Abstract
Epstein-Barr virus (EBV) nuclear antigen (EBNA) 2 (EBNA2) is involved in upregulating the expression of both EBNAs and latency-associated membrane proteins. Transcription of the six EBNA genes, which are expressed in EBV-immortalized primary B cells, arises from one of two promoters, Cp and Wp, located near the left end of the viral genome. Wp is exclusively used to drive EBNA gene transcription during the initial stages of infection in primary B cells; induction of transcription from Cp follows. We previously have mapped an EBNA2-dependent enhancer upstream of Cp (M. Woisetschlaeger et al., Proc. Natl. Acad. Sci. USA 88:3942–3946, 1991) and, more recently, have demonstrated that deletion of this enhancer results in EBV-immortalized lymphoblastoid cell lines (LCLs) that are heavily biased toward the use of Wp to drive transcription of the EBNA genes (L. Yoo et al., J. Virol. 71:9134–9142, 1997). To assess the immortalizing capacity of this mutant EBV and to monitor the early events after infection of primary B cells, B cells isolated from cottontop marmosets were used to generate LCLs immortalized with the Cp EBNA2 enhancer deletion mutant virus. As previously reported, all EBV-infected marmoset LCLs examined could be triggered to produce significant levels of virus. Infection of human B cells with wild-type or Cp EBNA2 enhancer mutant viruses recovered from marmoset B-cell lines demonstrated that (i) the Cp EBNA2 enhancer mutant virus immortalizes primary human B cells nearly as efficiently as wild-type virus and (ii) the Cp EBNA2-dependent enhancer plays an important role in the induction of Cp activity during the early stages of infection. The latter is consistent with the phenotype of LCLs immortalized with the Cp EBNA2 enhancer mutant EBV. Finally, using an established LCL in which EBNA2 function is regulated by β-estradiol, we showed that the loss of EBNA2 function results in an ∼4-fold decrease in the steady-state levels of Cp-initiated transcripts and a concomitant increase in the steady-state levels of Wp-initiated transcripts. Taken together, these results provide strong evidence that EBNA2 plays an important role in regulating Cp activity. These results also demonstrate that diminished induction of Cp activity does not appear to affect the ability of EBV to immortalize primary B cells in cultures. Finally, as shown here, infection of marmoset B cells with immortalization-competent mutants of EBV provides a convenient reservoir for the production of mutant viruses.
Epstein-Barr virus (EBV) infection of resting human B lymphocytes results in the differentiation of infected B cells into lymphoblastoid cell lines (LCLs), which can be passaged indefinitely. These LCLs have an activated B-cell phenotype and express a subset of viral genes which are crucial for the transformation of the cells. The viral genes known to be critical for transformation are EBV nuclear antigens (EBNAs) 1, 2, 3A, and 3C and latency-associated membrane protein 1 (4, 8, 9, 11, 20).
Expression of the EBNAs in LCLs is driven by the BamHI C promoter (Cp) and the BamHI W promoter (Wp) (Fig. 1). Studies of virus promoter usage at early times postinfection have shown that Wp activity is readily apparent by 18 h postinfection, whereas Cp activity is not detectable until 48 to 72 h postinfection (18, 21). When Cp activity rises, Wp activity begins to decline (22). One possible explanation for the decrease in Wp-initiated transcripts is that upstream transcription initiation from Cp may interfere with transcription initiation from Wp (transcriptional interference). This hypothesis has been supported by the finding that, in transient transfection assays with reporter constructs containing both Cp and Wp, Wp activity is induced when Cp is either deleted or inverted (17).
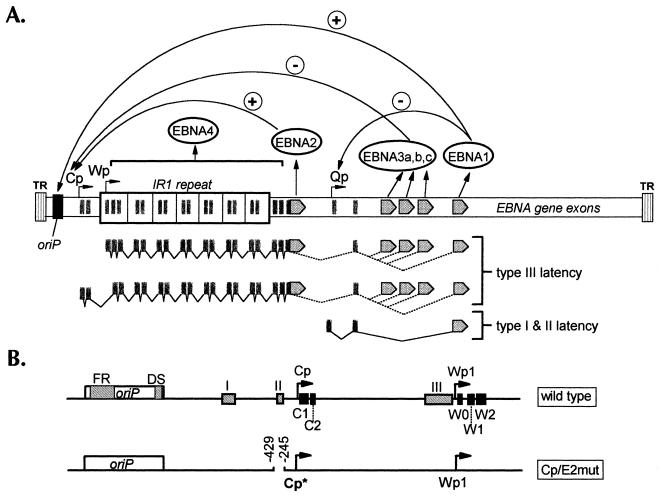
FIG. 1.
