Abstract
Objective:
This meta-analysis aims to pool the results of existing studies to obtain more precise estimates on the diagnostic efficiency of the Fourier transform infrared (FTIR) spectroscopy in detecting CRC using blood-based samples.
Methods:
A comprehensive database search identified 4,931 studies that were screened for eligibility. Relevant data were then extracted and collated and analyzed using Meta-DiSc 1.4 to measure the pooled diagnostic accuracy, sensitivity, specificity, likelihood ratio, and diagnostic odds ratio and presented in forest plots.
Results:
The pooled sensitivity across all six data entries was 86.10% (p = 0.20), and the specificity was 91.2% (p < 0.001). The pooled positive likelihood ratio was 9.84 (p < 0.001), indicating a strongly moderate diagnostic value, while the negative likelihood ratio was 0.16 (0.12), suggesting moderately decreased efficacy of FTIR spectroscopy in ruling out the disease. The pooled AUC was found to be at 0.94 which indicate excellent discriminating potential of FTIR of the method.
Conclusion:
Overall, the study suggests that FTIR spectroscopy has potential as minimally invasive diagnostic method for CRC using plasma samples.
Key Words: FTIR spectroscopy, blood, colorectal cancer (CRC), diagnostic performance, meta-analysis
Introduction
Colorectal cancer (CRC), also known as colorectal adenocarcinoma, is a prevalent and deadly disease. According to the GLOBOCAN 2020 report, which is published by the International Agency for Research on Cancer (IARC), there were over 900,000 deaths and more than 1.93 million cases of CRC in 2020. The report also states that CRC is the third most lethal cancer and the fourth most commonly diagnosed cancer worldwide [1]. The incidence of CRC is higher in developed countries, with a three to four-fold increase compared to developing nations. The occurrence and development of CRC are largely attributed to various risk factors such as personal habits, lifestyle choices, chronic diseases, and age [2]. These factors include sedentary lifestyle, smoking, alcohol consumption, and history of chronic diseases. Additionally, even in developing countries, the rise in sedentary lifestyle and excessive consumption of red meat and alcohol have contributed to the global epidemiological status of CRC cases [3,4].
Recent advancements in the treatment and diagnostic methods for CRC have been reported to enhance the survival rates of patients, which vary based on factors such as race, geographical location, and the stage at which the cancer was diagnosed [3,5]. The conventional screening modality is colonoscopy, while tissue biopsy is widely regarded as the reference standard for diagnosis. Nevertheless, both procedures are invasive and time-consuming, with biopsy also being susceptible to the issue of observer subjectivity [6]. This is because biopsy heavily depends on the microscopic evaluation of stained tissue specimens by licensed pathologists to identify morphological abnormalities [7]. Thus, the cancer research community is actively working towards the development of innovative screening and diagnostic techniques that are highly sensitive and specific, minimally invasive, affordable, and time efficient.
FTIR spectroscopy is a widely used analytical technique that allows the characterization of chemical compounds based on the absorption of infrared radiation by their functional groups. The resulting absorption spectrum, known as the signature spectral fingerprint, provides valuable information about the specific vibration energy states of the functional groups present in the substance, thereby allowing for its chemical composition to be identified [8]. FTIR spectroscopy is a promising approach in the field of cancer diagnosis, offering a minimally invasive method for analyzing both tissue and liquid biological samples. By examining the absorption spectrum of functional groups in these samples, this tool provides a distinctive spectral fingerprint that can be used to identify the chemical composition and presence of cancerous cells [9].
Several studies have proven the potential use of FTIR spectroscopy in distinguishing cancerous from healthy tissues with high sensitivity, specificity, and accuracy [10–14].This technique not only enhances the accuracy and reproducibility of cancer diagnosis, but it also significantly reduces the processing time required for tissue analysis. Besides tissues, it can also be used for other biological materials like blood, urine, bladder wash, bile, and sputum [9]. Thus, FTIR spectroscopy has the potential to revolutionize the field of cancer diagnosis, particularly with regards to the analysis of biological samples such as blood. Its non-destructive and minimally invasive approach offers numerous benefits over traditional methods, including the ability to facilitate early detection and improve patient outcomes through improved clinical decision-making [15].
Various modes of FTIR spectroscopy have been used to precisely discriminate biological samples by determining the changes in their molecular conformations, bonding types, and intermolecular interactions of the various functional groups corresponding to nucleic acids, proteins, lipids, and carbohydrates [15,16]. FTIR spectroscopy, in combination with statistical and machine learning algorithms, effectively overcomes the challenges posed by the intricacies of biomolecular profiles. This approach allows the analysis of spectral data to uncover chemico-physical changes that occur as healthy biological samples transition to a pathological state [14-16]. The effectiveness of this technology in differentiating malignancies from benign and healthy biological samples has led to the development of the term “spectral biomarkers,” which refer to the recognizable peaks and clustering patterns found in the infrared fingerprint region [12]. Although not yet approved for clinical use, this technology has the potential to revolutionize the early screening process for CRC by enabling clinicians to detect the onset and progression of cancer cells at the molecular level, even before morphological changes are evident [9].
