Abstract
Background:
Respiratory syncytial virus (RSV) poses a substantial threat to infants, often leading to challenges in hospital capacity. With recent pharmaceutical developments to be used during the prenatal and perinatal periods aimed at decreasing the RSV burden, there is a pressing need to identify infants at risk of severe disease. We aimed to stratify the risk of developing a clinically severe RSV infection in infants under 1 year of age.
Methods:
This retrospective observational study was conducted at the Hospices Civils de Lyon, France, involving infants born between 2014 and 2018. This study focused on infants hospitalized with severe and very severe acute lower respiratory tract infections associated with RSV (SARI-WI group). Data collection included perinatal information and clinical data, with machine-learning algorithms used to discriminate SARI-WI cases from nonhospitalized infants.
Results:
Of 42,069 infants, 555 developed SARI-WI. Infants born in November were very likely (>80%) predicted SARI-WI. Infants born in October were very likely predicted SARI-WI except for births at term by vaginal delivery and without siblings. Infants were very unlikely (<10%) predicted SARI-WI when all the following conditions were met: born in other months, at term, by vaginal delivery and without siblings. Other infants were possibly (10–30%) or probably (30–80%) predicted SARI-WI.
Conclusions:
Although RSV preventive measures are vital for all infants, and specific recommendations exist for patients with high-risk comorbidities, in situations where prioritization becomes necessary, infants born just before or within the early weeks of the epidemic should be considered as a risk group.
Keywords: severe infection, hospitalization, stratification
Respiratory syncytial virus (RSV) is the most common cause of viral acute lower respiratory tract infections in children younger than 5 years of age worldwide and is also associated with infant mortality in low-income countries.1 The ubiquitous nature of this infection is evident from population studies, showing that about 90% of children contract RSV by the age of 3 years.2 In high-income countries, RSV ranks among the top causes of outpatient visits for children and leads to hospitalization in 1%–3% of all infants during their first year of life.3–5 This substantial number of hospital admissions during RSV epidemics places immense strain on hospital resources, especially when coupled with other concurrent respiratory epidemics such as influenza and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).6
The significant global burden of RSV-related morbidity underscores the need for preventive strategies, encompassing both nonpharmaceutical interventions (NPI), which have shown efficacy during the SARS-CoV-2 pandemic, and pharmaceutical interventions.7 Recent pharmaceutical advancements offer promising avenues for reducing the impact of RSV in infants, including passive immunization utilizing a long-lasting monoclonal antibody (nirsevimab) as well as vaccination during pregnancy.8,9 Given their administration during the prenatal and perinatal periods, it becomes crucial to identify infants at high risk of severe disease as soon as the maternity stay for the optimal and targeted deployment of interventions.
Classical statistics have limited power to build predictive models based on events occurring in 1%–3% of the entire population. In light of this, machine-learning analysis emerges as an invaluable tool for investigating individual risk factors for severe RSV disease.10
The objective of this study was to stratify the perinatal risk of severe RSV hospitalizations within a large birth cohort from a public hospital in France, with the aim of providing a tool to guide targeted prevention. As a novelty, we used the standard severity definitions developed by the World Health Organization (WHO) to identify the most severe RSV cases, and employed machine-learning-based techniques to address the inherent imbalance in the event distribution within the birth cohort.
METHODS
Study Population
We conducted an observational study on a historical birth cohort of newborns delivered at the Hospices Civils de Lyon (HCL). The HCL is the only public tertiary hospital serving the city of Lyon, France, and its surrounding urban area (1.4 million inhabitants).11 All infants born at HCL between January 1, 2014, and December 31, 2018 to parents residing in the Lyon urban area were included in the cohort.12
Multiplex RSV-reverse transcription polymerase chain reaction (RT-PCR) is routinely done in the HCL pediatric wards during the winter epidemic season (October–March) for all infants hospitalized with respiratory symptoms.13 Infants born at the HCL and admitted to the HCL pediatric wards with a positive RSV-RT-PCR within their first year of life were identified. Perinatal characteristics of the infants in the cohort were extracted from the hospital birth database. Risk factors explored in the birth cohort were defined according to availability in the electronic health records, French health authority guidelines and the literature.12,14–16 Missing perinatal risk factors from the birth database were manually retrieved when possible. All medical files from the RSV-infected infants were reviewed by 2 pediatricians; for training purposes, 20% of their first medical files were double-reviewed to ensure convergence of their ratings. Vital measurements, clinical observations, and critical event descriptions were collected. Extreme values of respiratory rate and transcutaneous oxygen saturation (SpO2) were rigorously cross-referenced with the individual clinical histories to avoid inconsistent values and were considered if consistent with the clinical picture.
