Abstract
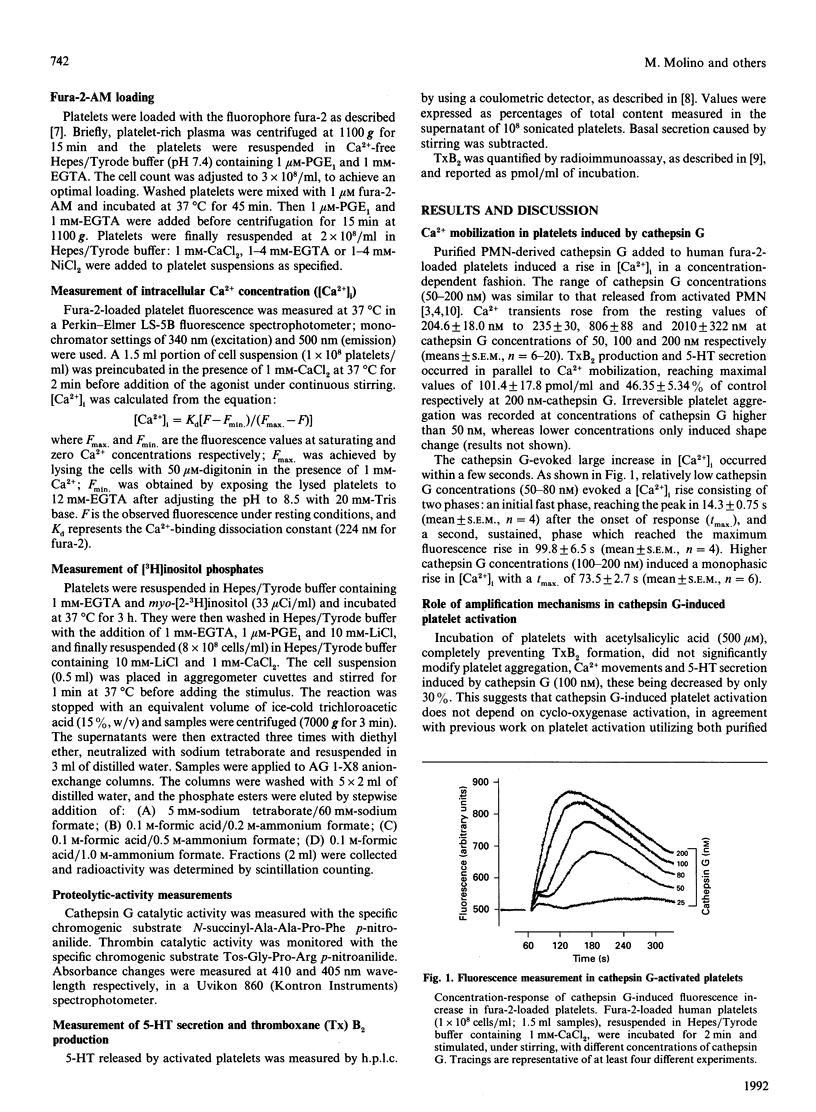
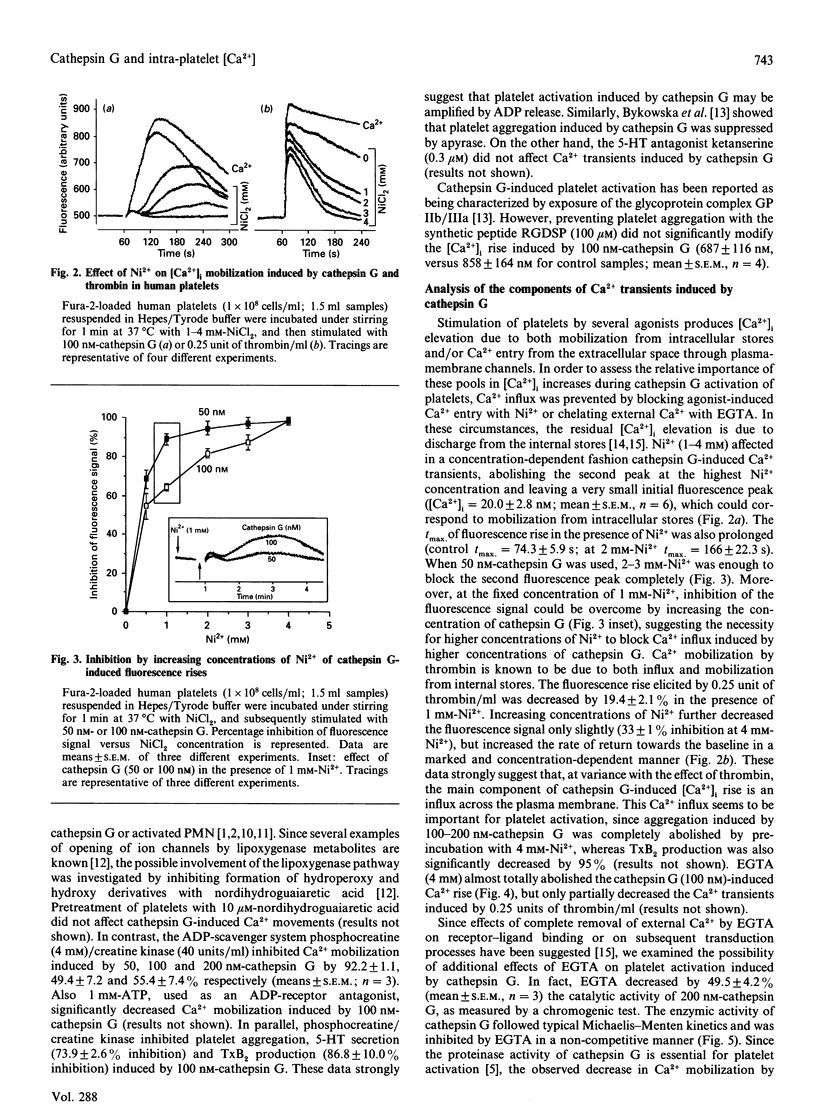
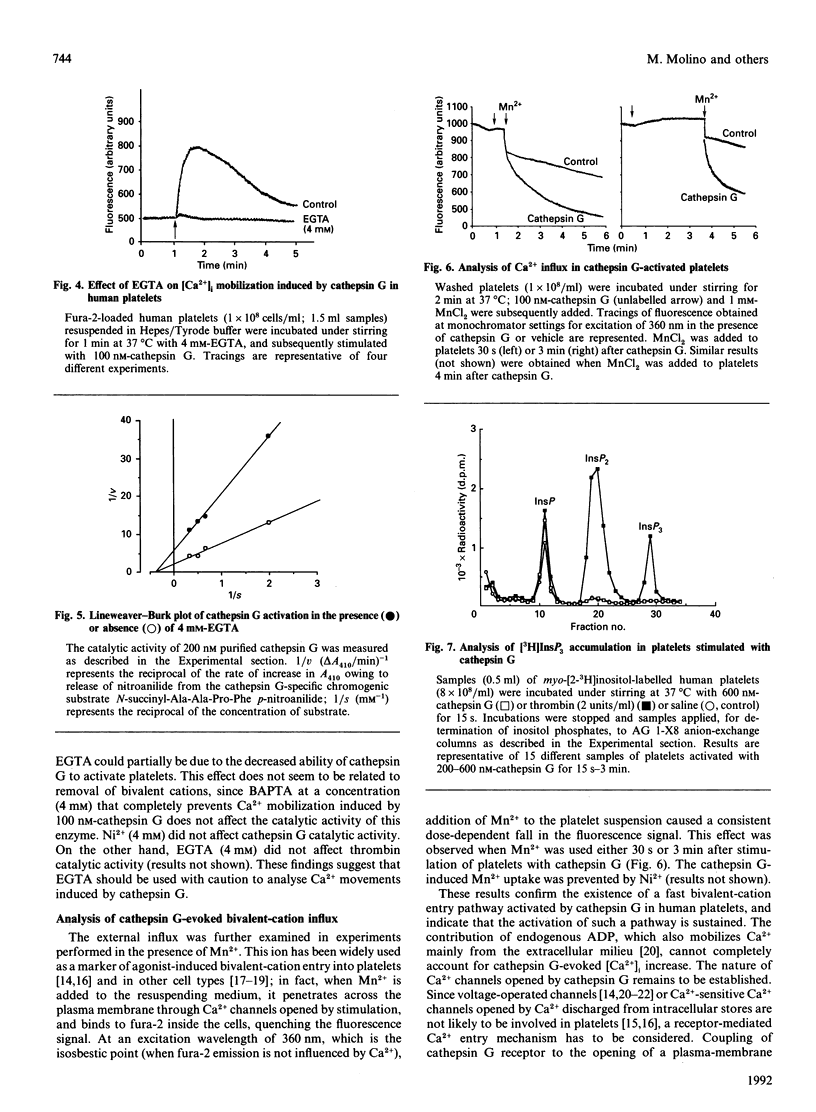
Cathepsin G, a serine protease released by polymorphonuclear-leucocyte azurophilic granules upon stimulation, activates human platelets, inducing an increase in intra-platelet Ca2+ concentration ([Ca2+]i) in a concentration-dependent manner (50-200 nM). The [Ca2+]i rises elicited by low (50-80 nM) cathepsin G concentrations in fura-2-loaded platelets showed a biphasic mode, with a first small peak followed by a greater and more prolonged Ca2+ transient. Higher (100-200 nM) cathepsin G concentrations induced a monophasic increase in intracellular Ca2+. Acetylsalicylic acid, nordihydroguaiaretic acid and ketanserin did not affect platelet activation by cathepsin G, whereas the ADP-scavenger system phosphocreatine/creatine kinase significantly decreased Ca2+ mobilization, platelet aggregation and 5-hydroxytryptamine secretion by cathepsin G. Preventing cathepsin G-induced platelet aggregation with the synthetic peptide RGDSP (Arg-Gly-Asp-Ser-Pro) did not significantly affect cathepsin G-induced Ca2+ transients. Ni2+ (4 mM), a bivalent-cation-channel inhibitor, decreased the cathepsin G-induced fluorescence rise by more than 90%. This effect was reversed by either decreasing Ni2+ or increasing cathepsin G concentration. Preventing Ca2+ influx across the plasma membrane with 4 mM-EGTA totally abolished Ca2+ transients. However, EGTA also strongly decreased catalytic activity of cathepsin G, which is essential for platelet activation. Evidence of a rapid and sustained bivalent-cation channel opening in the platelet membrane was obtained by adding Mn2+ to the platelet suspension 30 s or 3 min after cathepsin G. No accumulation of InsP3 could be detected when platelets were stimulated with cathepsin G. All these data indicate that cathepsin G induces a [Ca2+]i increase mainly