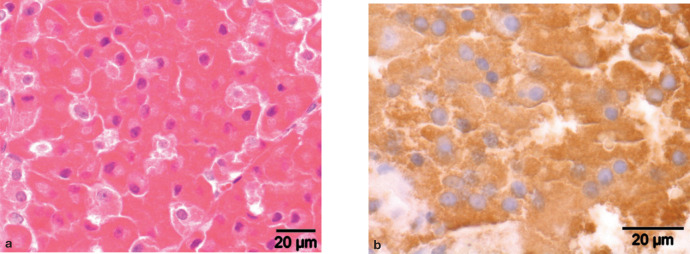
Microscopic examination of haematoxylin and eosin-stained sections of specimen 1 revealed two uniform adenomatous nodules of acidophilic cells with an infiltrative growth pattern in pre-existent pituitary tissue (Fig 6a). Specimen 2 contained unaffected adenohypophyseal and neurohypophyseal tissue. Immunohistochemical staining was performed by the avidin-biotin technique using a monoclonal mouse antibody to synthetic ACTH(1–24) (Department of Infectious Diseases and Immunology Faculty of Veterinary Medicine, Utrecht University, NL), a polyclonal rabbit antibody to synthetic human α-MSH (PU060-UP, Biogenex Laboratories, San Remon, CA, USA) and a polyclonal rabbit antibody to porcine GH (source 4750–3959, Biogenesis, Poole, UK). 8 Normal canine pituitary tissue served as control tissue. The dilution factor for the antibodies was 1:100 for ACTH, 1:5000 for GH and 1:600 for α-MSH. The nodules in specimen 1 stained immunohistochemically positive for GH (Fig 6b), whereas cells in the pre-existent pituitary tissue showed sporadic positive immunostaining for GH and ACTH.
FIG 6.
Somatotroph pituitary adenoma in a cat with acromegaly. (a) Adenomatous nodule comprised of acidophilic cells with uniform nuclei and a low mitotic index; haematoxylin and eosin staining. (b) Positive immunohistochemical staining of the adenomatous tissue for GH
The insulin requirement was reduced by 95% in the first week after hypophysectomy.
Recovery from surgery was uncomplicated; the cat started to eat and drink in the days after surgery. The insulin demand decreased from 25 IU q6h before surgery to 2.5 IU q12h at 7 days after surgery when the cat was discharged from the hospital. Medication after surgery consisted of desmopressin (Minrin; Ferring), one drop (5 μg) three times daily (q8h) in the conjunctival sac for 3 weeks, cortisone acetate (Cortisoni acetas; Genfarma), 0.3 mg q12h PO lifelong, and L-thyroxine (L-thyroxine; Aesculaap), 15 μg/kg q12h PO lifelong, as described previously.6,7 Polydipsia resolved at home. By week 3 after surgery insulin administration was no longer required (Fig 2) and 18 months after surgery the cat was still free of insulin medication. The cat resumed its normal behaviour at home and developed a healthy full hair coat (Fig 7). Basal urinary corticoid/creatinine ratios, measured in two consecutive morning urine samples collected at home 6 months after surgery, were low (2.6 and 2.7 × 10-6, reference range 8–42 × 10-6), confirming impaired adrenocortical function and the necessity for lifelong cortisol replacement therapy. 9 One year after surgery remission of acromegaly was confirmed by normalisation of plasma concentrations of GH (2.4 μg/l) and IGF-1 (113 μg/l) (Table 1). The appetite of the cat remained excessive, leading to a gain in body weight (9.4 kg).
FIG 7.

The cat 1 year after hypophysectomy, no longer requiring insulin medication. It has resumed its normal behaviour at home and has developed a healthy full hair coat
Discussion
The cat had been presented with insulin-resistant diabetes mellitus, a ravenous appetite, coarse facial features and a dull hair coat, a combination of features that is well recognised in feline acromegaly. The diagnosis was confirmed by documenting elevated plasma GH and IGF-1 levels.2,10 Pituitary imaging supported the diagnosis of a pituitary adenoma, which was eventually confirmed using histopathology. Dynamic CT enables the visualisation of different enhancements of the neurointermediate lobe (NIL) and anterior lobe (AL) as a consequence of the differences in blood supply; that is, an early and strong enhancement of the NIL (‘pituitary flush’) via the caudal hypophyseal artery, branching off from the internal carotid artery, and a delayed (less strong) enhancement of the peripheral AL by way of the long portal vessels. 11 The cat also exhibited this ‘pituitary flush’ and dynamic CT showed that it was displaced to the right, indicating the presence of an adenoma on the left.
