Abstract
Dual inhibition of vascular endothelial growth factor and epidermal growth factor receptor (EGFR) signaling pathways offers the prospect of improving the effectiveness of EFGR-targeted therapy. In this phase 3 study (ClinicalTrial.gov: NCT04028778), 315 patients with treatment-naïve, EGFR-mutated, advanced non-small cell lung cancer (NSCLC) were randomized (1:1) to receive anlotinib or placebo plus gefitinib once daily on days 1–14 per a 3-week cycle. At the prespecified final analysis of progression-free survival (PFS), a significant improvement in PFS was observed for the anlotinib arm over the placebo arm (hazards ratio [HR] = 0.64, 95% CI, 0.48–0.80, P = 0.003). Particularly, patients with brain metastasis and those harboring EGFR amplification or high tumor mutation load gained significant more benefits in PFS from gefitinib plus anlotinib. The incidence of grade 3 or higher treatment-emergent adverse events was 49.7% of the patients receiving gefitinib plus anlotinib versus 31.0% of the patients receiving gefitinib plus placebo. Anlotinib plus gefitinib significantly improves PFS in patients with treatment-naïve, EGFR-mutated, advanced NSCLC, with a manageable safety profile.
Subject terms: Oncology, Cancer
Introduction
Lung cancer remains a principal cause of cancer death in both sexes globally.1 China is experiencing an increasing burden of lung cancer, with approximately 820,000 new cases and 720,000 deaths in 2020, accounting for 40% of global lung cancer death.2 Approximately 60% of lung cancer cases harbor driver alterations and targeting actionable oncogenic driver alterations remains a cornerstone in targeted therapy for non-small cell lung cancer (NSCLC),3 which accounts for the majority of lung cancer cases.
The epidermal growth factor receptor (EGFR) gene is the most frequent genetic driver in metastatic NSCLC,4 and, therefore, a rational therapeutic target. Several advanced clinical trials have demonstrated that EGFR tyrosine kinase inhibitors (TKIs), including gefitinib,5,6 the first or second EGFR TKIs, and osimertinib, a third generation EGFR-TKI,7 confer progression-free survival (PFS) benefit in patients with NSCLC harboring EGFR mutation. EGFR-TKIs have been established as standard first-line treatments for EGFR-mutated NSCLC.8 However, acquired resistance to EGFR-TKIs inevitably develops and NSCLC patients ultimately experience disease progression,9–11 highlighting the importance of exploring novel EGFR-TKIs or therapeutic agents exhibiting biological synergy with EGFR-TKIs for advanced EGFR mutated NSCLC.12
The vascular endothelial growth factor (VEGF) pathway plays a critical role in driving oncoangiogenesis in lung cancer and dual inhibition of VEGF signaling and EGFR signaling pathways offers the prospect of improving the effectiveness of EFGR-targeted therapy and overcoming EGFR-TKI resistance.13
Anlotinib is an oral multikinase inhibitor and suppresses oncoangiogenesis and tumor growth via blocking VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and c-Kit14 while bevacizumab only targets the VEGFR signaling pathway.15 The antitumor activities of anlotinib is supported by its remarkable anti-angiogenic activities compared with other TKIs, with a low IC50 for VEGFR-2 (0.2 nmol/L vs. lenvatinib 4 nmol/L and sorafenib 90 nmol/L) and VEGFR-3 (0.7 nmol/L vs. lenvatinib 5.2 nmol/L and sorafenib 20 nmol/L).15–19 Currently, it is the only approved anti-angiogenic drug for lung cancer in China and has been recommended as third and later-line treatment for advanced NSCLC based on the ALTER 0303 trial showing anlotinib significantly extending the overall survival (OS) (HR 0.68, 95% CI 0.54–0.87) and PFS (HR 0.25, 95% CI 0.19–0.31) of advanced NSCLC patients progressing upon second or further line treatment.20,21 A subgroup analysis of the ALTER 0303 trial showed that anlotinib led to a 79% reduction in the risk of progression (hazard ratio [HR] 0.21; 95% confidence interval [CI]: 0.13–0.32) a 41% reduction in the risk of death (HR 0.59; 95% CI: 0.38–0.94) versus placebo in treatment-naïve NSCLC patients and this benefit was independent of EGFR mutation status.22 Anlotinib also showed promising antitumor activities in the first-line setting for advanced NSCLC in combination with another EGFR-TKI23 and immune checkpoint inhibitor sintilimab.24
Anlotinib offers convenient oral dosing compared to intravenous infusion for currently available anti-angiogenic inhibitors and has been studied in the first-line setting for NSCLC plus chemotherapy.25 We hypothesized that anlotinib in combination with an EGFR-TKI would be more effective than EGFR-TKI monotherapy for advanced NSCLC in the first-line setting. In this study, we sought to investigate the efficacy and safety of gefitinib plus anlotinib for previously untreated Chinese patients with EGFR-mutated advanced NSCLC.
Results
Patient characteristics
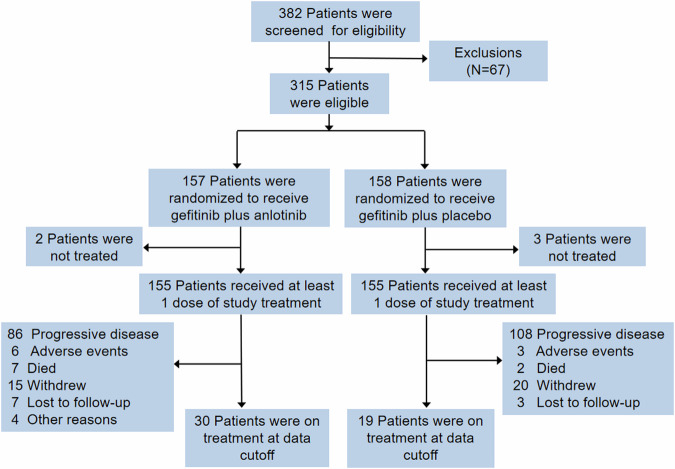
Between April 2019 and August 2021, 382 patients were screened for eligibility and 315 patients were eligible for the study and underwent randomization to receive gefitinib plus anlotinib (n = 157) or gefitinib plus placebo (n = 158) (Fig. 1). One hundred fifty-five patients in each group received at least one dose of the study medication and were included in the FAS. At the data cutoff (July 31, 2022), treatment was still ongoing for 30 (19.1%) patients in the gefitinib plus anlotinib group and 19 (12.0%) in the gefitinib plus placebo group.
Fig. 1.
The study flowchart and patient disposition. Enhancement MRI was performed during screening to evaluate each patient for brain metastasis and MRI/CT scans were undertaken in patients with brain metastasis at baseline during follow up and in other patients at the discretion of the investigators
The patients had a median age of 59 years and 43.9% were male. Most patients (92.9%) had clinical stage IV disease. Thirty-four (11.0%) patients had an ECOG performance status of 0 and 263 (84.8%) had an ECOG performance status of 1. Ninety-nine (31.9%) patients had brain metastasis and 42 (13.5%) had liver metastasis; at least 2 organs were involved in 196 (63.2%) patients. One hundred sixty-one (51.9%) patients harbored EGFR ex19del mutation and 149 (48.1%) harbored EGFR ex21L858R mutation (Table 1).
Table 1.
