Abstract
4-GU-DANA (zanamivir) (as well as DANA and 4-AM-DANA) was found to inhibit the neuraminidase activity of human parainfluenza virus type 3 (HPF3). The viral neuraminidase activity is attributable to hemagglutinin-neuraminidase (HN), an envelope protein essential for viral attachment and for fusion mediated by the other envelope protein, F. While there is no evidence that HN's neuraminidase activity is essential for receptor binding and syncytium formation, we found that 4-GU-DANA prevented hemadsorption and fusion of persistently infected cells with uninfected cells. In plaque assays, 4-GU-DANA reduced the number (but not the area) of plaques if present only during the adsorption period and reduced plaque area (but not number) if added only after the 90-min adsorption period. 4-GU-DANA also reduced the area of plaques formed by a neuraminidase-deficient variant, confirming that its interference with cell-cell fusion is unrelated to inhibition of neuraminidase activity. The order-of-magnitude lower 50% inhibitory concentrations of 4-GU-DANA (and also DANA and 4-AM-DANA) for plaque area reduction and for inhibition in the fusion assay than for reducing plaque number or blocking hemadsorption indicate the particular efficacy of these sialic acid analogs in interfering with cell-cell fusion. In cell lines expressing influenza virus hemagglutinin (HA) as the only viral protein, we found that 4-GU-DANA had no effect on hemadsorption but did inhibit HA2b-red blood cell fusion, as judged by both lipid mixing and content mixing. Thus, 4-GU-DANA can interfere with both influenza virus- and HPF3-mediated fusion. The results indicate that (i) in HPF3, 4-GU-DANA and its analogs have an affinity not only for the neuraminidase active site of HN but also for sites important for receptor binding and cell fusion and (ii) sialic acid-based inhibitors of influenza virus neuraminidase can also exert a direct, negative effect on the fusogenic function of the other envelope protein, HA.
Sialic acid is the receptor determinant for the human parainfluenza virus type 3 (HPF3) hemagglutinin-neuraminidase (HN) glycoprotein, the molecule responsible for binding of the virus to cell surfaces. In addition to binding receptor and contributing to fusion promotion, the HPF3 HN molecule contains receptor-destroying (sialidase) activity (11). The putative active sites are in the extracellular domain of this type II integral membrane protein; however, since the crystal structure of HPF3 HN is not available, the locations of these sites, as well as the structural requirements for binding to the cellular receptor(s), are unknown.
In the case of influenza virus, studies of the neuraminidase molecule as a potential target of antiviral therapy led to the synthesis of potent inhibitors of this enzyme (31). One of these unsaturated sialic acid analogs, 4-GU-DANA (4-guanidino-Neu5Ac2en; zanamivir), has recently been shown to be a clinically effective anti-influenza virus agent (6, 19). The common mechanism whereby such transition state analogs of sialic acid are thought to block the spread of infection is inferred from extensive information about the functions of the two influenza virus envelope proteins, hemagglutinin (HA) and neuraminidase (NA). HA, which recognizes the sialic acid moiety on the cell surface receptor, mediates both binding of the virus to the cell surface and fusion of the viral envelope with the endosomal membrane; NA is not involved in these processes but is necessary for promoting the release of newly formed virions from the cell surface by removing receptors for the virus. Thus, restriction by neuraminidase inhibitors of the number of virions available for infecting neighboring uninfected cells is believed to underlie the decrease in plaque size and, in the case of even more severe restriction, reduction of plaque number in the presence of an inhibitor (14, 30, 33).
Our interest in examining the possible effect of 4-GU-DANA on HPF3 stems from our observations for another unsaturated sialic acid analog, DANA (Neu5Ac2en) (13). After demonstrating the inhibitory potential of DANA on HPF3 neuraminidase, we showed that the analog also interferes with viral attachment and fusion. Notably, DANA blocked hemadsorption of HPF3-infected cells at a temperature where neuraminidase is inactive. In addition, in our assay system for quantitating HN-receptor interaction, DANA inhibited the fusion of persistently infected cells with uninfected cells (13). As possible interpretations of these results, we proposed that (i) binding of DANA to the neuraminidase active site of HN induces an inactivating change in the protein at the site(s) with receptor-binding and fusion-promoting function; (ii) there is one site responsible for both neuraminidase and receptor binding; or (iii) alternatively, DANA may bind independently to both the neuraminidase- and receptor-binding sites.
In this study, aimed primarily at separating HN's binding and enzymatic properties, we showed that 4-GU-DANA is a more effective, and 4-AM-DANA (4-amino-Neu5Ac2en) is a less effective, HPF3 neuraminidase inhibitor than DANA. We compared their effects on HN-mediated processes in several test systems and extended the inquiry to a newly isolated neuraminidase-deficient HPF3 variant (M. Porotto and A. Moscona, unpublished data). The results provided evidence in support of the ability of transition-state sialic acid analogs to not only block the neuraminidase site but also interfere with HN functions that are necessary for fusion.
In beginning to explore the possible relevance of these findings to influenza viruses, we used cell lines (HAb2 and HA300) that express only one of the influenza virus proteins, HA. We examined the interaction of these HA-expressing cells with human red blood cells (RBC) labeled with fluorescent dyes and obtained evidence that membrane fusion (lipid mixing) as well as content mixing was inhibited by 4-GU-DANA. The simplest explanation of the data is that these neuraminidase inhibitors also have affinity for HA, thus inhibiting its fusogenic functions required for viral entry. While the effects of 4-GU-DANA on influenza virus have been ascribed to neuraminidase inhibition preventing viral release, the results presented here suggest that the antiviral mechanism of action of 4-GU-DANA may be broader than suspected.