(A) Schematic diagram of the linearized EBV genome. EBNA open reading frames are indicated as gray arrows. The functions of the various EBNAs for regulating viral promoter activity are indicated with plus and minus signs. Viral transcription programs during type I, II, and III latencies are indicated below the genome diagram. TR, terminal repeat; IR, internal repeat; Qp, Q promoter. (B) Diagram of regulatory regions controlling Cp and Wp activities. The top diagram shows wild-type viral DNA sequences. oriP, latency origin of replication. Gray box I indicates the location of the glucocorticoid response element. Box II indicates the location of the Cp EBNA2-responsive enhancer. Box III indicates the location of the shared Cp-Wp enhancer. The C1, C2, W0, W1, and W2 exons are shown as black boxes. The bottom diagram represents the targeting plasmid used to incorporate the Cp EBNA2-responsive enhancer deletion into the viral genome. Cp*, tagged Cp in which the C1 exon contains a nucleotide sequence tag as previously described (23).
The kinetics of Cp upregulation after B-cell infection may be explained in part by the action of the EBNA2-dependent enhancer located 350 bp upstream of Cp. Consistent with this hypothesis, we have previously shown that LCLs harboring a Cp EBNA2 enhancer deletion mutant virus use Wp almost exclusively to drive EBNA transcription, whereas most wild-type (wt) EBV-infected LCLs predominantly use Cp to drive transcription of the EBNA genes (23). A separate characterization of LCLs harboring a mutant EBV in which the RBP-Jκ binding site in the Cp EBNA2-dependent enhancer was mutated revealed a more modest impact on Cp activity (6); this result may reflect either the ability of the other cis elements in the EBNA2 enhancer to function in the absence of a functional RBP-Jκ site or residual activity of the mutated RBP-Jκ site.
To date, it has not been possible to examine the initial stages of infection of resting B cells with the Cp EBNA2 enhancer deletion mutant virus or to rigorously determine whether this virus is impaired for B-cell immortalization, because of the lack of productively infected cell lines harboring this mutant. Here we report the generation of cottontop marmoset LCLs harboring either wt EBV or the Cp EBNA2 enhancer mutant virus and demonstrate the general utility of such cell lines for producing stocks of EBV that can be used to monitor early events after infection of primary B cells. This approach was used to critically compare the immortalizing capacity of the Cp EBNA2 enhancer mutant virus to that of wt EBV as well as to determine the role of the Cp EBNA2-dependent enhancer in upregulating Cp-initiated transcription during the establishment of latency.
MATERIALS AND METHODS
Generation of marmoset LCLs.
Cp EBNA2 enhancer deletion mutant-infected marmoset LCLs (mE2mut) were generated as follows. One day prior to infection, HS68 fibroblasts were irradiated with 3,000 rads and plated in 96-well plates. Human LCLs (clone 19-112) (7 × 106) harboring the Cp EBNA2 enhancer mutant virus were resuspended in RPMI 1640 medium, 10% fetal calf serum, 1 μg of tetradecanoyl phorbol acetate (TPA) per ml, and 1 μM ionomycin. On the day of infection, 2 to 3 ml of heparinized cottontop marmoset (Saguinus oedipus) blood (generously provided by Ronald Desrosiers) was diluted 1:1 in phosphate-buffered saline, and mononuclear cells were isolated on a Histopaque (Sigma) gradient. Platelets were removed by spinning the cells at 1,000 × g through a cushion of fetal calf serum and recovering the pellet. Erythrocytes were lysed by TAC treatment (9 parts 140 mM NH4Cl, 1 part 17 mM Tris). Marmoset peripheral blood mononuclear cells (PBMCs) were washed and resuspended in cold (4°C) complete RPMI 1640 medium. Human LCLs which had been treated with TPA-ionomycin were washed twice in complete RPMI 1640 medium, irradiated with 7,000 rads, and cocultured with marmoset PBMCs in 96-well plates in the presence of 1 μg of cyclosporin A per ml (3). Both the human LCLs and the marmoset PBMCs were plated at densities of 5 × 104 cells/well. Wells were monitored for LCL formation over several months, and LCLs were expanded on feeder layers until they were well established.
mB95 cells were generated by purifying marmoset PBMCs as described above, infecting them with 10 μl of B95.8 virus stock for 2 h, washing them, and plating them on a feeder layer in the presence of cyclosporin A.