Patient demographic and baseline characteristics-full analysis set
| Characteristics | Gefitinib plus anlotinib (n = 155) | Gefitinib plus placebo (n = 155) |
|---|---|---|
| Age, years | ||
| Median (range) | 59.0 (29.0–77.0) | 59.0 (32.0–76.0) |
| <65 | 119 (76.8) | 108 (69.7) |
| ≥65 | 36 (23.2) | 47 (30.3) |
| Male sex | 68 (43.9) | 67 (43.2) |
| ECOG performance status score | ||
| 0 | 19 (12.3) | 15 (9.7) |
| 1 | 130 (83.9) | 133 (85.8) |
| Unknown | 6 (3.4) | 7 (4.5) |
| Smoking status | ||
| Never smokers | 118 (76.1) | 121 (78.1) |
| Former smokers | 26 (16.8) | 24 (15.5) |
| Current smokers | 10 (6.5) | 9 (5.8) |
| Unknown | 1 (0.7) | 1 (0.7) |
| Stage of disease | ||
| IIIB | 10 (6.5) | 4 (2.6) |
| IV | 142 (91.6) | 148 (95.5) |
| Unknown | 3 (1.9) | 3 (1.9) |
| Tumor histologic subtypes | ||
| Adenocarcinoma | 153 (98.7) | 150 (96.8) |
| Others | 2 (1.3) | 5 (3.2) |
| Type of EGFR mutations | ||
| Exon 19 deletion | 80 (51.6) | 81 (52.3) |
| L858R | 75 (48.4) | 74 (47.7) |
| Metastatic sites | ||
| ≤2 | 54 (34.8) | 58 (37.4) |
| >2 | 101 (65.2) | 95 (61.3) |
| Unknown | 0 (0.0) | 2 (1.3) |
| Brain metastasis | ||
| Yes | 49 (31.6) | 50 (32.3) |
| No | 106 (68.3) | 105 (67.7) |
| Liver metastasis | ||
| Yes | 20 (12.9) | 22 (14.2) |
| No | 135 (87.1) | 133 (85.8) |
Data are expressed as number (%) unless otherwise indicated
Eastern Cooperative Oncology Group (ECOG) performance-status (PS) scores range from 0 to 5, with higher numbers indicating increasing impairment in activities of daily living
Definitions: Never smokers, defined as smoking <100 cigarettes/lifetime; former smokers, defined as abstinence from smoking for at least 15 years on the day before the start of therapy; current smokers, defined as smoking >100 cigarettes/lifetime, or smoking >100 cigarettes/lifetime but abstinence from smoking for less than one year on the day before the start of therapy
Efficacy analysis
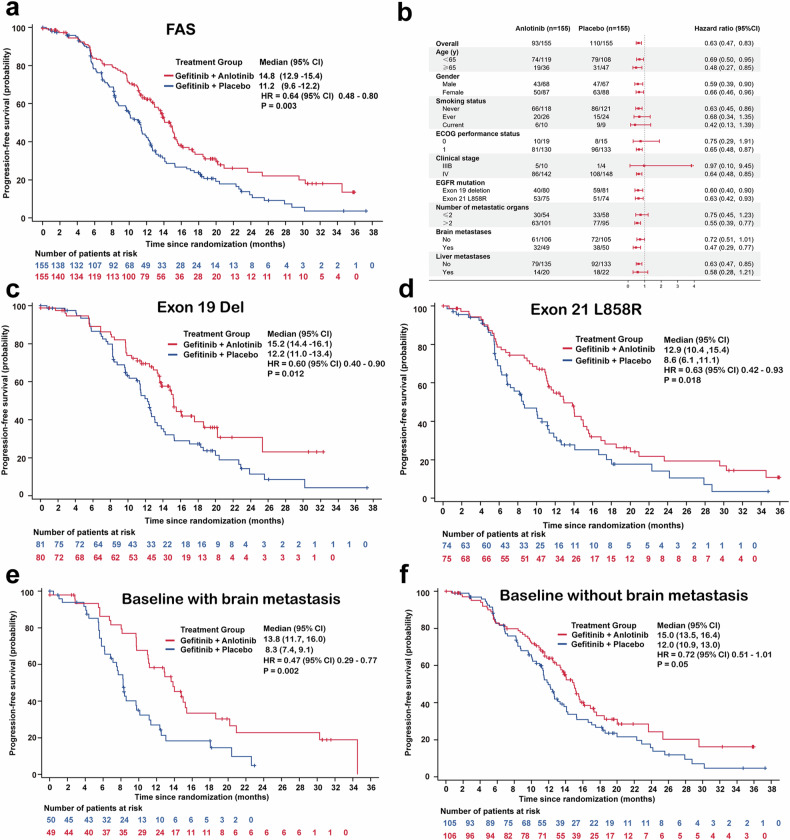
Patients were followed up for median duration of 18.5 (95% CI 15.9–20.1) months in the gefitinib plus anlotinib group and 18.2 months (95% CI 15.5–19.9) in the gefitinib plus placebo group. As of the data cutoff date, 99 and 114 independent review committee (IRC)-confirmed PFS events had occurred in the gefitinib plus anlotinib group and the gefitinib plus placebo group, respectively. The median PFS was 14.8 months (95% CI, 12.9–15.4) in the gefitinib plus anlotinib group versus 11.2 months (95% CI, 9.6–12.2) in the gefitinib plus placebo group (HR = 0.64, 95% CI 0.48–0.80; stratified log rank test, P = 0.003) (Fig. 2a and Supplementary table 1). Diverse groups of NSCLC patients gained significant benefit in PFS from gefitinib plus anlotinib (Fig. 2b). In patients with EGFR ex19del, gefitinib plus anlotinib conferred significantly greater benefit in PFS than gefitinib plus placebo (15.2 months, 95% CI, 14.4–16.1 vs. 12.2 months, 95% CI, 11.0–13.4; HR = 0.60, 95% CI, 0.40–0.90) (Fig. 2c). Furthermore, patients harboring EGFR ex21L858R gained significant PFS benefit from gefitinib plus anlotinib than gefitinib plus placebo (12.9 vs. 8.6 months, HR = 0.63, 95% CI, 0.42–0.93) (Fig. 2d). Notably, among patients with brain metastasis, gefitinib plus anlotinib extended the median PFS by 5.5 months versus gefitinib plus placebo, with a 53% reduction in the risk of progression (13.8 vs. 8.3 months; HR = 0.47, 95% CI, 0.29–0.77; log rank test P = 0.002) (Fig. 2e). Besides, patients without brain metastasis receiving gefitinib plus anlotinib tended to have a longer PFS than those receiving gefitinib plus placebo (15.0 vs. 12.0 months; HR = 0.72, 95% CI, 0.51–1.01; log rank test P = 0.05) (Fig. 2f).
Fig. 2.
Gefitinib plus anlotinib improves progression-free survival (PFS) of advanced NSCLC patients. a The Kaplan–Meier curves PFS of advanced NSCLC patients treated with gefitinib plus anlotinib or gefitinib plus placebo in the FAS. b Forest plots for PFS. The Kaplan–Meier curves of PFS of patients harboring EGFR Exon 19 Del c or EGFR Exon 21 L858R d treated with gefitinib plus anlotinib or gefitinib plus placebo. e The Kaplan–Meier curves of PFS of patients with e or without f brain metastasis treated with gefitinib plus anlotinib or gefitinib plus placebo
Thirty-eight deaths occurred in the gefitinib plus anlotinib group, and 33 deaths were reported in the gefitinib plus placebo group. Gefitinib plus anlotinib did not significantly improved OS versus gefitinib plus placebo in advanced NSCLC patients (HR = 1.12, 95% CI, 0.70–1.78). The median OS was 31.2 months (95% CI, 25.7-not estimable [NE]) in patients receiving gefitinib plus anlotinib and not reached in the gefitinib plus placebo group (95% CI, 27.1-NE; stratified log rank test, P = 0.644) (Supplementary Fig. 1a). No statistical difference was observed in OS across all subgroups of the two groups (Supplementary Fig. 1b).
In the FAS population, the objective response rate was significantly higher with gefitinib plus anlotinib (76.1%, 95% CI, 68.6–82.6%) versus gefitinib plus placebo (64.5%, 95% CI, 56.4–72.0%) (chi-square test, P = 0.025) (Supplementary Table 1 and Supplementary Fig. 2). The median duration of response was remarkably longer with gefitinib plus anlotinib (12.5 months, 95% CI, 11.1–16.2) versus that with gefitinib plus placebo (9.5 months, 95% CI 7.0–10.3) (log rank test P < 0.001).
