Abstract
Intraflagellar transport (IFT) involves the coordinated transport of molecular motors and other proteins and is required for ciliogenesis and ciliary maintenance. The C. elegans IFT protein OSM-5 /IFT88 is expressed in a majority of the ciliated neurons in the animal, and osm-5 mutants exhibit structurally defective cilia. The osm-5 promoter is commonly used to express genetic constructs in the ciliated neurons. In this study, we show that brightness of osm-5p- driven constructs is altered in mutants of the tubulin deglutamylase ccpp-1 and the NIMA-related kinase nekl-4 . This raises the possibility that osm-5 expression levels may be regulated by ccpp-1 and nekl-4 .
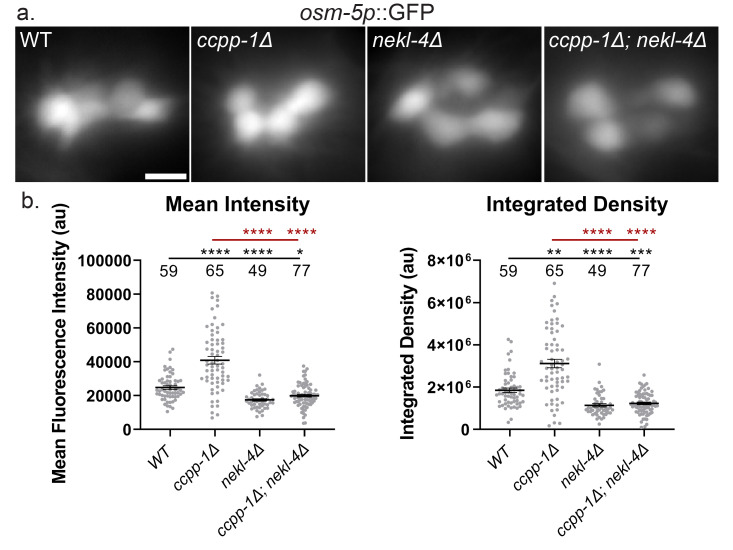
Figure 1. osm-5p- driven soluble GFP differs in brightness in ccpp-1Δ and nekl-4Δ mutant ciliated neurons .
a. Widefield images of soluble osm-5p ::GFP in the phasmid soma, sum intensity projections. Scale bar = 5 µm. b. Quantification of mean fluorescence intensity and integrated density of osm-5p ::GFP fluorescence in the phasmid soma. au = arbitrary unit. Mean ± SEM; * indicates p ≤ 0.05, ** indicates p ≤ 0.01, *** indicates p ≤ 0.001, **** indicates p ≤ 0.0001 by Kruskal-Wallis one-way ANOVA with post hoc Dunn's correction for multiple comparisons. n is indicated above each genotype. Black indicates significance relative to WT, red indicates significance relative to ccpp-1Δ.
Description
Cilia are built and maintained by the coordinated transport of molecular motors and other proteins, collectively named intraflagellar transport (IFT) (Reiter and Leroux, 2017) . C. elegans OSM-5 is an IFT-B protein homologous to mammalian IFT88, which is required for primary cilium assembly (Pazour et al., 2000; Qin et al., 2001) . Sensory cilia are severely shortened and deformed in osm-5 mutants, and expectedly, OSM-5 is expressed in a majority of the ciliated neurons in C. elegans (Perkins et al., 1986; Haycraft et al., 2001) . Due to its pan-ciliary expression and short (240 bp) length, the osm-5 promoter is commonly used to drive expression of transgenic constructs in the ciliated neurons. In this study, we uncovered differential brightness levels of osm-5p- driven constructs that may indicate transcriptional regulation of osm-5 by the cilia-related proteins CCPP-1 and NEKL-4 .
Previously, we examined localization and brightness of multiple osm-5p -driven extrachromosomal constructs to study ciliary and mitochondrial functions of the tubulin deglutamylase CCPP-1 and the Never-In-Mitosis A (NIMA)-related kinase NEKL-4 (Power et al., 2024) . In both ccpp-1Δ and nekl-4Δ mutants, we observed differences in the brightness of osm-5p -driven TOMM-20 ::tagRFP and roGFP. When quantified, total levels of roGFP in the phasmid soma were significantly increased in ccpp-1Δ mutants and decreased in nekl-4Δ and ccpp-1Δ; nekl-4Δ mutants (Power et al., 2024, Figure S5a). To determine if this phenomenon was array-specific, we quantified the fluorescence intensity of soluble osm-5p ::GFP in the phasmid soma of ccpp-1Δ and nekl-4Δ mutants. Sum intensity projections showed a slight increase in brightness in ccpp-1Δ mutants, and a slight decrease in nekl-4Δ single and ccpp-1Δ; nekl-4Δ double mutants ( Fig 1a ). When quantified, both the mean fluorescence intensity and integrated density were significantly increased in ccpp-1Δ mutants and significantly decreased in nekl-4Δ and ccpp-1Δ; nekl-4Δ mutants ( Fig 1b ). This may indicate that nekl-4 promotes transcription of osm-5 while ccpp-1 represses osm-5 transcription, and that nekl-4 is epistatic to ccpp-1 with respect to osm-5 expression regulation.