Nucleic acid hybridization blots.
Southern blotting was performed as previously described (23). To quantitate the amount of viral DNA in virus stocks, 100 μl of virus stock was treated with DNase I by mixing with 100 μl of 2× DNase I incubation buffer (100 mM Tris [pH 7.5], 20 mM MgCl2, 100 μg of bovine serum albumin per ml) and 280 ng of DNase I to remove unpackaged DNA. The samples were incubated for 30 min at 37°C, mixed, and incubated for an additional 5 min, followed by the addition of 5 μl of 0.5 M EDTA (pH 8) to stop DNase I digestion. Virions were subsequently lysed by mixing with 200 μl of 2× lysis buffer (2% Sarkosyl, 0.5% sodium dodecyl sulfate [SDS], 40 mM Tris [pH 7.5], 200 mM NaCl, 20 mM EDTA, 100 μg of proteinase K per ml) and incubation for 1 h at room temperature. Samples were extracted with 1:1 phenol-CHCl3 and ethanol precipated.
Dilutions of viral DNA were denatured in 0.4 N NaOH–10 mM EDTA (pH 8), heated for 5 min at 37°C, mixed with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and loaded into wells of a Bio-Dot SF apparatus (Bio-Rad). Hybond membranes (Amersham) were used for sample binding. Prehybridization, hybridization, and wash protocols were performed as for Southern blotting. An oriP-specific probe (bp 7316 to 9135) was used, and signal intensity was quantified with a PhosphorImager (Molecular Dynamics).
S1 nuclease protection.
RNA was prepared as previously described. S1 nuclease protection assays were performed as previously described with 10 μg of RNA from each sample in all experiments, except for the β-estradiol titration experiment with the er/eb 2-5 cell line in which 40 μg of RNA was used. The sequences of the W0-W1 and C1 S1 nuclease probes have been previously published. The sequence of the C1-C2 S1 nuclease probe was 5′-ACGTGCAGGAGGCTGTTTCTTCAGTCGGTTTAGATGATTTGGTATCGGAGCTGGACCTA-3′. The sequence of the BHLF1 S1 nuclease probe was 5′-GCTGGG AGGTGTGCACCCCCCGAGCGTCTGGACGAGCTGGCGAGCCGGGCCGG CTCGCC-3′.
Transformation assay.
Partially purified human peripheral B cells were prepared from leukopaks by E-rosette depletion of T cells (13). For each virus stock, 100, 10, 1, and 0.1 μl of virus was used to infect 3 × 106 human B lymphocytes in a 1-ml volume for 2 h at 37°C. Infected cells were plated on irradiated fibroblast feeder layers (HS68) in 96-well plates at a density of 5 × 104 cells/well. After 3.5 weeks, plates were screened on a weekly basis for the presence of LCLs. At 10 weeks, final counts were made to determine the transforming titer of the virus stocks at the appropriate dilutions.
RESULTS
Production of marmoset LCLs.
PBMCs were purified from 2- to 3-ml samples of heparinized cottontop marmoset (S. oedipus) blood. Marmoset LCLs were generated using one of two protocols. For the generation of marmoset LCLs infected with the B95.8 strain of EBV, marmoset PBMCs were directly infected with a high-titer B95.8 cell-free viral preparation for 2 h, followed by washing and plating of the cells on irradiated fibroblasts in 96-well plates. This method, however, was not successful in generating marmoset LCLs that harbor the Cp EBNA2 enhancer deletion mutant virus. The latter result was presumably due to the fact that the human LCLs immortalized with the Cp EBNA2 enhancer deletion mutant virus are tightly latent; thus, it is difficult to induce reactivation. In addition, marmoset B cells are more difficult to immortalize with EBV than human B cells. As an alternative method, we used cocultivation of marmoset B cells with human LCLs that had been treated with TPA and ionomycin for 24 h, washed, and irradiated with 7,000 rads. These irradiated LCLs were then cocultured with marmoset PBMCs on fibroblast feeder layers in 96-well plates in the presence of cyclosporin A. This method was successfully used to generate multiple marmoset LCLs harboring the Cp EBNA2 enhancer deletion mutant virus.