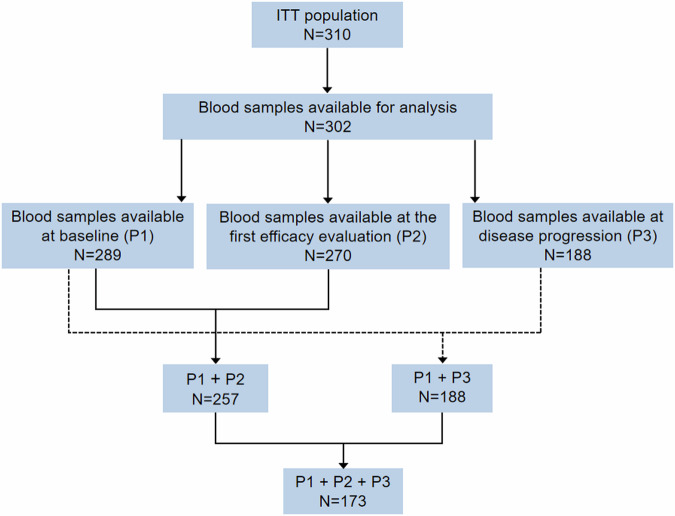
Dynamic analysis of peripheral blood ctDNA
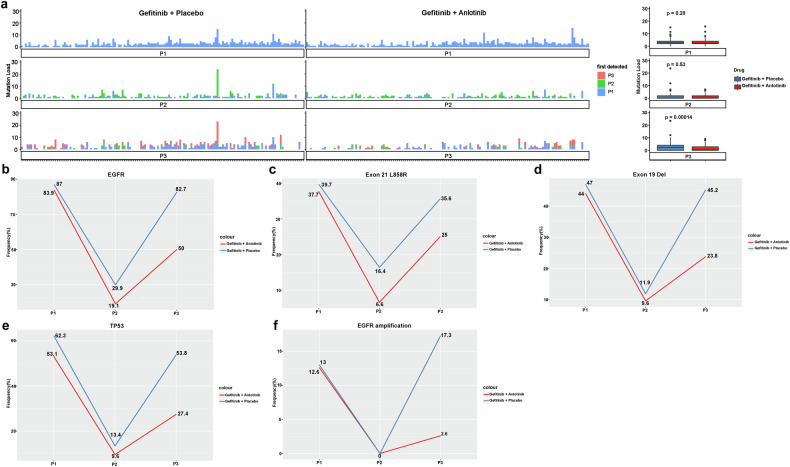
We hypothesized that dual inhibition of VEGF and EGFR signaling pathways could be more effective in suppressing the frequencies of mutated key driver genes than TKI monotherapy. We analyzed the genomic data based on blood samples for circulating tumor DNA (ctDNA) analysis which were available for 289 patients at baseline, 270 at the first posttreatment efficacy evaluation and 188 at the time of disease progression (Fig. 3). Next generation sequencing (NGS) revealed no notable difference in the median tumor mutational load (TML) between the two groups at baseline (gefitinib plus anlotinib 3.0 vs. gefitinib plus placebo 3.0, P = 0.30, single nucleotide variation) and the first posttreatment efficacy evaluation (gefitinib plus anlotinib 1.0 vs. gefitinib plus placebo 1.0, P = 0.53). At the time of progression, patients in the gefitinib plus anlotinib group had a significantly lower TML than patients in the gefitinib plus placebo group (2.0 vs. 4.0, P < 0.001) (Fig. 4a).
Fig. 3.
The flowchart for next generation sequencing (NGS) analysis. P1, baseline, P2, first posttreatment efficacy evaluation and P3, progressive disease
Fig. 4.
Dual inhibition of VEGF and EGFR signaling pathways reduces the tumor mutational load and the frequencies of key driver gene mutations. a Tumor mutational load in individual patients at baseline (P1), the first posttreatment efficacy evaluation (P2) and disease progression (P3) (left panel). Each column represents one patient in efficacy evaluable patients. Boxplots of tumor mutational load in patients in the gefitinib plus anlotinib group and the gefitinib plus placebo group are shown on the right. Temporal changes in the rates of mutated EGFR gene b, EGFR Exon 21 L858R c and Exon 19 Del d, mutated TP53 gene e and copy number variations (amplification) f in the two groups from baseline to disease progression
EGFR mutations were detectable by peripheral blood ctDNA in 83.9% of the patients receiving gefitinib plus anlotinib vs. 87.0% of those receiving gefitinib plus placebo at baseline. At the first posttreatment efficacy evaluation, the rate of mutated EGFR with gefitinib plus anlotinib was lower than that with gefitinib plus placebo (19.1% vs. 29.9%). Meanwhile, upon disease progression, it approached the level before treatment in the gefitinib plus placebo group (82.7%) while remaining subdued in the gefitinib plus anlotinib group (50.0%) (Fig. 4b). A similar trajectory of changes was observed in EGFR ex21L858R and ex19del (Fig. 4c, d).
The presence of mutated TP53 is reported to be an adverse predictor of TKI therapeutic outcome in NSCLC.26 We next examined the temporal changes in the mutational profiles of TP53, an established tumor initiator gene. The rate of mutated TP53 also showed a similar trajectory of changes to that of mutated EGFR, with a notable reduction in mutated TP53 rate in both groups at the first posttreatment efficacy evaluation (gefitinib plus anlotinib 9.6% from 53.1% at baseline vs. gefitinib plus placebo 13.4% from 62.3% at baseline). The rate of mutated TP53 approached the baseline level in patients receiving gefitinib plus placebo at the time of disease progression but only showed a modest rise in patients receiving gefitinib plus anlotinib (Fig. 4e).
In addition, EGFR amplification occurred in 12.6% of the patients in the gefitinib plus anlotinib group at baseline and was not detected at the first efficacy evaluation and rose to 2.6% at PD. Meanwhile, EGFR amplification occurred in 13.0% of the patients in the gefitinib plus placebo group at baseline and were undetected at the first efficacy evaluation; upon disease progression, it increased to 17.3%. The rate of EGFR amplification at the time of PD was significantly higher in the gefitinib plus placebo group than the gefitinib plus anlotinib group (P < 0.001) (Fig. 4f).
Biomarkers analysis
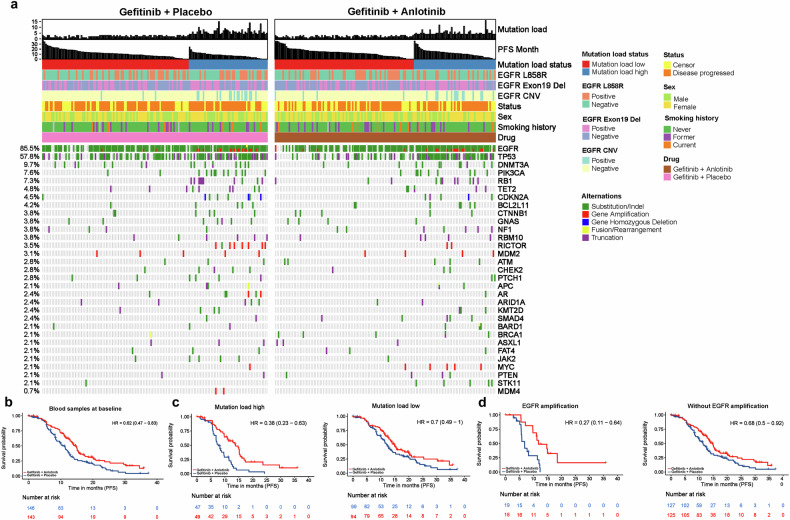
The mutational profiles at baseline of EGFR-mutated, advanced NSCLC patients receiving gefitinib and anlotinib and gefitinib plus placebo are shown in Fig. 5a. Dual therapy with gefitinib plus anlotinib significantly reduced the risk of progression in patients with blood sample available at baseline compared to gefitinib plus placebo (HR = 0.62, 95% CI 0.47–0.83, P = 0.001) (Fig. 5b). Currently, there are no established molecular biomarkers that can reliably predict clinical response or resistance to anti-angiogenic agents. We evaluated TML as a biomarker to estimate survival benefit associated with the combination of gefitinib and anlotinib. The median TML was 3.0 at baseline in both groups and was used to define patients with high and low TML. Gefitinib plus anlotinib extended the median PFS by 6.2 months versus gefitinib plus placebo (13.8 months, 95% CI 10.9–15.5 vs. 7.6 months, 95% CI 6.8–9.8), with a 62% reduction in the risk of progression in patients with high TML at baseline (HR = 0.38, 95% CI 0.23–0.63, P < 0.001) (Fig. 5c, left). Anlotinib added to gefitinib led to a 30% reduction in the risk of progression in patients with low TML at baseline (HR = 0.70, 95% CI 0.49–1.00; log rank test, P = 0.050), with a 2.5-month extension of PFS (14.7 months, 95% CI 12.9–20.0 vs. 12.2 months, 95% CI 10.6–14.3) (Fig. 5c, right).
Fig. 5.
Dual inhibition of VEGF and EGFR signaling pathways derives more benefits in patients with EGFR amplification or a high tumor mutational load. a Heatmaps show frequent somatic mutations (≥2%) in pretreatment NSCLC samples. Left, gefitinib plus placebo, right, gefitinib plus anlotinib. b The Kaplan–Meier PFS curves of advanced NSCLC patients with blood sample available at baseline c The Kaplan–Meier PFS curves of patients with high (left) and low tumor mutational load (right) treated with gefitinib plus anlotinib versus gefitinib plus placebo. d The Kaplan–Meier PFS curves of patients with (left) or without EGFR amplification (right) who were treated with gefitinib plus anlotinib versus those who were treated with gefitinib plus placebo
Copy number variation (CNV) could predict response to EGFR TKI therapy in patients with advanced NSCLC.27,28 However, the impact of EGFR amplification on the efficacy of dual therapy with an anti-angiogenic agent and EGFR TKI remains undefined and was explored in this study. Patients harboring EGFR amplification at baseline experienced a 73% reduction in the risk of progression with gefitinib plus anlotinib (HR = 0.27, 95% CI, 0.11–0.64; log rank test, P = 0.002), extending the median PFS by 4.9 months vs. gefitinib plus placebo (11.7 months, 95% CI 9.8-NE vs. 6.8 months, 95% CI 5.7–12) (Fig. 5d, left). Gefitinib plus anlotinib also lowered the risk of progression in patients without EGFR amplification at baseline (HR = 0.68, 95% CI, 0.50–0.92; log rank test, P = 0.012) (Fig. 5d, right and Supplementary Fig. 3).