Variables Definition
Categorical birth variables were: multiparity, mode of delivery, preterm birth, small for gestational age (SGA), multiple birth and birth month. Term delivery was defined as a birth occurring after 37 weeks of gestational age (GA), and moderate and very preterm deliveries were defined as births occurring at age ≥32 GA to <37 GA, and <32 GA, respectively. Small for GA was defined as a birth weight under the 10th percentile for the GA. Continuous variables were GA, number of weeks between birth and onset of seasonal epidemic (birth to epidemic delay), and birth weight expressed in Z-score for GA.
For the RSV-hospitalized infants, the main severity was assessed according to the WHO severity definitions: Upper respiratory tract infections (URTI) defined as cough or breathing difficulties; lower respiratory tract infections (LRTI) defined as age-adjusted tachypnea or SpO2 <95%; Severe LRTI (S-LRTI) defined as SpO2 <93% or chest-wall indrawing; and very severe LRTI (VS-LRTI) defined as SpO2 <90%, or inability to feed, or failure to respond/unconscious;17 the latter was functionally translated as neurological impairment gathering clinically significant apnea—apnea/bradycardia associated with unconsciousness, and/or marked change in skin and muscle tone. To summarize, criteria for S-/VS-LRTI were: neurological impairment, a SpO2 <93% or chest-wall indrawing (i.e. respiratory impairment) and inability to feed (i.e. nutritional impairment). Infants with any of the 3 functional impairments thus defined represented the group of infants with significant clinical severity, referred to here “severe acute respiratory infection with functional impairment” (SARI-WI).
Infants with missing information that could not be retrieved, with nosocomial infection (for whom the respiratory symptoms started after 2 days of hospitalization), without respiratory symptoms (including sudden infant death syndrome), hospitalized less than 24 hours before a transfer to another hospital (only the infants with less severe infection were transferred), and infants whose parents refused to be part of the study, were excluded. Admitted infants with URTI and nonsevere LRTI were excluded from the main analysis.
Analysis
The control group for SARI-WI prediction were infants who were never hospitalized during their first year of life. No statistical tests were performed to compare the SARI-WI and the nonhospitalized populations as the results would be affected by large and imbalanced sample sizes. Machine-learning algorithms for classification were applied to discriminate between SARI-WI cases and nonhospitalized infants. The birth cohort data set was randomly split so that information from 75% of the infants was used to train the algorithms and the remaining 25% served as a test set. Several models were applied and compared, all implemented in the caret R package: random forests (ranger),18 bagging (Adabag),19 gradient boosting machine (gbm),20 support vector machine (SVM) with radial kernel (svmPoly) or polynomial kernel (svmRadial)21 and logistic regression with elastic-net penalization (glmnet).22 For each model, a grid search was conducted to find the optimal parameters maximizing the mean area under the receiving operating curve over 20 repetitions of a 5-fold cross-validation procedure. In addition, to avoid that predictions would be affected by the extreme imbalance in the size of both groups, down sampling for non-hospitalized infants was applied to each fold to obtain equal size in both groups. Other techniques of subsampling were tested, unsuccessfully given the nature of the data (not shown). The model with the highest sensitivity on the train set was retained as the final model for interpretation. Sensitivity and specificity of the models on the test set were also evaluated, to anticipate the performances of the models on completely unseen data. The weight of each variable was measured individually through a filtering approach using the area under the receiving operating curve, scaled from 0 to 100. This measure, called variable importance (VI), was evaluated separately for each covariate to avoid misleading interpretations due to correlations between covariates. The final model was interpreted with the frequencies of SARI-WI predictions in groups formed by the combination of the most important variables. Four categories of SARI-WI predictions were created: very likely (more than 80% of the cases in the group), probable (between 30 and 80% of cases in the group), possible (between 10 and 30% of cases in the group) and very unlikely (<10% of cases in the group).