The presence of the Cp EBNA2-dependent enhancer deletion in the marmoset LCLs established with mutant virus recovered from human clone LCL 19-112 (23) was verified by Southern blot analyses (data not shown). We investigated the spontaneous lytic activity and TPA-ionomycin inducibility of the marmoset LCLs. The analysis of independently derived marmoset LCLs demonstrated that, as a general rule, marmoset LCLs are significantly more inducible than human LCLs (data not shown), and most of the newly established marmoset LCLs were more inducible than the established B95.8 marmoset LCL.
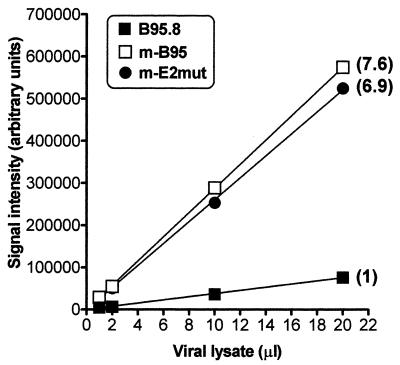
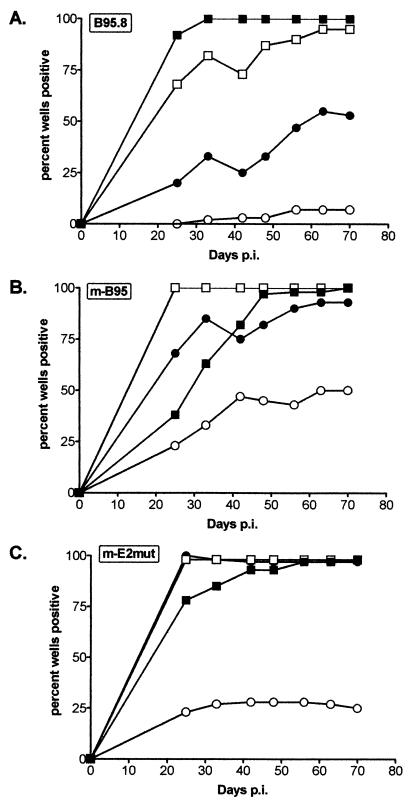
Virus stocks were generated by resuspending 4 × 108 cells in 1.2 liters of complete medium in a roller bottle and leaving the culture for 11 days without further feeding. The cells were subsequently spun down, the supernatant was recovered, and the virus was concentrated 100-fold by centrifugation at 14,000 × g for 2 h. The resulting virus stocks were quantified using a DNA dot blot (Fig. 2), and titers were determined using a B-cell immortalization assay (Fig. 3). Both assays revealed higher viral titers in the new marmoset LCL (mB95) stock than in the stock prepared from the established B95.8 cell line (B95.8). The viral DNA dot blot, done with a probe containing oriP (the latency origin of replication), demonstrated that the mB95 stock had about eightfold more viral DNA than the B95.8 stock (Fig. 2). Consistent with this analysis, the transformation assay demonstrated that there was a ninefold higher level of immortalizing units in the mB95 stock than in the B95.8 stock (Fig. 3). Notably, at higher input virus levels with the mB95 stock and, to a lesser extent with the mE2mut stock, there was a delay in the appearance of LCLs, suggesting that either higher virus titers or some substance present in these stocks may mildly delay LCL outgrowth (Fig. 3). Based on the viral DNA dot blot, the mE2mut stock had about sevenfold more virus that the B95.8 stock (Fig. 2). Importantly, the mE2mut stock demonstrated about fivefold more immortalizing units than the B95.8 stock (Fig. 3). Based on the good correlation between viral DNA content and immortalization titer observed with the B95.8 and mB95 stocks, estimating virus titer by measuring the amount of viral DNA present in a virus stock appears reasonable. This has been confirmed by additional analyses of B-cell immortalization with other mB95 and mutant virus stocks (unpublished data). Although the mE2mut virus and the B95.8 virus are not isogenic, a previous study has shown that when an EBNA2 gene from the B95.8 genome is recombined into the P3HR1 genome, the resulting intertypic virus has a transforming efficiency similar to that of the B95.8 virus (4). The mE2mut virus is just such an intertypic virus, except that it harbors the Cp EBNA2-dependent enhancer deletion as well. These data therefore indicate that little or no reduction in B-cell immortalization is caused by deletion of the Cp EBNA2-dependent enhancer.