EGFR mutations and the associated gene pathways at PD
At the time of PD, EGFR ex21L858R, EGFR ex19del and secondary EGFRT790M mutation accounted for 32.8%, 31.3% and 28.1% of EGFR mutations in patients receiving gefitinib plus anlotinib and 22.6%, 28.7%, and 31.1% of EGFR mutations in patients receiving gefitinib plus placebo, respectively (Supplementary Fig. 4a). Meanwhile, EGFR amplification accounted for 3.1% and 11.0% of EGFR mutations in patients receiving gefitinib plus anlotinib and those receiving gefitinib plus placebo, respectively. In addition, the rates of mutated EGFR, TP53, and RICTOR were significantly lower in patients receiving gefitinib plus anlotinib than those on gefitinib plus placebo (Supplementary Fig. 4b).
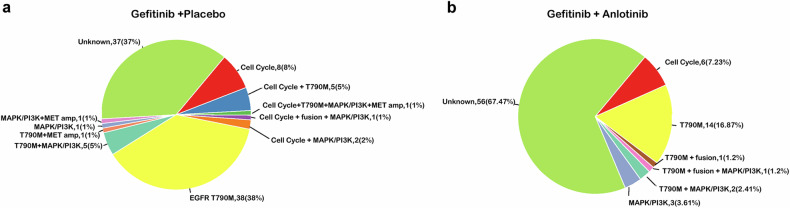
Gene pathway analysis showed that the mutated genes at the time of disease progression were involved in multiple cellular signaling pathways. The gefitinib plus placebo group had significantly higher rates of gene mutations implicated in EGFR signaling, p53 signaling, cell cycle progression, checkpoint factor (CPF) and RICTOR signaling than the gefitinib plus anlotinib group (Supplementary Fig. 4c). Secondary EGFRT790M mutations were the predominant change in both groups, occurring in 16.9% of patients receiving gefitinib plus anlotinib vs. 38.0% of patients receiving gefitinib plus placebo. This was followed by aberrant cell cycle signaling (gefitinib plus anlotinib 8.0% vs. gefitinib plus placebo 7.2%) (Fig. 6). However, resistance mechanisms remained undefined in 67.5% of the patients in the gefitinib plus anlotinib group and 37.0% of the patients in the gefitinib plus placebo group. At the data cutoff date, among 107 patients with treatment records after disease progression, 68% (30/44) went on to receive a third generation TKI in the anlotinib arm and 78% (49/63) in the placebo arm.
Fig. 6. Possible resistance mechanisms at the time of disease progression.
a The gefitinib plus placebo group; b The gefitinib plus anlotinib group
Safety
The safety set included 155 patients in the gefitinib plus anlotinib group and 155 patients in the gefitinib plus placebo group. Treatment was interrupted in 50 (32.3%) patients receiving gefitinib plus anlotinib and 34 (21.9%) patients receiving gefitinib plus placebo.
Treatment emergent AEs (TEAEs) led to dose reductions in 48 (31.0%) patients receiving gefitinib plus anlotinib and 21 (13.6%) patients receiving gefitinib plus placebo and treatment termination in 16 (10.3%) patients receiving gefitinib plus anlotinib and 7 (4.5%) patients receiving gefitinib plus placebo. Two (1.3%) patients receiving gefitinib plus anlotinib died, due to cerebral infarction and progressive tumor. One (0.7%) patient receiving gefitinib plus placebo died due to compression of the pulmonary artery. Subsequent antitumor therapies are provided in Supplementary Table 2.
Any grade TEAEs occurred in 99.4% of the patients receiving gefitinib plus anlotinib and 97.4% of the patients receiving gefitinib plus placebo. Grade 3 or higher TEAEs were reported in 49.7% of the patients receiving gefitinib plus anlotinib and 31.0% of those receiving gefitinib plus placebo. The most frequent any-grade TEAEs are shown in Table 2. The 3 most frequent any-grade TEAEs were diarrhea (66.5%), rash (65.8%) and hypertension (65.2%) in the gefitinib plus anlotinib group, and rash (52.9%), elevated alanine aminotransferase (ALT, 48.4%) and aspartate aminotransferase (AST, 48.4%) in the gefitinib plus placebo group. The most frequent grade 3 or higher TEAEs in the gefitinib plus anlotinib arm were hypertension (29.7%) and elevated ALT (6.5%). Meanwhile, the most frequent grade 3 or higher TRAEs in the gefitinib plus placebo arm were elevated ALT (12.3%) and AST (7.1%).
Table 2.
Treatment-emergent adverse events reported in at least 10% of the patients in the two treatment arms
| Gefitinib + anlotinib (N = 155) |
Gefitinib + placebo (N = 155) |
|||
|---|---|---|---|---|
| Any grade | Grade 3 or higher | Any grade | Grade 3 or higher | |
| Any TEAEs | 154 (99.4) | - | 151 (97.42) | - |
| Grade ≥ 3 TEAEs | - | 77 (50.0) | - | 48 (31.0) |
| Serious TEAEs | 17 (11.0) | - | 9 (5.8) | - |
| TEAEs leading to dose interruption, any drug | 50 (32.3) | - | 34(21.9) | - |
| TEAEs leading to dose reduction, any drug | 48 (31.0) | - | 21 (13.6) | - |
| Discontinued treatment due to TEAEs | 16 (10.3) | - | 7 (4.5) | - |
| $ TEAEs leading to death | 2 (1.3) | - | 1 (0.7) | - |
| TEAEs (≥ 10%) | ||||
| Diarrhea | 103 (66.5) | 0 (0.0) | 63 (40.7) | 2 (1.3) |
| Rash | 102 (65.8) | 5 (3.23) | 82 (52.90) | 0 (0.0) |
| Hypertension | 101 (65.2) | 46 (29.68) | 42 (27.10) | 8 (5.2) |
| ALT increased | 73 (47.1) | 10 (6.45) | 75 (48.39) | 19 (12.3) |
| AST increased | 70 (45.2) | 5 (3.23) | 75 (48.39) | 11 (7.1) |
| Proteinuria | 68 (43.9) | 3 (1.94) | 38 (24.52) | 0 (0.0) |
| Hypertriglyceridemia | 65 (41.9) | 4 (2.58) | 54 (34.84) | 2 (1.3) |
| Occult blood positive | 58 (37.4) | 0 (0.00) | 58 (37.42) | 0 (0.0) |
| Palmoplantar redness syndrome | 51 (32.9) | 3 (1.94) | 24 (15.48) | 0 (0.0) |
| Hypercholesterolemia | 43 (27.7) | 0 (0.00) | 28 (18.06) | 0 (0.0) |
| Dysphonia | 42 (27.1) | 0 (0.00) | 1 (0.65) | 0 (0.0) |
| Urine occult blood positive | 41 (26.5) | 0 (0.00) | 35 (22.58) | 2 (1.29) |
| Cough | 35 (22.6) | 0 (0.00) | 34 (21.94) | 0 (0.00) |
| Anorexia | 35 (22.6) | 0 (0.00) | 16 (10.32) | 1 (0.65) |
| Dizzy | 35 (22.6) | 0 (0.00) | 25 (16.13) | 1 (0.65) |
| Urine white blood cell positive | 31 (20.0) | 1 (0.65) | 23 (14.84) | 1 (0.65) |
| Mouth ulcer | 29 (18.7) | 0 (0.00) | 17 (10.97) | 0 (0.00) |
| Gingival bleeding | 29 (18.7) | 0 (0.00) | 14 (9.03) | 0 (0.00) |
| Weakness | 28 (18.1) | 0 (0.00) | 14 (9.03) | 0 (0.00) |
| Nosebleed | 26 (16.8) | 1 (0.65) | 17 (10.97) | 0 (0.00) |
| Headache | 26 (16.8) | 0 (0.00) | 14 (9.03) | 0 (0.00) |
| Hypokalemia | 25 (16.1) | 2 (1.29) | 12 (7.74) | 1 (0.65) |
| Oral mucositis | 25 (16.1) | 3 (1.94) | 8 (5.16) | 0 (0.00) |
| Hematuria | 25 (16.1) | 1 (0.65) | 15 (9.68) | 0 (0.00) |
| Vomiting | 24 (15.5) | 1 (0.65) | 15 (9.68) | 0 (0.00) |
| Hyperuricemia | 23 (14.8) | 0 (0.00) | 27 (17.42) | 0 (0.00) |
| Pruritus | 23 (14.8) | 0 (0.00) | 27 (17.42) | 0 (0.00) |
| Urine red blood cell positive | 22 (14.2) | 0 (0.00) | 15 (9.68) | 0 (0.00) |
| Back pain | 21 (13.6) | 0 (0.00) | 27 (17.42) | 0 (0.00) |
| Paronychia | 21 (13.6) | 1 (0.65) | 20 (12.90) | 0 (0.00) |
| Joint pain | 20 (12.9) | 0 (0.00) | 14 (9.03) | 0 (0.00) |
| Chest pain | 18 (11.6) | 0 (0.00) | 23 (14.84) | 0 (0.00) |
| Nasopharyngitis | 17 (11.0) | 0 (0.00) | 17 (10.97) | 0 (0.00) |
| Constipation | 17 (11.0) | 0 (0.00) | 15 (9.68) | 0 (0.00) |
| Hemoptysis | 17 (11.0) | 2 (1.29) | 14 (9.03) | 0 (0.00) |
| Fever | 16 (10.3) | 0 (0.00) | 6 (3.87) | 0 (0.00) |
| Abdominal pain | 16 (10.3) | 0 (0.00) | 8 (5.16) | 0 (0.00) |
| Blood bilirubin increased | 16 (10.3) | 0 (0.00) | 22 (14.19) | 1 (0.65) |
| Gingival pain | 16 (10.3) | 0 (0.00) | 5 (3.23) | 0 (0.00) |
| Limb pain | 16 (10.3) | 0 (0.00) | 13 (8.39) | 1 (0.65) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAEs, treatment emergent adverse events
Treatment-emergent adverse events were evaluated throughout the treatment period and to 30 days post the final dose using NCI CTC AE version 4.0 and coded using MedDRA 24.0
Gefitinib + anlotinib: 1 patient reported multiple cerebral infarctions leading to death, and 1 patient reported dyspnea leading to death
