Abstract
A minority of initial multiple sclerosis (MS) presentations clinically or radiologically resemble other central nervous system (CNS) pathologies, acute disseminated encephalomyelitis (ADEM) or tumefactive demyelination (atypical demyelination presentations). With the aim of better defining the long-term outcomes of this group we have performed a retrospective cohort comparison of atypical demyelination versus ‘typical’ MS presentations. Twenty-seven cases with atypical presentations (both first and subsequent demyelinating events) were identified and compared with typical MS cases. Disease features analysed included relapse rates, disability severity, whole brain and lesion volumes, lesion number and distribution. Atypical cases represented 3.9% of all MS cases. There was considerable overlap in the magnetic resonance imaging (MRI) features of ADEM-like and tumefactive demyelination cases. ADEM-like cases tended to be younger but not significantly so. Atypical cases showed a trend towards higher peak expanded disability severity score (EDSS) score at the time of their atypical presentation. Motor, cranial nerve, cerebellar, cerebral and multifocal presentations were all more common in atypical cases, and less likely to present with optic neuritis. Cerebrospinal fluid (CSF) white cell counts were higher in atypical cases (p = 0.002). One atypical case was associated with peripheral blood myelin oligodendrocyte glycoprotein (MOG) antibodies, but subsequent clinical and radiological course was in keeping with MS. There was no difference in long-term clinical outcomes including annualised relapse rates (ARR), brain volume, lesion numbers or lesion distributions. Atypical demyelination cases were more likely to receive high potency disease modifying therapy early in the course of their illness. Despite the severity of initial illness, our cohort analysis suggests that atypical demyelination presentations do not confer a higher risk of long-term adverse outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12349-6.
Keywords: Multiple sclerosis, Tumefactive, Acute disseminated encephalomyelitis, Prognosis
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS which often presents with recurrent episodes of focal neurological deficit in the absence of encephalopathy or fever [1, 2]. A small number of cases present with atypical clinical or radiological features suggestive of acute disseminated encephalomyelitis (ADEM) [3, 4] or cerebral neoplasia (tumefactive demyelination) [5]. The largest case series to date, retrospectively reviewing tumefactive demyelinating lesions over a period of 30 years at the Mayo Clinic, identified 183 cases meeting criteria for MS [6]. These atypical presentations are uncommon [7], pose a diagnostic dilemma, and data regarding treatment and prognosis are limited [8, 9]. Despite these difficulties, a recent review proposed that combinations of imaging and paraclinical findings can be used to diagnose tumefactive demyelinating lesions [10]
ADEM typically presents in childhood and features include altered level of consciousness, seizures, fever or focal/multifocal neurological deficits. These clinical features are accompanied by widespread, poorly demarcated predominantly white matter lesions of the same age. These features have been collated into criteria for childhood ADEM [11] however similar criteria for adult presentations are yet to be defined, and previous investigations commonly define their own criteria, leading to issues of heterogeneity in case definition. MRI of the brain and spine typically shows simultaneous multifocal demyelination throughout the brain and spine and up to 50% of cases are positive for MOG antibodies. ADEM-like presentations of MS are seen in adults but typically they do not have all of the clinical features of the childhood form and MOG antibody prevalence has been less well studied [12, 13]. Tumefactive demyelination is defined as lesions at least two centimetres in diameter and featuring gadolinium (Gd) enhancement [7–9, 14, 15]. Incomplete peripheral Gd-enhancement (‘broken ring’) is considered unique to this form of MS [8, 15]. Whilst initially described as being mono-focal, multifocal lesions feature in many series [8]. Cases with antibodies to AQP4 and MOG have been described [12, 13, 16–19]. Expert opinion and case series analysis has led to plasma exchange and immunosuppressive therapy being advocated for atypical forms of MS [13].
With the aim of further adding to knowledge of the long-term outcomes for atypical MS presentations in adults we conducted a retrospective cohort comparison study comparing clinical and radiological outcomes with age- and sex-matched typical MS cases. Our hypotheses were: (1) atypical MS cases would have worse outcomes than typical cases in terms of disability, lesion load and brain atrophy and (2) that a proportion of atypical cases would be positive for AQP4 or MOG antibodies.
Methods
Ethics oversight and approval
Ethics approval was sought and obtained through the Griffith University and Gold Coast Hospital and Health Service, Human Research Ethics Committees. Written informed consent was provided by all participants.
Case ascertainment and data collection
Atypical MS presentations (both initial and subsequent) were identified through systematic review of medical records of patients under the care of the CNS inflammatory diseases clinic at the Gold Coast University Hospital. Cases were enrolled if they featured atypical clinical presenting symptoms (fever, seizure, encephalopathy, severe multifocal neurological deficits) and/or atypical MRI findings (see lesion definitions below). Typical cases matched for sex and age at onset were identified from a register of cases seen at the same clinic. We attempted to match up to three typical cases for every atypical case. Typical cases met the 2017 McDonald criteria for MS and atypical cases were also assessed against these criteria. Cases (atypical or typical) were excluded if there was insufficient data (clinical or MRI) to confirm a diagnosis of MS or provide a minimum dataset (demographics, disability, and relapse information).
The following clinical details were collected from available records and direct interview with cases: current age, sex, age at onset, relapse history, relapse frequency, time to first relapse (following initial presentation), time to expanded disability status scale (EDSS) score 6.0, final EDSS (last review), MS treatment, CSF cell counts, CSF protein, oligoclonal bands and MRI data (see below for details). Annualised relapse rate (ARR), EDSS and MRI parameters were recorded for the 2-year, 5-year and most recent clinical review available following disease onset. Clinical and MRI data were included if they were available within 6 months of each time point.
Serological testing
Testing for AQP4 antibodies was performed by Pathology Queensland Immunology Laboratory, Brisbane using a combination of tissue-based immunofluorescence as previously described [20] and fixed cell-based assay (Eurommun®). MOG antibodies were tested by Westmead Immunology Laboratory, Sydney using a live-cell fluorescence activated cell sorting technique as previously described [20].
Radiological lesion definitions
ADEM-like MS was defined as multiple (> 10), large (>6 mm maximum diameter in any single plane), irregularly shaped, or poorly demarcated lesions of high intensity on T2 FLAIR MRI of the brain and spine that were of the same age on DWI and Gd-enhancing sequences [21]. Tumefactive MS lesions were defined as very large (> 2 cm) lesions identified on T2 FLAIR sequences, spanning the peri-ventricular to subcortical white matter, with or without a surrounding oedema or Gd-gadolinium enhancement [21].
MRI analysis
MRI were assessed using eFilm Workstation® 4.2.3, IBM Watson Health software on Eizo® RadiForce MX270W 68 cm monitors. MRI parameters included the number of T2 FLAIR hyperintense lesions, the neuroanatomical location of these lesions, the number of large lesions (defined as >6 mm in diameter in at least one plane), the presence and number of gadolinium-enhancing lesions, and the presence and number of T1 hypointense lesions (black holes).
The following criteria were used to determine if lesions were of the same age; No established T1 black holes (minor T1 hypointensity was permitted as can be seen in acute lesions), all large lesions (> 6 mm) showed diffusion restriction or T1 Gd-enhancement and all lesions had a poorly demarcated boarder.
Volumetric analysis was performed using the open source software 3DSlicer v4.10.2 (http://www.slicer.org) [22]. Following importation of DICOM format imaging, cranial vault and soft tissue imaging was removed using the Swiss Skull Stripper module v4.1(https://www.slicer.org/wiki/Documentation/Nightly/Modules/SwissSkullStripper, Institute for Surgical Technology and Biomechanics, University of Bern, Switzerland). Whole brain and lesion volumes were measured using the Editor module v4. 1 (https://www.slicer.org/w/index.php/Documentation/4.3/Modules/Editor, National Alliance for Medical Imaging Computing, Harvard University, US).