FIG. 2.
PhosphorImager analysis of a dot blot measuring the quantity of viral DNA in viral stocks. B95.8, mB95, and mE2mut stocks (1, 2, 10, and 20 μl) were lysed, blotted onto a nitrocellulose membrane, and hybridized with a probe specific for oriP. Relative signal quantitation is indicated in parentheses.
FIG. 3.
B-cell immortalization determining the transformation titer of viral stocks. Viral stocks at 100 (■), 10 (□), 1 (●), or 0.1 μl (○) were used to infect 3 × 106 primary B cells for 2 h. Cells were then plated in 96-well plates and examined for LCL formation over a period of 10 weeks. LCL-positive wells were scored on a weekly basis. p.i., postinfection.
Examination of promoter usage at early times postinfection.
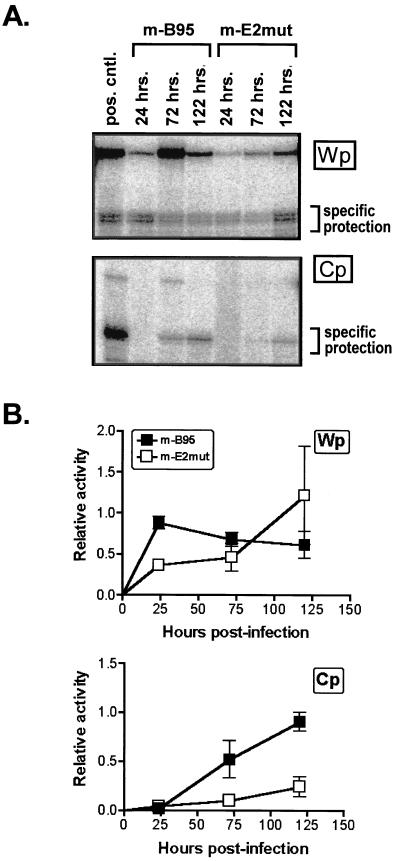
Equivalent amounts of mB95 and mE2mut viruses, as determined by the viral DNA dot blot, were used to infect 2 × 108 purified human B cells. Cells were harvested at 24, 72, and 120 h postinfection, and RNA was prepared. S1 nuclease protection assays were performed using probes specific for transcripts initiating from Cp or Wp (Fig. 4). The Cp-specific probe spans the C1-C2 exon junction, while the Wp-specific probe hybridizes to the 5′ end of the W0 exon through the W0-W1 exon junction. We did not assay for the presence of W0-W1′ spliced transcripts, since previous analyses of early viral transcription during the establishment of B-cell latency indicated that (i) the majority of Wp-initiated transcripts at early times contain the W0-W1 exon splicing pattern and (ii) there is no apparent change in the relative ratio of W0-W1 and W0-W1′ spliced transcripts over time (1).
FIG. 4.
S1 nuclease protection analysis of promoter activity at early times after infection with viral stocks. Peripheral blood lymphocytes were infected with mB95 or mE2mut virus, and cells were harvested for RNA at 24, 72, and 122 h postinfection. RNA (10 μg) was probed for Cp- and Wp-initiated transcripts. pos. cntl., positive control. (A) Representative experiment. (B) Summary of four experiments. Error bars indicate standard deviations.
Analysis of the infection time courses showed that while relatively high levels of Cp-initiated transcripts were detectable by 120 h after infection with the mB95 virus, only a low level of Cp activity was detectable by 120 h after infection with the mE2mut virus (Fig. 4). In contrast, the mE2mut virus continued to exhibit high levels of Wp-initiated transcripts at 120 h postinfection, whereas in mB95-infected B cells, the levels of Wp-initiated transcripts routinely started to fall by this time (Fig. 4). These data are consistent with the previously observed bias of LCLs immortalized with the Cp EBNA2 enhancer deletion mutant virus toward the use of Wp to drive EBNA gene transcription and suggest that the Cp EBNA2-dependent enhancer plays an important role during the establishment of latency in the upregulation of transcription from Cp. The strong “protection” of the full-length Wp probe observed at 72 h after infection with the mB95 virus (Fig. 4A) was not observed in other experiments and thus likely represents a small amount of probe which was not exposed to S1 nuclease digestion.