Gefitinib + placebo: 1 patient reported Pulmonary hypertension leading to death
Discussion
This multicenter double-blind phase III trial demonstrated that addition of anlotinib to gefitinib significantly reduced the risk of progression in treatment-naïve EGFR-mutated, advanced NSCLC patients, conferring PFS benefits among multiple subgroups of NSCLC patients, especially those with brain metastasis and broadly active against NSCLC of diverse genomic profiles. The combination therapy showed an acceptable safety profile. The findings support continued development of gefitinib plus anlotinib in the frontline setting for advanced NSCLC patients with EGFR activating mutations.
In the current trial, more than half of the population were women, the majority were never smokers, most had lung adenocarcinoma and approximately one third of them had brain metastasis upon presentation. The study demonstrated that additional anti-angiogenic therapy with anlotinib, which offers convenient oral dosing instead of intravenous infusion as with bevacizumab, provides a clear benefit in terms of PFS versus gefitinib monotherapy for patients with advanced NSCLC harboring EGFR activating mutations. In the study, gefitinib plus anlotinib extended PFS by 3.6 months versus gefitinib plus placebo, with a 36% reduction in the risk of progression (HR = 0.64, 95% CI 0.48–0.80). The PFS gain is consistent with the Okayama Lung Cancer Study Group Trial 1001 in Japanese patients with previously untreated EGFR-mutated advanced NSCLC,29 showing that bevacizumab added to gefitinib extended PFS by 4–5 months over historical controls receiving gefitinib monotherapy.5,30 This benefit in PFS is also similar to the ARTEMIS-CTONG1509 study31 showing a significant reduction in the risk of progression with bevacizumab added to erlotinib versus erlotinib alone (HR = 0.55; 95% CI, 0.41–0.73). One strength of the current study is a double-blind design while the ARTEMIS-CTONG1509 study31 and many other trials have an open-label design in which investigators might have bias in deciding treatment continuation.32 Of note, a recent phase 2 study failed to exhibit the superiority of osimertinib, a third-generation irreversible EGFR TKI, plus bevacizumab over osimertinib monotherapy in improving the PFS as a front-line treatment for patients with NSCLC harboring EGFR mutations.32 Osimertinib has demonstrated superior efficacy as first-line therapy compared with first-generation TKIs erlotinib and gefitinib for EGFR-mutated, advanced NSCLC patients in the FLAURA trial and in combination with chemotherapy versus osimertinib monotherapy in the FLAURA2 trial.7 It also significantly extended the PFS of patients of previously untreated, EGFR mutation-positive advanced NSCLC compared to gefitinib (18.9 months vs. 10.2 months; HR for disease progression or death, 0.46; 95% CI, 0.37–0.57).7 However, resistance to first line osimertinib eventually emerges and the resistance mechanism are mostly non-targetable. Apart from inconvenient intravenous administrations every 3 weeks, the PFS benefit of osimertinib plus chemotherapy comes at increased toxicities. Combination strategies including amivantamab plus lazertinib in the MARIPOSA study33 that could overcome emerging resistance in the first-line setting for NSCLC with sensitive EGFR mutations remain to be defined.
The PFS benefit in our study did not translate into any gain in the OS of the patients across all subgroups. This is consistent with the ARTEMIS-CTONG1509 study31 and the NEJ026 trial.34 The current trial was powered for PFS as the primary endpoint, which relies on radiological assessment of disease progression and OS was still immature at the data cutoff. In addition, OS may be heavily influenced by subsequent 2nd or later line treatment after disease progression.35,36
Our subgroup analysis demonstrated that gefitinib plus anlotinib provided significant benefits in PFS for patients with EGFR ex19del and EGFR ex21L858R versus gefitinib plus placebo. EGFR ex21L858R is a known adverse predictor of PFS.29 Gefitinib plus anlotinib extended the PFS of patients harboring EGFR ex21L858R by 4.3 months (HR = 0.63, 95% CI 0.42–0.93). Twenty to 30% of NSCLC patients have brain metastases at the time of initial diagnosis.37 The NEJ026 study allowed inclusion of patients with brain metastasis but demonstrated no PFS benefit with erlotinib plus bevacizumab versus erlotinib alone for this subgroup38 (HR = 0.78, 95% CI 0.42–1.43). A post hoc analysis of the phase 3 ALTER0303 trial showed that anlotinib as a second or later line treatment significantly reduced the risk of progression in advanced NSCLC patients with brain metastasis versus placebo (HR = 0.29, 95% CI 0.15–0.56), suggesting intracranial antitumor activities of anlotinib.39 In this trial, gefitinib plus anlotinib led to a 53% reduction in the risk of progression or death versus gefitinib plus placebo in patients with brain metastasis (HR = 0.47, 95% CI, 0.29–0.77), which is consistent with the ARTEMIS-CTONG1509 study.31 Similar to the ALTER0303 trial and the ARTEMIS-CTONG1509 study, a recent phase 2 trial showed osimertinib plus bevacizumab failed to provide any PFS benefit over osimertinib monotherapy as a front-line treatment for EGFR mutated patients with brain metastasis.32
As we know, monitoring the dynamic changes of mutation load in peripheral blood can serve as an effective indicator of treatment efficacy. But the impact of EGFR TKI and anti-angiogenic therapy on TML remains undefined. Our study found no notable difference in TML at baseline and the first posttreatment efficacy evaluation. But there is an apparently greater reduction in the rates of total EGFR and EGFR ex21L858R treated with gefitinib plus anlotinib at the first posttreatment efficacy evaluation. The lower mutation rate of key driver genes indicates stronger tumor inhibition and anti-tumor activity. Meanwhile, upon disease progression, the TML of patients receiving gefitinib plus placebo was significantly higher than patients receiving gefitinib plus anlotinib, suggesting that TML rises coincident with disease progression. Dual therapy with an EGFR TKI and an anti-angiogenic agent suppresses TML to a greater extent than EGFR TKI monotherapy. Similar change trajectories were observed in EGFR ex21L858R, ex19del and amplification. A similar study had also proved that dual therapy with ramucirumab and erlotinib could suppress EGFR-activating mutation allele count in EGFR-mutated, advanced NSCLC.40 Tracking temporal changes in TML and the frequencies of key driver gene mutations in individual patients could better delineate the dynamics of gene mutations during disease progression. Consistent with other studies, mutated TP53 was enriched in EGFR-mutated NSCLC, occurring in 57.8% of the patients at baseline, which falls within the reported range of 54.6%%-64.6% for mutated TP53.41–43 The presence of mutated TP53 is reported to be an adverse predictor of TKI therapeutic outcome in NSCLC.26 In this study, dual inhibition likely contributed to the reduction in the occurrence of TP53 mutations, resulting in a lower rate of mutated TP53 at the first posttreatment efficacy evaluation and at the time of disease progression. Above all, our study further showed that dual inhibition of VEGF and EGFR signaling pathways was more effective in suppressing the TML and the frequencies of mutated key driver genes than TKI monotherapy.