Analyzes used R statistical software, version 4.2.2. (R CoreTeam. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/).
Ethics
The study protocol was approved by the Comité Éthique et Scientifique des HCL (21_502 21_5502) and registered on ClinicalTrials.gov (NCT05348616). In accordance with French regulations, parents were informed of the study by postal mail, and any refusal expressed by the parents was excluded. The database was registered to the French data protection agency according to the MR004-protocol (CNIL- n°21_5502, September 2021, 28). STROBE guidelines were followed to report the study.
RESULTS
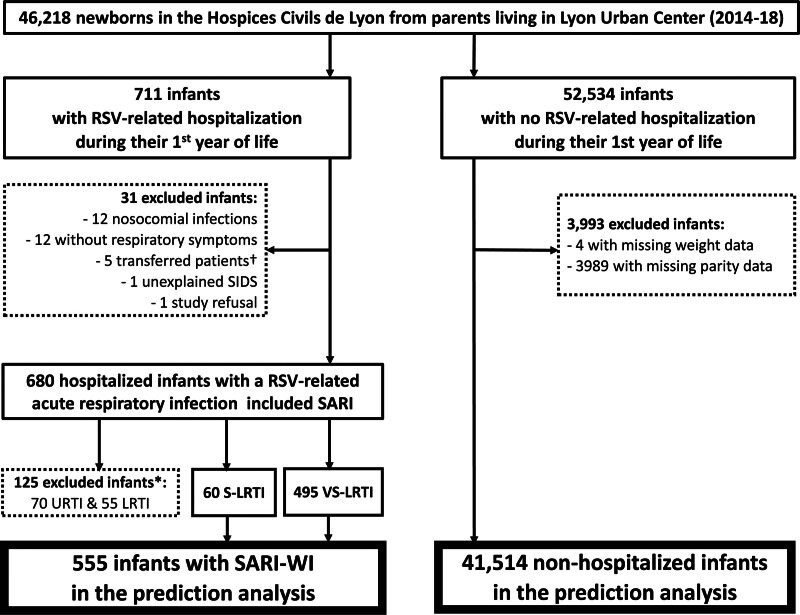
Overall, 46,218 newborns were identified in the birth cohort database, that is, 44% (46,218/104,366) of all the Lyon urban area births from January 1, 2014, to December 31 2018.11 Of these, 4021 were excluded from the analyses, mostly due to missing parity data (n = 3989, other reasons are shown in Fig. 1). As such, 42,069 infants were included in the prediction analysis: 41,514 were nonhospitalized infants and 555 were SARI-WI cases (Fig. 1), with an incidence rate of 1337 SARI-WI cases per 100,000 infants/year.
FIGURE 1.
Study flow chart. *125 infants with URTI/LRTI were excluded after a sensitivity analysis showed no significant difference when the 125 cases were included in the nonhospitalized group. †Transfers-out were used for lower severity and lower-risk infants in case of bed saturation. LRTI indicates lower respiratory tract infection (WHO-definition); RSV, respiratory syncytial virus; SARI, severe acute respiratory infection (WHO-definition); SARI-WI, SARI with functional impairment; SIDS, sudden infant death syndrome; S-LRTI, severe lower respiratory tract infection (WHO-definition); URTI, upper respiratory tract infection (WHO-definition); VS-LRTI, very severe lower respiratory tract infection (WHO-definition).
Table 1 summarizes the population characteristics among SARI-WI and nonhospitalized infants.
TABLE 1.