Effect of EBNA2 on promoter usage in an established LCL.
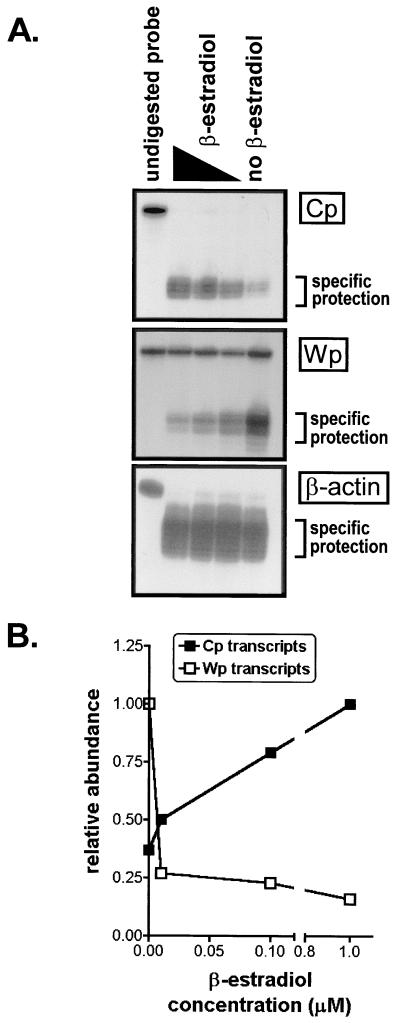
The early-time-point S1 nuclease protection assay, as well as previous studies of established LCLs harboring the Cp EBNA2 enhancer deletion mutant virus, have shown that the EBNA2-responsive enhancer is critical for Cp activity. Previous studies from our laboratory have also provided evidence that Cp-initiated transcription may directly serve to downregulate Wp activity by interfering with Wp-initiated transcription (17). To further investigate this hypothesis, promoter activity was examined in the EBNA2-conditional LCL er/eb 2-5. This cell line contains an estrogen receptor-EBNA2 fusion protein which renders EBNA2 function dependent on the presence of β-estradiol (10). It has been shown that this cell line growth arrests and dies within a few days upon withdrawal of β-estradiol. Thus, the er/eb 2-5 cell line is normally passaged in 1 μM β-estradiol, since full EBNA2 function is present at this concentration. Control of β-estradiol levels in the cell medium allowed us to manipulate Cp activity via the EBNA2-responsive enhancer while simultaneously monitoring Wp activity. Cells were incubated for 48 h in 1, 0.1, 0.01, or 0 μM β-estradiol and then harvested for preparation of RNA. Notably, no cell death was evident at this time. In addition, we determined that the presence of β-estradiol did not affect the levels of Cp- or Wp-initiated transcripts in three independently generated LCLs harboring wt EBV (data not shown). RNA samples from the er/eb 2-5 cell line were assayed for promoter activity by an S1 nuclease protection assay, using probes which hybridized to the C1 exon or the W0-W1 exon junction, to identify Cp- or Wp-initiated transcripts, respectively. As expected, Cp activity correspondingly decreased as β-estradiol concentrations were decreased, since EBNA2 function was diminished (Fig. 5). Furthermore, Wp activity significantly increased as β-estradiol concentrations were decreased, again demonstrating a direct relationship between decreased Cp activity and induction of Wp activity.
FIG. 5.
S1 nuclease protection assay of er/eb 2-5 cells incubated with various concentrations of β-estradiol for 48 h. RNA was harvested and probed for Cp- and Wp-initiated transcription. Undigested probe is in the left lane of each panel. β-estradiol concentrations, from left to right, were 1, 0.1, 0.01, and 0 μM, respectively. (A) Actual S1 nuclease assay autoradiograph. (B) PhosphorImager quantitation of data from panel A.
DISCUSSION
An increasing number of engineered EBV mutants have been generated in the past decade. Some of the strategies used to produce these mutants have involved infecting human B cells with pools of virus from transfected P3HR1 cells and then screening resulting clonal LCLs for viral genomes with the desired recombinant mutation. Once the desired virus is latent in these cells, however, it is difficult to generate large quantities of viral particles because human LCLs are refractory to lytic induction with currently known methods. TPA, sodium butyrate, ionomycin, 5-azacytidine, and dexamethasone (2, 12, 14, 24) are examples of chemicals with which we have treated human LCLs in order to boost lytic induction. However, in our experiments, none of these methods induced newly established LCLs to sufficient levels to produce high-titer viral stocks.