Statistical analysis
Statistical comparisons between the atypical MS cohort, and whole MS database, and the age- and sex-matched typical MS cohort were performed. The first comparison used a database of person with MS (pwMS) fulfilling the 2017 revised McDonald’s criteria [23] seen at Gold Coast University Hospital over the past 17 years. These data were used to compare demographics and disease course of the atypical MS cases against an unmatched cohort. The second comparison group was an age- and sex-matched cohort of typical MS cases identified from the same database as described. Comparison of categorical data were performed using a Χ2 test and continuous data with the Kruskal–Wallis test. The effect of baseline characteristics on outcomes was assessed using forward stepwise linear regression analysis with p < 0.05 as the cut off for inclusion in the model. Survival analysis was undertaken using Kaplan–Meier curves and Cox proportional hazard modelling including significant predictors identified from the regression analysis of outcomes [24]. All statistical analyses were performed using the Statistical Package for Social Science (SPSS®) v25 (IBM®; Chicago, US).
Results
Case ascertainment
A total of 28 cases were identified on clinical or radiological grounds as meeting our criteria for atypical demyelination. One case was only ever seen once in our clinic, sometime after their atypical presentation and was excluded due to lack of clinical and imaging data. This left 27 included atypical MS cases. All these cases met the McDonald criteria for MS (excluding one case in regards to requirement for an alternative diagnosis—see below). There were 712 cases in the MS Clinic database. This gives a relative frequency of 28/712 (3.9% [95%CI 2.6–5.6%]) We identified 76 age- and sex-matched typical MS cases from the database. One of these cases was also excluded due to a lack of clinical and MRI data, leaving 75 included in the analysis.
Atypical cases
Table 1 gives the demographic information, initial clinical features, MRI data, CSF results and antibody results for individual atypical MS cases. There were 13 ADEM-like cases and 14 tumefactive cases. We determined ADEM-like cases to be atypical demyelinating presentations rather than traditional ADEM on the basis of ADEM-like cases demonstrating combinations of CSF oligoclonal band positive status (6/8), remote MRI T1 black holes on initial MRI suggesting previous demyelinating events (8/13), presence of periventricular lesions (13/13) or subsequent relapses (6/13). Atypical presentations occurred at the onset of disease (first attack) in the majority of (23/27 (85%) cases), but a small number occurred as the second (1 case) or third attack (3 cases). When monophasic cases were excluded the number of atypical presentations occurring as first events 12/16 (75%) was higher than the expected number of 5/16 (34%) based on the mean number of relapses observed (p < 0.01). One case had 6 tumefactive relapses affecting both hemispheres and posterior fossa. Atypical clinical features were seen in 12/27 (44%) of atypical MS cases. Cognitive impairment at first presentation was seen in 7/13 (54%) of ADEM-like cases compared with 2/14 (14%) of tumefactive cases (p = 0.077). Depressed level of consciousness was seen in 4/13 (31%) of ADEM cases and none of the tumefactive cases. Two ADEM cases featured headache (7%) and one presentation involved fever in (4%). The remaining atypical MS cases (15/27 [56%]) were identified on the basis of radiological features and in some cases the symptoms were relatively mild. Lesions meeting our criteria for tumefactive demyelination were also seen in 6/13 (46%) of ADEM-like cases. The median (range) of total T2 brain lesions was greater (p = 0.029) for ADEM-like presentations 22 (3–85) than for tumefactive cases 2.5 (1–81). Gd-enhancement was seen in 7/11 (64%) ADEM-like and 10/12 (83%) tumefactive MS cases where contrast was administered (p = 0.549).
Table 1.
Summary of clinical features from atypical MS cases
| Case | Type | Demographics | Relapse | Prior | Clinical features | MRI brain T2 lesions | MRI brain T1 | MRI spine T2 lesions | CSF examination | Antibodies | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset age (years) | Sex | Symp episode | Infect (wks) | Cog Imp | Obtund | H/A | Focal Neurol | Tumefactive | Large (≥ 6 mm) | Small (< 6 mm) | Total | Black | Cervical | Thoracic | WCC (× 106/L) | Protein (mg/dL) | OCB | AQP4 | MOG | ||||||||||
| N | DWI | Gd | N | DWI | Gd | N | DWI | Gd | N | Holes | N | Gd | N | Gd | |||||||||||||||
| 1 | ADEM-like | 24 | F | 1 | − | + | + | + | 0 | 19 | + | − | 62 | + | − | 81 | 9 | 0 | 1 | − | 3 | 340 | Local | − | |||||
| 2 | ADEM-like | 15 | F | 1 | UTI (8) | + | + | + | 3 | + | + | 0 | 56 | + | + | 59 | 26 | 1 | + | 0 | 10 | 630 | Local | − | − | ||||
| 3 | ADEM-like | 51 | M | 1 | URTI (8) | + | + | 3 | + | + | 36 | + | − | 18 | + | + | 57 | 31 | 0 | 1 | + | 2 | 380 | Negative | − | − | |||
| 4 | ADEM-like | 25 | F | 1 | − | + | 0 | 32 | + | + | 15 | + / − | + | 47 | 7 | 5 | + / − | 3 | + / − | − | − | ||||||||
| 5 | ADEM-like | 16 | F | 1 | Fever (0) | + | + | − | 0 | 35 | − | + | 11 | − | + | 46 | 3 | 1 | + | 0 | 14 | 350 | Local | − | − | ||||
| 6 | ADEM-like | 34 | F | 1 | − | + | 4 | + | + / − | 13 | + | + / − | 26 | + | + / − | 43 | 8 | 0 | 0 | − | + | ||||||||
| 7 | ADEM-like | 25 | F | 1 | − | + | 0 | 13 | + | + / − | 16 | + | + / − | 29 | 6 | 0 | 0 | − | − | ||||||||||
| 8 | ADEM-like | 17 | F | 1 | URTI (2) | + | + | + | + | 5 | + | + | 11 | + | + | 6 | + | + | 22 | 19 | 0 | 1 | + | 43 | 240 | Local | − | ||