Pituitary adenomas in cats show a greater diversity in cell characteristics than their canine counterparts. In cats, corticotroph (ACTH cell) adenomas cause pituitary-dependent hypercortisolism or Cushing's disease 6 and somatotroph (GH cell) adenomas cause acromegaly. 1 In addition, a melanotroph (α-MSH cell) adenoma 12 and double (ACTH and GH cell) adenomas3,13 have been described in cats. Finally, endocrinologically non-functioning pituitary tumours occur in older cats and comprise 9% of feline intracranial neoplasms leading to neurological signs due to the tumour mass effect on the brain. 14
In the current case the insulin requirement was reduced by 95% in the first week after hypophysectomy. The explanation for this dramatic response may be the immediate decrease in plasma GH levels, which became evident from the 5 h postoperative GH profile. The prior elevated plasma GH and IGF-1 levels apparently caused no irreversible damage to the insulin responsiveness of pancreatic endocrine and peripheral tissues. This is in sharp contrast to insulin-resistant diabetes mellitus caused by Cushing's disease in dogs, which is usually irreversible.
Increased food intake may lead to obesity, which in turn causes peripheral insulin resistance and ultimately may lead to diabetes mellitus. Therefore, the weight gain in the cat after surgery is a concern. It may be hypothesised that the central hypothalamic regulation of food intake has been disturbed in this cat by the enlarged pituitary.
In humans transsphenoidal selective adenomectomy and medical treatments with somatostatin analogues are the main therapeutic options for acromegaly. As illustrated by the present case, transsphenoidal hypophysectomy is an effective treatment for cats with acromegaly caused by a GH-producing pituitary adenoma. Hypophysectomy led to normalisation of plasma GH and IGF-1 levels, reversal of insulin resistance, and complete cessation of diabetes mellitus. Cryohypophysectomy has been reported in two cats. In one cat plasma IGF-1 levels remained unchanged, insulin resistance persisted and residual tumour tissue was detected on MRI 2 months after cryohypophysectomy; 15 later, this cat showed neurological deterioration and blindness that was attributed by the authors to enrofloxacin medication. 16 Likewise, in the other cat cryohypophysectomy resulted neither in a significant lowering of the plasma IGF-1 concentration nor a resolution of the insulin resistance. 17
Transsphenoidal hypophysectomy is an effective treatment for acromegaly caused by a GH-producing pituitary adenoma. In this cat it led to normalisation of plasma GH and IGF-1 levels, reversal of insulin resistance and complete cessation of diabetes mellitus.
The most frequently reported treatment for feline acromegaly has been radiation therapy. In five cases cobalt 60 (gamma) radiation lowered the insulin requirement transiently and reduced the size of the pituitary tumour.1,18 In one cat in which linear accelerator (high-energy X-ray) radiation was used, insulin resistance was reduced but the plasma IGF-1 concentration remained elevated and acromegaly continued as an active disease process. 19 Beta radiation reduced the insulin requirement only slightly in one cat but linear accelerator radiation reduced the insulin dose in another cat by half. 20 Radiation therapy is variably successful, primarily because of the partial and delayed effect of radiation necrosis.1,18,19,21
Medical treatment with the somatostatin analogue octreotide 1 and a dopamine agonist 22 was not successful in reducing the insulin requirements in cats with acromegaly. Depending on the receptor profile of the tumour, somatostatin analogues are effective in a high percentage of humans with acromegaly, reducing both plasma GH and IGF-1 levels and the size of the tumour. 23 In one cat treated with the somatostatin analogue octreotide, plasma GH concentration was normalised, 24 but in four others octreotide had little or no effect on serum GH levels. 1 A screening test with a single intravenous injection of octreotide was introduced recently to evaluate the potential effectiveness of octreotide treatment in acromegalic cats. 3 Those responding favourably might be candidates for long-acting release (LAR) octreotide treatment.
The recently introduced GH receptor antagonist pegvisomant has been reported to be effective, safe, and well tolerated in humans with acromegaly. 25 However, given there are no species-specific antagonists, this approach is not yet an option for dogs and cats.
