Abstract
Key Clinical Message
Venous spasm is an important reason for complicated or failed implantations of cardiac implantable electronic devices. Prevention or risk reduction of venous spasm during cardiac implantable electronic device implantation may be achieved by ultrasound or fluoroscopic imaging prior to puncture, cephalic vein cut‐down, sufficient pre‐ and perioperative hydration, nitroglycerin injection and effective sedation, and analgesia.
Abstract
This case report with literature review focuses on venous spasm as a potential cause for complicated implantations of cardiac implantable electronic devices. The case report is clinically relevant as it describes a progressive spasm affecting the axillary and the subclavian vein. A 66‐year‐old female complained of symptomatic atrial fibrillation (AF) and atypical atrial flutter despite interventional and medical treatment. As an ultimate treatment, she was scheduled for pacemaker implantation and atrioventricular node ablation. Several puncture attempts of the axillary vein failed. Despite venous blood aspiration, no guidewires could be advanced into the axillary vein. We performed a first venogram revealing significant spasm of the axillary vein. Another failed venous puncture occurred after change of access site to the subclavian vein. A second venogram displayed progression of the spasm, now affecting both the axillary and the subclavian veins. Normal saline perfusion was administered as well as intravenous isosorbide. Unfortunately, a repeated venogram after 15 min waiting time showed persistence of the spasm, still affecting both veins. The procedure was discontinued as the patient became uncomfortable. Venous spasm is an important reason for complicated or failed implantations of cardiac implantable electronic devices. Commonly used medical prevention and treatment are intravenous fluids and nitroglycerin. Prevention or risk reduction of venous spasm during cardiac implantable electronic device implantation may be achieved by ultrasound or fluoroscopic imaging prior to puncture, cephalic vein cut‐down, sufficient pre‐ and perioperative hydration, nitroglycerin injection and effective sedation and analgesia.
Keywords: axillary vein, cardiac implantable electronic device, implantation, pacemaker, subclavian vein, venous spasm
Venogram during permanent pacemaker implantation with different stages of venous spasm Figure 1A: First venogram, realized after some attempts to puncture axillary vein, showing significant spasm of the axillary vein. Figure 1B: Second venogram, realized after changing of access site using subclavian vein, showing progression of the spasm affecting both axillary and subclavian veins. Figure 1C: Third venogram realized after waiting for 15 minutes after giving normal saline perfusion and intravenous isosorbide dinitrate, showing persistence of the spasm of both veins (axillary and subclavian). Figure 1D: Hydrophilic guidewire inside cephalic vein. AV: axillary vein; CV: cephalic vein; SV: subclavian vein; SVC: superior vena cava; HG: Hydrophilic guidewire
1. INTRODUCTION
Vascular spasm is a well‐known condition in arteries, as it may cause access problems to the radial artery during coronary angiographies, or it can lead to Prinzmetal angina when it occurs in the coronary artery. Capillary spasm has also been described, for example as Raynaud's phenomenon, which is caused by altered microcirculation. 1 , 2 , 3 , 4
Venous spasm, on the other hand, seems to be a less known condition. Relevant venous spasm is rarely encountered during cardiac implantable electronic device (CIED) implantation. As venograms are not routinely performed during CIED implantations, it is probably underreported. Nevertheless, as this case of a failed pacemaker implantation illustrates, it may lead to more complicated, prolonged, or even failed procedures. We performed a review of the literature, as important aspects of venous spasm during CIED implantation such as definition, incidence, risk factors, treatment, and prevention remain poorly elucidated.
2. CASE REPORT
A 66‐year‐old female (BMI = 28) was scheduled for pacemaker implantation and subsequent atrioventricular node ablation. She had highly symptomatic atrial fibrillation (AF) and atypical atrial flutters with several external cardioversions and two AF ablations with arrhythmia recurrence during the follow‐up despite additional medical treatment.