This study aimed to evaluate the diagnostic efficacy of blood based FTIR spectroscopy in the detection of CRC through a systematic review of the available literature. To determine the diagnostic accuracy of FTIR spectroscopy, we conducted a statistical analysis of the existing data and findings from previous studies with 60 CRC patients and 73 healthy control individuals. The performance metrics of FTIR spectroscopy, including sensitivity, specificity, positive and negative likelihood ratios (LR), diagnostic odds ratio (DOR), and area under the receiver operating characteristic curve (AUC), were compared with the current diagnostic standard, which consists of a combination of colonoscopy and microscopic examination of biopsied tissue specimens.
Materials and Methods
Study selection, data extraction, and methodological quality assessment
The meta-analysis study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. A comprehensive search strategy was implemented to identify relevant studies, which were then screened and selected based on the criteria outlined in the PRISM guidelines. Literature search was conducted across four databases: PubMed, ScienceDirect, Google Scholar, and Cochrane Library. The search was unrestricted as regards to publication date.
The keywords used were a combination of terms divided into 3 components: FTIR spectroscopy (eg, FTIR, ATR-FTIR, spectroscopy, and ATR-FTIR spectroscopy), colorectal cancer diagnostics (eg. cancer diagnosis, cancer identification, cancer discrimination, and cancer screening), and blood/plasma/serum. The literature search was conducted from September 30 through December 30, 2022. The abstracts of the published articles were then sorted using Mendeley reference management software. An evaluation of the titles and abstracts of each study was performed to determine if they met the criteria for full-text analysis. The studies were included in the analysis if: (1) the study’s primary objective is to diagnose colorectal cancer using FTIR spectroscopy, (2) the use of blood samples; and (3) the availability of data on sensitivity, specificity, accuracy, or confusion matrices in each study. Studies that did not adhere to these parameters were disregarded for further analysis. Furthermore, studies that employed animal models, lacked extractable data for the confusion matrix, and were review articles were excluded from further assessment. The studies that met the eligibility criteria were compiled into a comprehensive list, which included details such as the surname of the first author, country of
conduct, sample size and age range, study design, FTIR and FTIR-based techniques used, performance measures of specificity, selectivity, accuracy, and confusion matrices. The relevant data was analyzed, gathered, and organized into a customized spreadsheet for further summarization and tabulation. Studies and their associated data were collected and screened by GMM, GEL, ERA, FLN, MIG, MRC, and JAM. PMA, RET, and JAM verified the accuracy of the included studies and data.
The quality of the research articles was determined using the Newcastle-Ottawa Scale (NOS), a validated instrument for evaluating the quality of non-randomized studies. The NOS grading criteria are based on a scale of 0 to 9, where grades of 7-9 indicate high-quality studies, grades of 5-6 indicate moderate quality, and grades of 0-4 indicate low-quality studies.
Data synthesis and analysis
The data was analyzed using Meta-DiSc 1.4, a Windows-based software designed for meta-analysis of diagnostic test accuracy. The analyses conducted included calculations for sensitivity and specificity, positive and negative likelihood ratios, diagnostic odds ratio, and threshold analysis. Heterogeneity between the studies was evaluated using the inconsistency index (I²), Cochran-Q test, and tau-squared (T²). Random-effects models were used to synthesize the results, while a summary receiver operating characteristic curve plot and threshold analysis were generated to assess the threshold effect. The test accuracy was determined using the AUC index and the index Q*.
Results
Characteristics of the included studies
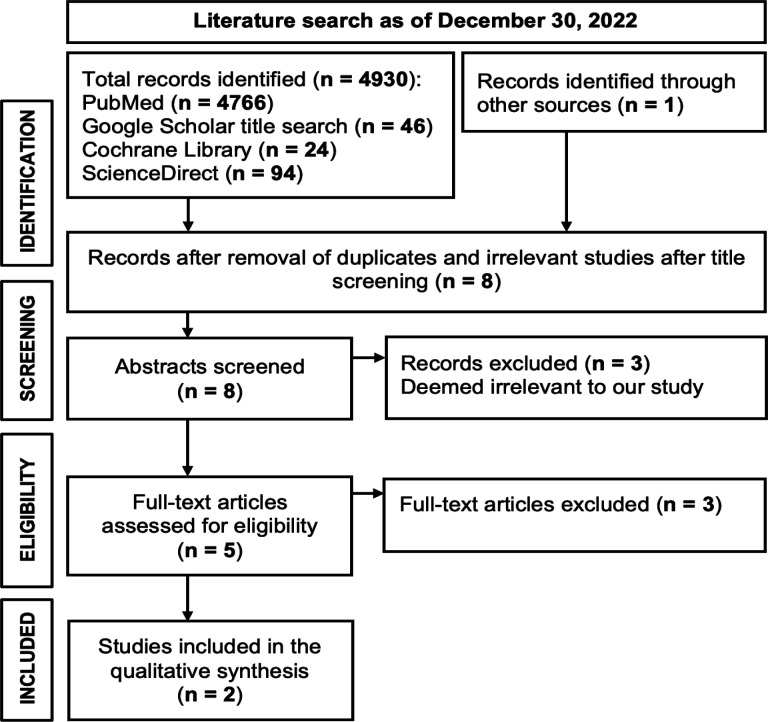
A comprehensive database search was conducted using the various keyword combinations and as of December 30, 2022, the search resulted in the identification of 4,931 relevant studies (Figure 1). After removing duplicate entries and screening out irrelevant studies based on title and abstract, the remaining studies underwent further analysis, where eight (8) full-text articles were meticulously evaluated. Of the eight full-text articles, six (6) were excluded primarily due to inadequate sample size and lack of diagnostic metrics such as sensitivity, specificity, accuracy, or any dataset to extract a confusion matrix. Thus, two (2) articles, which were all case-control studies, were selected for the meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Flow Diagram of Included Studies
Table 1 provides a summary of the key characteristics of the included studies. Our analysis included a total of 133 participants, comprising 60 individuals diagnosed with colorectal cancer (CRC), and 73 healthy control individuals. Age at diagnosis for the CRC cases ranged from 35 to 93 years, while the healthy control group was aged between 22 and 90 years. Our sample comprised participants from two countries (Turkey and the Czech Republic).
