Abstract
The POU domain transcription factor, Oct-2, is essential for the B cell-specific expression of CD36 in mouse B cells. In order to determine how Oct-2 mediates expression of CD36 in B cells, we cloned and analysed the mouse CD36 promoter. In contrast to the human CD36 promoter, the mouse promoter contains a consensus octamer element of the type ATGCTAAT. This octamer element can be bound by either Oct-1 or Oct-2 but requires the expression of Oct-2 to activate transcription in B cells. Mutation of the octamer element renders the CD36 promoter refractory to activation by Oct-2. Furthermore, we demonstrate that the CD36 octamer element does not support recruitment of the B cell-specific co-activator OBF-1 and that CD36 expression is unaffected in primary B cells derived from obf-1–/– mice. We conclude that Oct-2 activates CD36 gene expression in mouse B cells via the octamer element in the promoter. Our data also demonstrate that CD36 is the first example of an Oct-2-dependent gene whose expression in B cells is independent of OBF-1. These findings support the notion that Oct-2 regulates gene transcription by both OBF-1-dependent and -independent mechanisms.
INTRODUCTION
The octamer motif ATGC(A/T)AAT is a conserved DNA element found in the promoters and enhancers of a diverse range of genes (reviewed in 1–3). Genes that are transcriptionally regulated by octamer motifs can be either ubiquitously expressed or cell type specific. For example, the octamer motif is required for the ubiquitous expression of the small nuclear RNA (snRNA) genes, the cell cycle regulation of the histone H2B gene and the B cell-specific expression of immunoglobulin (Ig) genes (reviewed in 1–4). The ability of the octamer motif to differentially regulate transcription is determined by the octamer binding proteins Oct-1 and Oct-2 (reviewed in 1–4). Oct-1 and Oct-2 are members of the POU domain family of transcription factors (reviewed in 2 and 3). The POU domain is a conserved bipartite DNA-binding domain consisting of two subdomains: a POU-specific domain (POUS) and a homedomain (POUH), separated by a flexible linker.
Oct-1 is ubiquitously expressed whereas Oct-2 expression is restricted to a small number of cell types, including B cells (reviewed in 1; 4–6). The differential expression of some octamer-dependent genes can be explained partly by the interaction between octamer-binding proteins and promoter-selective co-activators that are themselves either cell type specific or ubiquitous. In the case of snRNA genes, ubiquitous expression is achieved by the association of Oct-1 with the SNAPc complex (7). B cell-restricted expression of Ig genes requires association of the B cell-specific co-activator OBF-1/OCA-B/Bob-1 (referred to here as OBF-1) with either Oct-1 or Oct-2 (8–12). Interaction of OBF-1 with Oct-1 or Oct-2 is mediated by direct contacts with their POU domains (9–16). However, the formation of a stable complex is also determined by the specific sequence of the octamer element, suggesting that OBF-1 targets a subset of octamer-regulated genes (13–16). In obf-1–/– mice the two most obvious defects are the absence of germinal centres and a profound deficiency in serum Ig of secondary isotypes (17,18).
The presence of Oct-1 in cell types that do not express Oct-2 suggests that Oct-1 determines the transcriptional activation of octamer-dependent genes in these cells. However, in B cells, where both Oct-1 and Oct-2 are present, it is not clear how each of these transcription factors targets individual octamer-dependent genes. Analysis of B cells in Oct-2-deficient mice demonstrated that whilst Oct-2 was dispensable for the antigen-independent phase of B cell differentiation, it was required for antigen-dependent differentiation and proliferation (19,20). These observations suggest that there are genes whose transcription cannot be activated by Oct-1 but which are critically dependent upon Oct-2. Analysis of the regulatory elements in Oct-2-specific genes should therefore reveal how Oct-2 can regulate genes that Oct-1 cannot.
The first bona fide Oct-2 target gene to be identified encodes the scavenger receptor, CD36. CD36 is expressed in a number of cell types in both humans and mice, including monocytes/macrophages, platelets and adipocytes (21,22). However, CD36 expression in B cells appears to be specific to mice (21). Nevertheless, the expression of CD36 in mouse B cells is critically dependent upon Oct-2, and is therefore an ideal model to investigate Oct-2-specific transcriptional regulation in B cells (21). The human CD36 promoter has been characterized and, consistent with the lack of expression of CD36 in human B cells, does not contain recognisable octamer elements (23,24). The promoter of the mouse CD36 gene has not been characterised previously and the mechanism by which Oct-2 regulates CD36 expression in mouse B cells remains to be elucidated. Moreover, of the small number of additional Oct-2 target genes that have since been identified, only the crisp-3 and blr1 promoters have been investigated (25,26). Two low-affinity octamer sites in the crisp-3 promoter mediate Oct-2-specific transcription of the crisp-3 gene, whereas a single non-consensus octamer mediates the Oct-2-dependent transcriptional activation of the blr1 gene (25,26).