through an influx across the plasma membrane. This massive Ca2+ entry is probably due to opening of receptor-operated channels and is amplified by endogenous ADP release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Buczko W., Invernizzi R., de Gaetano G. High pressure liquid chromatography microassay for platelet serotonin. Thromb Haemost. 1984 Feb 28;51(1):131–131. [PubMed] [Google Scholar]
- Bykowska K., Kaczanowska J., Karpowicz M., Stachurska J., Kopeć M. Effect of neutral proteases from blood leukocytes on human platelets. Thromb Haemost. 1983 Dec 30;50(4):768–772. [PubMed] [Google Scholar]
- Cerletti C., Gambino M. C., Garattini S., de Gaetano G. Biochemical selectivity of oral versus intravenous aspirin in rats. Inhibition by oral aspirin of cyclooxygenase activity in platelets and presystemic but not systemic vessels. J Clin Invest. 1986 Jul;78(1):323–326. doi: 10.1172/JCI112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignard M., Selak M. A., Smith J. B. Direct evidence for the existence of a neutrophil-derived platelet activator (neutrophilin). Proc Natl Acad Sci U S A. 1986 Nov;83(22):8609–8613. doi: 10.1073/pnas.83.22.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maschio A., Evangelista V., Rajtar G., Chen Z. M., Cerletti C., De Gaetano G. Platelet activation by polymorphonuclear leukocytes exposed to chemotactic agents. Am J Physiol. 1990 Mar;258(3 Pt 2):H870–H879. doi: 10.1152/ajpheart.1990.258.3.H870. [DOI] [PubMed] [Google Scholar]
- Doyle V. M., Rüegg U. T. Lack of evidence for voltage dependent calcium channels on platelets. Biochem Biophys Res Commun. 1985 Feb 28;127(1):161–167. doi: 10.1016/s0006-291x(85)80139-x. [DOI] [PubMed] [Google Scholar]
- Ernst E., Hammerschmidt D. E., Bagge U., Matrai A., Dormandy J. A. Leukocytes and the risk of ischemic diseases. JAMA. 1987 May 1;257(17):2318–2324. [PubMed] [Google Scholar]