Currently, there are no established enough molecular biomarkers that can reliably predict clinical response or resistance to anti-angiogenic agents, although patients with EGFR ex21L858R or baseline brain metastasis derive more benefits from dual inhibition in ARTEMIS-CTONG1509 study.18 We observed the trajectory of TML changes and further evaluated TML as a biomarker to explore survival benefit associated with the dual inhibition. Anlotinib added to gefitinib led to a 6.2-month extension in PFS compared to gefitinib alone, with a 62% reduction in the risk of progression in patients with high TML, proving that patients with high TML could benefit more from gefitinib plus anlotinib. The presence of CNV of resistance-related genes including EGFR was associated with a poorer response to osimertinib in advanced EGFR-mutated lung adenocarcinoma patients.28 The current study found that anlotinib added to gefitinib led to a 4.9-month extension in PFS compared to gefitinib alone, with a 73% reduction in the risk of progression. In short, except for patients with brain metastases, those with high TML or EGFR amplification gain more benefits from combined TKI therapy and anti-angiogenic therapy.
The current study excluded patients with EGFRT790M-mutated NSCLC at baseline. Upon disease progression, the rate of secondary EGFRT790M-mutation rose to 38% in patients on gefitinib plus placebo and 17% in patients treated with gefitinib plus anlotinib. This is lower than 40–50% in patients who develop secondary EGFRT790M mutations as a result of treatment with first/second-generation EGFR TKIs.44 The rate of EGFRT790M mutations is related to tumor burden and TML. The low rate of this mutation in our patients suggests that gefitinib plus anlotinib was more effective in reducing tumor burden and TML. In this study, mutations were detected using peripheral blood samples, which tend to be less frequently detected in carcinoma tissues, which may partially explain the low rate of EGFRT790M mutations in our patients.
The toxicity profile of gefitinib plus anlotinib is consistent with that of gefitinib and anlotinib monotherapy. Rash and diarrhea are common in patients treated with gefitinib5; any grade diarrhea and rash occurred in 65.8% and 65.2% of the patients receiving gefitinib plus anlotinib versus 39.0% and 52.6% of those receiving gefitinib plus placebo, respectively. The rate of liver abnormalities is reportedly higher with gefitinib in Asian patients than non-Asian patients45 and ALT and AST elevations occurred in 48.4% of our patients in both groups. Hepatic impairment can be resolved by dose reductions or treatment interruptions. Grade 3 or higher hypertension (28.4% vs. 5.2%) and proteinuria (1.9% vs. 0%), two main AEs of anti-VEGF therapy, were more frequent in patients receiving gefitinib plus anlotinib than patients receiving gefitinib plus placebo and are largely consistent with those of anlotinib therapy for other tumors.46 The rate of grade 3 or higher hypertension and proteinuria is lower than that (hypertension 37–60% and proteinuria 7–8%) reported for erlotinib plus bevacizumab in other trials.38,47–49 Overall, gefitinib plus anlotinib had a manageable toxicity profile in advanced NSCLC patients.
The major limitations should be addressed in this study. Third generation EGFR-TKIs, which are being increasingly employed in the treatment of NSCLC, were not investigated in the current trial. At the time the current trial was started (April 2019), osimertinib was not available in China. The agent was approved in September 2019 in China as first line treatment for EGFR mutated NSCLC.50 It remains to be investigated whether anlotinib added to a third-generation EGFR-TKI would confer similar or greater benefit on EGFR-mutated, advanced NSCLC patients. The study enrolled mostly Han Chinese patients, and it still needs to be explored whether the study findings are applicable to non-Asian patients. Though the study findings suggest that high TML and EGFR gain appeared to be potential predictors for anlotinib benefit. However, given the small sample size in these subgroups in this study, the findings remain exploratory. This benefit with regards to objective response was observed in advanced NSCLC patients with high TML treated with anlotinib plus immunotherapy (≥10 Muts/Mb 85.7% vs. <10 Muts/Mb 63.6%) in a phase 1 study24 and was also reported in pretreated advanced biliary tract cancer patients.51 However, the mechanism whereby anlotinib favors patients with high genomic instability and the prognostic significance of TML and EGFR gain remain to be investigated.
In conclusion, the combination of EGFR TKI with gefitinib and anti-angiogenic therapy with anlotinib significantly improved PFS with a manageable safety profile for patients with untreated advanced NSCLC harboring EGFR activating mutations. Particularly, patients with brain metastases, those with high TML, and patients harboring EGFR amplification gain more benefits from gefitinib plus anlotinib compared with gefitinib monotherapy. The findings support further investigation of the third generation TKI plus antiangiogenic agent in advanced stage trials in the frontline and later line setting for advanced NSCLC patients with EGFR activating mutations. Studies on anlotinib plus third generation TKIs for advanced NSCLC are currently ongoing (NCT04770688; NCT06043973).
Materials and methods
Ethics approval and consent to participate
The trial protocols were approved by the independent ethics committee at each participating center and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects. The studies were conducted according to the International Conference on Harmonisation guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent prior to the study. This report followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial is registered with ClinicalTrials.gov (NCT04028778).
Study design and participants
FL-ALTER, a multicenter, randomized, double-blind, phase III trial, was conducted at 18 hospitals (Supplementary Appendix I) in the People’s Republic of China. It enrolled adult patients (aged above 18 years) with histologically confirmed American Joint Committee on Cancer (AJCC) stage IIIB or IV NSCLC. Patients with an ex19del or ex21L858R EGFR mutation were eligible. Patients had received no prior chemotherapy or targeted therapy. They should have at least one measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version52 1.1, adequate organ function, and an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1. We excluded patients with EGFR T790M-mutated NSCLC. Patients were ineligible if they had hypertension requiring at least 2 types of antihypertensive medications, prior or current interstitial lung disease, and abdominal fistulation, gastrointestinal perforation, or abdominal abscess in the preceding 6 months before enrollment. Patients with symptomatic, unstable brain metastases, arterial or venous embolism within 12 months of enrollment, or at a higher risk of bleeding were also excluded. Full eligibility criteria are provided in the study protocol (Data Supplement).
Randomization and treatments
The participants were randomized in a 1:1 ratio to treatment with gefitinib plus anlotinib or gefitinib plus placebo using the Interactive Web Response System (IWRS). Randomization was stratified by sex (male vs. female), ECOG performance status (0 vs. 1), EGFR mutation subtype (ex19del vs. ex21L858R) and pathologic types (adenocarcinoma vs. others). Both patients and investigators were blinded to treatment allocation.
Gefitinib 250 mg and anlotinib 12 mg (Chia Tai Tianqing Pharmaceutical Group Co.) were taken orally once daily on days 1–14 per cycle in a two weeks on, one week off schedule, which lasted for 3 weeks. Anlotinib treatment could be interrupted due to toxicities but should be resumed at the same or a lower dose level. Anlotinib treatment was discontinued if patients experienced severe toxicities (gastrointestinal perforation [any grade], arterial thromboembolism [any grade], venous thromboembolism [grade 4], hypertensive crisis or cerebral hemorrhage [any grade], leukoencephalopathy syndrome [any grade], pulmonary hemorrhage [grade ≥2] or other hemorrhages [grade ≥3], and renal, hepatic, cardiac or neurologic toxic effects [grade 4]). Treatment was also discontinued if patients had not recovered from a toxic effect requiring treatment interruptions (hematologic or nonhematologic toxicities [grade ≥3]). Two levels of dose reduction (12 mg/d to 10 mg/d and 10 mg/d to 8 mg/d) were allowed for anlotinib and treatment with anlotinib was discontinued if more than two levels of dose reduction were required. Patients who were intolerant of anlotinib were permitted to continue gefitinib treatment. Gefitinib treatment was discontinued upon acute onset or aggravation of respiratory symptoms, new onset ocular symptoms or diagnosis of interstitial lung disease. Dose reductions of gefitinib were not allowed. They were allowed to continue anlotinib treatment at the discretion of investigators. Both drugs were continued until disease progression, intolerable toxicities, death, withdrawal of consent or termination at the discretion of investigators. Although treatment should be terminated upon occurrence of progressive disease (PD) per the study protocol, patients were allowed to continue the study treatment beyond radiological progression when deemed clinically beneficial by the investigators.