In order to determine how Oct-2 mediates expression of CD36 in B cells, we cloned and analysed the mouse CD36 promoter. In contrast to the human CD36 promoter, the mouse promoter contains a consensus octamer element of the type ATGCTAAT. This octamer element can be bound by either Oct-1 or Oct-2 but requires the expression of Oct-2 to activate transcription in B cells. Mutation of the octamer element renders the CD36 promoter refractory to activation by Oct-2. Furthermore, we demonstrate that the CD36 octamer element does not support recruitment of OBF-1 and that CD36 expression is unaffected in primary B cells derived from obf-1–/– mice. We conclude that Oct-2 activates CD36 gene expression in mouse B cells via the octamer element in the promoter. Our data also demonstrate that CD36 is the first example of an Oct-2-dependent gene whose expression in B cells is independent of OBF-1. These findings support the notion that Oct-2 regulates gene transcription by both OBF-1-dependent and -independent mechanisms.
MATERIALS AND METHODS
Cloning of the mouse CD36 promoter
The mouse CD36 promoter sequence was cloned using the mouse Promoter Finder DNA Walking kit according to the manufacturer’s instructions (Clontech). PCRs were carried out using CD36-specific primers corresponding to the 5′ region of the published cDNA sequence (27) and the supplied adapter primers. The sequences of the gene-specific primers were CD36GSP1, 5′-ACCATCCACCAGTTGCTCCACACATTTCAG-3′ and CD36GSP2, 5′-GGCAGCTGTGAAGAAGAAAAAGTCCTCAGT-3′. A single ∼600 bp PCR product from the secondary reactions was ligated directly into the T-vector pT7blue (Novagen). Nine of the clones were sequenced and found to have identity with the published human CD36 promoter (27). One of these clones was subsequently used as a probe to obtain larger genomic clones from a 129/Sv mouse genomic library in Lambda FIX II (Stratagene) using standard hybridisation protocols (28). A single positive genomic clone of ∼19 kb was obtained and partially sequenced by the dideoxy chain termination method. The presence of DNA matching the published mouse CD36 cDNA was confirmed by a BLAST search of the NCBI sequence database. DNA sequence alignment was done using Align Plus 4 software.
Plasmid constructs
An ∼2.6 kb EcoRI fragment from the mouse CD36 genomic clone that contains the region with homology to the human CD36 promoter (see Fig. 1), and extends ∼2.3 kb further upstream, was ligated into pBluescript II KS (Stratagene) to produce pBS2.6CD36. The CD36 promoter reporter plasmid, pGLCD36, was constructed by PCR amplification of the region spanning –615 to +20 bp (primers 5′-GATCGAGCTCTATAAGCATATCACCATC-3′; 5′-GATCAAGCTTGAATTCCTCACTACTTATGT-3′). The PCR product was ligated into the SacI and HindIII sites of pGL2-Basic (Promega) to produce the plasmid pGLCD36. The octamer site in pGLCD36 was mutated using a two-step PCR protocol as described previously (29). The octamer was mutated using the primers 5′-GATCGAGCTCTATAAGCATATCACCATC-3′ and the mutagenic primer 5′-TTAAAAAATTCAATGTCAGCGGCGAATTTGTGGTAGGTTGCCAA-3′ in the first round PCR and 5′-GATCCAAGCTTGAATTCCTCACTACTTATGT-3′ in the second round. The subsequent PCR product was ligated into the SacI and HindIII sites of pGL2-Basic to produce pGLCD36mOct. The sequence encoding the POU domain of Oct-2 (30) was amplified by PCR using the primers 5′-GATCGGATCCCCGGAGGAGCCCAGCGATCTG-3′ and 5′-GATCGAATTCATCACGCACTGCAAGGGTTGAT-3′ and ligated into the BamHI and EcoRI sites of pGEX-2TK (Pharmacia Biotech) to produce the plasmid pGEXPOU-2, which encodes the POU domain of Oct-2. The sequences of constructs generated by PCR were verified by automated dideoxy sequencing.