Population Characteristics of the Birth Cohort
| SARI-WI (N = 555) |
Nonhospitalized (N = 41,514) |
Total (N = 42,069) |
|
|---|---|---|---|
| Moderate preterm* | 78; (14) | 2758; (7) | 2836; (7) |
| Very preterm* | 29; (5) | 638; (2) | 667; (2) |
| Small for gestational age* | 40; (7) | 2641; (6) | 2681; (6) |
| Cesarean section delivery* | 151; (27) | 5928; (14) | 6079; (14) |
| Twin birth* | 48; (9) | 1993; (5) | 2041; (5) |
| Male* | 305; (55) | 20,916; (50) | 21,221; (50) |
| Multiparity* | 406; (73) | 24,010; (58) | 24,416; (58) |
| Birth month* | |||
| January | 23; (4) | 3553; (9) | 3576; (9) |
| February | 6; (1) | 3128; (8) | 3134; (7) |
| March | 11; (2) | 3410; (8) | 3421; (8) |
| April | 14; (3) | 3384; (8) | 3398; (8) |
| May | 20; (4) | 3534; (9) | 3554; (8) |
| June | 20; (4) | 3565; (9) | 3585; (9) |
| July | 22; (4) | 3652; (9) | 3674; (9) |
| August | 32; (6) | 3584; (9) | 3616; (9) |
| September | 55; (10) | 3464; (8) | 3519; (8) |
| October | 128; (23) | 3502; (8) | 3630; (9) |
| November | 141; (25) | 3387; (8) | 3528; (8) |
| December | 83; (15) | 3351; (8) | 3434; (8) |
| Z-score at birth† | 0.3 (−0.5 to 1.1) | 0.2 (−0.4 to 0.9) | 0.2 (−0.4 to 0.9) |
| Birth to epidemic delay† | −3.0 (−8.0 to 1.0) | 0.0 (−13.0 to 13.0) | 0.0 (−13.0 to 13.0) |
| Gestational age† | 39.0 (37.5–40.0) | 39.0 (38.0–40.0) | 39.0 (38.0–40.0) |
Data presented as n; (%).
Data presented as median (interquartile range).
SARI-WI indicates severe acute respiratory infection with functional impairment.
The performances of the 6 machine-learning algorithms evaluated with their optimal parameters on the train set are displayed in Table 2. The retained final model was the bagging, with an 80.8% sensitivity (the highest) and a 70.2% specificity (ranked second). As expected, all sensitivities decreased when models were applied to the test set, with a maximum discrepancy of 2.9%.
TABLE 2.
Sensitivity and Specificity of the Machine-learning Algorithms Evaluated With Their Optimized parameters on the Train and Test Sets
| Train Set | Test Set | |||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| Random forests | 80.3 | 68.1 | 72.5 | 68.3 |
| Bagging | 80.8 | 70.2 | 72.5 | 70.4 |
| Gradient boosting machine | 78.9 | 68.6 | 73.2 | 68.9 |
| SVM—radial kernel | 77.0 | 68.1 | 73.2 | 68.7 |
| SVM—polynomial kernel | 77.0 | 67.7 | 73.9 | 68.3 |
| Penalized logistic regression | 74.1 | 70.3 | 71.0 | 70.6 |
SVM, support vector machine.
Bold values indicate the values for the selected algorithm.
The VI for predictors in the classification of SARI-WI cases exhibited a leading group of 6 variables above 50. GA had the highest VI (VI = 100), followed by births in October (VI = 74) or November (VI = 72), parity (VI = 69), type of delivery (VI = 59) and the birth to epidemic delay (VI = 59). However, since this last variable was directly linked to the month of birth, it was discarded for the interpretation of the bagging model (see Figure, Supplemental Digital Content 1, http://links.lww.com/INF/F542).