Her prior medical history consisted of an atrial septal defect (ostium secundum); diagnosed and closed in 2017 with a percutaneous closure system (Amplatzer device). Before the Amplatzer device implantation, she had undergone pulmonary vein (PV) isolation and cavo‐tricuspid isthmus ablation. After several external electrical cardioversions with symptomatic arrhythmia recurrences during the following years and amiodarone associated hyperthyroidism, she underwent a second AF ablation in 2023, revealing an extensively scarred left atrium (80% with a low voltage <0.2 mV) with isolated PV and multiple left and right atrial flutters with different cycles lengths and coronary sinus activation patterns. After successful ablation of a septal flutter and induction of other unstable atrial flutters and AF, further ablation was discontinued because of probable futility. The procedure was terminated in sinus rhythm after electrical cardioversion.
During the follow‐up visit 3 months after the second ablation, she complained of dyspnea and palpitations. Her heart rate was 130 bpm on treatment with bisoprolol 10 mg. The ECG showed AF. Serum electrolytes, renal function, hemoglobin, and thyroid parameters were within the normal range. Transthoracic echocardiography revealed a normal left ventricular ejection fraction (LVEF), a dilated left atrium (83 mL/m2) and significant mitral regurgitation and tricuspid regurgitation.
Due to the recurrence of symptomatic AF despite maximal dosage of bisoprolol, we explained pros and cons of the treatment options (intensified medical rate control, another ablation attempt, “pace and ablate” strategy, repeated electrical cardioversion). The patient finally agreed to an implantation of a single‐chamber pacemaker with left‐bundle branch area pacing (LBBAP) and subsequent atrioventricular node ablation during one procedure.
Prior to the procedure, lidocaine as local anesthesia was subcutaneously injected. She additionally received 5 mg nalbuphine chlorhydrate and 2 mg midazolam. As she continued to be anxious and in pain during the procedure, she received a cumulative dose of 15 and 7 mg respectively.
We proceeded with the skin incision in the left deltopectoral groove and prepared the subcutaneous pre‐pectoral pocket for device placement. As two relatively large sheaths were required for the planned procedure (7 Fr for the LBBAP sheath and 8 Fr for the ablation catheter), we did not prepare the cephalic vein and primarily attempted an axillary venous puncture using the second rib bone as fluoroscopic landmark. However, despite several attempts with venous blood aspiration, neither a standard guidewire nor a hydrophilic guidewire could be advanced into the axillary vein. To better delineate the venous drainage of the left upper limb, intravenous contrast agent (iodixanol 320 mg /mL; 5 mL diluted by 5 mL normal saline) was injected through the ipsilateral peripheral venous access. The venogram revealed significant spasm of the axillary vein. The subclavian vein was of a normal caliber, draining into the left brachiocephalic vein and then into superior vena cava (Figure 1A, Video S1). Consequently, we changed access site and attempted to puncture the left subclavian vein, this time without aspiration of venous blood despite several attempts by an experienced operator. Subsequently, a second venogram was performed. It displayed progression of the spasm, now affecting both the axillary and the subclavian veins (Figure 1B, Video S1). Normal saline perfusion was administered as well as two sequential boluses of intravenous isosorbide dinitrate (1 mg followed by 1 mg 5 min later). Unfortunately, a repeat venogram after 15 min waiting time showed persistence of the spasm, still affecting the axillary and the subclavian veins (Figure 1C, Video S1). We prepared the cephalic vein and canulated it with a hydrophilic guidewire (Figure 1D, Video S1). Unfortunately, it was not possible to advance even a small 5 Fr sheath into the subclavian vein over the guidewire. As our patient became uncomfortable due to back pain and pain at the operation site despite local and systemic analgesia, the procedure was discontinued. The patient agreed to intensified medical treatment by additional digoxin 0.125 mg/day. The need for another implantation attempt will be discussed with her during the next follow‐up.
FIGURE 1.