MATERIALS AND METHODS
Virus.
Stocks of wild-type (wt) and variant HPF3 were made in CV-1 cells from virus that was plaque purified four times. Virus was collected 36 to 48 h postinfection and stored at −80°C. Virus titer was determined by a plaque assay with CV-1 cells. HPF3 variants were isolated during growth of virus in neuraminidase-treated cells as previously described (22). For isolation of the variant C28a, supernatant fluid from cultures infected with C28 was collected and used in plaque assays. Large plaques were picked and plaque purified four times, and a single plaque was used to infect each CV-1 cell monolayer for preparation of stocks of variant virus.
Chemicals.
DANA was obtained from Sigma Chemical Co. (St. Louis, Mo.). 4-GU-DANA and 4-AM-DANA were gifts from Glaxo Wellcome Research and Development Ltd. (Stevenage, United Kingdom).
Cells.
HeLa-CD4-LTR-βgal cells and HeLa-tat cells were obtained through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. HeLa cell lines and CV-1 (African green monkey kidney) cells were maintained with Eagle minimal essential medium supplemented with 10% fetal bovine serum and antibiotics. HAb2 cells, a line of stably transfected NIH 3T3 fibroblasts expressing the HA of the A/Japan/305/57 strain of influenza virus (4), were cultured as described elsewhere (18). HA300 cells (CHO-K1 cells expressing the HA of influenza virus strain X:31) were grown as described by Kemble et al. (10).
RBC.
RBC were freshly obtained from whole human blood. Labeling of RBC with either the fluorescent lipid-soluble dye PKH26-GL (Sigma) or with both this dye and the water-soluble dye carboxyfluorescein was done as described by Chernomordik et al. (2).
Neuraminidase assay.
The fluorimetric assay of neuraminidase in sonicated HPF3 preparations was based on the methods of Warner and O'Brien (32) and Potier et al. (27). Reaction mixtures, containing 100 mM malate buffer (pH 4.75) and the indicated concentrations of MUNANA (4-methylumbelliferyl-α-d-N-acetylneuraminate) in a total volume of 25 to 50 μl, were incubated at 37°C for 15 to 20 min. To determine the rate of product formation, which was constant during these periods, samples were taken at four to five time points, mixed with 100 mM methylenediamine, and read in a Sequoia-Turner fluorimeter at 365-nm excitation wavelength and 450-nm emission wavelength. The amount of reaction product denoted by these readings was determined from fluorescence versus concentration curves determined with commercially obtained 4-methylumbelliferone. Fluorescence resulting from the spontaneous hydrolysis of the substrate, corrected for as described by Potier et al. (27), was always less than 25% of the total. Enzyme activity is reported as nanomoles of product formed per minute per milligram of protein.
Cell fusion assay.
HeLa-CD4-LTR-βgal cells were persistently infected with HPF3, using our methods developed for CV-1 cells (13, 20, 21), at a multiplicity of infection sufficient to infect all cells in the culture (>5 PFU per cell). The cell fusion assay was performed as described previously (13). Briefly, persistently infected HeLa-CD4-LTR-βgal cells were plated in 96-well plates, after 24 h uninfected HeLa-tat cells (3 × 104 per well) were added to the adherent cells, and fusion was allowed to proceed for 6 h at 37°C. β-Galactosidase (β-Gal) activity was then measured as described elsewhere (13, 23).
Plaque reduction assay and measurement of plaque size.
Effects of the various compounds on plaque number and size were assessed by a plaque reduction test, performed as described elsewhere (13). Briefly, CV-1 cell monolayers grown in plastic dishes of 3.5-cm diameter were inoculated with 20 to 100 PFU of HPF3 (wt or variant) and then incubated in the presence of various concentrations of inhibitors. After 90 min, Eagle minimum essential medium containing 0.5% agarose was added to the dishes, which were then incubated for 24 to 48 h. For experiments to determine the effects of the time of addition of inhibitors, the compounds were either (i) added at the time of infection and removed after the 90-min adsorption period by three washes in Eagle minimum essential medium or (ii) added after the 90-min adsorption period only, dissolved in the agarose overlay to achieve the desired final concentration. After removal of the agarose overlay, the cells were immunostained for plaque detection (7, 13). Plaques in the control and experimental wells were counted. To determine plaque area, plaque diameters were measured at a magnification of ×7 to ×45 using a zoom stereomicroscope equipped with a micrometer.
Hemadsorption assay.
Monolayers of persistently infected cells were washed with cold medium lacking serum and then incubated with human RBC at 4°C for 120 min in the presence of various concentrations of compounds. Nonadherent cells were removed by washing with cold medium; the extent of RBC adsorption was estimated. For quantitation of hemadsorption, the adherent RBC were lysed in 50 mM NH4Cl and transferred into 96-well plates, and the optical density at 540 nm (OD540) was read on a Biotek Instruments ELISA reader.
Binding and fusion of HA cells with RBC.
HAb2 and HA300 cells were treated, and their interaction with fluorescence-labeled RBC was studied, as previously described (2). All incubations were at room temperature (20 to 22°C). To study binding, HA cells were incubated with RBC for 15 min; unbound RBC were removed by four washes with phosphate-buffered saline (PBS), and the number of bound RBC per cell was determined by inspection of at least 500 cells per plate. In HA cells with prebound RBC, fusion was triggered by PBS titrated to pH 4.9 with iso-osmotic citrate buffer; replacement (2 min later) of this acid medium by PBS at pH 7.4 was followed by a 20-min incubation. The resulting fusion, defined as dye redistribution from the RBC, was quantified by fluorescence microscopic determination of the ratio of dye-redistributed, bound RBC to the total number of bound RBC as previously described (2).