Further studies, including transcriptomics and quantitative PCR, are necessary to definitively determine the effects of nekl-4 and ccpp-1 mutation on osm-5 transcription, as well as the effects of these mutations on the expression of other ciliary genes. It has been shown that expression levels of nekl-4 are tightly regulated via RNA editing in order to prevent overexpression of NEKL-4 and other kinases to prevent ciliary instability (Li et al., 2021) . Therefore, it is possible that NEKL-4 protein levels influence transcription of osm-5 and other ciliary genes. The osm-5 promoter contains an X-box motif, which is found in the promoters of many mammalian and C. elegans genes involved in cilia and ciliopathies (Chen et al., 2006) . This presents the possibility that nekl-4 and ccpp-1 may affect expression of other cilia-related genes with X-box-containing promoters, such as dyf-5 , bbs-5 , and che-11 . Further experiments with these mutants would provide valuable insight into the complex regulatory mechanisms that underlie ciliary protein expression.
Methods
Widefield imaging
Live animals were anesthetized with 10 mM levamisole and mounted on 10% agarose pads for imaging at room temperature. Widefield images were acquired on a Zeiss Axio Observer with Colibri 7 LEDs and ZenBlue software (Carl Zeiss Microscopy, Oberkochen, Germany) using a Photometrics Prime 95B sCMOS camera (Teledyne Photometrics, Tucson, AZ). A 63x/1.4 Oil Plan-Apochromat objective was used for imaging. Acquisition settings were identical for all genotypes.
Quantification of GFP brightness
Image files were imported into Fiji/ImageJ with the BioFormats Importer plugin and sum intensity projections including the entire set of phasmid soma were created. Images were duplicated and used to create ROIs including only the phasmid soma by thresholding. The mean fluorescence intensity and integrated density within the ROI were measured. Kruskall-Wallis one-way ANOVA analysis and posthoc Dunn's multiple comparison test were performed in Prism (Graphpad Software).
Reagents
PT2700 : pha-1 ( e2123 )III; him-5 ( e1490 )V; myEx819 [osm-5p::GFP + pBx]
PT3826 : pha-1 ( my82 [ pha-1 ( e2123 )+SnaBI]) nekl-4 ( tm4910 )III; him-5 ( e1490 )V; myEx819 [osm-5p::GFP + pBx]
PT3837 : ccpp-1 ( ok1821 )I; pha-1 ( e2123 )III; him-5 ( e1490 )V; myEx819 [osm-5p::GFP + pBx]
PT3838 : ccpp-1 ( ok1821 )I; pha-1 ( my82 [ pha-1 ( e2123 )+SnaBI]) nekl-4 ( tm4910 )III; him-5 ( e1490 )V; myEx819 [osm-5p::GFP + pBx]
Acknowledgments
Acknowledgments
We thank the members of the Barr laboratory for their feedback on this research, particularly Inna Nikonorova, Juan Wang, and Katie Jacobs. We also thank Gloria Androwski for technical assistance, and Keora Power for support. We thank WormBase (U41 HG002223) and WormAtlas (R24 OD010943) for valuable online resources.
References
- Chen Nansheng, Mah Allan, Blacque Oliver E, Chu Jeffrey, Phgora Kiran, Bakhoum Mathieu W, Hunt Newbury C Rebecca, Khattra Jaswinder, Chan Susanna, Go Anne, Efimenko Evgeni, Johnsen Robert, Phirke Prasad, Swoboda Peter, Marra Marco, Moerman Donald G, Leroux Michel R, Baillie David L, Stein Lincoln D. null. Genome Biology. 2006;7(12):R126–R126. doi: 10.1186/gb-2006-7-12-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft Courtney J., Swoboda Peter, Taulman Patrick D., Thomas James H., Yoder Bradley K. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms . Development. 2001 May 1;128(9):1493–1505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- Li Dongdong, Liu Yufan, Yi Peishan, Zhu Zhiwen, Li Wei, Zhang Qiangfeng Cliff, Li Jin Billy, Ou Guangshuo. RNA editing restricts hyperactive ciliary kinases. Science. 2021 Aug 27;373(6558):984–991. doi: 10.1126/science.abd8971. [DOI] [PubMed] [Google Scholar]
- Pazour Gregory J., Dickert Bethany L., Vucica Yvonne, Seeley E. Scott, Rosenbaum Joel L., Witman George B., Cole Douglas G. Chlamydomonas IFT 88 and Its Mouse Homologue, Polycystic Kidney Disease Gene Tg 737, Are Required for Assembly of Cilia and Flagella . The Journal of Cell Biology. 2000 Oct 30;151(3):709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins Lizabeth A., Hedgecock Edward M., Thomson J.Nichol, Culotti Joseph G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Developmental Biology. 1986 Oct 1;117(2):456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Power Kaiden M., Nguyen Ken C., Silva Andriele, Singh Shaneen, Hall David H., Rongo Christopher, Barr Maureen M. NEKL-4 regulates microtubule stability and mitochondrial health in ciliated neurons. Journal of Cell Biology. 2024 May 20;223(9) doi: 10.1083/jcb.202402006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Hongmin, Rosenbaum Joel L., Barr Maureen M. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Current Biology. 2001 Mar 1;11(6):457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Reiter Jeremy F., Leroux Michel R. Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology. 2017 Jul 12;18(9):533–547. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]