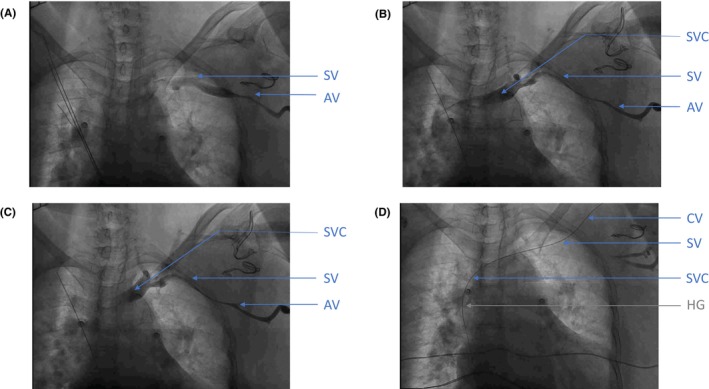
Venogram during permanent pacemaker implantation with different stages of venous spasm. (A) First venogram, realized after some attempts to puncture axillary vein, showing significant spasm of the axillary vein. (B) Second venogram, realized after changing of access site using subclavian vein, showing progression of the spasm affecting both axillary and subclavian veins. (C) Third venogram realized after waiting for 15 minutes after giving normal saline perfusion and intravenous isosorbide dinitrate, showing persistence of the spasm of both veins (axillary and subclavian). (D) Hydrophilic guidewire inside cephalic vein. AV, axillary vein; CV, cephalic vein; SV, subclavian vein; SVC, superior vena cava; HG, hydrophilic guidewire.
3. METHODS
This study provides a narrative review rather than a systematic review of the literature that focuses on the description of venous spasm during CIED implantation. It included articles published in English language on MEDLINE/PubMed database from January 1, 1965 to August 31, 2023. We included the following terms: {“venous” AND “spasm” OR “vasospasm”} AND {“pacemaker” OR “device” OR “cardiac electronic device” OR “ICD” OR “defibrillator” OR “implantation”}. A total of 1708 potentially relevant articles were identified. After screening the titles and abstracts, 12 articles were selected for a full analysis. Finally, the references of the included papers were screened for additional relevant literature and two more articles were identified for further analysis.
4. RESULTS AND DISCUSSION
4.1. Definition
Venous spasm can be diagnosed by venogram after injection of contrast dye into the ipsilateral peripheral vein. Lumen caliber reduction of the vein, together with contrast opacification, indicate the presence of venous spasm. 5 , 6 According to our knowledge, there are no broadly endorsed guidelines for the evaluation of venous spasm. Duan et al. defined mild venous spasm and severe venous spasm as a reduction in lumen caliber of 50%–90% and ≥ 90% respectively. 5 , 6
Central veins directly drain into the superior vena cava, the inferior vena cava or the right atrium. The veins of the upper extremity consist of a superficial and a deep system. The veins of the superficial system, the basilic and cephalic veins drain blood from the skin and the superficial fascia. The deep veins of the upper extremity drain blood from the deeper fascia, muscles, and bones via the brachial vein, to the extra‐thoracic axillary vein and then to the intra‐thoracic subclavian vein. 7
4.2. Incidence
Central venous spasm was first described during right cardiac catheterization in 1965. 8 More cases of central venous spasm during right cardiac catheterization were observed in the following years. 9 , 10 , 11 Peripheral venous spasm was also described. 12 Older studies on right heart catheterizations or central venous catheter placement found incidence rates from 2% to 5%. 8 , 10 , 11
As venograms are not routinely performed in clinical practice, the incidence of venous spasm is likely to be underreported. 13 An observational study performed venograms in cases of difficult puncture and found an incidence of venous spasm of 3%. 14 In contrast, when venograms were systematically performed, mild venous spasms could be observed in 27.8%–29.7% and severe venous spasms in 5.85%–8.1%. 5 , 15
Venous spasm can be suspected when access cannot be easily obtained. Without successful venous access within the first 3 min of attempted puncture, venous spasm was found in 66.6% of patients and in 100% when the procedure had to be abandoned. 5 This observation was confirmed by another prospective study. 15
Different methodologies in venous access technique, imaging and diagnostic criteria explain the different incidences of venous spasms between 3% and 29.7%.