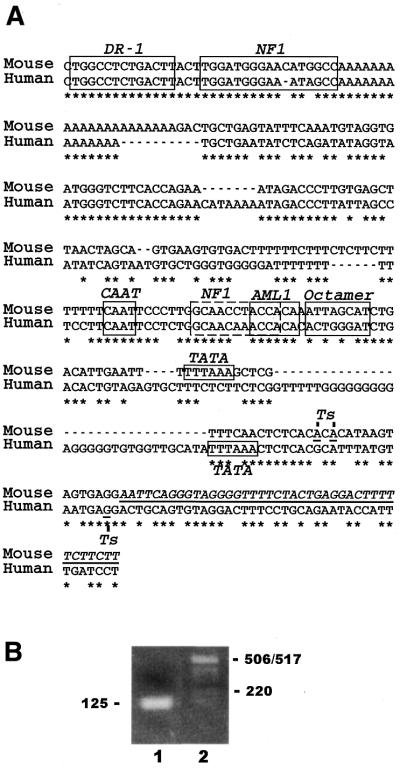
Figure 1.
Sequence alignment of the mouse and human CD36 proximal promoters. (A) The mouse CD36 5′-proximal promoter sequence is shown above the human sequence (23). Asterisks indicate identical nucleotides. Nucleotides corresponding to the known mouse cDNA are underlined. Ts denotes the transcription start sites. The transcription factor binding sites are boxed. (B) Agarose gel showing a single band of ∼125 bp from the 5′-RACE reaction (lane 1). The band was excised, cloned and 12 separate clones were sequenced.
Proteins
The POU domain of Oct-2 was expressed in Escherichia coli as a fusion protein with glutathione S-transferase from the plasmid pGEXPOU-2. The proteins were purified as described previously for the Elk-1 ETS domain (31). Oct-1, Oct-2 and OBF-1 were produced by coupled transcription–translation using TNT rabbit reticulocyte lysates (Promega) from plasmids pHA-Oct-1, pHA-Oct-2 (32) and pCATCH-Bob1 (10), respectively. Nuclear extracts from cell lines were prepared as described previously (33).
Cell culture, cell transfection and reporter assays
B cell lines Abl1.1, Abl/Oct-2-ER (kindly provided by T. Wirth, Universität Würzburg, Germany) and WEHI231 were grown in standard conditions (21) and transfected with 10–20 µg of plasmid using the DEAE/Dextran method (34). Abl1.1 and Abl/Oct-2-ER cells were stimulated with β-estradiol as described previously (21).
For luciferase assays, cells were harvested 24 h after transfection, lysed with reporter lysis buffer and the enzyme activity determined using the luciferase assay system according to the manufacturer’s recommendations (Promega). Transfection of the B cell lines was performed in duplicate or triplicate and normalised for total cellular protein using the Bradford assay reagent (Bio-Rad). β-Estradiol-stimulated and -unstimulated cultures were derived from the same transfection by dividing the culture into two, 8 h after transfection. An equal amount of protein from each culture was used in the luciferase assay 18 h after stimulation. Northern blot analysis was performed by hybridisation using the CD36 cDNA according to standard protocols (27,28).
Gel retardation assays
Gel retardation assays were performed as described previously (33). Synthetic oligonucleotides were radiolabelled by incorporation of [α-32P]dATP or [α-32P]dCTP using the Klenow fragment of DNA polymerase I according to standard protocols (28). The following pairs of complimentary oligonucleotides were used to make radiolabelled probes: CD36, 5′-GATCAATTCAATGTCAGATGCTAATTTGTGGTAGGT-TG-3′ and 5′-GATCCAACCTACCACAAATTAGCATCTGACATTGAATT-3′; CD36mOct, 5′-GATCAATTCAATGTCAGCGGCGAATTTGTGGTAGGTTG-3′ and 5′-GATCCAACCTACCACAAATTCGCCGCTGACATTGAATT-3′; CD36 Oct, 5′-CTAGCAAATTAGCATCTG-3′ and 5′-CTAGCAGATGCTAATTTG-3′; IgK Oct, 5′-CAGTGGGTATGCAAATTATT-3′ and 5′-CAGTAATAATTTGCATACCC-3′ (13); VHOct, 5′-GATCCTTAATAATTTGCATACCCTCA-3′ and 5′-GATCTGAGGGTATGCAAATTATTAAG-3′ (35).
All DNA-binding sites were purified on 10% non-denaturing polyacrylamide gels. In standard reactions, proteins and labelled DNA were incubated in a total of 10 or 12 µl volumes. Reactions were incubated at room temperature for 20 min prior to loading onto a 5% non-denaturing polyacrylamide gel. Gels were fixed, dried and visualised by autoradiography.