RESULTS
Inhibition of neuraminidase activity by 4-GU-DANA, DANA, and 4-AM-DANA.
To continue our studies of neuraminidase inhibition in HPF3, we adapted the fluorimetric assay of neuraminidase (27, 32), which had not previously been applied to HPF3, for use in preparations of this virus. Incubations with the substrate MUNANA were carried out at 37°C at a pH (4.7) optimal for HPF3 neuraminidase (22). Samples taken at four to six time points during the 15- to 30-min incubation period were routinely assayed to ensure that the reaction rate remained constant and proportional to the amount of enzyme added.
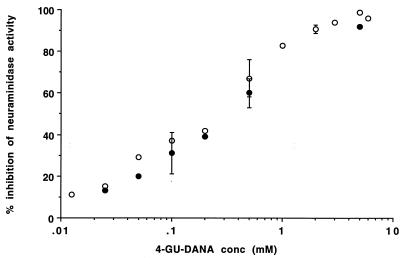
Previous measurement of HPF3 neuraminidase demonstrated inhibition by DANA (13). After confirming these results with the fluorimetric assay, we extended the study to two other unsaturated derivatives of sialic acid, 4-GU-DANA and 4-AM-DANA, and found that these also inhibited HPF3 neuraminidase. The experiments showed that these inhibitory effects were not enhanced by preincubation of the sonicated HPF3 preparations with 4-GU-DANA, DANA, or 4-AM-DANA. These substances were thus added to the reaction mixture at time zero, and the concentration of the fluorescent reaction product was determined in samples taken at 5-min intervals. The lower rates of product formation in the experimental than control (inhibitor-free) mixtures were apparent from the first 5-min samples and remained constant for at least 25 min. Figure 1 depicts the inhibition of HPF3 neuraminidase activity as a function of the concentration of 4-GU-DANA. Similar inhibition was found at 2.5 and 40 mM MUNANA (Fig. 1). This result is in accord with additional experiments that showed that raising the substrate concentration from 2.5 mM to saturating (25 mM) or higher than saturating levels did not diminish the percent inhibition caused by either 4-GU-DANA or the less effective inhibitor DANA. However, 4-AM-DANA caused significantly greater inhibition in the presence of 2.5 than 40 mM MUNANA, so that plots like that in Fig. 1 yielded two curves for the two substrate concentrations. The IC50-Ns (concentrations required for 50% inhibition of neuraminidase activity) for 4-AM-DANA determined at these two substrate concentrations were 3.2 and 8.0 mM, respectively. Both values for 4-AM-DANA are higher than the IC50-N for DANA, 2.1 mM, and much higher than that for 4-GU-DANA, 0.25 mM (Table 1). The finding that the IC50-N for DANA is 10 times higher than that for 4-GU-DANA contrasts with published data on HPF2 (8), where 4-GU-DANA was found to be a less effective neuraminidase inhibitor than DANA. In the case of influenza A and B viruses, the IC50-N for DANA is several orders of magnitude higher than that for 4-GU-DANA (14, 33), compared to our finding of only a 12-fold difference for HPF3.
FIG. 1.
Inhibition of HPF3 neuraminidase activity by 4-GU-DANA. Viral preparations were assayed at substrate concentrations of 2.5 mM (open circles) and 40 mM (closed circles) in the absence and presence of 4-GU-DANA at the indicated concentrations. The data points, showing percent inhibition of neuraminidase activity (nanomoles per minute per milligram of protein) as a function of log millimolar 4-GU-DANA, are averages of the results of duplicate experiments or means of results of three to four experiments (bars denote SD).
TABLE 1.
IC50s of three inhibitors in different test systemsa
| Inhibitor | IC50 (mM) for:
|
|||
|---|---|---|---|---|
| Neuraminidase activity | Cell fusion | Plaque no. | Plaque area | |
| 4-GU-DANA | 0.25 | 0.19 | 0.8 | 0.025 |
| DANA | 2.1 | 3.2 | 5.3 | 0.62 |
| 4-AM-DANA | 3.2–8.0 | 4.0 | 16.0 | 1.03 |
Use of a fusion assay to assess the effects of neuraminidase inhibitors on HN-receptor interaction.
Based on our finding that cells persistently infected with HPF3 do not fuse with one another but fuse with uninfected cells (20), we developed an assay for quantifying HN-receptor interaction (13). Employing persistently infected HeLa-CD4-LTR-βgal cells, whose fusion with HeLa-tat cells results in β-Gal production, this assay was found to provide a simple quantitative method of screening for substances that may interfere with the HN-receptor interaction required for fusion. The neuraminidase inhibitor DANA was identified as such a substance (13), and we now report that 4-GU-DANA and 4-AM-DANA also interfere with cell-cell fusion in this assay.
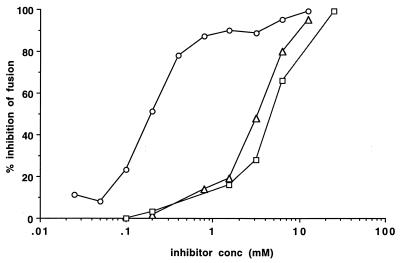
Figure 2 shows the results of a fusion assay carried out in the presence of the three inhibitors. 4-GU-DANA was most effective of the three compounds. Its IC50-F (concentration required for 50% inhibition of fusion) was 0.19 mM, compared to 3.2 and 4.0 mM for DANA and 4-AM-DANA, respectively (Fig. 2; Table 1).