| 9 | ADEM-like | 28 | F | 1 | − | + | 0 | 16 | − | − | 6 | − | − | 22 | 0 | 1 | − | − | |||||||||||
| 10 | ADEM-like | 19 | F | 3 | − | − | 2 | + | − | 0 | 18 | + | − | 20 | 0 | 2 | − | 1 | − | − | − | ||||||||
| 11 | ADEM-like | 42 | F | 1 | − | + | + | 0 | 0 | 16 | − | − | 16 | 0 | 1 | 190 | Local | − | |||||||||||
| 12 | ADEM-like | 21 | F | 1 | − | + | + | 0 | 10 | + / − | − | 0 | 10 | 3 | 3 | − | 4 | − | Negative | − | − | ||||||||
| 13 | ADEM-like | 38 | M | 1 | − | + | 4 | + | − | 4 | + | − | 0 | 8 | 3 | 0 | 0 | 32 | 360 | Local | − | − | |||||||
| 14 | Tumefactive | 31 | F | 3 | URTI (2) | + | 1 | + | + | 40 | + / − | − | 44 | + / − | − | 85 | 29 | 1 | − | 3 | − | ||||||||
| 15 | Tumefactive | 25 | F | 1 | − | + | 1 | + | + | 21 | + / − | − | 28 | + / − | − | 50 | 16 | 1 | − | 0 | 3 | 200 | Local | − | − | ||||
| 16 | Tumefactive | 26 | M | 3 | − | + | 1 | + | + | 7 | + | − | 1 | + | − | 9 | 0 | 0 | 0 | 12 | 330 | Local | − | ||||||
| 17 | Tumefactive | 36 | F | 1 | − | + | 1 | + | + | 1 | − | − | 1 | − | − | 5 | 0 | 0 | 1 | − | 5 | 280 | Local | − | |||||
| 18 | Tumefactive | 36 | M | 1* | + | + | + | 1 | + | + | 1 | − | − | 3 | − | − | 5 | 0 | 121 | 1800 | Negative | − | |||||||
| 19 | Tumefactive | 39 | F | 1 | − | + | 1 | + | + | 2 | − | − | 1 | − | − | 4 | 0 | 0 | 0 | − | − | ||||||||
| 20 | Tumefactive | 28 | F | 1 | − | + | 2 | + | − | 1 | + | + | 0 | + | − | 3 | 2 | 0 | 0 | 9 | 210 | Local | − | − | |||||
| 21 | Tumefactive | 41 | F | 1 | − | + | 2 | + | − | 1 | + | − | 0 | 3 | 0 | 0 | 0 | 0 | 240 | Negative | − | − | |||||||
| 22 | Tumefactive | 28 | F | 1 | − | + | 1 | + | + | 1 | − | − | 1 | − | − | 3 | 2 | 0 | 0 | 10 | 340 | Local | − | ||||||
| 23 | Tumefactive | 23 | F | 1 | − | + | + | 1 | + | + | 2 | + | + | 0 | 3 | 2 | 0 | 0 | 16 | 310 | Local | − | − | ||||||
| 24 | Tumefactive | 44 | F | 1 | URTI (8) | + | + | 1 | + | + | 0 | 2 | − | − | 3 | 0 | 1 | − | 0 | 2 | 210 | Negative | − | − | |||||
| 25 | Tumefactive | 38 | F | 1 | − | + | + | 2 | + | + | 0 | 1 | − | − | 3 | 0 | 0 | 0 | 250 | Systemic | − | − | |||||||
| 26 | Tumefactive | 31 | M | 1 | URTI (2) | + | + | 1 | + | − | 1 | − | − | 0 | 2 | 0 | 3 | 550 | Local | − | − | ||||||||
| 27 | Tumefactive | 31 | M | 1 | − | + | 1 | − | − | 1 | + | − | 0 | 2 | 1 | 1 | − | 3 | − | − | − | ||||||||
Symp Ep symptomatic episode; Cog Imp cognitive impairment; Obtund obtundation; Focal focal neurological deficit; N number; DWI diffusion weighted imaging; Gd Gadolinium enhancement; WCC white cell count; OCB oligoclonal bands; ADEM acute disseminated encephalomyelitis; F female; M male; + present and/or present in all/nearly all; − absent and/or absent in all; + / − present in some lesions; Local locally synthesized gamma-globulin found only in CSF; Systemic systemically synthesized gamma-globulin found in serum and CSF
MRI of atypical presentations
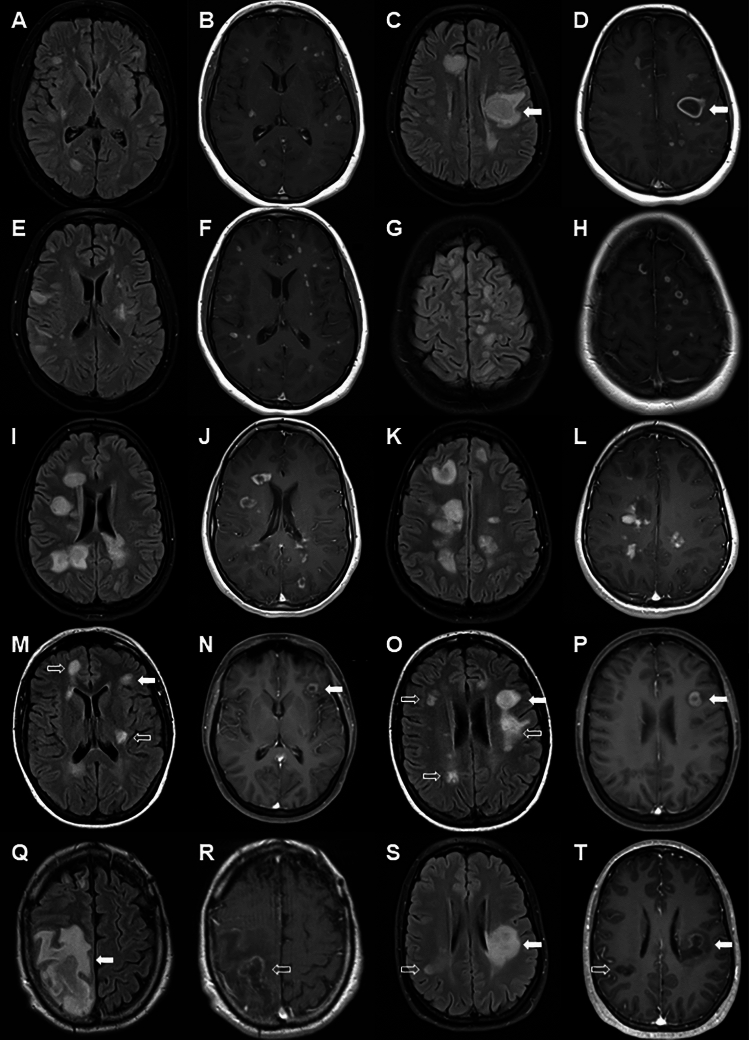
Illustrative MRI features for ADEM-like and tumefactive presentations of MS are given in Fig. 1. Particular features of note included multiple enhancing lesions in ADEM-like presentations (Fig. 1B, F, J, D, H and L), peri-lesional T1 hypointensity (Fig. 1D and P), perilesional oedema (Fig. 1Q), central hypo-intensity on T1 (Fig. 1D, P, R and T), complete ring-enhancement (Fig. 1D, J and P), incomplete ring enhancement (Fig. 1R and T), homogeneous enhancement (Fig. 1l, O and S) and heterogeneous enhancement (Fig. 1K and Q). We noted three patterns of ADEM-like lesion as shown in Fig. 2 which appeared to be independent of timing of the scans in relation to onset of clinical symptoms. In the first pattern there was confluent T2 hyperintensity on FLAIR imaging matched by homogeneous hyperintensity on DWI sequences and hypodensity on T1 sequences without Gd-enhancement. In the second pattern T2 hyperintense lesions on FLAIR imaging showed central relative hypo-intensity, which was matched by similar, but more pronounced changes on DWI sequences and a clear pattern of ring-enhancement with central hypo-intensity on T1 sequences. The third pattern showed patchy central T2 hyperintensities on FLAIR imaging matched by similar changes on DWI and Gd-enhanced T1 sequences. A summary of MRI features in atypical cases is given in Supplementary Table 1.
Fig. 1.
MRI of ADEM-like and tumefactive MS cases. Images are paired (matched slices) with FLAIR images in first and third vertical panels and Gd-enhanced T1 weighted sequences in second and fourth vertical panels. Case of ADEM-like lesions with small and large lesions which all show Gadolinium enhancement (A–D), one larger lesion (D arrow) shows ring-enhancement with central hypointensity and peri-lesional hypointensity with surrounding oedema. Case of ADEM-like lesions with a multitude of smaller lesions all of which show either homogeneous or ring enhancement (E–H). Case of ADEM with multiple large lesions showing both ring enhancement and heterogeneous enhancement. Case of ADEM-like lesions showing multiple small and large lesions (M–N). Some lesions are non-enhancing, with some having central hypointensity on T1 sequences (M and O open arrows) whilst other lesions show ring-enhancement (open arrows). Case of recurrent tumefactive MS showing large incomplete ring-enhancing lesion with central hypointensity and surrounding oedema (solid arrow) with mass effect (S and T). Case of tumefactive MS with a large incomplete ring-enhancing lesion (solid arrow) with central and perilesional hypointensity and a second non-enhancing lesion (open arrow) with central hypointensity (S and T)
Fig. 2.