References
- 1.Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med 1990; 4: 192–201. [DOI] [PubMed] [Google Scholar]
- 2.Niessen SJ, Petrie G, Gaudiano F, et al. Feline acromegaly: an underdiagnosed endocrinopathy? J Vet Intern Med 2007; 21: 899–905. [DOI] [PubMed] [Google Scholar]
- 3.Slingerland LI, Voorhout G, Rijnberk A, Kooistra HS. Growth hormone excess and the effect of octreotide in cats with diabetes mellitus. Domest Anim Endocrinol 2008; 35: 352–61. [DOI] [PubMed] [Google Scholar]
- 4.Norman EJ, Mooney CT. Diagnosis and management of diabetes mellitus in five cats with somatotrophic abnormalities. J Feline Med Surg 2000; 2: 183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javadi S, Slingerland LI, van de Beek MG, et al. Plasma renin activity and plasma concentrations of aldosterone, cortisol, ACTH and α-MSH in healthy household cats. J Vet Intern Med 2004; 18: 625–31. [DOI] [PubMed] [Google Scholar]
- 6.Meij BP, Voorhout G, van den Ingh TS, Rijnberk A. Transsphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in 7 cats. Vet Surg 2001; 30: 72–86. [DOI] [PubMed] [Google Scholar]
- 7.Meij BP, Voorhout G, Rijnberk A. Progress in transsphenoidal hypophysectomy for treatment of pituitary-dependent hyperadrenocorticism in dogs and cats. Mol Cell Endocrinol 2002; 197: 89–96. [DOI] [PubMed] [Google Scholar]
- 8.Diaz Espiñeira MM, Mol JA, van den Ingh TSGAM, van der Vlugt-Meijer RH, Rijnberk A, Kooistra HS. Functional and morphological changes in the adenohypophysis of dogs with induced primary hypothyroidism: loss of TSH hypersecretion, hypersomatotropism, hypoprolactinemia, and pituitary enlargement with transdifferentiation. Domest Anim Endocrinol 2008; 35: 98–111. [DOI] [PubMed] [Google Scholar]
- 9.De Lange MS, Galac S, Trip MR, Kooistra HS. High urinary corticoid/creatinine ratios in cats with hyperthyroidism. J Vet Intern Med 2004; 18: 152–55. [DOI] [PubMed] [Google Scholar]
- 10.Berg RI, Nelson RW, Feldman EC, Kass PH, Pollard R, Refsal KR. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med 2007; 21: 892–98. [DOI] [PubMed] [Google Scholar]
- 11.Van der Vlugt-Meijer RH, Voorhout G, Meij BP. Imaging of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. Mol Cell Endocrinol 2002; 197: 81–87. [DOI] [PubMed] [Google Scholar]
- 12.Meij BP, van der Vlugt-Meijer RH, van den Ingh TSGAM, Flik G, Rijnberk A. Melanotroph pituitary adenoma in a cat with diabetes mellitus. Vet Pathol 2005; 42: 92–97. [DOI] [PubMed] [Google Scholar]
- 13.Meij BP, van der Vlugt-Meijer RH, van den Ingh TSGAM, Rijnberk A. Somatotroph and corticotroph pituitary adenoma (double adenoma) in a cat with diabetes mellitus and hyperadrencorticism. J Comp Pathol 2004; 130: 209–15. [DOI] [PubMed] [Google Scholar]
- 14.Troxel MT, Vite CH, Van Winkle TJ, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985–2001). J Vet Intern Med 2003; 17: 850–59. [DOI] [PubMed] [Google Scholar]
- 15.Abrams-Ogg ACG, Holmberg DL, Stewart WA, Claffey FP. Acromegaly in a cat: diagnosis by magnetic resonance imaging and treatment by cryohypophysectomy. Can Vet J 1993; 34: 682–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Abrams-Ogg ACG, Holmberg DL, Quinn RF, Keller C, Wilcock BP, Claffey FP. Blindness now attributed to enrofloxacin therapy in a previously reported case of a cat with acromegaly treated by cryohypophysectomy. Can Vet J 2002; 43: 53–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Blois SL, Holmberg DL. Cryohyophysectomy used in the treatment of a case of feline acromegaly. J Small Anim Pract 2008; 49: 596–600. [DOI] [PubMed] [Google Scholar]
- 18.Goossens MM, Feldman EC, Nelson RW, et al. Cobalt 60 irradiation of pituitary gland tumors in three cats with acromegaly. J Am Vet Med Assoc 1998; 213: 374–76. [PubMed] [Google Scholar]
- 19.Littler RM, Polton GA, Brearley MJ. Resolution of diabetes mellitus but not acromegaly in a cat with a pituitary macroadenoma treated with hypofractionated radiation. J Small Anim Pract 2006; 47: 392–95. [DOI] [PubMed] [Google Scholar]
- 20.Kaser-Hotz B, Rohrer CR, Stankeova S, Wergin M, Fidel J, Reusch C. Radiotherapy of pituitary tumours in five cats. J Small Anim Pract 2002; 43: 303–7. [DOI] [PubMed] [Google Scholar]
- 21.Mayer MN, Greco DS, LaRue SM. Outcomes of pituitary tumor irradiation in cats. J Vet Intern Med 2006; 20: 1151–54. [DOI] [PubMed] [Google Scholar]
- 22.Abraham LA, Helmond SE, Mitten RW, Charles JA, Holloway SA. Treatment of an acro-megalic cat with the dopamine agonist L-deprenyl. Aust Vet J 2002; 80: 479–83. [DOI] [PubMed] [Google Scholar]
- 23.Colao A, Pivonello R, Auriemma RS, et al. Growth hormone-secreting tumor shrinkage after 3 months of octreotide-long-acting release therapy predicts the response at 12 months. J Clin Endocrinol Metab 2008; 93: 3436–42. [DOI] [PubMed] [Google Scholar]
- 24.Meij BP, Kooistra HS, Rijnberk A. Hypothalamus-pituitary system. In: Rijnberk A, Kooistra HS, eds. Clinical endocrinology of dogs and cats. 2nd edn. Hannover: Schlütersche, 2010: 13–54. [Google Scholar]
- 25.Higham C, Chung TT, Lawrance J, Drake WM, Trainer PJ. Long term experience of pegvisomant therapy as a treatment for acromegaly. Clin Endocrinol 2009; 71: 86–91. [DOI] [PubMed] [Google Scholar]