FIG. 2.
Effects of 4-GU-DANA, DANA, and 4-AM-DANA on the fusion of persistently infected cells with uninfected cells. Percent inhibition of β-Gal production measured after the 6-h fusion period is shown as a function of log millimolar 4-GU-DANA (circles), DANA (triangles), or 4-AM-DANA (squares). For every point, the mean results in 10 experimental wells were compared with the mean of results in 10 control wells on the same 96-well plate.
To determine whether the inhibitory effect in the cell fusion assay is due only to interference with cellular attachment or involves a downstream step in fusion, we carried out experiments in which the incubation of persistently infected cells with uninfected cells at 37°C was preceded by a 1-h period at 4°C, so as to allow cellular attachment but not fusion. After 1 h at 4°C, all of the uninfected cells had adhered to the persistently infected monolayer but no fusion had occurred; at this point, inhibitors were added and the cells were transferred to 37°C. 4-GU-DANA, DANA, and 4-AM-DANA interfered with fusion even if added after the 1-h period in the cold, and the percents inhibition of fusion at various concentrations of these compounds were not significantly different from those obtained when the compounds were added before the 1-h period. For example, addition of 0.8 mM 4-GU-DANA before and after the 1-h period at 4°C caused 88 and 81% inhibition, respectively, and 96 and 84% were the corresponding values obtained at 6.25 mM DANA. These results suggest that the inhibitory effect of 4-GU-DANA, DANA, and 4-AM-DANA in this assay system results from interference with the cell fusion process at a step subsequent to attachment.
Characterization of HPF3 variants deficient in neuraminidase activity.
In previous studies on the role of neuraminidase in the HPF3 life cycle, we isolated a variant, C28, with half as much neuraminidase activity as in wt (9). Cloning and sequencing of the fusion protein (F) and HN genes revealed a single amino acid change in the HN protein, with no alterations in the F sequence. This variant was characterized by a delay in the release of virus particles into the supernatant, by the formation of large plaques, and by causing more extensive fusion through infected cell monolayers. The addition of exogenous bacterial neuraminidase enhanced the release of viral particles, indicating that HPF3 viral neuraminidase activity is important for the release of newly formed virions from infected cells.
We subsequently isolated a variant of C28 which preserved the mutation at nucleotide 724 but had an additional point mutation in HN. The neuraminidase activity of this variant, C28a, is insignificant; i.e., less than 3% of wt activity, as measured by either the thiobarbituric acid assay or the fluorimetric assay used in this study (Porotto and Moscona, unpublished). Analysis of its growth properties showed a severely decreased release of viral particles from infected cells to the medium (≥6-log-lower titer in the supernatant fluid) which was reversed, resulting in wt levels of release, by the addition of Clostridium perfringens neuraminidase to the infected cells after the adsorption period. This variant has been used in plaque studies on neuraminidase inhibitors.
Effects of neuraminidase inhibitors on plaque number and size.
The effects of the three compounds were next assessed by a plaque reduction test in which we tested the ability of each compound to interfere with plaque formation by HPF3. We found that 4-GU-DANA and 4-AM-DANA, as well as DANA (13), were active in the plaque reduction assay. 4-GU-DANA, causing a significant inhibition at 0.3 mM, was more effective than the other two compounds. The IC50-PNs (concentrations required for a 50% decrease in plaque number), determined from the S-shaped curves obtained by plotting percent decrease in plaque number against log inhibitor concentration, were 0.8, 5.3, and 16 mM for 4-GU-DANA, DANA, and 4-AM-DANA, respectively (Table 1).
As we have shown for DANA (13), the decreases in plaque number were the same if the inhibitors (added at time zero) were removed after the 90-min adsorption period, and there was no deficit in plaque number if the addition of 4-GU-DANA or 4-AM-DANA was delayed until after the 90-min adsorption period. These findings suggest that reduction of plaque number resulted from interference with viral binding and/or entry. However, infection is not the only process that these compounds can inhibit. Table 2 shows that 0.5 mM 4-GU-DANA (which reduced plaque number but not plaque size if present during the adsorption period only) caused a 97% decrease in plaque area, but no deficiency in plaque number, if added after the 90-min adsorption period. Moreover, plaque area was also strikingly reduced by 4-GU-DANA concentrations (0.125 mM or less) that were too low to reduce plaque number (when added at the time of infection). The same results were obtained (though at higher concentrations) with DANA and 4-AM-DANA. Experiments carried out at various concentrations of the three inhibitors (added at 90 min) showed that the IC50-PAs (concentrations required for a 50% decrease in plaque area) were 0.025, 0.62, and 1.03 mM for 4-GU-DANA, DANA, and 4-AM-DANA, respectively. For all three inhibitors, these values were lower than their IC50s in the other test systems (Table 1); for example, the effectiveness of 4-GU-DANA in reducing plaque area was 32 times greater than its effectiveness in reducing plaque number and 10 times greater than that for inhibiting neuraminidase activity.
TABLE 2.