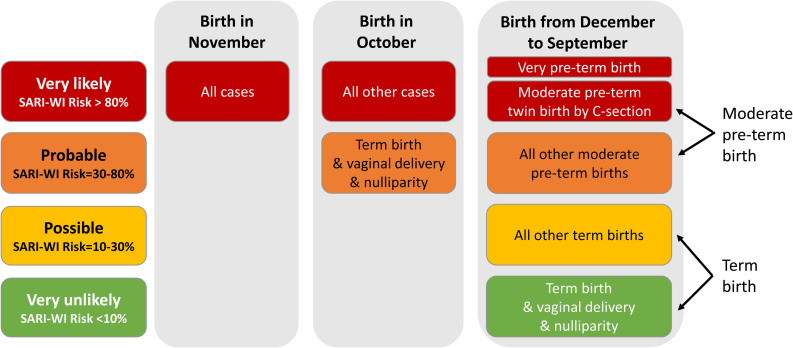
The bagging algorithm allowed to express a class of SARI-WI prediction using these 5 variables. Infants born in November were all very likely (>80%) predicted SARI-WI. Infants born in October were all very likely predicted SARI-WI except for births at term by vaginal delivery and without siblings (i.e. born to nulliparous mother), for whom the risk to be a SARI-WI case was probable (30%–80%). Infants born from December to September were very likely predicted SARI-WI if very preterm, or moderate preterm from a multiple birth pregnancy delivered by cesarean section, and probably predicted SARI-WI for other moderate preterm births. Other infants born in these months were very unlikely (<10%) predicted SARI-WI cases when all the following conditions are met: born at term, by vaginal delivery and without siblings. They were possibly (10%–30%) predicted SARI-WI for other births at term (Fig. 2). For infants born from December to September, the presence of siblings increased SARI-WI risk up to 17%, and a cesarean delivery increased SARI-WI risk up to 23% (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/F543).
FIGURE 2.
Prediction of SARI with functional impairment (SARI-WI) frequency classes using the bagging algorithm. Red is the category of infants who should be prioritized for preventive interventions. Orange, yellow, and green are the next categories. The risk of SARI-WI for births in October and November are at the highest levels of risk. For infants born during the other months, SARI-WI risk may be estimated more precisely according to perinatal variables of importance (see Table, Supplemental Digital Content 2. http://links.lww.com/INF/F543).
Sensitivity analyses were conducted with the creation of a class for missing parity and imputation of missing parity values (using the “ranger” R package); results remained the same using these strategies (data not shown). Another sensitivity analysis was done including the 125 RSV-infected infants classified as URTI and nonsevere LRTI in the non-hospitalized group; results were unchanged (data not shown).
DISCUSSION
The risk of developing clinically severe RSV infection (SARI-WI) was stratified using perinatal variables available at the time of birth in a large birth cohort in France. Our model’s output generated a risk distribution based on the classes of frequencies of SARI-WI predictions (Fig. 2). This type of chart holds practical significance, as it can be effectively employed within maternity settings to identify mothers of high-risk infants promptly, facilitating the application of both pharmaceutical interventions and NPIs. Our work is complementary to existing recommendations for high-risk infants, such as previous palivizumab indications for bronchopulmonary dysplasia or congenital heart disease; we aimed to give a practical risk gradation in the general population for prioritization of existing or incoming pharmaceutical and nonpharmaceutical preventive interventions. Our findings highlighted birth month as the main driver influencing the likelihood of severe RSV disease in our setting. This observation aligns with previous reports exploring factors associated with RSV infection,12,23,24 and highlights the notion that infants born during the initial weeks of the epidemic face a higher risk due to the absence of passive transplacental immunity naturally acquired by their mothers, as their mothers have not recently encountered the virus.2 In our setting, November births were at the highest risk of severe RSV disease, with the RSV epidemic usually starting at week 48 and lasting for 14–15 weeks.25 It is important to note that this chart is tailored for countries where RSV circulation follows seasonal patterns. Local adaptations will be necessary to align with the specific temporal distribution trends in each region. For infants born outside of the early epidemic period, GA at birth, delivery method and existence of siblings were important variables to predict SARI-WI.