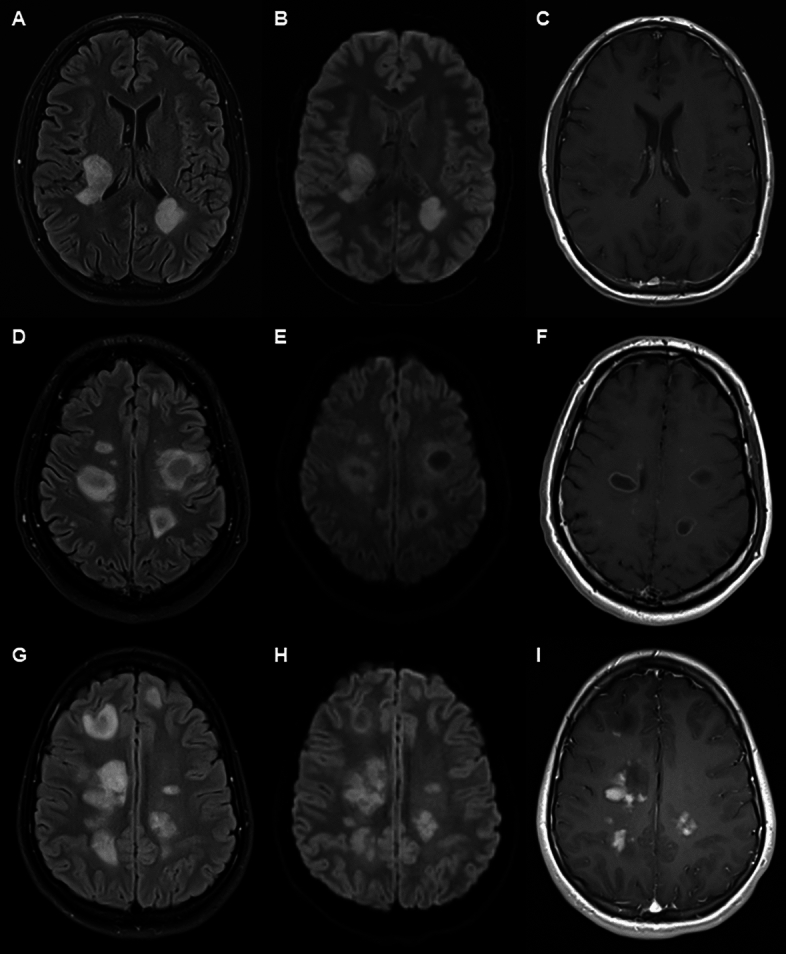
Three patterns of lesion in ADEM-like lesions. Vertical panels show FLAIR sequences (right), diffusion weighted images (centre) and T1 with contrast (left). Horizontal panels show individual cases. Upper panel (A–C) shows case with FLAIR and DWI hyperintensity with T1 hypointensity, but no Gd-enhancement. Middle panel (D–F) shows ring pattern hyperintensity on FLAIR and DWI with central hypointensity and ring-enhancement on T1 sequence. Lower panel (G–I) shows predominantly heterogeneous FLAIR and DWI hyperintensity with heterogeneous Gd-enhancement. There are additional lesions showing central T1 hypointensity and no enhancement.
Comparison of baseline characteristics
Comparison of baseline characteristics between the MS database cohort, the age/sex matched typical MS cohort and the atypical MS cases, as well as between ADEM-like and tumefactive cases are shown in Table 2. There was no difference in the sex distribution of any of the groups. Whilst overall there was no difference in the age of onset between the atypical cases and MS database cases, the ADEM-like cases showed a trend towards a younger age of onset (20.5 [15–51] years) compared to tumefactive cases (29.5 [23–44] years, p = 0.068) and were younger than typical MS database cases (35 [14–71] years, p = 0.038). Peak EDSS (during the index presentation) was higher in the ADEM-like group, but this difference was not statistically significant. There was no difference in the median age of atypical and the age-matched typical MS cases. A history of recent infection was noted in 7/25 (28%) of the atypical cases and 7/54 (13%) of typical MS cases, but this difference was not statistically significant (p = 0.19). Atypical MS cases were more likely to have motor (p = 0.035), cranial nerve (p = 0.003), cerebellar (p = 0.005) and cerebral (p < 0.001) features in their atypical episode. They were also more likely to have multifocal attacks (p < 0.001), particularly in the ADEM-like group (p = 0.046) and were less likely to have optic neuritis (p = 0.037). CSF white cell count was higher in the atypical MS cases when compared to both typical MS cohorts (p = 0.002 for the databases and p = 0.052 for the matched cohort). This difference appeared to be principally driven by the tumefactive MS cases (Supplementary Fig. 1). No significant differences in CSF protein and presence of oligoclonal bands were seen. Antibodies to AQP4 were tested in 24/27 (89%) atypical cases and all were negative. MOG antibodies were tested in 21/27 (78%) of atypical cases and were positive in 1/21 (5%). This case has been treated with rituximab and MOG antibodies were negative on repeat serum testing 2 years later. MRI in this case shows features typical for MS (total of 21 white matter brain lesions, periventricular lesions, Dawson finger lesions, juxta-cortical lesions and inferior temporal lobe lesions). There had been new lesions over time, but no Gd-enhancing lesions since the ADEM-like presentation and no lesions typical for MOGAD (no lesions of the optic nerve, spinal cord, brainstem or cerebellum).
Table 2.
Comparison of demographic and clinical features at baseline between typical and atypical MS cohorts
| Typical | Atypical | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Clinical characteristic | Database (A) | Matched (B) | All (C) | ADEM-like (D) | Tumefactive (E) | A vs C | B vs C | D vs E |
| N | 712 | 75 | 27 | 13 | 14 | |||
| Sex (female)—n/N (%) | 568 (80) | 56 (74) | 21 (78) | 11 (85) | 10 (71) | ns | ns | ns |
| Age at disease onset (years)—median (range) | 35 (14–71) | 33 (13–56) | 28 (15–51) | 20.5 (15–51) | 29.5 (23–44) | ns | ns | 0.068 |
| Deficits at presentation—n/N (%) | ||||||||
| Sensory | 29 (39) | 13 (48) | 7 (54) | 6 (43) | ns | ns | ||
| Motor | 14 (19) | 11 (41) | 6 (46) | 5 (36) | 0.035 | ns | ||
| Optic neuritis | 17 (23) | 1 (4) | 1 (8) | 0 (0) | 0.037 | ns | ||
| Cranial nerve | 11 (15) | 12 (44) | 7 (54) | 5 (36) | 0.003 | ns | ||
| Incoordination | 5 (7) | 8 (30) | 3 (23) | 5 (36) | 0.005 | ns | ||
| Bladder/bowel dysfunction | 5 (7) | 2 (7) | 0 (0) | 2 (8) | ns | ns | ||
| Cerebral | 0 (0) | 9 (33) | 7 (54) | 2 (14) | < 0.001 | 0.046 | ||
| Multifocal | 4 (5) | 15 (56) | 7 (54) | 8 (57) | < 0.001 | ns | ||
| CSF analysis | ||||||||
| Protein (mg/L)—median (range) | 360 (110–1700) | 340 (190–1085) | 320 (190–1800) | 290 (190–380) | 320 (210–1800) | ns | ns | ns |
| WCC (× 106/mL)—median (range) | 2 (0–390) | 4 (0–390) | 7 (0–121) | 3 (1–43) | 10 (0–121) | 0.002 | ns | ns |
| Local synthesis of oligoclonal bands—n/N (%) | 168/224 (75) | 28/37 (76) | 13/19 (68) | 6/8 (77) | 7/11 (64) | ns | ns | ns |
| Serology—n/N (%) | ||||||||
| AQP4 antibody positive | 0/52 (0) | 0/24 (0) | 0/13 (0) | 0/11 (0) | ns | ns | ||
| MOG antibody positive | 0/10 (0) | 1/21 (5) | 1/12 (8) | 0/9 (0) | ns | ns | ||
Statistical comparisons undertaken were Atypical MS vs Typical MS or Database MS and Tumefactive vs ADEM-like
MS multiple sclerosis; ADEM acute disseminated encephalomyelitis; ns not significant; OCB oligoclonal bands; AQP4 aquaporin-4; MOG myelin oligodendrocyte glycoprotein
Comparison of long-term clinical outcomes
A comparison of clinical outcomes is given in Table 3. The period of follow up for typical MS cases and consequently age at last review were higher than the atypical cases (p < 0.001). This affects several time dependent outcomes. In view of this we would be circumspect about the finding of a higher rate of monophasic/CIS disease in the atypical cohorts compared to both typical MS cohorts. With longer follow up this rate would be likely to fall (see time to event analysis below). Similarly, final EDSS was lower for the atypical MS cases. There was no difference in any of the disease duration standardised scores (e.g. 2 year and 5-year EDSS) and the final MSSS, which corrects for disease duration. Atypical MS cases were more likely to have subsequent motor (p = 0.025) and cerebral (p = 0.001) relapses and less likely to have optic neuritis (p = 0.009). Atypical cases were more likely to have been commenced on highly effective disease modifying therapy as their initial treatment compared to typical MS cases (p < 0.001). Subsequent escalation of treatment was conversely more common in the typical MS cohort (p = 0.001). This may also reflect the greater duration of follow up for the typical MS cases and a lower availability of highly effective therapies at the time of their original diagnosis.
Table 3.