To circumvent this limitation, we have developed a method for generating marmoset LCLs that allows the production of relatively large quantities of mutant virus which can be used for analysis of the early events in B-cell immortalization. This approach is limited to EBV mutants that retain immortalizing capacity, since it is dependent on the immortalization of marmoset B cells. Previous studies have shown that marmoset LCLs are more spontaneously lytic than human LCLs and therefore might be useful for virus production (5, 15, 16). However, marmoset B cells are relatively difficult to transform with EBV, perhaps due to high levels of spontaneous lytic activity during initial infection. Using cocultivation of irradiated human LCLs with marmoset PBMCs, we have been able to generate marmoset LCLs harboring the virus strain of interest.
We have used this approach to analyze the behavior of the Cp EBNA2 enhancer deletion mutant virus during the early stages of infection of primary B cells. We have demonstrated, using a robust and quantitative immortalization assay, that deletion of the Cp EBNA2-dependent enhancer does not diminish the immortalizing capacity of this virus in vitro. However, this mutation does affect the induction of Cp-initiated transcription during the establishment of EBV latency. The latter observation demonstrates that EBNA2 plays an important role in the induction of Cp activity and is consistent with our previous analyses of LCLs harboring the deletion virus, which exhibited only low levels of Cp-initiated transcription. Based on the current analyses, as well as previous characterizations of the kinetics of induction of Cp-initiated transcription during the early stages of B-cell infection (18, 21), it appears that both EBNA2 and EBNA1 are required for efficient induction of Cp-initiated transcripts.
Previous analyses of EBNA gene transcription indicated that Wp activity is directly linked to Cp activity. Notably, here we have shown that the failure to efficiently induce Cp-initiated transcription during the early stages of primary infection leads to a continued high level of transcription from Wp. Thus, upregulation of transcription from the distal EBNA gene promoter Cp results in diminished transcription from the proximal EBNA gene promoter Wp, consistent with transcriptional interference. In support of this hypothesis, we have previously shown, using reporter constructs, that inversion or deletion of Cp results in maximal Wp activity (17). Here we have extended this analysis by examining the impact of diminished EBNA2 activity on transcription initiation from Cp and Wp. As predicted from the analysis of the Cp EBNA2 enhancer deletion mutant virus, diminished EBNA2 activity led to decreased levels of Cp-initiated transcripts and increased levels of Wp-initiated transcripts. Thus, all the available data strongly argue that transcription initiation from Cp directly diminishes the level of Wp-initiated transcription.
Several independent lines of evidence indicate that diminished, or absent, Cp activity does not adversely affect EBV immortalization of primary B cells in vitro (19, 23). The current data from the analysis of EBNA gene transcription demonstrate that Wp-initiated EBNA gene transcripts can fully substitute for Cp-driven EBNA gene transcripts. Still unresolved is the importance of Cp during EBV infection in vivo. Analysis of the sequences around Cp in two primate lymphocryptoviruses has demonstrated the preservation of all the known cis elements involved in regulating Cp (7). This finding argues that the functions of Cp that distinguish it from Wp are well conserved among the related primate viruses and suggests that Cp plays a critical role in the pathogenesis of these viruses. While Wp-initiated transcription appears to be regulated largely by cellular factors, Cp-initiated transcription has evolved to be tightly regulated by EBNA gene products. Thus, there may be a situation in vivo where this feedback regulation of EBNA gene expression plays a critical role.
ACKNOWLEDGMENTS
We thank George Bornkamm and Bettina Kempkes for the er/eb 2-5 cell line. We also thank David Leib, Peggy MacDonald, Lynda Morrison, Skip Virgin, and members of their laboratories for helpful discussions during weekly laboratory meetings.
This research was supported by NIH grant R01 CA43143.