Effects of 4-GU-DANA on wt HPF3 and variant C28a plaque number and areaa
| 4-GU-DANA added | Plaque no.b | Plaque area (mm2)c | % Inhibition |
|---|---|---|---|
| wt | |||
| None | 72 ± 4 | 0.58 ± 0.14 | |
| 0.5 mM at 0 min | 34 ± 1 | 0.59 ± 0.11 | 0 |
| 0.5 mM at 90 min | 70 ± 4 | 0.015 ± 0.01 | 97 |
| 0.125 mM at 0 min | 71 ± 3 | 0.57 ± 0.09 | 0 |
| 0.125 mM at 90 min | 77 ± 6 | 0.08 ± 0.01 | 87 |
| 0.063 mM at 90 min | 0.19 ± 0.02 | 67 | |
| 0.016 mM at 90 min | 0.40 ± 0.07 | 31 | |
| C28a | |||
| None | 35 ± 8 | 1.23 ± 0.26 | |
| 12.5 mM at 0 min | 33 ± 6 | 1.24 ± 0.25 | |
| 12.5 mM at 90 min | 26 ± 4 | 0.015 ± 0.009 | 98 |
| 3.1 mM at 90 min | 28 ± 5 | 0.145 ± 0.01 | 88 |
| 0.4 mM at 90 min | 29 ± 6 | 0.69 ± 0.21 | 44 |
Cell monolayers were infected with wt HPF3 or the variant C28a. The indicated concentrations of 4-GU-DANA were added either at the time of infection (0 min) or at the end of the 90-min adsorption period.
Mean ± SD of counts on three to four plates.
Mean ± SD of measurements of 20 to 30 plaques on two to three plates.
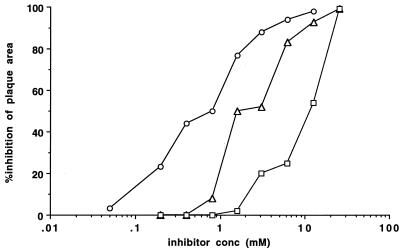
Results for the neuraminidase-deficient variant C28a are shown in Table 2 and Fig. 3. No significant reduction in plaque number resulted from the presence of 12.5 mM 4-GU-DANA during the adsorption period (Table 2), and the same was true for 12.5 to 25 mM DANA and 4-AM-DANA (not shown). However, plaque area was strikingly reduced by addition after the adsorption period of 12.5, 3.1, or 0.4 mM 4-GU-DANA (Table 2). Experiments comparing the effects on plaque area of various concentrations of neuraminidase inhibitors are shown in Fig. 3. The IC50-PAs for C28a determined from these experiments were 0.5, 2.3, and 11.0 for 4-GU-DANA, DANA, and 4-AM-DANA, respectively. Thus, in this neuraminidase-deficient variant, the order of effectiveness of the three inhibitors with respect to reducing plaque area was the same as in wt.
FIG. 3.
Effects of neuraminidase inhibitors on the area of plaques formed by the HPF3 variant C28a with no detectable neuraminidase activity. Monolayers of CV-1 cells were infected with C28a. The agarose overlay, added after the 90-min adsorption period, contained the indicated concentrations of 4-GU-DANA (circles), DANA (triangles), or 4-AM-DANA (squares). Plaque area was determined 42 h later. For each point, percent inhibition was determined by comparing the mean area of 10 to 20 plaques on two experimental and two control plates. (For variability, see SDs of means in Table 2).
Effect on hemadsorption.
The effects of sialic acid analogs on receptor binding were assessed by a hemadsorption assay. The assay, which consists of determining and quantitating RBC adherence to cells persistently infected with HPF3, was carried out at a temperature (4°C) where neuraminidase is inactive but the binding function is intact. Table 3 shows that 4-GU-DANA and 4-AM-DANA, as well as DANA (13), caused a concentration-dependent inhibition of hemadsorption. 4-GU-DANA was more effective than the other two compounds.
TABLE 3.
Effects of GU-DANA, 4-AM-DANA, and DANA on RBC adherence to persistently infected cellsa
| Compound concn (mM) | Mean HAD (102 OD540) ± SD and % binding in presence of:
|
||
|---|---|---|---|
| 4-GU-DANA | DANA | 4-AM-DANA | |
| 5.0 | 0.1 ± 0.16 (1b) | 1.2 ± 0.64 (11) | 3.4 ± 1.20 (30) |
| 2.5 | 0.1 ± 0.15 (1) | 2.3 ± 0.60 (20) | 2.3 ± 0.60 (20) |
| 1.25 | 0.0 ± 0.00 (0) | 2.1 ± 0.21 (18) | 6.6 ± 1.40 (58) |
| 0.5 | 0.1 ± 0.23 (1) | 6.6 ± 1.80 (58) | 6.3 ± 0.60 (56) |
| 0.25 | 0.9 ± 0.40 (8) | 6.0 ± 0.84 (53) | 9.5 ± 1.40 (84) |
| 0.125 | 1.7 ± 0.46 (15) | 9.1 ± 1.90 (80) | 8.2 ± 0.20 (73) |
| 0.05 | 4.3 ± 0.45 (38) | 9.1 ± 1.20 (80) | 11.8 ± 0.70 (104) |
| 0.025 | 6.4 ± 1.25 (57) | 11.8 ± 1.60 (104) | 11.3 ± 1.50 (100) |
| 0 | 11.3 ± 1.20 (100) | 11.3 ± 1.55 (100) | 11.3 ± 1.38 (100) |
4-GU-DANA, 4-AM-DANA, and DANA, in concentrations ranging from 0.025 to 5.0 mM, were added to confluent monolayers of persistently infected cells immediately before the addition of a 1% solution of human RBC. Hemadsorption activity (HAD) was determined after 2 h of incubation at 4°C, by lysis and spectrophotometric quantitation of bound RBC.