Comparison of disease outcomes in typical and atypical MS cases
| Typical MS | Atypical MS | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Clinical outcome | Database (A) | Matched (B) | All (C) | ADEM-like (D) | Tumefactive (E) | A vs C | B vs C | D vs E |
| N | 712 | 75 | 27 | 13 | 14 | |||
| age at last follow up (years)—median (range) | 49 (19–73) | 35 (17–57) | 30 (17–57) | 42.5 (24–51) | < 0.001 | ns | ||
| follow-up (years)—median (range) | 16.0 (2.0–39.7) | 5.8 (0.3–15.3) | 5.8 (0.3–15.2) | 6.4 (0.4–15.3) | < 0.001 | ns | ||
| Clinical course—n (%) | ||||||||
| Monophasic/CIS | 53 (7) | 6 (8) | 9 (33) | 6 (46) | 3 (21) | < 0.001 | 0.01 | ns |
| Relapsing remitting | 422 (59) | 55 (73) | 16 (59) | 7 (54) | 9 (64) | |||
| Secondary progressive | 168 (23) | 10 (13) | 2 (7) | 0 (0) | 2 (14) | |||
| Primary progressive | 69 (10) | 4 (5) | 0 (0) | 0 (0) | 0 (0) | |||
| Time to first relapse (years)—median (95% CI) | 3.0 (1.8–4.2) | 2.0 (0.4–3.6) | 2.0 (0.5–3.5) | 2.0 (0.0–10.3) | ns | ns | ||
| Annualised relapse rate—median (range) | ||||||||
| To Year 2 | 0.0 (0.0–2.0) | 0.5 (0.0–1.0) | 0.0 (0.0–1.0) | 0.5 (0.0–0.5) | ns | ns | ||
| To Year 5 | 0.2 (0.0–0.8) | 0.2 (0.0–0.6) | 0.2 (0.0–0.6) | 0.2 (0.0–0.4) | ns | ns | ||
| Final | 0.1 (0.0–0.4) | 0.1 (0.0–2.4) | 0.0 (0.0–0.5) | 0.1 (0.0–2.4) | ns | ns | ||
| Subsequent relapse symptoms–n (%) | ||||||||
| Sensory | 41/64 (64) | 7/17 (41) | 5/10 (50) | 2/7 (29) | ns | ns | ||
| Motor | 27/64 (42) | 13/17 (77) | 8/10 (80) | 5/7 (71) | 0.025 | ns | ||
| Optic neuritis | 28/64 (44) | 1/17 (6) | 1/10 (10) | 0/7 (0) | 0.009 | ns | ||
| Brainstem | 22/64 (34) | 7/17 (41) | 5/10 (50) | 2/7 (29) | ns | ns | ||
| Cerebellar | 8/64 (13) | 3/17 (18) | 3/10 (30) | 0/7 (0) | ns | ns | ||
| Bladder/bowel dysfunction | 19/64 (30) | 3/17 (18) | 3/10 (30) | 0/7 (0) | ns | ns | ||
| Cerebral | 1/64 (2) | 5/17 (30) | 1/10 (10) | 4/7 (57) | 0.001 | ns | ||
| EDSS—median (range) | ||||||||
| Peak at presentation | 2.5 (0.0–8.0) | 3.0 (1.0–9.0) | 3.0 (2.0–9.0) | 2.75 (1.0–7.0) | ns | ns | ||
| Year 2 | 2.5 (0.0–7.0) | 1.75 (0.0–5.0) | 1.5 (0.0–5.0) | 1.25 (0.0–2.0) | ns | ns | ||
| Year 5 | 1.5 (0.0–4.0) | 1.5 (1.0–6.0) | 1.25 (0.0–6.0) | 1.25 (0.0–4.0) | ns | ns | ||
| Final EDSS | 2.5 (0.0–7.5) | 1.0 (0.0–6.5) | 0.0 (0.0–2.0) | 1.0 (0.0–6.5) | 0.018 | ns | ||
| Disability | ||||||||
| Time to EDSS 6.0 (years)—mean (95% CI) | 24.1 (23.3–30.4) | 13.5 (11.8–15.3) | 13.1 (11.3–14.9) | 13.1 (10.5–15.6) | ns | ns | ||
| Progression rate—median (range) | 0.12 (0.00–0.97) | 0.13 (0.00–3.54) | 0.00 (0.00–0.93) | 0.18 (0.00–3.54) | ns | ns | ||
| MSSS—median (range) | 1.64 (0.03–8.49) | 1.07 (0.10–8.49) | 0.56 (0.10–8.49) | 1.53 (0.23–7.95) | ns | ns | ||
| Initial treatment—n (%) | ||||||||
| No treatment | 15 (20) | 6 (22) | 1 (8) | 5 (36) | < 0.001 | ns | ||
| Low efficacya | 36 (48) | 4 (15) | 1 (8) | 3 (21) | ||||
| Moderate efficacya | 12 (16) | 1 (4) | 1 (8) | 0 (0) | ||||
| High efficacya | 12 (16) | 16 (59) | 10 (77) | 6 (43) | ||||
| Escalation of treatment | 27 (36) | 1 (4) | 0 (0) | 1 (7) | 0.001 | ns | ||
Statistical comparisons undertaken were Atypical MS vs Typical MS or Database MS and Tumefactive vs ADEM-like
MS multiple sclerosis; ADEM acute disseminated encephalomyelitis; ns not significant; CIS clinically isolated syndrome; EDSS expanded disability status scale; N/A not applicable
aSee text for definitions of efficacy
Regression analysis of baseline data on outcome (final MSSS) showed that both male sex (β 2.087 [95% CI 0.981–3.193] and a higher total number of FLAIR T2 hyperintense lesions on MRI brain (β 0.035 [0.012–0.058]) were associated with a worse outcome (Supplementary Table 2 and Supplementary Fig. 2), although the effect of FLAIR T2 lesions was small (R2 = 0.047). Initial treatment did not significantly affect final MSSS within the atypical MS cohort but was associated with MSSS outcomes (Supplementary Fig. 3) across the whole cohort (p = 0.016), with more efficacious therapies being associated with worse outcomes for final MSSS, but not ARR.
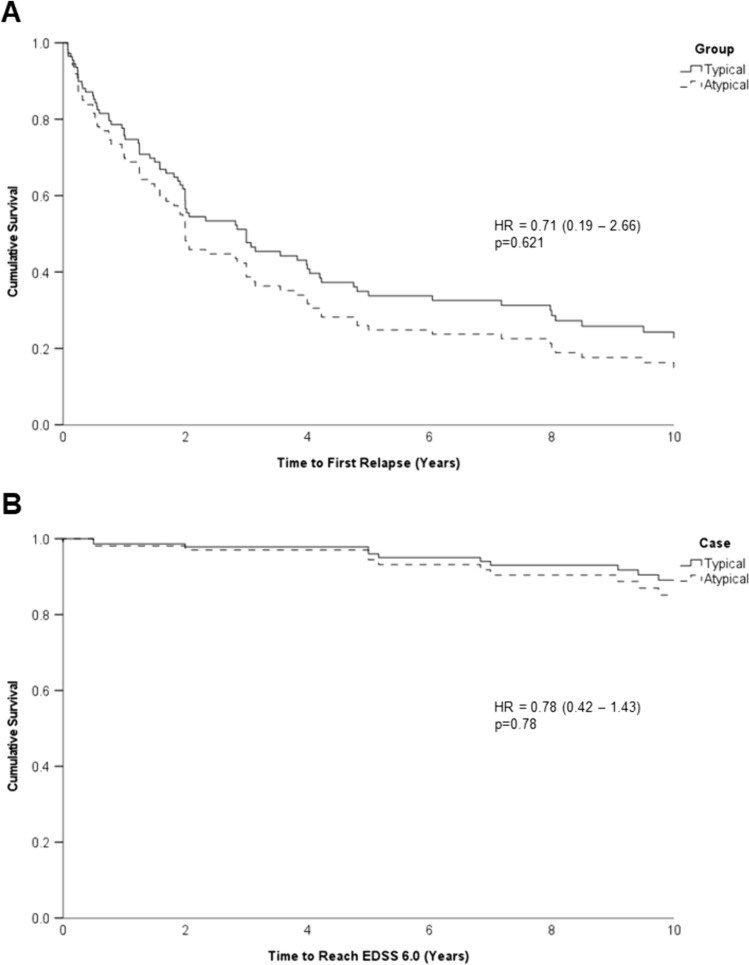
Survival analysis
Cox proportional hazards survival analysis was used for both time to first relapse and time to EDSS 6.0 from first attack (Fig. 3). Only age at onset proved to be statistically significant in this analysis for time to EDSS 6.0 using a forward stepwise approach. However, because of the baseline regression analysis, age, sex and initial treatment were included in the models for both analyses (Supplementary Tables 3 and 4). There were no significant differences in these outcome measures for atypical MS cases compared to typical MS cases. A subgroup analysis looking at ADEM-like and tumefactive cases separately in a Kaplan–Meier analysis (Supplementary Fig. 4) similarly showed no differences in outcomes.