4.3. Risk factors
Severe axillary venous spasm was found to be more frequent in elderly patients (age ≥ 70 years), female sex and with right‐sided puncture. 5 , 14 , 15
4.4. Pathophysiology
The exact mechanism of venous spasm is not clearly understood and would benefit from further studies, such as in vitro bioengineered tissue models or in vivo animal models. Potential factors raised are mechanical vessel injury, such as direct injury by needle punctures and guidewire placements, as well as indirect compression of the tissue surrounding the vessel by needle maneuvers, chemical irritation by local anesthesia or intravenous contrast agent and temperature changes. 14 , 16 , 17 , 18 , 19 An unyielding valve in the subclavian vein—leading to repeated needle punctures and guidewire manipulations with a resulting spasm–was reported as an exceptional anatomical condition. 20
Anatomically, axillary and subclavian veins belong to the deep veins of the upper extremity. Their tunica media contains circularly arranged smooth muscle fibers that mediate vascular contraction or relaxation. Participation of the sympathetic nervous system in local autonomic fibers has been evoked in cases of venous spasm as well as local metabolic factors. 12 , 14 Increased catecholamine levels may be induced by vessel injury and compression of the surrounding tissue. This might have been the case in our patient, in addition to the fact that she was anxious and uncomfortable during the procedure, with a possibly increased level of catecholamines.
Platelet and mast cell activation have been evoked as potential causes for vasospasm in saphenous venous grafts. 16 , 17 , 21 Damaged endothelium may cause the release of histamine and cytokines, with vasoconstriction and platelet aggregation. 22 , 23 , 24 A similar pathophysiological mechanism may also explain venous spasm in subclavian and axillary veins during CIED implantations.
Venous spasms seem to typically propagate both proximally and distally along the vessel. Thereafter, irrespective of the initial direction of spasm propagation, the subsequent vascular wall relaxation simultaneously occurs in the entire spasm‐affected venous segment. In cases of spasm‐related failed axillary puncture, subclavian puncture could be successfully achieved without evidence of subclavian venous spasm. 14 Our case describes a progressive venous spasm, first affecting the axillary vein (Figure 1A), then the axillary and the subclavian vein (Figure 1B,C) without resolution during a waiting period of 15 min.
4.5. Differential diagnoses
A reduced venous caliber may be caused by spasm, local hematoma, thrombosis, and dissection. 17 It may be difficult to distinguish those entities by two‐dimensional contrast fluoroscopy.
4.6. Treatment
The management of venous spasm is challenging. As additional puncture attempts may cause worsening of the spasm, they should be avoided if possible. 18 Intravenous fluids from the ipsilateral venous access may support the resolution of the venous spasm. 18 Intravenous nitroglycerin, injected into an ipsilateral venous access may also relieve the venous spasm. Usually, incremental doses of 100–200 μg of intravenous nitroglycerin are given with a repeat venogram after a waiting period of 10 min to demonstrate the resolution of the spasm, before reattempting another puncture. 18 The incidence of mild or severe venous spasm was lower in the nitroglycerin group than in the control group (3/20 vs. 11/20, p = 0.019) in a prospective randomized study. 6 Whereas reduction of venous spasms with nitroglycerin has been shown in this study, there is no study comparing the treatment of venous spasm between nitroglycerin and other substances. 6
As intravenous nitroglycerin is not available in France, we injected a similar substance, isosorbide dinitrate. In our case, the treatment with 1 mg of intravenous isosorbide dinitrate, repeated after 5 min, could not resolve the venous spasm after a waiting time of 15 min. No other treatment attempt with intravenous isosorbide dinitrate has been described in literature. Unsuccessful treatment of venous spasms during CIED implantations was also observed with nitroglycerin in some cases. 13 , 16 , 17 , 25 , 26 In other cases, nitroglycerin relieved peripheral venous spasms or even central venous spasm, with only one case of axillary vein spasm. 11 , 18 , 27 , 28 Venous spasms may also spontaneously resolve without medical treatment after some waiting time. 14 , 29
Another trouble shooting option is the change of access site to a more medial or lateral part of the axillary vein, 16 the subclavian vein, 26 or a contralateral venous access. 13 , 17 , 19 , 25 , 29 A more medial access can lead to an increased risk of complications, as pneumothorax or subclavian crush syndrome. 17 Due to lower complication rates, cephalic and axillary veins are recommended as the first‐line access sites by current guidelines. 30 , 31 Cephalic vein access was reported as a good bail‐out option when dealing with axillary venous spasm. 25 Nonetheless, cephalic vein spasm may also occur. 32 In our case, we were able to advance a hydrophilic guidewire via a tiny cephalic vein to the right atrium but we could not advance a sheath into the subclavian vein, most likely due to excessive venous spasm or a valve (Figure 1D).