Percent RBC binding, 100% being the value obtained in the absence of compounds.
Effect on cells expressing influenza virus HA.
Influenza virus attaches to host cells by the binding of its envelope protein HA to the sialic acid residues on the cell surface receptors, and it enters the cell via the endocytic pathway. HA undergoes a conformational change in the low-pH environment of the endosome and then mediates fusion between the viral and the endosomal membrane, with the consequent release of the nucleocapsid into the cytosol (12). Interaction of HA-expressing cells with RBC has been used for elucidating the underlying mechanisms (4, 17, 28, 34).
We used such a system to examine whether unsaturated sialic acid derivatives that inhibit influenza virus NA activity would also interfere with HA-mediated binding or fusion. We tested for binding of RBC to HA2b or HA300 cells and found that binding was unaffected by 10 mM 4-GU-DANA or DANA. However, fusion was almost completely blocked by these agents. For a more detailed examination of fusion at different 4-GU-DANA concentrations we used HA2b cells, which do not require neuraminidase pretreatment for binding to RBC (2).
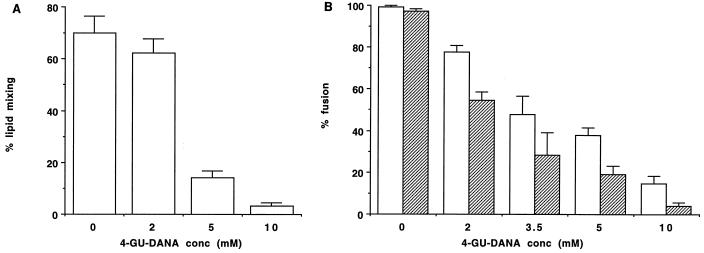
The results obtained for HAb2 cells with prebound, PKH26-GL-labeled RBC are illustrated in Fig. 4A. Lipid mixing was strikingly reduced by 10 or 5 mM 4-GU-DANA, with a smaller inhibition seen at 2 mM. For subsequent experiments, RBC were double labeled so as to distinguish lipid mixing from the ensuing content mixing. In the control plates, >95% of HAb2 cells with prebound RBC exhibited both lipid mixing and content mixing, and 4-GU-DANA exerted a concentration-dependent inhibition on both processes (Fig. 4B). At each 4-GU-DANA concentration, content mixing was inhibited by a greater extent than lipid mixing. This may be related to the finding that content mixing is more sensitive to the surface density of activated HA trimers (2).
FIG. 4.
Inhibition of influenza virus HA-mediated fusion by 4-GU-DANA. Fusion assays on HAb2 cells with prebound, labeled RBC (see Materials and Methods) were carried out in the absence and presence of the indicated concentrations of 4-GU-DANA (abscissae). The RBC were labeled with the lipid-soluble dye PKH26-GL (A) or with both PKH26 and the water soluble dye carboxyfluorescein (B). Using fluorescence microscopy, the numbers of fused (i.e., dye-redistributed) and unfused HA2b cells with prebound RBC were counted in 30 to 40 fields (totaling about 30 cells per field); the results (ordinates) are means ± standard errors. Light and dark columns represent cells exhibiting lipid mixing and content mixing, respectively.
DISCUSSION
In view of the demonstrated role of influenza virus neuraminidase in the release of progeny virions from infected cells, the interference of DANA and its analogs with plaque formation by influenza virus has been attributed to the neuraminidase-inhibitory effect of these compounds (15, 24, 33). The same mechanism has been postulated for the paramyxovirus Newcastle disease virus (NDV) (25). Meindl et al. reported in 1974 that another unsaturated sialic acid analog, FANA (2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid), blocked hemagglutination by NDV and simian virus 5, suggesting that neuraminidase inhibitors might also interfere with HN-receptor interaction (16). The 1974 report by Palese et al. then showed, however, that FANA did not block the binding of NDV to chicken embryo fibroblasts and, in demonstrating the quantitative relation of the neuraminidase inhibition to the plaque-reducing potency of different sialic acid analogs, concluded that the antiviral effect of these agents is mediated by specific inhibition of viral neuraminidase activity (25). In the ensuing years, little consideration has been given to the possibility that such sialic acid analogs may exert a negative effect on the interaction of parainfluenza virus HN or influenza virus HA with the cellular receptor. However, since both neuraminidase activity and receptor binding necessitate the recognition of a sialoside group, it is reasonable to postulate that the same molecule could inhibit (to different degrees) both neuraminidase activity and receptor interaction.
Experimental support for this idea came from our 1999 study (13) showing that DANA, in addition to inhibiting HPF3 neuraminidase activity, also inhibited HN-receptor interaction in our cell fusion assay. This assay system utilizes persistently infected cells which do not form syncytia because their surface is deficient in receptors but can fuse with uninfected cells that possess the necessary receptors (20). Accordingly, pretreatment of the uninfected cells with exogenous neuraminidase prevents fusion of these cells with persistently infected cells (13), and it initially seemed paradoxical that an inhibitor of neuraminidase could also block fusion. However, this finding and the fact that DANA blocked hemadsorption were consistent with our hypothesis that the molecule might interfere with functions that do not depend on HN's neuraminidase activity. The present report provides stronger evidence for this hypothesis.
The finding that the presence of 4-GU-DANA during the adsorption period reduced the number of plaques formed in a plaque reduction assay is unlikely to be due to neuraminidase inhibition; there is no evidence to suggest that the neuraminidase activity of HN is necessary for viral entry. (Note that in this discussion, “4-GU-DANA” will stand for all three sialic acid analogs, since in each experimental assay 4-GU-DANA exerted the same effects as DANA or 4-AM-DANA and did so at lower concentrations.)