Fig. 3.
Survival curves from cox-proportional hazard models for time to first relapse (A) and time to reach EDSS 6.0 (B). Age, sex and initial treatment (low, medium or high efficacy) were included in the model. EDSS expanded disability status scale
MRI analysis
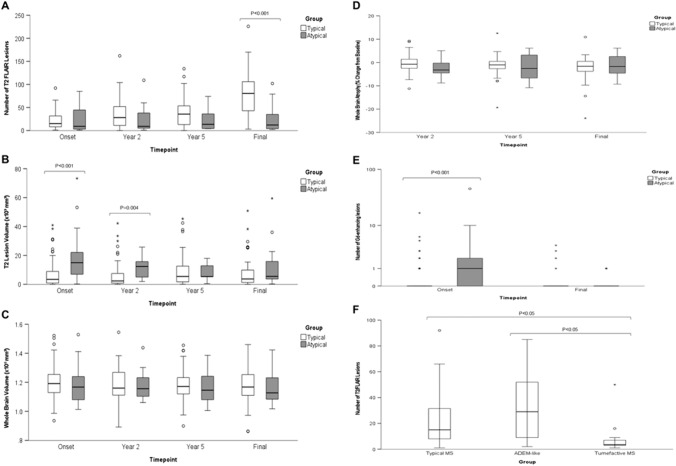
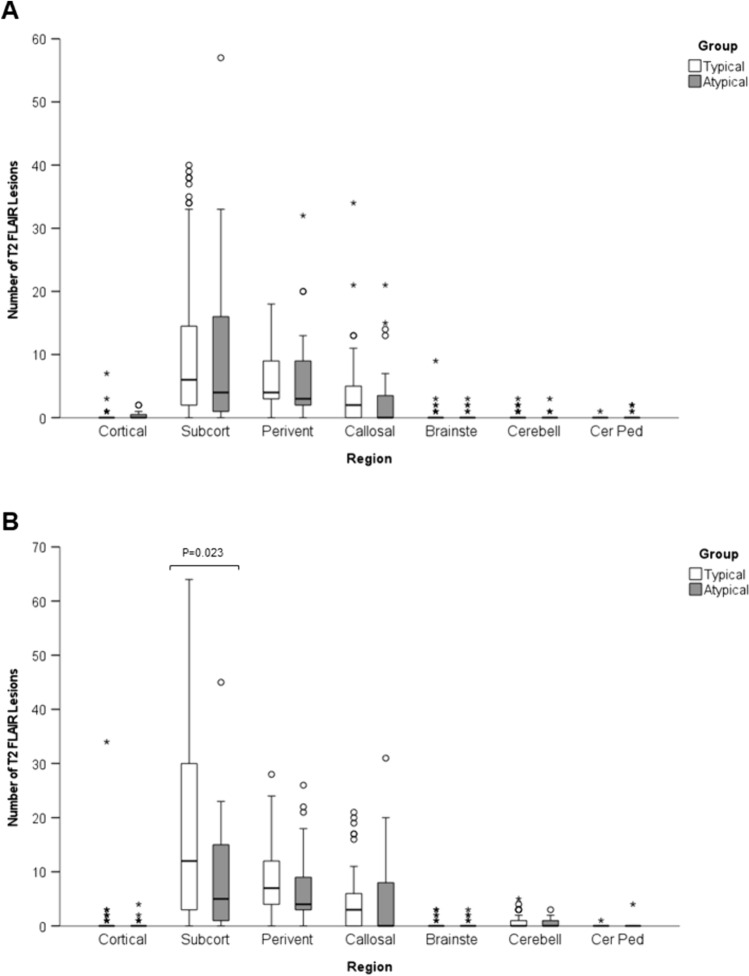
Analyses of MRI brain with lesion counts, lesion volume and whole brain volume are shown in Fig. 4A–C and Supplementary Table 4. This analysis indicates no significant difference in the number of T2 lesions for typical and atypical cases. More Gd-enhancing lesions were seen in the atypical cases than typical cases (p < 0.001) at presentation (Supplementary Table 5. More lesions were evident for the last available MRI in typical MS cases (p < 0.001), but this likely reflects the longer period of follow up. T2 lesion volume for atypical cases was higher at disease onset (p < 0.001) and at Year 2 (0 = 0.004). However, subsequently there was no significant difference suggesting possible regression to the mean and similar final outcomes. There were no significant differences in whole brain volume or percentage change from baseline in whole brain volume at any timepoint (Fig. 4D and Supplementary Table 4). The number of Gd-enhancing lesions was greater (p < 0.001) in the atypical cases than typical MS cases at presentation (Fig. 4E and Supplementary Table 4). As expected, there were fewer T2/FLAIR lesions at presentation in the tumefactive MS group (Fig. 4F). There were more FLAIR lesions at onset in the ADEM-like group compared to typical MS but this difference was not statistically significant. There were no differences in the number of large T2 lesions (> 6 mm) and T1 hypointense lesions (‘old black holes’) at onset or final MRI (Supplementary Fig. 5and Supplementary Table 5). The anatomical distribution of T2 brain lesions showed no significant difference at disease onset (Fig. 5A). Subcortical lesions were more frequent in the typical MS cases at final follow up (Fig. 5B), but this perhaps reflects the greater duration of follow and age of this group. There were no statistically significant differences in the frequency of different lesion features between ADEM-like and tumefactive presentations.
Fig. 4.
Box and whisker plots of number of T2/FLAIR lesions (A), T2 lesion volume (B) and whole brain volume (C) at presentation of atypical attack (Onset), 2 years, 5 years and last MRI (Final), change in whole brain volume (brain atrophy) compared to baseline at 2 year, 5 year and final follow up (D), Number of Gadolinium enhancing lesions at onset and final follow up (E), and number of T2/FLAIR lesions at onset for MS, ADEM-like and tumefactive cases (F). Significant differences between atypical and typical MS cases are indicated. Central bar shows median, box shows interquartile range and whiskers indicate range. Outliers indicated by circles; extreme outliers indicated by asterisks
Fig. 5.
Box and whisker plots of distribution of T2/FLAIR lesions at presentation (A) and last follow up (B). Significant differences between atypical and typical MS cases are indicated. Central bar shows median, box shows interquartile range and whiskers indicate range. Outliers indicated by circles; extreme outliers indicated by asterisks. Subcort subcortical; Perivent periventricular; Brainste brainstem; Cerebell Cerebellar; Cer Ped cerebellar peduncle
Discussion
The identified cohort of 28 MS cases with atypical presentations represents approximately 3.9% (95%CI 2.6–5.6%) of cases under the care of GCUH. This prevalence of atypical MS in adults is similar to prior studies (1–5%) [12, 13, 18, 19]. A disproportionately high number of atypical presentations were disproportionally first events (75%) versus what would be expected by chance (34%) amongst those with a relapsing course (p < 0.01). This tendency has been noted for tumefactive MS [25], but we observed this pattern in both ADEM-like and tumefactive MS. Recurrent tumefactive lesions were seen in one case, a phenotype that has been previously noted [26]. Frequency of preceding infective symptoms was higher in the atypical group, but this difference was not significant. Previous studies have noted the prevalence of prior infective symptoms, but these prior investigations had no comparison group [13, 27–31]. Atypical clinical features were seen in less than half of the atypical MS cases. The most common presenting symptom in both typical (38%) and atypical (48%) cohorts was sensory deficit, contrasting with previous studies in which motor deficits were the most common presentation in tumefactive MS and ADEM-like presentations [8, 32]. In keeping with previous investigations, multifocal presentations were more common in atypical (56%) compared to typical (5%) MS cases (p < 0.001) [13, 29, 32, 33].