Table 1.
Characteristics of the Included Studies
| Author, year, and country | Sample size |
FTIR spectroscopic technique | Specimen | Diagnostic performance |
Confusion matrix | NOS assessment |
|---|---|---|---|---|---|---|
| Miskovicova et al., 2020; Czech Republic | CRC: 30 | ATR-FTIR | Blood plasma | FTIR only | FTIR only: | High Quality |
| Healthy: 33 | Sn: 73.3% | TP: 22 | ||||
| Sp: 81.8% | FP: 6 | |||||
| Acc: 77.8% | FN: 8 | |||||
| TN: 27 | ||||||
| FTIR combined with ROA, ECD, Raman | FTIR combined with ROA, ECD, Raman | |||||
| Sn: 90% | TP: 27 | |||||
| Sp: 75.8% | FP: 8 | |||||
| Acc: 82.5% | FN: 3 | |||||
| TN: 25 | ||||||
| Toraman et al., 2019; Turkey | CRC: 30 | ATR-FTIR | Blood plasma | MLPNN: | MLPNN: | High Quality |
| Healthy: 40 | ||||||
| 1300-1000 cm-1 | 1300-1000 cm-1 | |||||
| Sn: 93.3% | TP: 28 | |||||
| Sp: 97.5% | FP: 1 | |||||
| Acc: 95.7% | FN: 2 | |||||
| TN: 39 | ||||||
| 1800-1300 cm-1 | ||||||
| Sn: 83.3% | 1800-1300 cm-1 | |||||
| Sp: 100% | TP: 25 | |||||
| Acc: 92.9% | FP: 0 | |||||
| FN: 5 | ||||||
| TN: 40 | ||||||
| SVM: | SVM: | |||||
| 1300-1000 cm-1 | 1300-1000 cm-1 | |||||
| Sn: 93.3% | TP: 28 | |||||
| Sp: 95.0% | FP: 2 | |||||
| Acc: 94.3% | FN: 2 | |||||
| TN: 38 | ||||||
| 1800-1300 cm-1 | ||||||
| Sn: 83.3% | 1800-1300 cm-1 | |||||
| Sp: 92.5% | TP: 25 | |||||
| Acc: 88.6% | FP: 3 | |||||
| FN: 5 | ||||||
| TN: 37 |
Two studies were included in our analysis. One study [17] analyzed 63 plasma specimens from CRC cases (n=30) and healthy controls (n=33) using ATR-FTIR. Another study [18] also used ATR-FTIR to analyze 70 plasma specimens from CRC cases (n=30) and healthy controls (n=40). Both studies received high-quality ratings according to the NOS assessment.
Diagnostic efficiency of FTIR in detecting CRC using blood-based samples
Our analysis comprised six data entries from the two studies. Miskovicova et al. provided two datasets that were divided into FTIR only and FTIR combined with conventional spectroscopic techniques, such as Raman spectroscopy, Raman optical activity (ROA), and electronic circular dichroism (ECD). Toraman et al. contributed four datasets, which were divided into the multilayer perceptron neural network (MLPNN) and support vector machine (SVM) models. Both machine learning techniques were employed to assess the diagnostic potential of FTIR spectroscopy within the 1,800-1,300 cm-1 and 1,300-1,000 cm-1 ranges. Meta-DiSc 1.4 software [19] was utilized to measure the accuracy of each dataset and to visually present their diagnostic metrics, including sensitivities, specificities, likelihood ratios (LR), and diagnostic odds ratios (DOR), in forest plots.