4.7. Prevention
Whereas the efficacy of nitroglycerin is proven for the prevention and treatment of arterial spasm, 1 , 2 , 33 its effect on venous spasm is less certain.
To prevent venous spasm, different empirical strategies were described in case reports, including intravenous nitroglycerin, calcium channel blocker, procaine 1%, and sedative agents such as diazepam (Table 1). 12 , 17 , 34
TABLE 1.
Overview and main findings of publications on venous spasm during cardiac electronic device implantation.
| Author | Number of patients | Study type | Main finding |
|---|---|---|---|
| Chan and Leung 16 | 1 | Case report | Axillary venous spasm. Nitroglycerin IV (incremental doses 200 and 400 μg): no effect. Successful more lateral puncture of the axillary vein |
| Cooper et al. 29 | 1 | Case report | Subclavian venous spasm. Nitroglycerin: not tried. Contralateral access, venous spasm again but spontaneous resolution of venous spasm and successful puncture |
| Duan et al. 13 | 1 | Case report | Axillary venous spasm. Nitroglycerin (dose not mentioned): no effect. Successful puncture after contralateral access |
|
Koza et al. 26 |
1 | Case report | Subclavian venous spasm. Nitroglycerin (dose not mentioned): no effect. Partial reversal of the spasm and successful puncture of the subclavian vein |
| Krishnappa et al. 20 | 1 | Case report | Subclavian venous spasm. Nitroglycerin: not tried (hypotension). Successful more medial puncture of the subclavian vein |
| Vemuri et al. 17 | 1 | Case report | Axillary and subclavian venous spasm. Nitroglycerin IV (incremental doses 200 and 400 μg): no effect. Successful puncture after contralateral access |
| Steckiewicz et al. 19 | 1 | Case report | Cephalic, axillary and subclavian venous spasm. Nitroglycerin: not tried. Successful puncture after contralateral access |
| Hiruma et al. 25 | 1 | Case report | Axillary venous spasm. Nitroglycerin IV (1000 μg): no effect. Contralateral access, venous spasm again, successful puncture via cephalic vein |
| Bhasin et al. 18 | 1 | Case report | Axillary venous spasm. Normal saline + Nitroglycerin IV (dose not mentioned): resolution after 10 min. Successful puncture after resolution of venous spasm |
| Steckiewicz et al. 14 | 403 (337 + 66) | Observational retrospective | Axillary or subclavian venous puncture (377) ± cephalic vein cut‐down (66). Venogram performed in cases of difficult puncture (proportion not mentioned). Axillary vein spasm = 3% |
| Steckiewicz et al. 32 | 146 | Observational retrospective | Cephalic vein cut‐down as first line approach ± axillary or subclavian vein puncture. Any vasoconstriction observed during venography or via vessel exposure in the clavipectoral groove has been documented with captured images. Cephalic vein spasm = 7.5% |
| Al‐Gburi 15 | 137 | Observational prospective |
Axillary venous puncture. Contrast venogram systematically performed prior to puncture and after puncture. Axillary spasm: Mild 27.8%, Severe 5.8% Associated with: age >70 years, female |
| Duan et al. 5 | 74 | Observational prospective |
Axillary vein puncture. Contrast venogram systematically performed prior to puncture and after puncture. Axillary spasm: Mild = 29.7%, Severe = 8.1% Venous spasm associated with: age >70 years, right‐side implantation |
| Duan et al. 6 | 40 (20 + 20) |
Interventional prospective randomized |
Prevention of axillary spasm: nitroglycerin IV (200 μg 3 min prior to puncture) group versus control group. Incidence of axillary spasm: 3/20 versus 11/20 (p = 0.019). Mean degree of axillary spasm: 23% versus 45.5% (p = 0.018) |
Note: Success = success to implant the lead and the electronic cardiac device.