Another effect of 4-GU-DANA, even when added after the adsorption period at concentrations much lower than those required to block entry, was a striking reduction of plaque area (but no reduction of plaque number). The neuraminidase activity of HN has been shown to aid the efficient release of newly formed virions from infected cells (9). However, it is unlikely that the plaque size-reducing effect of 4-GU-DANA is attributable to neuraminidase inhibition, since for HPF3, plaque enlargement involves cell-cell fusion and does not require the release of virions from the infected cell to enter neighboring cells. Indeed, our results show that 4-GU-DANA also reduced the area of plaques formed by the neuraminidase-deficient HPF3 variant, C28a, providing further evidence that neuraminidase inhibition does not form the basis of this reduction.
Interpretation of the effects of 4-GU-DANA on HPF3 in terms of molecular mechanisms is made difficult by the lack of X-ray crystallographic information about the structures and locations of the active sites on HN, as well as by the fact that a second envelope protein, F, and its cofunction with receptor-bound HN are necessary for fusion (11). For influenza virus, in contrast, fusion is attributable entirely to HA, an envelope protein that has been extensively characterized in terms of its structure and mechanism of action (12). For these reasons, and because of the demonstrated anti-influenza virus efficacy of 4-GU-DANA in vivo, we extended our studies to influenza virus HA-expressing cells and found that while 4-GU-DANA did not inhibit RBC binding, it strikingly inhibited HA-mediated fusion. A minimum 4-GU-DANA concentration of 2 mM was required for fusion inhibition. Note that 2.8 and 4.2 mM are the dissociation constants for the binding of sialic acid analogs to influenza virus HA found in nuclear magnetic resonance studies by Sauter et al. (29) and Hanson et al. (5).
The most straightforward explanation of our influenza virus HA data is that 4-GU-DANA, sharing chemical features with sialic acid, can bind to both the enzyme active site of NA, at high affinity, and to the receptor-binding site of HA, at low affinity. If the majority of binding sites on HA were occupied by 4-GU-DANA rather than by the sialic acid receptor, then binding to RBC would be blocked, precluding fusion. If (as indicated by uninhibited RBC binding) the affinity of 4-GU-DANA is not high enough to prevent receptor binding, it can still inhibit fusion, because the local density of receptor-bound HA trimers is not high enough for fusion complex formation, thought to require aggregates of six or more trimers (1–3, 26). It does not take as many trimers to mediate binding, and the trimers responsible for binding may be spread through the surface, with individual trimers moving on and off the binding site at equilibrium. Thus, it may take almost complete occupation of HA by 4-GU-DANA to inhibit binding. On the other hand, due to the multiplicity of HA trimers required for fusion complex formation, even partial occupancy of adjacent trimers may be sufficient to block fusion. A possible alternative to this straightforward explanation is that 4-GU-DANA has no affinity for the receptor-binding site of HA but rather interacts with a site that is exposed only when fusion is triggered.
In the case of HPF3, too, 4-GU-DANA appears to be more effective in inhibiting fusion than attachment; we found that processes dependent on cell fusion (plaque enlargement and fusion of persistently infected with uninfected cells) were inhibited by lower 4-GU-DANA concentrations than was hemadsorption or plaque number. It is thus possible that in both influenza virus and HPF3, the difference between inhibition of binding and fusion can be explained by the difference in the number of viral glycoprotein-receptor contacts that are needed for the two processes (21).
In conclusion, the experiments reported here on HPF3 and a neuraminidase-deficient variant suggest that 4-GU-DANA, which we found to be an inhibitor of HN's neuraminidase activity, interferes with HN functions that do not involve neuraminidase. We postulate that unsaturated sialic acid derivatives like 4-GU-DANA have affinity not only for the neuraminidase active site but also for the site(s) whereby HN binds to the sialic acid receptor and executes its necessary role in cell fusion. The data indicating that 4-GU-DANA blocks the fusion of influenza virus HA-expressing cells with RBC constitute the first evidence that sialic acid-based inhibitors of influenza virus NA can also exert a direct effect on the function of the other envelope protein, HA. The implications of the findings in this report remain to be examined in various in vitro and in vivo experimental paradigms for virus-cell interaction. One intriguing question is whether the ability of 4-GU-DANA to interfere with HA functions contributes to the clinically demonstrated anti-influenza virus potency of this neuraminidase inhibitor.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 31971 to A.M. from the National Institutes of Health.
We thank Rob Fenton, Glaxo Wellcome Research and Development Ltd. (Stevenage, United Kingdom), for helpful discussions and for providing zanamivir.