Sensitivity and Specificity
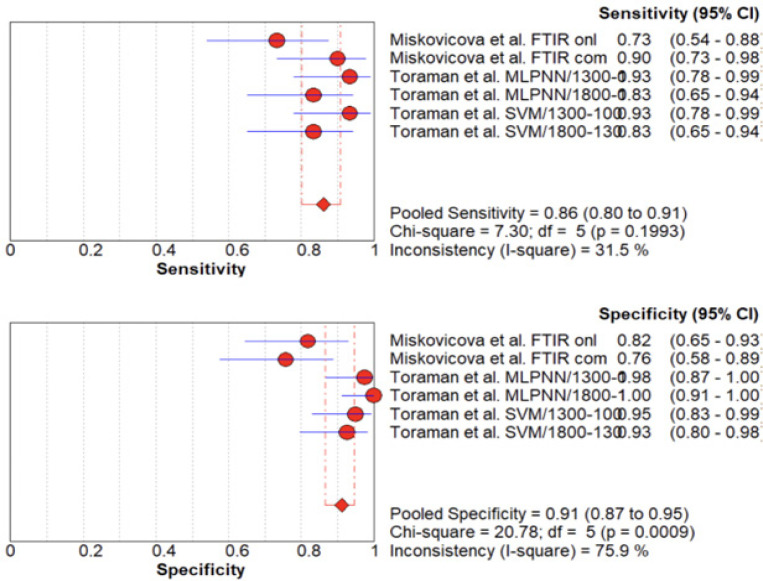
FTIR spectroscopy when used to detect CRC in plasma samples has an overall sensitivity ranging from 73% to 93% (Figure 2). Miskovicova et al. reported a sensitivity of 73.3% using ATR-FTIR spectral data, which increased to 90% after combining ATR-FTIR with spectral data from other spectroscopic techniques. Toraman et al. utilized machine learning algorithms, namely MLPNN and SVM, and reported the highest sensitivity of 93.3%. The pooled sensitivity across all six data entries was 86.10% (P > 0.05; df = 5), which was considered optimal.
Figure 2.
Forest Plot of Sensitivity (A) and Specificity (B) of FTIR Spectroscopy in Detecting Colorectal Cancer Using Blood Plasma.
The specificity varied from 76% to 100%, with the highest value of 98-100% achieved using MLPNN to analyze spectral data from the 1,800-1,300 cm-1 range. The pooled specificity across all six data entries was 91.2% (P < 0.05; df = 5). The I2 values for sensitivity and specificity were 31.5% and 75.9%, respectively, indicating moderate heterogeneity for sensitivity and substantial heterogeneity for specificity.
Likelihood Ratios
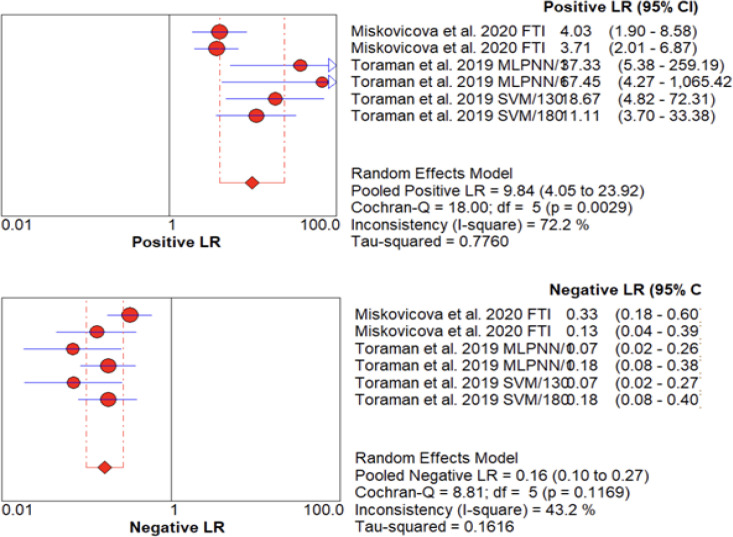
Likelihood ratios (LRs) are statistical measures used to evaluate the diagnostic accuracy of a test. A positive LR >10 and a negative LR <0.1 are considered to provide strong evidence for the presence or absence of a disease, respectively. In this study, the pooled positive LR (LR+) for FTIR spectroscopy in detecting colorectal cancer (CRC) was 9.84 (95% CI: 4.05-23.92), indicating a strongly moderate diagnostic value and conclusive evidence to rule in CRC. Conversely, the pooled negative LR (LR-) was 0.16 (95% CI: 0.10-0.27), suggesting a moderately decreased likelihood of CRC and decreased efficacy of FTIR spectroscopy in ruling out the disease (Figure 3).
Figure 3.
Forest Plot Showing Likelihood Ratios (LRs) with 95% Confidence Intervals (CIs) for positive (A) and negative (B) test results in detecting CRC using plasma analyzed by FTIR spectroscopy.
To assess the heterogeneity of the LR+ and LR-, we used the I2 statistic, Cochran-Q test, and T2. The LR+ exhibited high heterogeneity (I2 =72.2%; P> 0.05; T2 = 0.7760), while the LR- showed medium heterogeneity with significant variation between studies (I2 =43.2%; P> 0.05; T2 = 0.1616). Our findings suggest that FTIR spectroscopy has variable diagnostic accuracy across studies, highlighting the need for further research to address the sources of heterogeneity.