One prospective randomized trial demonstrated the preventive efficacy of 200 μg intravenous nitroglycerin 3 min before the axillary puncture. 6 As expected, it caused a decreased blood pressure but no symptomatic hypotension or other significant side effects. There are no other comparative studies on preventive measures. Empirical approaches focus on reducing vessel trauma prior to puncture by target vein visualization by fluoroscopic contrast venogram or ultrasound imaging. A puncture prior to skin incision or microneedle access may also reduce vessel trauma, thus the risk of venous spasm.
4.8. Impact of venous spams and trouble shooting
An important consequence of venous spasm might be failure to implant the CIED in cases without spasm resolution, in the absence of an alternative venous access. Access to another venous access site also increases the risk of complications, e.g., pneumothorax, hematoma, vessel injury and infection as well as the operating and fluoroscopy time. In cases of contralateral venous access, a second operation might be required with possibly prolonged hospital stay and increased costs.
The clinical impact of venous spasm mainly depends on the indication of CIED implantation. A venous spasm in an urgent pacemaker implantation of a pacing dependent patient may have different consequences than in an elective implantable cardioverter defibrillator (ICD) implantation. Thus, handling of venous spasm in clinical practice will differ depending on the initial indication and on patient's clinical characteristics. In cases of pacing dependent patients, temporary external transthoracic pacing may be required. Pacing via the jugular or femoral vein is a frequently used temporary solution until a permanent solution has been found. Noteworthy, venous access of the femoral vein will limit patients' autonomy due to the necessary supine position with restricted hip flection. Another issue are unstable pacing parameters of pacing leads without screw fixation.
Subcutaneous lead tunneling to a contralateral pacemaker or ICD pocket may be used in cases with lack of ipsilateral venous access. Femoral vein access—or rarely internal jugular vein access—may permit the implantation of leadless pacemakers as an alternative to traditional pacemaker leads. Epicardial pacing leads—implanted after surgical transthoracic access—can be used in cases of lacking transvenous access. Subcutaneous defibrillator (S‐ICD) may be an option when ICD are indicated without pacing indication.
On the other hand, if CIED implantation is successful after initial venous spasm, long‐term prognosis of patient's and CIAD's functional status may be unaltered but this issue deserves further investigation.
4.9. Outlook into future research
There are numerous limitations and gaps of knowledge on venous spasms in the setting of CIED implantation: for example, classification of the extent of the spasm, highly variable incidence depending on the study and the systematic search for spasm. As far as pathophysiology is concerned, there are mainly hypotheses but no proof.
An important issue is to explore the difference between local hematoma and venous spasm with different imaging techniques such as ultrasound, magnetic resonance angiography, or computed tomography to define diagnostic criteria and to improve the understanding of pathophysiology.
Further studies are warranted to explore treatment options, using in vitro or in vivo models.
5. CONCLUSION
Venous spasm during CIED implantation may lead to difficult or failed procedures as illustrated by our initial case report. The scarce literature consists of several case reports, two non‐systematic, retrospective observational studies and three systematic prospective studies. An incidence of more than 30% for axillary vein spasm in systematic studies emphasizes the importance of this phenomenon. Potential causes for venous spasm are trauma by needle puncture, chemical reaction by drug infusion or contrast agent and individual susceptibility enhanced by adrenergic activation. Commonly used medical prevention and treatment are intravenous fluids and nitroglycerin. Prevention or risk reduction of venous spasm during CIED implantation may be achieved by ultrasound or fluoroscopic imaging prior to puncture, cephalic vein cut‐down, sufficient pre‐ and perioperative hydration, nitroglycerin injection and effective sedation and, analgesia. Venous spasm may also spontaneously resolve during the procedure.
Future experimental and systematic prospective studies may further elucidate this important issue.
AUTHOR CONTRIBUTIONS
Amelie Venet: Conceptualization; data curation; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Romain Vergier: Conceptualization; validation. Kurlene Cenac: Writing – review and editing. Jocelyn Inamo: Validation. Andreas Müssigbrodt: Conceptualization; project administration; validation; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Supporting information
Video 1. This video presents a sequence of four different venograms during the surgery. The first venogram A reveals significant spasm of the axillary vein after failed puncture (fluoroscopic landmarks guided). The second venogram B displays venous spasm after failed puncture of the subclavian vein. The third venogram C shows progression of the spasm affecting both axillary and subclavian veins. The fourth venogram D confirms the persistence of the extensive venous spasm after a waiting period of 15 min, with cephalic vein cut‐down, insertion of a hydrophilic guidewire, normal saline perfusion, and intravenous isosorbide dinitrate injection.