AQP-4 antibodies were not detected in our cohort. One ADEM-like case tested positive for MOG antibodies, out of the 21 cases available anti-MOG tested (5%). We acknowledge that this is an incomplete serological data set. Unfortunately, this deficit could not be rectified some of patients were lost to follow-up prior to MOG antibody testing being available. This finding is consistent with prior studies indicating low seroprevalence of AQP4 and anti-MOG antibodies in tumefactive and ADEM-like MS in adults [27, 30, 34–36]. One study suggested a higher prevalence (36%) of AQP4 antibodies in adult tumefactive MS [33]. The low frequency of antibodies contrasts with paediatric cases of ADEM, where MOG antibodies are found to be present in approximately one-half of cases [37]. We acknowledge the controversy of diagnosing a MOG-positive case as atypical multiple sclerosis The primary rationale for inclusion is the subsequent clinical course and radiological progression was more in line with MS than MOG. Potential explanations for this clinical course include treated MOGAD, MS with a false positive MOG antibody or co-incident MOGAD and MS.
We found that, compared to age- and sex-matched typical MS controls, and correcting for follow-up duration, atypical MS showed no difference in long-term outcomes (ARR, MSSS, time to first relapse, time to EDSS 6.0, and number of T2 lesions, T2 lesion volume and brain atrophy at 5 years). This contrasts with some differences seen with the unmatched cohort and measures that were not duration of follow-up adjusted (e.g. final EDSS). This highlights the importance of identifying suitable controls and adjusting for duration of follow up in such studies.
Atypical MS cases were more often commenced on high-efficacy therapy. This likely reflects prognostic concerns in the face of alarming radiological and clinical changes. Interestingly, initial treatment choice did not influence the survival analyses. However, the possibility that differences in initial efficacy of treatment choice may have mitigated natural history differences in long-term outcomes for the two forms of MS needs to be considered [38]. As seen in the majority of prior studies of MS, male sex was associated with greater likelihood of reaching EDSS 6.0 sooner. With a median follow up of 6 years we observed in patients with atypical MS, a conversion to MS on clinical grounds in 17/27 (63%), by MRI criteria in 18/27 (67%) and by both clinical and MRI criteria in 23/27 (85%).
Higher lesion burden within the first 5 years of diagnosis is recognised as conferring increased risk of more severe long term disability in MS [39]. We observed higher T2 lesion volume at disease onset and year 2 in the atypical MS cases (Fig. 4). By year 5 there were no differences and whilst the effect of the number of T2/FLAIR lesions at onset on the time to reach EDSS 6.0 was significant the effect size was small. More lesions were Gd-enhancing at first atypical presentation than in typical MS cases consistent with the florid acute presentations that are commonly seen.
The strengths of this study were that cases were ascertained through a systematically collected single centre database of demyelinating disease cases and comparisons were made with age- and sex-matched typical MS cases selected at random from the same database. In addition, data for typical and atypical cases were collected in the same manner and time-factored outcome measures have been utilised. The weaknesses of this study were that it was retrospective and that whilst matched for age at onset there was a significant difference in the duration of follow up. The inclusion of cases that pre-dated the routine use of volumetric MRI sequences necessitated the use of a less reliable tool for measuring brain volumes. The lack of histopathological correlation is another limitation. However, brain biopsy for evaluation of cerebral lesions has become increasingly rare in clinical practice, given the inherit high risk of complication and the potential of use of imaging characteristics and paraclinical information to identify likely demyelinating lesions pre-biopsy. Furthermore, given the length of follow-up for atypical cases, the presence of alternative diagnoses such as cerebral malignancy would have declared itself clinically or radiologically.
ADEM-like and tumefactive presentations of MS are uncommon. Comparison of clinical features and outcomes with a cohort of typical MS suggests that despite the initial severity of neuro-inflammatory changes, atypical MS presentations result in similar clinical and radiological outcomes (including brain atrophy) to the wider MS population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the study participants and would like to thank the support of the members of the Australian and New Zealand Association of Neurologists and Multiple Sclerosis Nurses Australia who assisted with data collection.
Abbreviations
- ADEM
Acute disseminated encephalomyelitis
- AQP4
Aquaporin-4
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DICOM
Digital imaging and communications in medicine
- EDSS
Expanded disability severity score
- FLAIR
Fluid-attenuated inversion and recovery
- GCUH
Gold Coast University Hospital
- MOG
Myelin oligodendrocyte glycoprotein
- MRI
Magnetic resonance imaging
- MS
Multiple Sclerosis
- pwMS
Persons with multiple sclerosis
- MSSS
Multiple sclerosis severity score
- NMOSD
Neuromyelitis optica spectrum disorder
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflicts of interest
SAB has received honoraria for attendance at advisory boards and travel sponsorship from Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis, and Sanofi-Genzyme, has received speakers honoraria from Biogen-Idec and Genzyme, is an investigator in clinical trials sponsored by Biogen Idec, Novartis and Genzyme, and was the recipient of an unencumbered research grant from Biogen-Idec. Dr Sudarshini Ramanathan has received research funding from the National Health and Medical Research Council (Australia), the Brain Foundation (Australia), the Royal Australasian College of Physicians, the Petre Foundation, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for Biogen, Excemed, and Limbic Neurology. SVA has accept financial support to attend conferences supported by Biogen. KP, SR, SB, FB report no relevant financial disclosures.
Ethics approval
Ethics approval was sought and obtained through the Griffith University and Gold Coast Hospital and Health Service, Human Research Ethics Committees. Written informed consent was provided by all participants.
References
- 1.Gajofatto A, Calabrese M, Benedetti MD, Monaco S (2013) Clinical, MRI, and CSF markers of disability progression in multiple sclerosis. Dis Markers 35:687–699 10.1155/2013/484959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 3.Eckstein C, Saidha S, Levy M (2012) A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol 259:801–816 10.1007/s00415-011-6240-5 [DOI] [PubMed] [Google Scholar]
- 4.Pohl D, Alper G, Van Haren K, Kornberg AJ, Lucchinetti CF, Tenembaum S, Belman AL (2016) Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology 87:S38-45 10.1212/WNL.0000000000002825 [DOI] [PubMed] [Google Scholar]
- 5.Karussis D (2014) The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun 48–49:134–142 10.1016/j.jaut.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 6.Fereidan-Esfahani M, Decker PA, Weigand SD, Lopez Chiriboga AS, Flanagan EP, Tillema JM, Lucchinetti CF, Eckel-Passow JE, Tobin WO (2023) Defining the natural history of tumefactive demyelination: a retrospective cohort of 257 patients. Ann Clin Transl Neurol 10:1544–1555 10.1002/acn3.51844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Algahtani H, Shirah B, Alassiri A (2017) Tumefactive demyelinating lesions: a comprehensive review. Mult Scler Relat Disord 14:72–79 10.1016/j.msard.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Thomsen K, Mandrekar J, Altintas A, Erickson BJ, Konig F, Giannini C, Lassmann H, Linbo L, Pittock SJ, Bruck W (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain 131:1759–1775 10.1093/brain/awn098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederick MC, Cameron MH (2016) Tumefactive demyelinating lesions in multiple sclerosis and associated disorders. Curr Neurol Neurosci Rep 16:26 10.1007/s11910-016-0626-9 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez P, Chan F, Hardy TA (2021) Tumefactive demyelination: updated perspectives on diagnosis and management. Expert Rev Neurother 21:1005–1017 10.1080/14737175.2021.1971077 [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study G (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19:1261–1267 10.1177/1352458513484547 [DOI] [PubMed] [Google Scholar]