Diagnostic Odds Ratio
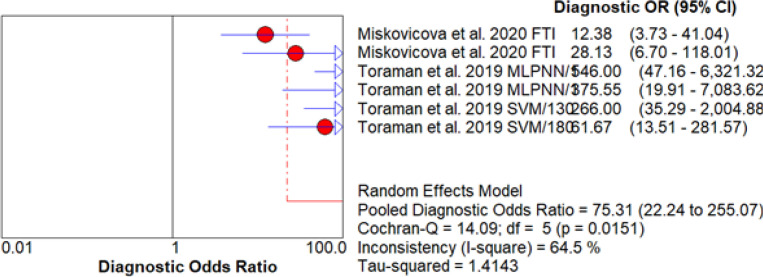
The DOR is a measure of the diagnostic accuracy of a test. In this study, the DOR values from all six datasets were greater than 10, which is considered favorable according to current standards. The MLPNN and the 1,300-1,000 cm-1 datasets exhibited high DOR values (>500), indicative of high sensitivity and specificity ratios with a low chance of false positives and false negatives (Figure 4).
Figure 4.
Forest Plot of Diagnostic Odds Ratio (DOR) for CRC Detection in Blood Plasma by FTIR Spectroscopy
The heterogeneity analysis showed that the six datasets were statistically different with a p-value of 0.015 (CI = 95%). The I-square statistic of 64.5% suggested substantial heterogeneity in the diagnostic specificity. The Cochran Q test also showed a significant difference between the datasets (p-value = 0.0151). To account for the heterogeneity, a random-effects model (REM) was used to calculate the pooled DOR of 75.314, suggesting that blood-based infrared spectroscopy is a robust method for early detection of CRC.
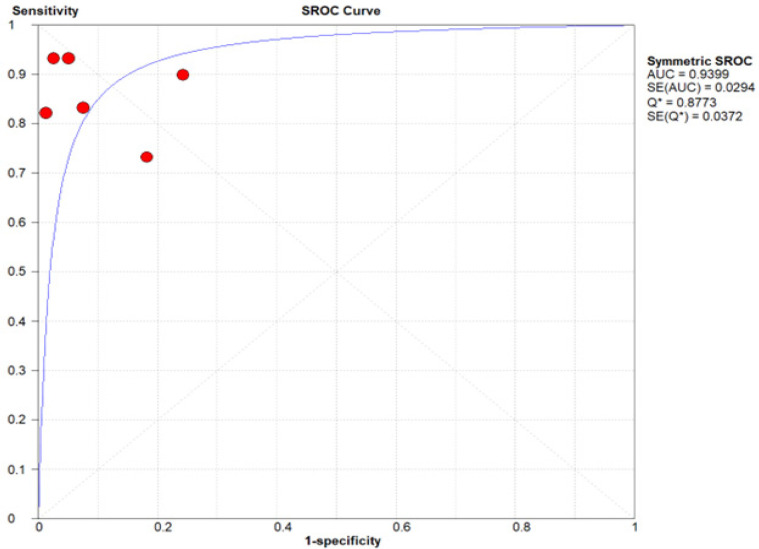
The sensitivity and specificity values of all six datasets were plotted on a summary receiver operating characteristic (SROC) curve, resulting in an AUC value of 0.9399 (SE = 0.0294), indicating that blood-based infrared spectroscopy is an accurate method for early detection of colorectal cancer (Figure 5). The Q* index value was calculated at 0.8773 (SE = 0.0372), which suggesting that blood-based infrared spectroscopy is efficient in differentiating patients with CRC from healthy ones.
Figure 5.
Diagnostic Accuracy Summary Receiver Operating Characteristic (SROC) Curve for FTIR to Detect CRC Using Blood Plasma.
Discussion
Detection of CRC in plasma samples using FTIR spectroscopy was examined in two countries (Turkey and the Czech Republic). The study found the overall sensitivity of the technique to range from 73% to 93%, with a pooled sensitivity of 86.10%. Specificity ranged from 76% to 100%, with a pooled specificity of 91.2%. Moreover, it proved a strongly moderate diagnostic value for detecting CRC as indicated by a pooled positive likelihood ratio (LR) of 9.84, while the pooled negative LR of 0.16 suggests decreased efficacy in ruling out the disease.
Effective early detection of CRC presents many challenges, including the limited resources for diagnosis due to lack of specific symptomatology. The most common diagnostic procedure, colonoscopy, is invasive and time-consuming, leading to delays in diagnosis that can be detrimental to patients. FTIR spectroscopy using blood samples may offer a diagnostic alternative with a simpler, minimally invasive sampling method. The advantages of FTIR spectroscopy make it a promising technique for early detection of colorectal cancer.