Venet A, Vergier R, Cenac K, Inamo J, Müssigbrodt A. Axillary and subclavian venous spasm during pacemaker implantation – A case report and literature review. Clin Case Rep. 2024;12:e9309. doi: 10.1002/ccr3.9309
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen C‐W, Lin C‐L, Lin T‐K, Lin C‐D. A simple and effective regimen for prevention of radial artery spasm during coronary catheterization. Cardiology. 2005;105:43‐47. [DOI] [PubMed] [Google Scholar]
- 2. Dharma S, Shah S, Radadiya R, Vyas C, Pancholy S, Patel T. Nitroglycerin plus diltiazem versus nitroglycerin alone for spasm prophylaxis with transradial approach. J Invasive Cardiol. 2012;24:122‐125. [PubMed] [Google Scholar]
- 3. Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774‐1782. [DOI] [PubMed] [Google Scholar]
- 4. Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatology. 2005;44:587‐596. [DOI] [PubMed] [Google Scholar]
- 5. Duan X, Ling F, Shen Y, Yang J, Xu H. Venous spasm during contrast‐guided axillary vein puncture for pacemaker or defibrillator lead implantation. Eurospace. 2012;14:1008‐1011. [DOI] [PubMed] [Google Scholar]
- 6. Duan X, Ling F, Shen Y, Yang J, Xu H, Tong X. Efficacy and safety of nitroglycerin for preventing venous spasm during contrast‐guided axillary vein puncture for pacemaker or defibrillator leads implantation. Eurospace. 2013;15:566‐569. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen JD, Duong H. Anatomy, Shoulder and Upper Limb, Veins. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 8. Wennevold A, Christiansen I, Lindeneg O. Complications in 4,413 catheterizations of the right side of the heart. Am Heart J. 1965;69:173‐180. [DOI] [PubMed] [Google Scholar]
- 9. Morgan MG. Letter: Venospasm during cardiac catheterization. Am Heart J. 1974;88:813. [DOI] [PubMed] [Google Scholar]
- 10. Barber CJ. Central venous catheter placement for intravenous digital subtraction angiography: an assessment of technical problems and success rate. Br J Radiol. 1989;62:599‐602. [DOI] [PubMed] [Google Scholar]
- 11. Dalvi BV, Gupta KG, Munsi SC, Vengsarkar AS. Entrapment of venous catheter following inferior vena caval spasm. Catheter Cardiovasc Diagn. 1989;17:161‐163. [DOI] [PubMed] [Google Scholar]
- 12. Cunningham E, Korbon GA. Venospasm preventing peripheral venous access. Anesthesiology. 1983;59:141‐142. [DOI] [PubMed] [Google Scholar]
- 13. Duan X, Ling F, Shen Y, Xu H. Venous spasm during pacemaker implantation. Anadolu Kardiyol Derg AKD Anatol J Cardiol. 2011;11:E24. [DOI] [PubMed] [Google Scholar]
- 14. Steckiewicz R, Górko D, Świętoń EB, Szparecki G, Stolarz P. Axillary vein spasm during cardiac implantable electronic device implantation. Folia Morphol (Warsz). 2016;75:543‐549. [DOI] [PubMed] [Google Scholar]
- 15. Al‐Gburi A. Axillary vein spasm during real‐time venography‐guided puncture for cardiac electronic device implantation. J Babol Univ Med Sci. 2023;25:10‐19. [Google Scholar]
- 16. Chan N‐Y, Leung W‐S. Venospasm in contrast venography‐guided axillary vein puncture for pacemaker lead implantation. Pacing Clin Electrophysiol. 2003;26:112‐113. [DOI] [PubMed] [Google Scholar]
- 17. Vemuri KS, Parashar N, Bootla D, et al. Refractory axillary venous spasm during permanent pacemaker implantation. Egypt Heart J. 2020;72:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhasin D, Dhir A, Sharma YP. Axillary vein spasm during permanent pacemaker implantation. J Invasive Cardiol. 2022;34:E824. [DOI] [PubMed] [Google Scholar]
- 19. Steckiewicz R, Stolarz P, Zieliński A. Two problems during one pacemaker implantation procedure: axillary vein spasm and subclavian vein compression, or ‘every cloud has a silver lining’. Folia Cardiol. 2021;16:402‐406. [Google Scholar]