- 12.Codjia P, Ayrignac X, Carra-Dalliere C, Cohen M, Charif M, Lippi A, Collongues N, Corti L, De Seze J, Lebrun C, Vukusic S, Durand-Dubief F, Labauge P (2019) Multiple sclerosis with atypical MRI presentation: results of a nationwide multicenter study in 57 consecutive cases. Mult Scler Relat Disord 28:109–116 10.1016/j.msard.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 13.de Seze J, Debouverie M, Zephir H, Lebrun C, Blanc F, Bourg V, Wiertlewski S, Pittion S, Laplaud D, Le Page E, Deschamps R, Cabre P, Pelletier J, Malikova I, Clavelou P, Jaillon V, Defer G, Labauge P, Gout O, Boulay C, Edan G, Vermersch P (2007) Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol 64:1426–1432 10.1001/archneur.64.10.1426 [DOI] [PubMed] [Google Scholar]
- 14.Zaheer K, Ajmeri AN, Singh M, Suliman MS, Teka S (2018) Tumefactive multiple sclerosis, a rare variant presenting as multiple ring-enhancing lesions in an immunocompetent patient: a case report. Cureus 10:e3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Yu WY, Liauw L, Chander RJ, Soon WE, Lee HY, Tan K (2017) Clinicoradiologic features distinguish tumefactive multiple sclerosis from CNS neoplasms. Neurol Clin Pract 7:53–64 10.1212/CPJ.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara T, Beck G, Baba K, Mihara M, Okuno T, Sumi H, Nakatsuji Y, Mochizuki H (2016) Tumefactive brain lesion with rapid cavity formation associated with anti-aquaporin-4 antibody. Neurol Neuroimmunol Neuroinflamm 3:e230 10.1212/NXI.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsuse K, Kurihara M, Sugiyama Y, Kodama S, Takahashi M, Momose T, Yumoto M, Kaneko K, Takahashi T, Kubota A, Hayashi T, Toda T (2019) Aphasic status epilepticus preceding tumefactive left hemisphere lesion in anti-MOG antibody associated disease. Mult Scler Relat Disord 27:91–94 10.1016/j.msard.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Balloy G, Pelletier J, Suchet L, Lebrun C, Cohen M, Vermersch P, Zephir H, Duhin E, Gout O, Deschamps R, Le Page E, Edan G, Labauge P, Carra-Dallieres C, Rumbach L, Berger E, Lejeune P, Devos P, N’Kendjuo JB, Coustans M, Auffray-Calvier E, Daumas-Duport B, Michel L, Lefrere F, Laplaud DA, Brosset C, Derkinderen P, de Seze J, Wiertlewski S (2018) Inaugural tumor-like multiple sclerosis: clinical presentation and medium-term outcome in 87 patients. J Neurol 265:2251–2259 10.1007/s00415-018-8984-7 [DOI] [PubMed] [Google Scholar]
- 19.Liao MF, Huang CC, Lyu RK, Chen CM, Chang HS, Chu CC, Hsu WC, Wu YR, Kuo HC, Cheng MY, Hung PC, Chou ML, Lin KL, Hsieh MY, Ro LS (2011) Acute disseminated encephalomyelitis that meets modified McDonald criteria for dissemination in space is associated with a high probability of conversion to multiple sclerosis in Taiwanese patients. Eur J Neurol 18:252–259 10.1111/j.1468-1331.2010.03114.x [DOI] [PubMed] [Google Scholar]
- 20.Prain K, Woodhall M, Vincent A, Ramanathan S, Barnett MH, Bundell CS, Parratt JDE, Silvestrini RA, Bukhari W, Australian, New Zealand NMOC, Brilot F, Waters P, Broadley SA (2019) AQP4 antibody assay sensitivity comparison in the era of the 2015 diagnostic criteria for NMOSD. Front Neurol 10:1028 10.3389/fneur.2019.01028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin SE, Callen DJ (2013) The magnetic resonance imaging appearance of monophasic acute disseminated encephalomyelitis: an update post application of the 2007 consensus criteria. Neuroimaging Clin N Am 23:245–266 10.1016/j.nic.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 24.Cox DR (1972) Regression models and life-tables. J Roy Stat Soc 34:187–220 10.1111/j.2517-6161.1972.tb00899.x [DOI] [Google Scholar]
- 25.Tremblay MA, Villanueva-Meyer JE, Cha S, Tihan T, Gelfand JM (2017) Clinical and imaging correlation in patients with pathologically confirmed tumefactive demyelinating lesions. J Neurol Sci 381:83–87 10.1016/j.jns.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vakrakou AG, Tzanetakos D, Argyrakos T, Koutsis G, Evangelopoulos ME, Andreadou E, Anagnostouli M, Breza M, Tzartos JS, Gialafos E, Dimitrakopoulos AN, Velonakis G, Toulas P, Stefanis L, Kilidireas C (2020) Recurrent fulminant tumefactive demyelination with Marburg-like features and atypical presentation: therapeutic dilemmas and review of literature. Front Neurol 11:536 10.3389/fneur.2020.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vakrakou AG, Tzanetakos D, Evangelopoulos ME, Argyrakos T, Tzartos JS, Anagnostouli M, Andreadou E, Koutsis G, Velonakis G, Toulas P, Gialafos E, Dimitrakopoulos A, Psimenou E, Stefanis L, Kilidireas C (2021) Clinico-radiologic features and therapeutic strategies in tumefactive demyelination: a retrospective analysis of 50 consecutive cases. Ther Adv Neurol Disord 14:17562864211006504 10.1177/17562864211006503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villarreal JV, Abraham MJ, Acevedo JAG, Rai PK, Thottempudi N, Fang X, Gogia B (2021) Tumefactive multiple sclerosis (TMS): a case series of this challenging variant of MS. Mult Scler Relat Disord 48:102699 10.1016/j.msard.2020.102699 [DOI] [PubMed] [Google Scholar]
- 29.Altintas A, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M, Saip S, Boz C, Aydin T, Arici-Duz O, Ozer F, Siva A (2012) Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler 18:1448–1453 10.1177/1352458512438237 [DOI] [PubMed] [Google Scholar]
- 30.Ketelslegers IA, Visser IE, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ (2011) Disease course and outcome of acute disseminated encephalomyelitis is more severe in adults than in children. Mult Scler 17:441–448 10.1177/1352458510390068 [DOI] [PubMed] [Google Scholar]
- 31.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B (2001) Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology 56:1313–1318 10.1212/WNL.56.10.1313 [DOI] [PubMed] [Google Scholar]
- 32.Koelman DL, Chahin S, Mar SS, Venkatesan A, Hoganson GM, Yeshokumar AK, Barreras P, Majmudar B, Klein JP, Chitnis T, Benkeser DC, Carone M, Mateen FJ (2016) Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology 86:2085–2093 10.1212/WNL.0000000000002723 [DOI] [PubMed] [Google Scholar]
- 33.Jeong IH, Kim SH, Hyun JW, Joung A, Cho HJ, Kim HJ (2015) Tumefactive demyelinating lesions as a first clinical event: clinical, imaging, and follow-up observations. J Neurol Sci 358:118–124 10.1016/j.jns.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 34.Koelman DL, Benkeser DC, Xu Y, Neo SX, Tan K, Katsuno M, Sobue G, Natsume J, Chahin S, Mar SS, Venkatesan A, Chitnis T, Hoganson GM, Yeshokumar AK, Barreras P, Majmudar B, Carone M, Mateen FJ (2017) Acute disseminated encephalomyelitis in China, Singapore and Japan: a comparison with the USA. Eur J Neurol 24:391–396 10.1111/ene.13220 [DOI] [PubMed] [Google Scholar]
- 35.Fereidan-Esfahani M, Decker PA, Eckel Passow JE, Lucchinetti CF, Flanagan EP, Tobin WO (2022) Population-based incidence and clinico-radiological characteristics of tumefactive demyelination in Olmsted County, Minnesota, United States. Eur J Neurol 29:782–789 10.1111/ene.15182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silsby M, Sanchez P, Spies JM, Frith J, Barton J, Beadnall HN, Barnett MH, Reddel SW, Hardy TA (2019) Investigation of tumefactive demyelination is associated with higher economic burden and more adverse events compared with conventional multiple sclerosis. Mult Scler Relat Disord 35:104–107 10.1016/j.msard.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 37.Selter RC, Brilot F, Grummel V, Kraus V, Cepok S, Dale RC, Hemmer B (2010) Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74:1711–1715 10.1212/WNL.0b013e3181e04096 [DOI] [PubMed] [Google Scholar]
- 38.Pardo G, Jones DE (2017) The sequence of disease-modifying therapies in relapsing multiple sclerosis: safety and immunologic considerations. J Neurol 264:2351–2374 10.1007/s00415-017-8594-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346:158–164 10.1056/NEJMoa011341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.