We expanded from a previous work that did not account for the severity of the disease and was limited to infants younger than 6 months.12 We benefited from the inclusion of a comprehensive birth cohort, which encompassed data from all public maternity hospitals in the Lyon area, mitigating selection bias. Furthermore, we conducted a thorough identification of all RSV-infected hospitalized infants. At the HCL, all infants admitted to a pediatric ward with respiratory symptoms undergo systematic in-hospital testing for respiratory viruses at the national reference laboratory. Consequently, we were able to retrospectively identify the infected infants. Notably, the HCL is the only hospital admitting severe cases, has the only pediatric intensive care unit in the Lyon urban area, and transfers to other hospitals occurred solely in instances of bed saturation for infants without risk factors, as described in the national guidelines, or when presenting less severe infections. This enabled us to undertake exhaustive case detection over 5 years. To ensure the accuracy of our findings and control for classification bias we employed in-depth file reviews conducted by 2 trained pediatricians, as well as the utilization of definitions recommended by WHO, the European Medicines Agency and the United States Food and Drug Administration to identify the most severe cases.17,26,27
The rationale for preventing RSV disease is underscored by the significant burden it places on healthcare systems, encompassing severe acute illness, nosocomial infections, and notable mortality rates.1,4,28,29 Preventing acute RSV disease is also expected to impact long-term disease, as infections in early life are linked to long-term respiratory health issues, including asthma, reduced lung function and possibly allergic sensitization, highlighting the multifaceted impact of this viral infection.30 The potential benefits of preventive measures were evident through the clinical trials of the aforementioned innovative pharmaceutical strategies.8,9 The confirmation of these benefits will become more apparent as their true cost-benefit impact in the real world will be assessed after deployment. Within this context, the use of the proposed risk-guided chart (Fig. 2) will optimize the utilization of these new products. In regions where these products may not be readily available, strategies centered on immediately accessible and cost-effective NPIs could be used.7 However, they require active engagement in terms of education that might be more relevant in a focused time period. The utilization of our proposed chart can thus significantly enhance the effectiveness of these strategies.
Utilizing a machine-learning approach allowed us to account for nonlinear relationships between the predictors, a feature not easily accommodated by standard logistic regression techniques used in previous RSV studies.31 In addition, machine-learning models tend to yield more accurate predictions.32 The downsampling of the class of nonhospitalized infants enabled us to deal with the relative rarity of SARI-WI cases. A limitation of the machine-learning strategy was that a postprocessing phase of the model needed to be conducted to understand its main characteristics, and easily interpretable results such as odds ratios cannot be presented. We chose to evaluate variable importance of each variable separately to avoid the measures being affected by other correlated variables. Another option, suitable in the case of noncorrelated variables, would be to use Shapley values or built-in importance measures from Adabag or Ranger models.18,19 Instead of a filtering approach to evaluate variable importance, a wrapper method for variable selection could have been envisioned, such as recursive feature elimination.33
This birth cohort included around half of all the births from 2014 to 2018 in the Lyon urban area and is representative of the public healthcare system of a main French metropole.11 Comparisons with private or peri-urban healthcare systems need, however, caution. The observed incidence of severe RSV hospitalization (1337 cases per 100,000 infants/year) is similar to other French and European settings.1,5 Furthermore, data from our setting have been shared with regional and international groups evaluating the burden of RSV disease.1,5 The selection of the risk factors to be evaluated in our analysis was based on the literature11–13 and on the available information collected at birth in the HCL birth registry. Variables related to family history previously reported to be associated with RSV lower respiratory infection, such as parental education level, maternal smoking during pregnancy and family history of asthma,23,34 were not available. Reassuringly, a recent clinical prediction model for the risk of RSV hospitalization in infants using large nationwide registers in Finland and Sweden, also found that predefined predictors from the literature were significantly associated with hospitalization. Additionally, the authors found that esophageal malformation and lower complexity congenital heart defects were strongly correlated with severe RSV infection.24 Our study is the first to explore clinical severity using the WHO severity definitions. Inter-study comparison is thus difficult, but our results support the overall literature on hospitalization and intensive care unit hospitalization risk factors.
The numerical thresholds used to categorize the classes of frequencies of SARI-WI prediction were defined according to our clinical point of view. We wanted to highlight extreme SARI-WI risk levels, whether minimal or near certain. Intermediate risk levels are broader, and their interpretation depends on the context of care. Although these thresholds can be argued, 1 advantage of the machine-learning algorithms lies in the flexibility of the definition of risk levels and interpretation.