REFERENCES
- 1.Blumenthal R, Sarkar D P, Durell S, Howard D E, Morris S J. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J Cell Biol. 1996;135:63–71. doi: 10.1083/jcb.135.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernomordik L V, Frolov V A, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danieli T, Pelletier S L, Henis Y I, White J M. Membrane fusion mediated by the influenza virus hemagglutinin requires the concerted action of at least three hemagglutinin trimers. J Cell Biol. 1996;133:559–569. doi: 10.1083/jcb.133.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doxsey S J, Sambrook J, Helenius A, White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985;101:19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson J E, Sauter N K, Skehel J J, Wiley D C. Proton nuclear magnetic resonance studies of the binding of sialosides to intact influenza virus. Virology. 1992;189:525–533. doi: 10.1016/0042-6822(92)90576-b. [DOI] [PubMed] [Google Scholar]
- 6.Hayden F G, Osterhaus A D, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 7.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzer C T, Von Itzstein M, Jin B, Pegg M S, Stewart W P, Wu W-Y. Inhibition of sialidases from viral, bacterial and mammalian sources by analogues of 2-deoxy-2,3-didehydro-N-acetylneuraminic acid modified at the C-4 position. Glycoconj J. 1993;10:40–44. doi: 10.1007/BF00731185. [DOI] [PubMed] [Google Scholar]
- 9.Huberman K, Peluso R, Moscona A. The hemagglutinin-neuraminidase of human parainfluenza virus type 3: role of the neuraminidase in the viral life cycle. Virology. 1995;214:294–300. doi: 10.1006/viro.1995.9925. [DOI] [PubMed] [Google Scholar]
- 10.Kemble G W, Danieli T, White J M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 11.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 12.Lamb R A, Krug R M. Fields virology, B. Fields, D. Knipe, and P. M. Howley (ed.) 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. Orthomyxoviridae: the viruses and their replication; pp. 1353–1395. [Google Scholar]
- 13.Levin Perlman S, Jordan M, Brossmer R, Greengard O, Moscona A. The use of a quantitative fusion assay to evaluate HN-receptor interaction for human parainfluenza virus type 3. Virology. 1999;265:57–65. doi: 10.1006/viro.1999.0024. [DOI] [PubMed] [Google Scholar]
- 14.McKimm-Breschkin J L, Sahasrabudhe A, Blick T J, McDonald M, Colman P M, Hart G J, Bethell R C, Varghese J N. Mutations in a conserved residue in the influenza virus neuraminidase active site decreases sensitivity to Neu5Ac2en-derived inhibitors. J Virol. 1998;72:2456–2462. doi: 10.1128/jvi.72.3.2456-2462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKimm-Breschkin J L, McDonald M, Blick T J, Colman P M. Mutation in the influenza virus neuraminidase gene resulting in decreased sensitivity to the neuraminidase inhibitor 4-guanidino-Neu5Ac2en leads to instability of the enzyme. Virology. 1996;225:240–242. doi: 10.1006/viro.1996.0595. [DOI] [PubMed] [Google Scholar]
- 16.Meindl P, Bodo G, Palese P, Schulman J, Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1974;58:457–463. doi: 10.1016/0042-6822(74)90080-4. [DOI] [PubMed] [Google Scholar]
- 17.Melikyan G B, Niles W D, Cohen F S. Influenza virus hemagglutinin-induced cell-planar bilayer fusion: quantitative dissection of fusion pore kinetics into stages. J Gen Physiol. 1993;102:1151–1170. doi: 10.1085/jgp.102.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melikyan G B, Niles W D, Ratinov V A, Karhanek M, Zimmerberg J, Cohen F S. Comparison of transient and successful fusion pores connecting influenza hemagglutinin expressing cells to planar membranes. J Gen Physiol. 1995;106:803–819. doi: 10.1085/jgp.106.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monto A S, Fleming D M, Henry D, de Groot R, Makela M, Klein T, Elliott M, Keene O N, Man C Y. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261. doi: 10.1086/314904. [DOI] [PubMed] [Google Scholar]
- 20.Moscona A, Peluso R W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991;65:2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moscona A, Peluso R W. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992;66:6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moscona A, Peluso R W. Relative affinity of the human parainfluenza virus 3 hemagglutinin-neuraminidase for sialic acid correlates with virus-induced fusion activity. J Virol. 1993;67:6463–6468. doi: 10.1128/jvi.67.11.6463-6468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reported gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 25.Palese P, Schulman J L, Bodo G, Meindl P. Inhibition of influenza and parainfluenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA) Virology. 1974;59:490–498. doi: 10.1016/0042-6822(74)90458-9. [DOI] [PubMed] [Google Scholar]
- 26.Plonsky I, Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potier M, Mameli L, Belislem M, Dallaire L, Melanxon S B. Fluorimetric assay of neuraminidase with a sodium 4-methylumbelliferyl-α-d-N-acetylneuraminidase substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar D P, Morris S J, Eidelman O, Zimmerberg J, Blumenthal R. Initial stages of influenza hemagglutinin-induced cell fusion monitored simultaneously by two fluorescent events: cytoplasmic continuity and lipid mixing. J Cell Biol. 1989;109:113–122. doi: 10.1083/jcb.109.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauter N K, Hanson J E, Glick G D, Brown J H, Crowther R L, Park S J, Skehel J J, Wiley D C. Binding of influenza virus hemagglutinin to analogs of its cell-surface receptor, sialic acid: analysis by proton nuclear magnetic resonance spectroscopy and X-ray crystallography. Biochemistry. 1992;31:9609–9621. doi: 10.1021/bi00155a013. [DOI] [PubMed] [Google Scholar]
- 30.Thomas G P, Forsyth M, Penn C R, McCauley J W. Inhibition of the growth of influenza viruses in vitro by 4-guanidino-2,4-dideoxy-N-acetylneuraminic acid. Antiviral Res. 1994;24:351–356. doi: 10.1016/0166-3542(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 31.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Dyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 32.Warner T G, O'Brien J S. Synthesis of 2′-(4-methylumbelliferyl)-alpha-d-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 33.Woods J M, Bethell R C, Coates J A, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, et al. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerberg J, Blumenthal R, Sarkar D P, Curran M, Morris S J. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J Cell Biol. 1994;127:1885–1894. doi: 10.1083/jcb.127.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]