REFERENCES
- 1.Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. doi: 10.1016/0042-6822(91)90893-g. . (Erratum, 185:946.) [DOI] [PubMed] [Google Scholar]
- 2.Ben Sasson S A, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28:131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- 3.Chang R S, Lung M L. A modified procedure for the propagation of wild-type Epstein-Barr virus in cultures of marmoset blood cells. J Virol Methods. 1994;46:167–178. doi: 10.1016/0166-0934(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J I, Wang F, Mannick J, Kieff E. Epstein-Barr virus nuclear protein 2 is a key determinant of lymphocyte transformation. Proc Natl Acad Sci USA. 1989;86:9558–9562. doi: 10.1073/pnas.86.23.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desgranges C, Lenoir G, de The G, Seigneurin J M, Hilgers J, Dubouch P. In vitro transforming activity of EBV. I. Establishment and properties of two EBV strains (M81 and M72) produced by immortalized Callithrix jacchus lymphocytes. Biomedicine. 1976;25:349–352. [PubMed] [Google Scholar]
- 6.Evans T J, Farrell P J, Swaminathan S. Molecular genetic analysis of Epstein-Barr virus Cp promoter function. J Virol. 1996;70:1695–1705. doi: 10.1128/jvi.70.3.1695-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuentes-Panana E M, Swaminathan S, Ling P D. Transcriptional activation signals found in the Epstein-Barr virus (EBV) latency C promoter are conserved in the latency C promoter sequences from baboon and rhesus monkey EBV-like lymphocryptoviruses (cercopithicine herpesviruses 12 and 15) J Virol. 1999;73:826–833. doi: 10.1128/jvi.73.1.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 9.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempkes B, Zimber-Strobl U, Eissner G, Pawlita M, Falk M, Hammerschmidt W, Bornkamm G W. Epstein-Barr virus nuclear antigen 2 (EBNA2)-oestrogen receptor fusion proteins complement the EBNA2-deficient Epstein-Barr virus strain P3HR1 in transformation of primary B cells but suppress growth of human B cell lymphoma lines. J Gen Virol. 1996;77:227–237. doi: 10.1099/0022-1317-77-2-227. [DOI] [PubMed] [Google Scholar]
- 11.Lee M A, Diamond M E, Yates J L. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J Virol. 1999;73:2974–2982. doi: 10.1128/jvi.73.4.2974-2982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 13.Madsen M, Johnsen H E, Hansen P W, Christiansen S E. Isolation of human T and B lymphocytes by E-rosette gradient centrifugation. Characterization of the isolated subpopulations. J Immunol Methods. 1980;33:323–336. doi: 10.1016/0022-1759(80)90003-4. [DOI] [PubMed] [Google Scholar]
- 14.Magrath I T, Pizzo P A, Novikovs L, Levine A S. Enhancement of Epstein-Barr virus replication in producer cell lines by a combination of low temperature and corticosteroids. Virology. 1979;97:477–481. doi: 10.1016/0042-6822(79)90360-x. [DOI] [PubMed] [Google Scholar]
- 15.Miller G, Coope D, Niederman J, Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis-derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976;18:1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller G, Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci USA. 1973;70:190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puglielli M T, Desai N, Speck S H. Regulation of EBNA gene transcription in lymphoblastoid cell lines: characterization of sequences downstream of BCR2 (Cp) J Virol. 1997;71:120–128. doi: 10.1128/jvi.71.1.120-128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlager S, Speck S H, Woisetschläger M. Transcription of the Epstein-Barr virus nuclear antigen 1 (EBNA1) gene occurs before induction of the BCR2 (Cp) EBNA gene promoter during the initial stages of infection in B cells. J Virol. 1996;70:3561–3570. doi: 10.1128/jvi.70.6.3561-3570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan S. Characterization of Epstein-Barr virus recombinants with deletions of the BamHI C promoter. Virology. 1996;217:532–541. doi: 10.1006/viro.1996.0148. [DOI] [PubMed] [Google Scholar]
- 20.Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woisetschlaeger M, Jin X W, Yandava C N, Furmanski L A, Strominger J L, Speck S H. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci USA. 1991;88:3942–3946. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woisetschlaeger M, Yandava C N, Furmanski L A, Strominger J L, Speck S H. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci USA. 1990;87:1725–1729. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo L I, Mooney M, Puglielli M T, Speck S H. B-cell lines immortalized with an Epstein-Barr virus mutant lacking the Cp EBNA2 enhancer are biased toward utilization of the oriP-proximal EBNA gene promoter Wp1. J Virol. 1997;71:9134–9142. doi: 10.1128/jvi.71.12.9134-9142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.zur Hausen H, O'Neill F J, Freese U K, Hecker E. Persisting oncogenic herpesvirus induced by the tumour promoter TPA. Nature. 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]