Patients were allowed to receive bisphosphonates for bone metastasis and palliative radiotherapy was allowed for uncontrollable metastasis-associated pain with the irradiation field confined to less than 5% of the bone marrow.
Assessments and outcomes
Responses were evaluated radiologically by investigators per RECIST v1.1 at baseline, at the end of each cycle for the first 4 cycles, and the end of every 2 cycles from cycle 5 until disease progression, intolerable toxicities, death, or withdrawal of consent. Complete (CR) and partial response (PR) had to be confirmed radiologically at least 4 weeks later and SD at least 8 weeks after an initial response. Treatment-emergent adverse events (TEAEs) were evaluated throughout the treatment period and to 30 days post the final dose using the Common Toxicity Standards of the National Cancer Institute (NCI CTC AE) version 4.0.
The primary endpoint was PFS (time from randomization to PD or death of any cause, whichever occurred earlier). The secondary endpoints included OS (time from randomization to death of any cause), objective response rate (proportion of patients who achieved CR or PR), disease control rate (proportion of patients who achieved CR, PR, or SD), duration of response (time from the first documented CR or PR to the first documented disease progression or relapse), and time from randomization to radiological progression.
Next generation sequencing
Blood samples for ctDNA analysis were collected from each patient at baseline, first evaluation, and PD, and transferred to Clinical Laboratory Improvement Amendments (CLIA)-certified/College of American Pathologists (CAP)-accredited laboratory of OrigiMed Co., Ltd. (Shanghai, China) for plasma extraction and genomic testing. CtDNA were prepared using the QIAGEN QIAamp Circulating Nucleic Axid Kit according to the manufacturer’s instructions.
Ten mL peripheral blood was withdrawn before treatment, at the first efficacy evaluation for detection of the effect of ctDNA clearance on efficacy and dynamic monitoring and upon documentation of PD to evaluate mechanisms of resistance. Dynamic detection of plasma ctDNA was performed using the NGS-based QiyuanTM 329-gene panel (OrigiMed, Shanghai, China). Circulating tumor DNA (ctDNA) analysis was performed on the basis of read depth, which was first normalized to sequencing depth and corrected for GC content to reduce technical bias. Subsequently, the relative change in ctDNA samples was quantified as a ratio using a baseline established from normal human cfDNA libraries and a log2 transformation was performed to obtain a log2 ratio for further analysis. The log2ratio was then segmented using the cyclic binary segmentation (CBS) algorithm to determine the log2 ratios of discrete fragments. The change in copy number for these segments was inferred as 2^(1 + log2ratio). The copy number ≥2.5 indicates gene amplifications whereas the copy number ≤1.2 indicates gene deletions. Genes were captured and sequenced at a mean depth of approximately 15,000× using an Novaseq 6000 (Illumina, CA, USA), followed by noise filtering and molecular tracking, and variant calling for single nucleotide variants (SNVs). Sequencing files were analyzed using Origimed’s internal bioinformatic pipelines to identify SNVs using MuTect (v1.17), copy number alterations (CNAs) using Control-FREEC (v9.7), insertion-deletion (indels) using PINDEL (v2.05), and gene fusion events. By comparing the ctDNA with matched white blood cell samples, germline mutations were filtered out and retained, and only somatic mutations were identified in the plasma samples during the analysis. Somatic mutations with the variant allelic fraction (VAF) were analyzed for tracking dynamic ctDNA levels over time relative to baseline. To enable relative change calculations between timepoints, if a variant was not detectable, the VAF percentage (VAF%) was set to the assay’s lowest detection limit of 0.2%. The functional impact of each genomic alteration was annotated using SnpEff3.0. The results were annotated to several databases, including the Reference Sequence (RefSeq), 1000 Genomes, Genome Aggregation Database (gnomAD), the Exome Aggregation Consortium (ExAC), NHLBI GO Exome Sequencing Project 6500 (ESP6500), Sorting Intolerant from Tolerant (SIFT), PolyPhen, and Catalogue of Somatic Mutations in Cancer (COSMIC) databases.
In addition, TML was estimated by dividing the total number of mutations by the total length of the coding sequence (CDS) region. Mutations were defined by the following criteria: (1) SNVs or insertions/deletions were located within the CDS region and (2) the mutation had an abundance of ≥0.25%.
Statistical analysis
We hypothesized that the PFS of patients treated with gefitinib plus anlotinib would be superior to that of patients treated with gefitinib plus placebo. Based on previous studies,5,30,53–57 we assumed a median PFS of 10 months for the gefitinib plus placebo group and 15 months for the gefitinib plus anlotinib group. A 5-month increase in the median PFS at 15 months was considered to be clinically meaningful to show superiority of gefitinib plus anlotinib over gefitinib plus placebo. To detect this improvement in PFS with 80% power at a two-sided 5% significance level, 192 events (88 with gefitinib plus anlotinib and 104 with gefitinib plus placebo) were required. Assuming an attrition rate of 20%, a sample size of 310 was planned.
All statistical analyses were prespecified and followed the intention-to-treat (ITT) principle and were conducted using SAS version 9.3 or above (SAS Institute Inc., Cary, NC, USA). An independent data monitoring committee (IDMC) carried out interim analyses. The Full Analysis Set (FAS) included all patients who were randomized and received at least one dose of the study medications and the Per Protocol Set (PPS) included all patients who were randomized and received at least one dose of the study medications and had at least one post treatment radiological evaluation. Efficacy analysis was based on the FAS. A Cox regression model was used to estimate HR stratified by sex, ECOG performance status, EGFR mutation subtype and pathologic types and its 95% CI. PFS, OS, time to PD, and duration of response and their 95% CI were estimated using the Kaplan–Meier method and compared using log-rank test. The ORR and DCR and their 95% CI were compared using Cochran-Mantel-Haenszel test or χ2 test. No imputation was done for missing data.
The Safety Set included all patients who had received at least one dose of the study medications and had post treatment safety data. AEs were mainly analyzed using descriptive statistics.
All tests were two-tailed with a level of significance set at P ≤ 0.05.
Supplementary information
Acknowledgements
We thank all the patients, their families and the site investigators who participated in this study. We thank Chia Tai Tianqing Pharmaceutical Group Co., Ltd. for sponsoring the trial medication, and Shanghai OrigiMed Co., Ltd for supporting genetic testing. The study was funded by the Chinese National Natural Science Foundation Project (Grant No. 82173101, 82373262, 82241232, 82272789, 82102864) and Guangzhou Basic and Applied Basic Research Foundation (2024A04J4082). This study was partly funded by the 308 Clinical Research Foundation of Sun Yat-sen University Cancer Center.
Author contributions
L.Z. and W.F. were the principal investigator and provided the study concept and design. H.Q.Z., Y.Z., G.C., Q.Y., and H.Z. were involved in data analysis and interpretation. All authors contributed to patient recruitment and data collection. All authors participated in drafting and reviewed the report and approved the final version for submission.
Data availability
The raw sequencing data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (https://ngdc.cncb.ac.cn/gsa-human). These data are accessible under the accession number: HRA007966. These data are under controlled access by human privacy regulations and are only available for research purposes. Access to the data can be granted following approval from the Data Access Committee of the GSA-human database. And other datasets used and/or analyzed during the current study are also available from the corresponding author on reasonable request.
Competing interests
L.Z. has received research support from Chia Tai Tianqing Pharmaceutical Group Co., Ltd, Eli Lilly, Novartis, Roche, and Bristol-Myers Squibb. Other authors declare no competing interests.
Footnotes
These authors contributed equally: Hua-Qiang Zhou, Ya-Xiong Zhang, Gang Chen, Qi-Tao Yu, Hua Zhang
Contributor Information
Li Zhang, Email: zhangli@sysucc.org.cn.
Wen-Feng Fang, Email: fangwf@sysucc.org.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-024-01927-9.