FTIR spectroscopy has been extensively studied for detecting CRC and other neoplasms using colonic tissue samples [20–22]. In this study, we investigated its potential to detect CRC using blood-based specimens. The chemical composition of a sample can be identified by measuring the absorption of infrared radiation at specific wavelengths using FTIR spectroscopy. Different chemical functional groups have characteristic absorption frequencies, which enable the identification of specific molecules in the sample. In plasma, each macromolecule has a unique set of absorption bands that correspond to its specific functional groups, which serve as spectral biomarkers for the detection of CRC and other neoplasms [23].
Caution should be exercised when interpreting the results due to the significant heterogeneity and low p-values among the studies. Possible factors that contribute to this heterogeneity include the minimal number of studies assessed, type of sample analyzed, and pre-analytical factors. The included articles displayed inconsistencies in patient selection and categorization. The lack of information regarding the specific diagnostic tests used to identify CRC in the samples analyzed by Toraman et al. limits the comparability of their results with other studies using FTIR spectroscopy to diagnose CRC via plasma analysis. This gap in knowledge also raises concerns about the reliability of their findings. Moreover, it was also observed that combining FTIR spectroscopy with other conventional spectroscopies or integrating machine learning models can improve diagnostic accuracy for CRC [17,18].
The criteria used to distinguish between healthy and CRC samples varied significantly across the studies. For example, Miskovicova et al. used a combination of spectroscopic methods including FTIR to discriminate between different clinical stages of CRC. Generally, a high accuracy rate for a diagnostic test is considered to be above 90% [24]. However, in their case, the overall accuracy rates achieved for differentiating the clinical stages of CRC using a combination of spectroscopic methods were below this threshold, ranging from 64% to 82%. Therefore, while the results suggest that spectroscopic methods could be a promising tool for identifying different stages of CRC, further research is needed to determine their effectiveness and limitations in this regard.
Meanwhile, Toraman et al. observed that although the spectral features extracted from several spectral bands showed promising differences between the two groups, using a t-test to compare the intensity values of absorption signals did not provide a significant distinction. Hence, they used more advanced analytical methods, such as machine learning algorithms to overcome this limitation. Based on their study, the spectral bands that were able to effectively discriminate between CRC and healthy controls were 1300 cm-1 to 1000 cm-1 using MLPNN (Sn: 93.3%, Sp: 97.5%, Acc: 95.7%) and SVM (Sn: 93.3%, Sp: 95.0%, Acc: 94.3%). The accuracy was slightly lower at 1800 cm-1 to 1300 cm-1 for both MLPNN (Sn: 83.3%, Sp: 100%, Acc: 92.9%) and SVM (Sn: 83.3%, Sp: 92.5%, Acc: 88.6%). These findings suggest that advanced analytical methods may be necessary to effectively distinguish between healthy and CRC plasma samples using ATR-FTIR spectroscopy.
Like other studies, the most significant spectral differences between healthy and colorectal plasma samples are observed in the spectral regions reflective of changes in protein secondary structure, with two dominant bands associated with the vibration of amide I and amide II [25,26]. Other spectral bands of interest for colorectal cancer correspond to deformation vibrations of methyl and methylene groups in the side chains of proteins and lipids, as well as stretching vibration of protein carboxyl groups of phospholipids and cholesterol. These molecular alterations may indicate protein misfolding and disordered lipid metabolism triggered by the pathogenesis of colorectal cancer.
Although histology remains the gold standard for cancer diagnosis, infrared spectroscopic analyses of biofluids have garnered increased interest in the scientific community [27]. This is mainly due to much easier and more efficient way of collecting, handling, and preparing biofluid samples for spectroscopic analysis [28]. With the rise of ATR accessories and modern data analytics, ATR-FTIR spectroscopy may provide diagnosis within minutes with small volumes of biofluid sample, particularly human blood serum or plasma [29]. The first study to analyze blood serum with FTIR spectroscopy reported more than 95% sensitivity and specificity for both machine learning classification methods – cluster analysis and artificial neural networks – in detecting breast cancer [30]. Since then, other studies have followed and have shown favorable results in diagnosing digestive tract cancers such as colorectal and gastric [31], breast cancer [13], brain cancer [32], and ovarian cancer [33] using blood-based FTIR spectroscopy. Thus, these studies may represent the utility of blood-based FTIR spectroscopy in the clinic particularly in early detection of cancer across almost all types of carcinomas.
In conclusion, we report in this meta-analysis the promising diagnostic efficiency of FTIR spectroscopy in detecting CRC using blood-based samples. While the findings suggest that FTIR spectroscopy has potential as a minimally invasive diagnostic method for CRC, which could improve early detection rates and patient outcomes, it has several limitations. The small number of studies analyzed may affect the overall results and the small sample sizes of the included studies may limit the statistical power of the analysis. The potential for publication bias is also a concern, as only studies published and available in the databases were included in the analysis. Further, the study did not provide a comparison of the diagnostic accuracy of FTIR spectroscopy with other minimally invasive diagnostic methods for CRC.