- Evangelista V., Rajtar G., de Gaetano G., White J. G., Cerletti C. Platelet activation by fMLP-stimulated polymorphonuclear leukocytes: the activity of cathepsin G is not prevented by antiproteinases. Blood. 1991 Jun 1;77(11):2379–2388. [PubMed] [Google Scholar]
- Gavin C. E., Gunter K. K., Gunter T. E. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990 Mar 1;266(2):329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Responses to adenosine diphosphate in human platelets loaded with the fluorescent calcium indicator quin2. J Physiol. 1985 Nov;368:131–146. doi: 10.1113/jphysiol.1985.sp015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kurachi Y., Ito H., Sugimoto T., Shimizu T., Miki I., Ui M. Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature. 1989 Feb 9;337(6207):555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Peterson M. W., Gruenhaupt D., Shasby D. M. Neutrophil cathepsin G increases calcium flux and inositol polyphosphate production in cultured endothelial cells. J Immunol. 1989 Jul 15;143(2):609–616. [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Romano M., Molino M., Cerletti C. Endotoxic lipid A induces intracellular Ca2+ increase in human platelets. Biochem J. 1991 Aug 15;278(Pt 1):75–80. doi: 10.1042/bj2780075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Selak M. A., Chignard M., Smith J. B. Cathepsin G is a strong platelet agonist released by neutrophils. Biochem J. 1988 Apr 1;251(1):293–299. doi: 10.1042/bj2510293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak M. A., Smith J. B. Cathepsin G binding to human platelets. Evidence for a specific receptor. Biochem J. 1990 Feb 15;266(1):55–62. doi: 10.1042/bj2660055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschauer A., van Breemen C., Bühler F. R., Nelson M. T. Calcium channels in thrombin-activated human platelet membrane. Nature. 1988 Aug 25;334(6184):703–705. doi: 10.1038/334703a0. [DOI] [PubMed] [Google Scholar]