Identifying a population of newborns at high risk of severe disease is paramount for targeted prevention strategies as early as in maternity hospitals. In addition to birth predictors, it would be worth applying the same machine-learning methodology to predict the severity of bronchiolitis from clinical observations collected from the emergency department. The purpose would differ from our study, stratifying the severity risk among patients to guide clinical decisions. Description of the optimal immunization period through at-risk months, in the context of narrow epidemic seasons, could guide the distribution of pharmaceutical interventions and supplies.
Although RSV preventive measures are vital for all infants, and specific recommendations exist for patients with high-risk comorbidities, in situations where prioritization becomes necessary, infants born just before or within the early weeks of the epidemic should be considered as a group of interest. Prioritization should also be considered for preterm infants, and term infants born by cesarean delivery, or having siblings.
ACKNOWLEDGMENTS
The authors thank all the participants in the study.
The VRS Study Group in Lyon: Emilie Bard, Mehdi Benchaib, Sylvie Bin, Marine Butin, Regine Cartier, Jean-Sebastien Casalegno, Olivier Claris, Sandrine Couray-Targe, Lélia Duclaux-Loras, Antoine Duclos, Sylvie Fiorini, Julie Fort-Jacquier, Pascal Gaucherand, Alexandre Gaymard, Yves Gillet, Julie Haesebaert, Etienne Javouhey, Marine Jourdain, Rolf Kramer, Bruno Lina, Jerome Massardier, Elsa Masson, Mona Massoud, Yahia Mekki, Florence Morfin, Anne-Florence Myard Dury, Michelle Ottmann, Antoine Ouziel, Luc Panetta, Dominique Ploin, Stephanie Polazzi, Aurélie Portefaix, Nathalie Rivat, Olivier Terrier, Martine Valette, Philippe Vanhems.
Supplementary Material
Footnotes
M.C.N. reports grants from the Bill & Melinda Gates Foundation, European & Developing Countries Clinical Trials Partnership, Pfizer, AstraZeneca, and Sanofi; and personal fees Sanofi. The other authors have no funding or conflicts of interest to disclose.
Côme Horvat and Cécile Chauvel contributed equally as co-first authors
Dominique Ploin and Marta C. Nunes contributed equally as co-senior authors
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Côme Horvat, Email: come.horvat@chu-lyon.fr.
Cécile Chauvel, Email: cecile.chauvel@chu-lyon.fr.
Mehdi Benchaib, Email: mehdi.benchaib@chu-lyon.fr.
REFERENCES
- 1.Li Y, Wang X, Blau DM, et al. ; Respiratory Virus Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajo K, Nishiura H. Age-dependent risk of respiratory syncytial virus infection: a systematic review and hazard modeling from serological data. J Infect Dis. 2023;228:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildenbeest JG, Billard MN, Zuurbier RP, et al. ; RESCEU Investigators. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. 2023;11:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronchiolite. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/bronchiolite. Accessed October 6, 2023. [Google Scholar]
- 6.Abbasi J. “This Is Our COVID”—what physicians need to know about the pediatric RSV surge. JAMA. 2022;328:2096. [DOI] [PubMed] [Google Scholar]
- 7.Baker RE, Park SW, Yang W, et al. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117:30547–30553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammitt LL, Dagan R, Yuan Y, et al. ; MELODY Study Group. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–846. [DOI] [PubMed] [Google Scholar]
- 9.Kampmann B, Madhi SA, Munjal I, et al. ; MATISSE Study Group. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451–1464. [DOI] [PubMed] [Google Scholar]
- 10.Tso CF, Lam C, Calvert J, et al. Machine learning early prediction of respiratory syncytial virus in pediatric hospitalized patients. Front Pediatr. 2022;10:886212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.INSEE. Dossier complet - Intercommunalité - Métropole de Lyon. Published August 9, 2022. Available at: https://www.insee.fr/fr/statistiques/2011101?geo=EPCI-200046977#tableau-RFD_G1. Accessed August 9, 2022. [Google Scholar]