- 20. Krishnappa D, Sakaguchi S, Kasinadhuni G, Tholakanahalli VN. An unyielding valve leading to venous spasm during pacemaker implantation: a case report. Eur Heart J Case Rep. 2019;3:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cross KS, el‐Sanadiki MN, Murray JJ, Mikat EM, McCann RL, Hagen PO. Mast cell infiltration: a possible mechanism for vein graft vasospasm. Surgery. 1988;104:171‐177. [PubMed] [Google Scholar]
- 22. Charles AK, Gresham GA. Histopathological changes in venous grafts and in varicose and non‐varicose veins. J Clin Pathol. 1993;46:603‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ginsburg R, Bristow MR, Davis K, Dibiase A, Billingham ME. Quantitative pharmacologic responses of normal and atherosclerotic isolated human epicardial coronary arteries. Circulation. 1984;69:430‐440. [DOI] [PubMed] [Google Scholar]
- 24. Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct‐related coronary artery. Circulation. 1999;99:361‐369. [DOI] [PubMed] [Google Scholar]
- 25. Hiruma T, Nagase T, Inoue K, Nitta J, Isobe M. Cephalic vein cut‐down technique for severe venous spasm following axillary vein puncture at pacemaker implantation. J Cardiol Cases. 2022;26:245‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koza Y, Şimşek Z, Taş MH, Şenocak H. A challenging image during pacemaker implantation: venous spasm. Anatol J Cardiol. 2015;15(75):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zmyslowski WP. Treating peripheral venospasm. Anesthesiology. 1984;60:261. [DOI] [PubMed] [Google Scholar]
- 28. Clemens RK, Lillis AP, Alomari AI. Catheter‐induced venous spasm. Circulation. 2012;126:2363‐2365. [DOI] [PubMed] [Google Scholar]
- 29. Cooper RM, Krishnan U, Pyatt JR. Central venous spasm during pacemaker insertion. Heart. 2010;96:1484. [DOI] [PubMed] [Google Scholar]
- 30. Atti V, Turagam MK, Garg J, et al. Subclavian and axillary vein access versus cephalic vein cutdown for cardiac implantable electronic device implantation: a meta‐analysis. JACC Clin Electrophysiol. 2020;6:661‐671. [DOI] [PubMed] [Google Scholar]
- 31. Burri H, Starck C, Auricchio A, et al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter‐defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin‐American Heart Rhythm Society (LAHRS). Eurospace. 2021;23:983‐1008. [DOI] [PubMed] [Google Scholar]
- 32. Steckiewicz R, Świętoń EB, Bogdańska M, Stolarz P. Vasoconstrictive responses of the cephalic vein during first‐time cardiac implantable electronic device placement. Folia Morphol (Warsz). 2018;77:464‐470. [DOI] [PubMed] [Google Scholar]
- 33. Abrams J. Nitroglycerin and long‐acting nitrates. N Engl J Med. 1980;302:1234‐1237. [DOI] [PubMed] [Google Scholar]
- 34. Guragai N, Rampal U, Vasudev R, Patel H, Joshi MB, Shamoon F. A rare case of late onset saphenous vein graft spasm. J Community Hosp Intern Med Perspect. 2017;7:332‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. This video presents a sequence of four different venograms during the surgery. The first venogram A reveals significant spasm of the axillary vein after failed puncture (fluoroscopic landmarks guided). The second venogram B displays venous spasm after failed puncture of the subclavian vein. The third venogram C shows progression of the spasm affecting both axillary and subclavian veins. The fourth venogram D confirms the persistence of the extensive venous spasm after a waiting period of 15 min, with cephalic vein cut‐down, insertion of a hydrophilic guidewire, normal saline perfusion, and intravenous isosorbide dinitrate injection.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