Future studies should evaluate the diagnostic accuracy of FTIR spectroscopy in diverse patient populations, identify sources of heterogeneity that may affect accuracy, compare the diagnostic accuracy of FTIR spectroscopy with other non-invasive diagnostic methods for CRC, and investigate the potential for FTIR spectroscopy to distinguish between different stages or subtypes of CRC. Further research is also needed to assess the clinical utility of FTIR spectroscopy as a screening tool for early detection of CRC in asymptomatic populations, as well as its feasibility for incorporation into routine clinical practice and cost-effectiveness compared to other diagnostic methods. Larger sample sizes are recommended for future studies to improve the statistical power and generalizability of the results.
Acknowledgements
Ethical declaration
The study did not involve any human participants or any animal model. Hence, no informed consent was implemented.
Data availability
All data relevant to the paper is included in the main text.
List of Abbreviations
AUC: Area under the receiver operating characteristic curve; ATR-FTIR: Attenuated Total Reflectance - Fourier Transform Infrared; CRC: Colorectal cancer; DOR: Diagnostic odds ratio; ECD: Electronic circular dichroism; LR: Likelihood ratios; MLPNN: Multilayer perceptron neural network; NOS: Newcastle-Ottawa Scale; PBMC: Peripheral blood mononuclear cells; ROA: Raman optical activity; SVM: Support vector machine.
Conflict of interest
The authors declare no conflict of interest.
Author Contribution Statement
All authors contributed equally in this study.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:1. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ting FIL, Sacdalan DBL, Tampo MMT, Apellido RT, Monroy HJ, Sacdalan MDP, et al. Treatment outcomes of patients with colorectal cancer enrolled in a comprehensive benefits program of the national insurance system in the philippines: Data from the pilot site. JCO Glob Oncol. 2020;6:35–46. doi: 10.1200/JGO.19.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 6.Chan SCH, Liang JQ. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev Mol Diagn. 2022;22(4):449–60. doi: 10.1080/14737159.2022.2065197. [DOI] [PubMed] [Google Scholar]
- 7.Kuderer NM, Burton KA, Blau S, Rose AL, Parker S, Lyman GH, et al. Comparison of 2 commercially available next-generation sequencing platforms in oncology. JAMA Oncol. 2017;3(7):996–8. doi: 10.1001/jamaoncol.2016.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed MA, Jaafar J, Ismail AF, Othman MHD, Rahman MA. Fourier Transform Infrared (FTIR) Spectroscopy. Membrane characterization: Elsevier; 2017 . [Google Scholar]
- 9.Su KY, Lee WL. Fourier transform infrared spectroscopy as a cancer screening and diagnostic tool: a review and prospects. Cancers. 2020 ;12(1):115. doi: 10.3390/cancers12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangaoil R, Santillan A, Angeles LM, Abanilla L, Lim A, Ramos MC, et al. Atr-ftir spectroscopy as adjunct method to the microscopic examination of hematoxylin and eosin-stained tissues in diagnosing lung cancer. PLoS One. 2020;15(5):e0233626. doi: 10.1371/journal.pone.0233626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala A, Anderson DJ, Brennan PM, Butler HJ, Cameron JM, Jenkinson MD, et al. Biofluid diagnostics by ftir spectroscopy: A platform technology for cancer detection. Cancer Lett. 2020;477:122–30. doi: 10.1016/j.canlet.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Santillan A, Tomas RC, Bangaoil R, Lopez R, Gomez MH, Fellizar A, et al. Discrimination of malignant from benign thyroid lesions through neural networks using ftir signals obtained from tissues. Anal Bioanal Chem. 2021;413(8):2163–80. doi: 10.1007/s00216-021-03183-0. [DOI] [PubMed] [Google Scholar]
- 13.Sitnikova VE, Kotkova MA, Nosenko TN, Kotkova TN, Martynova DM, Uspenskaya MV. Breast cancer detection by atr-ftir spectroscopy of blood serum and multivariate data-analysis. Talanta. 2020;214:120857. doi: 10.1016/j.talanta.2020.120857. [DOI] [PubMed] [Google Scholar]
- 14.Villamanca JJ, Hermogino LJ, Ong KD, Paguia B, Abanilla L, Lim A, et al. Predicting the likelihood of colorectal cancer with artificial intelligence tools using fourier transform infrared signals obtained from tumor samples. Appl Spectrosc. 2022;76(12):1412–28. doi: 10.1177/00037028221116083. [DOI] [PubMed] [Google Scholar]
- 15.Fadlelmoula A, Pinho D, Carvalho VH, Catarino SO, Minas G. Fourier transform infrared (ftir) spectroscopy to analyse human blood over the last 20 years: A review towards lab-on-a-chip devices. Micromachines (Basel) 2022;13:2. doi: 10.3390/mi13020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabico LJ, Jaron JN, Mosqueda ME, Wu JJ, Tiongco RE, Albano PM. Diagnostic efficiency of serum-based infrared spectroscopy in detecting breast cancer: A meta-analysis. Lab Med. 2023;54(1):98–105. doi: 10.1093/labmed/lmac068. [DOI] [PubMed] [Google Scholar]