- 12.Jourdain M, Benchaib M, Ploin D, et al. ; On Behalf Of The Vrs Study Group In Lyon. Identifying the target population for primary respiratory syncytial virus two-step prevention in infants: Normative Outcome of Hospitalisation Assessment for Newborns (NOHAN). Vaccines. 2022;10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer R, Duclos A, Lina B, et al. ; VRS study group in Lyon. Cost and burden of RSV related hospitalisation from 2012 to 2017 in the first year of life in Lyon, France. Vaccine. 2018;36:6591–6593. [DOI] [PubMed] [Google Scholar]
- 14.Haute Autorité de Santé. Prise en charge du 1er épisode de bronchiolite aiguë chez le nourrisson de moins de 12 mois. Published online November 14, 2019. Available at: https://has-sante.fr/jcms/p_3118113/fr/prise-en-charge-du-1er-episode-de-bronchiolite-aigue-chez-le-nourrisson-de-moins-de-12-mois [Google Scholar]
- 15.Li T, Fang H, Liu X, et al. Defining RSV epidemic season in southwest China and assessing the relationship between birth month and RSV infection: a 10-year retrospective study from June 2009 to May 2019. J Med Virol. 2023;95:e28928. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen K, Fisker N, Haerskjold A, et al. Caesarean section and hospitalization for respiratory syncytial virus infection: a population-based study. Pediatr Infect Dis J. 2015;34:145–148. [DOI] [PubMed] [Google Scholar]
- 17.Modjarrad K, Giersing B, Kaslow DC, et al. ; WHO RSV Vaccine Consultation Expert Group. WHO consultation on respiratory syncytial virus vaccine development report from a world health organization meeting held on 23-24 march 2015. Vaccine. 2016;34:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright MN, Ziegler A. Ranger: a fast implementation of random forests for high dimensional data in C++ and R. J Stat Softw. 2017;77:1–17. [Google Scholar]
- 19.Alfaro E, Gámez M, García N. Adabag: an R package for classification with boosting and bagging. J Stat Softw. 2013;54:1–35. [Google Scholar]
- 20.Friedman JN. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001;29:1189–1232. [Google Scholar]
- 21.Karatzoglou A, Smola A, Hornik K, et al. Kernlab - an S4 package for kernel methods in R. J Stat Softw. 2004;11:1–20. [Google Scholar]
- 22.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Houben ML, Bont L, Wilbrink B, et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics. 2011;127:35–41. [DOI] [PubMed] [Google Scholar]
- 24.Vartiainen P, Jukarainen S, Rhedin SA, et al. Risk factors for severe respiratory syncytial virus infection during the first year of life: development and validation of a clinical prediction model. Lancet Digit Health. 2023;5:e821–e830. [DOI] [PubMed] [Google Scholar]
- 25.van Summeren J, Meijer A, Aspelund G, et al. ; VRS study group in Lyon. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter?. Euro Surveill. 2021;26:2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines. Published online January 1, 2017. Available at: https://www.who.int/publications/i/item/WHO-IVB-17.11 [Google Scholar]
- 27.FDA. Respiratory Syncytial Virus Infection: Developing Antiviral Drugs for Prophylaxis and Treatment Guidance for Industry. Published online June 10, 2017. Available at: https://www.fda.gov/media/108437/download [Google Scholar]
- 28.Löwensteyn YN, Willemsen JE, Mazur NI, et al. ; on behalf of the RSV GOLD Study Group. Nosocomial RSV-related In-hospital mortality in children <5 years: a global case series. Pediatr Infect Dis J. 2023;42:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheltema NM, Gentile A, Lucion F, et al. ; PERCH Study Group. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5:e984–e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosas-Salazar C, Chirkova T, Gebretsadik T, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet. 2023;401:1669–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo G, Nkoy FL, Gesteland PH, et al. A systematic review of predictive modeling for bronchiolitis. Int J Med Inf. 2014;83:691–714. [DOI] [PubMed] [Google Scholar]
- 32.Steyerberg EW. Clinical Prediction Models. New York: Springer; 2009. [Google Scholar]
- 33.Guyon I, Weston J, Barnhill S, et al. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46:389–422. [Google Scholar]
- 34.Stensballe LG, Kristensen K, Simoes EAF, et al. ; Danish RSV Data Network. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118:e1360–e1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.