References
- 1.Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin.71, 209–249 (2021). 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Cao, W. et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J.134, 783–791 (2021). 10.1097/CM9.0000000000001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanna, N. H. et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol.39, 1040–1091 (2021). 10.1200/JCO.20.03570 [DOI] [PubMed] [Google Scholar]
- 4.Akamatsu, H. et al. Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial. JAMA Oncol.7, 386–394 (2021). 10.1001/jamaoncol.2020.6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med.362, 2380–2388 (2010). 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 6.Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med.361, 947–957 (2009). 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 7.Soria, J. C. et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med.378, 113–125 (2018). 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 8.Hsu, W. H., Yang, J. C., Mok, T. S. & Loong, H. H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol.29, i3–i9 (2018). 10.1093/annonc/mdx702 [DOI] [PubMed] [Google Scholar]
- 9.Yu, H. A. et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res.19, 2240–2247 (2013). 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thress, K. S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med.21, 560–562 (2015). 10.1038/nm.3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlesi, F. et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet387, 1415–1426 (2016). 10.1016/S0140-6736(16)00004-0 [DOI] [PubMed] [Google Scholar]
- 12.Rocco, D., Della Gravara, L., Palazzolo, G. & Gridelli, C. The role of antiangiogenic monoclonal antibodies combined to EGFR-TKIs in the treatment of advanced non-small cell lung cancer with activating EGFR mutations: acquired resistance mechanisms and strategies to overcome them. Cancer Drug Resist.5, 1016–1024 (2022). 10.20517/cdr.2022.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi, S. H., Yoo, S. S., Lee, S. Y. & Park, J. Y. Anti-angiogenesis revisited: reshaping the treatment landscape of advanced non-small cell lung cancer. Arch. Pharm. Res.45, 263–279 (2022). 10.1007/s12272-022-01382-6 [DOI] [PubMed] [Google Scholar]
- 14.Shen, G. et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol.11, 120 (2018). 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, B. et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene654, 77–86 (2018). 10.1016/j.gene.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 16.Grande, E., Díez, J. J., Zafon, C. & Capdevila, J. Thyroid cancer: molecular aspects and new therapeutic strategies. J. Thyroid Res.2012, 847108 (2012). 10.1155/2012/847108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui, J. et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J. Cancer122, 664–671 (2008). 10.1002/ijc.23131 [DOI] [PubMed] [Google Scholar]
- 18.Tian, S. et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci.102, 1374–1380 (2011). 10.1111/j.1349-7006.2011.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie, C. et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci.109, 1207–1219 (2018). 10.1111/cas.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han, B. et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol.4, 1569–1575 (2018). 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinese expert consensus on Anlotinib Hydrochloride for advanced lung cancer. Zhonghua Zhong Liu Za Zhi. 42, 807–816 (2020). [DOI] [PubMed]
- 22.Zhao, Y. et al. The efficacy of anlotinib as third-line treatment for non-small cell lung cancer by EGFR mutation status: a subgroup analysis of the ALTER0303 randomized phase 3 study. Transl. Lung Cancer Res.11, 776–785 (2022). 10.21037/tlcr-22-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu, T. et al. Efficacy and safety of first-line anlotinib-based combinations for advanced non-small cell lung cancer: a three-armed prospective study. Transl. Lung Cancer Res.11, 1394–1404 (2022). 10.21037/tlcr-22-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu, T. et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J. Thorac. Oncol.16, 643–652 (2021). 10.1016/j.jtho.2020.11.026 [DOI] [PubMed] [Google Scholar]
- 25.Huang, M. et al. A phase I study of the tyrosine kinase inhibitor anlotinib combined with platinum/pemetrexed-based chemotherapy in untreated nonsquamous non-small-cell lung cancer. Invest. N. Drugs40, 308–313 (2022). 10.1007/s10637-021-01179-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderLaan, P. A. et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: Correlation with clinical outcomes. Lung Cancer106, 17–21 (2017). 10.1016/j.lungcan.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He, H. et al. Chromosomal Copy Number Variation Predicts EGFR-TKI Response and Prognosis for Patients with Non-Small Cell Lung Cancer. Pharmgenomics Pers. Med.16, 835–846 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buder, A. et al. Somatic Copy-Number Alterations in Plasma Circulating Tumor DNA from Advanced EGFR-Mutated Lung Adenocarcinoma Patients. Biomolecules11, 618 (2021). 10.3390/biom11050618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichihara, E. et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J. Thorac. Oncol.10, 486–491 (2015). 10.1097/JTO.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi, T. et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol.11, 121–128 (2010). 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 31.Zhou, Q. et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell39, 1279–91.e3 (2021). 10.1016/j.ccell.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 32.Kenmotsu, H. et al. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients With Nonsquamous NSCLC Harboring EGFR Mutations: WJOG9717L Study. J. Thorac. Oncol.17, 1098–1108 (2022). 10.1016/j.jtho.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 33.Cho, B. C., et al. LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. In Presented at the 2023 Annual Meeting of the European Society for Medical Oncology, (European Society for Medical Oncology, 2023).
- 34.Maemondo, M. et al. NEJ026: Final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. J. Clin. Oncol.38, 9506 (2020). 10.1200/JCO.2020.38.15_suppl.9506 [DOI] [Google Scholar]
- 35.Broglio, K. R. & Berry, D. A. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl Cancer Inst.101, 1642–1649 (2009). 10.1093/jnci/djp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawashima, Y. et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir. Med.10, 72–82 (2022). 10.1016/S2213-2600(21)00166-1 [DOI] [PubMed] [Google Scholar]
- 37.Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol.22, 2865–2872 (2004). 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- 38.Saito, H. et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol.20, 625–635 (2019). 10.1016/S1470-2045(19)30035-X [DOI] [PubMed] [Google Scholar]
- 39.Jiang, S. et al. The Impact of Anlotinib on Brain Metastases of Non-Small Cell Lung Cancer: Post Hoc Analysis of a Phase III Randomized Control Trial (ALTER0303). Oncologist25, e870–e874 (2020). 10.1634/theoncologist.2019-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishio, K. et al. Impact of ramucirumab plus erlotinib on circulating cell-free DNA from patients with untreated metastatic non-small cell lung cancer with EGFR-activating mutations (RELAY phase 3 randomized study). Transl. Lung Cancer Res.12, 1702–1716 (2023). 10.21037/tlcr-22-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu, H. A. et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin. Cancer Res.24, 3108–3118 (2018). 10.1158/1078-0432.CCR-17-2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blakely, C. M. et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet49, 1693–1704 (2017). 10.1038/ng.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frampton, G. M. et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov.5, 850–859 (2015). 10.1158/2159-8290.CD-15-0285 [DOI] [PubMed] [Google Scholar]
- 44.Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med.3, 75ra26 (2011). 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, K. W. & Chan, S. L. Hepatotoxicity of targeted therapy for cancer. Expert Opin. Drug Metab. Toxicol.12, 789–802 (2016). 10.1080/17425255.2016.1190831 [DOI] [PubMed] [Google Scholar]
- 46.Gao, Y., Liu, P. & Shi, R. Anlotinib as a molecular targeted therapy for tumors. Oncol. Lett.20, 1001–1014 (2020). 10.3892/ol.2020.11685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seto, T. et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol.15, 1236–1244 (2014). 10.1016/S1470-2045(14)70381-X [DOI] [PubMed] [Google Scholar]
- 48.Stinchcombe, T. E. et al. Effect of Erlotinib Plus Bevacizumab vs Erlotinib Alone on Progression-Free Survival in Patients With Advanced EGFR-Mutant Non-Small Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol.5, 1448–1455 (2019). 10.1001/jamaoncol.2019.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosell, R. et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med.5, 435–444 (2017). 10.1016/S2213-2600(17)30129-7 [DOI] [PubMed] [Google Scholar]
- 50.Tagrisso approved in China as a 1st-line treatment for EGFR-mutated non-small cell lung cancer. Available from: https://www.astrazeneca.com/media-centre/press-releases/2019/tagrisso-approved-in-china-as-a-1st-line-treatment-for-egfr-mutated-non-small-cell-lung-cancer-04092019.html (2019).
- 51.Zhou, J. et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology77, 65–76 (2023). 10.1002/hep.32548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer45, 228–247 (2009). 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 53.Landis, S. H., Murray, T., Bolden, S. & Wingo, P. A. Cancer statistics, 1999. CA Cancer J. Clin.49, 31 (1999). 10.3322/canjclin.49.1.8 [DOI] [PubMed] [Google Scholar]
- 54.Novello, S. & Le Chevalier, T. Chemotherapy for non-small-cell lung cancer. Part 1: Early-stage disease. Oncology17, 357–364 (2003). [PubMed] [Google Scholar]
- 55.Schiller, J. H. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N. Engl. J. Med.346, 92–98 (2002). 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- 56.Fukuoka, M. et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol.29, 2866–2874 (2011). 10.1200/JCO.2010.33.4235 [DOI] [PubMed] [Google Scholar]
- 57.Han, J. Y. et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J. Clin. Oncol.30, 1122–1128 (2012). 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequencing data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (https://ngdc.cncb.ac.cn/gsa-human). These data are accessible under the accession number: HRA007966. These data are under controlled access by human privacy regulations and are only available for research purposes. Access to the data can be granted following approval from the Data Access Committee of the GSA-human database. And other datasets used and/or analyzed during the current study are also available from the corresponding author on reasonable request.