- 17.Miskovicova M, Fryba V, Petruzelka L, Setnicka V, Synytsya A, Tatarkovic M, et al. Novel spectroscopic biomarkers are applicable in non-invasive early detection and staging classification of colorectal cancer. Neoplasma. 2020;67(6):1349–58. doi: 10.4149/neo_2020_200506N494. [DOI] [PubMed] [Google Scholar]
- 18.Toraman S, GİRgİN M, ÜStÜNdaĞ B, Turkoglu I. Classification of the likelihood of colon cancer with machine learning techniquesusing ftir signals obtained from plasma. Turk J Elec Eng Comp Sci. 2019;27:1765–79. [Google Scholar]
- 19.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-disc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong L, Sun X, Chao Z, Zhang S, Zheng J, Gurung R, et al. Evaluation of ftir spectroscopy as diagnostic tool for colorectal cancer using spectral analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2014;122:288–94. doi: 10.1016/j.saa.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Piva J, Piva C, Lucas J, Silva R, Raniero L, Silvia C, et al. Biochemical imaging of normal, adenoma, and colorectal adenocarcinoma tissues by fourier transform infrared spectroscopy (ftir) and morphological correlation by histopathological analysis: Preliminary results. Revista Brasileira de Engenharia Biomédica. 2015;31:10 . [Google Scholar]
- 22.Song CL, Kazarian SG. Effect of controlled humidity and tissue hydration on colon cancer diagnostic via ftir spectroscopic imaging. Anal Chem. 2020;92(14):9691–8. doi: 10.1021/acs.analchem.0c01002. [DOI] [PubMed] [Google Scholar]
- 23.Depciuch J, Kaznowska E, Koziorowska A, Cebulski J. Verification of the effectiveness of the fourier transform infrared spectroscopy computational model for colorectal cancer. J Pharm Biomed Anal. 2017;145:611–5. doi: 10.1016/j.jpba.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Pepe MS. The statistical evaluation of medical tests for classification and prediction. Oxford university press; 2003. [Google Scholar]
- 25.Nallala J, Gobinet C, Diebold MD, Untereiner V, Bouché O, Manfait M, et al. Infrared spectral imaging as a novel approach for histopathological recognition in colon cancer diagnosis. J Biomed Opt. 2012;17(11):116013. doi: 10.1117/1.JBO.17.11.116013. [DOI] [PubMed] [Google Scholar]
- 26.Nallala J, Piot O, Diebold MD, Gobinet C, Bouché O, Manfait M, et al. Infrared imaging as a cancer diagnostic tool: Introducing a new concept of spectral barcodes for identifying molecular changes in colon tumors. Cytometry A. 2013;83(3):294–300. doi: 10.1002/cyto.a.22249. [DOI] [PubMed] [Google Scholar]
- 27.Baker M, Hussain S, Lovergne L, Untereiner V, Hughes C, Lukaszewski RA, et al. Developing and understanding biofluid vibrational spectroscopy: A critical review. Chemical Society reviews. 2015:45 . doi: 10.1039/c5cs00585j. [DOI] [PubMed] [Google Scholar]
- 28.Shaw RA, Low-Ying S, Man A, Liu KZ, Mansfield C, Rileg CB, Vijarnsorn M. Infrared spectroscopy of biofluids in clinical chemistry and medical diagnostics. Biomedical vibrational spectroscopy. 2008 [Google Scholar]
- 29.Butler H, Smith B, Fritzsch R, Radhakrishnan P, Palmer D, Baker M. Optimised spectral pre-processing for discrimination of biofluids via atr-ftir spectroscopy. The Analyst. 2018:143. doi: 10.1039/c8an01384e. [DOI] [PubMed] [Google Scholar]
- 30.Backhaus J, Müller R, Formanski N, Szlama N, Meerpohl H-G, Eidt M, et al. Diagnosis of breast cancer with infrared spectroscopy from serum samples. Vibrational Spectroscopy. 2010;52:173–7. [Google Scholar]
- 31.Guo S, Wei G, Chen W, Lei C, Xu C, Guan Y, et al. Fast and deep diagnosis using blood-based atr-ftir spectroscopy for digestive tract cancers. Biomolecules. 2022;12:12. doi: 10.3390/biom12121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler HJ, Brennan PM, Cameron JM, Finlayson D, Hegarty MG, Jenkinson MD, et al. Development of high-throughput atr-ftir technology for rapid triage of brain cancer. Nat Commun. 2019;10(1):4501 . doi: 10.1038/s41467-019-12527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giamougiannis P, Morais CLM, Rodriguez B, Wood NJ, Martin-Hirsch PL, Martin FL. Detection of ovarian cancer (± neo-adjuvant chemotherapy effects) via atr-ftir spectroscopy: Comparative analysis of blood and urine biofluids in a large patient cohort. Anal Bioanal Chem. 2021;413(20):5095–107. doi: 10.1007/s00216-021-03472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the paper is included in the main text.