RT–PCR
Spleen cell suspensions were prepared from mice bearing mutations in the obf-1 gene (18), or from RAG-1-deficient mice reconstituted with oct-2+/+ or oct-2–/– fetal liver (19,20). The cells were cultured for 16 h in the presence of 10 µg/ml lipopolysaccharide (LPS), after which B cells (B220+) were harvested by sorting on a MoFlo cell sorter (Cytomation, Inc.). Cytoplasmic RNA was prepared and first-strand cDNA synthesised according to the manufacturer’s instructions (Pharmacia Biotech). Template cDNAs were titrated using serial dilutions and a β-actin-specific PCR (primers 5′-GTGGGCCGCTCTAGGCACCAA-3′; 5′-CTCTTTGATGTCACGCACGATTTC-3′), with 25 cycles (94°C for 1 min, 55°C for 1 min, 72°C for 1 min). Dilutions giving equivalent levels of β-actin product were used in PCR assays for either CD36 (primers 5′-GGATCTGAAATCGACCTTAAAG-3′; 5′-TAGCTGGCTTGACCAATATGTT-3′), Oct-2 (primers 5′-CCCGGGCTGGGCTTCCTACACAG-3′; 5′-GTAGCTGGTCGGCTTTCCCGGGCT-3′) or OBF-1 (primers 5′-CGGTGTTGACCTATGCTTCTCCACC-3′; 5′-GAGGGGCGCCTGGTGCTCGGGACCC-3′). The CD36 PCR was done using the β-actin programme. The annealing temperature for the Oct-2 and OBF-1 reactions was 70°C. Reaction products were resolved on agarose gels, transferred to filters and probed with the corresponding cDNAs according to standard protocols (28).
5′-RACE
5′-RACE was performed using the SMART RACE cDNA amplification kit according to the protocol provided. The RNA was total RNA from LPS-stimulated splenocytes from C57BL/6 mice. The CD36 gene-specific primers were 5′-CCAGTTGCTCCACACATTTCAGAAGGCAGC-3′ and 5′-CCTTTGAAAGGCTAGGAAACCATCCACCAGTTGC-3′. After the secondary PCR reaction, there was a single band on the gel of ∼150 bp. This was purified and ligated directly into pGEM-T Easy (Promega). Twelve clones were sequenced, and all revealed initiation sites at one of two positions; seven at the 5′-most A, and five at the 3′-most A (Fig. 1A).
RESULTS
Cloning of the mouse CD36 promoter
The CD36 gene was identified as an Oct-2-regulated gene by subtractive cDNA cloning (21). However, the sequence of the mouse promoter has not been reported and the molecular basis of Oct-2-dependent CD36 expression has not been elucidated. We therefore used the published sequence of the mouse CD36 cDNA to design PCR primers to amplify genomic DNA upstream of the start of the known cDNA sequence (27). The sequence of this fragment was determined and found to have identity with the human CD36 promoter (23). This PCR-derived genomic DNA fragment was used as a probe to obtain larger clones from a mouse genomic library. Restriction mapping and partial sequencing revealed that a single clone contained a 19 kb genomic fragment extending ∼3.5 kb upstream from the cDNA sequence (data not shown). The region upstream of the cDNA was sequenced and aligned with the reported human CD36 promoter (Fig. 1A). The transcription start sites in mouse primary B cells were determined by 5′-RACE. After the secondary PCR, there was a single band of ∼150 bp (Fig. 1B). Cloning and sequencing of 12 individual 5′-RACE products revealed initiation sites at one of the two positions indicated (Fig. 1B).
The human and mouse sequences are 62% identical and several transcription factor-binding sites are conserved (Fig. 1A). The CAAT box is conserved and there is a TATA element appropriately positioned with respect to the transcription start sites. The two NF1 sites and the AML-1 site identified previously in the human promoter are also conserved (23,24). In addition, the DR-1 element, which binds the PPARγ:RXRα complex, is perfectly conserved (36). The most striking difference is the presence of a consensus octamer element in the mouse promoter from –39 to –46 bp with respect to the 5′-most start site. This element is not conserved in the human promoter (Fig. 1). We therefore postulated that the presence of this octamer site in the mouse CD36 promoter determines the Oct-2-dependent expression of CD36 in mouse B cells.
Oct-1 and Oct-2 bind to the CD36 promoter
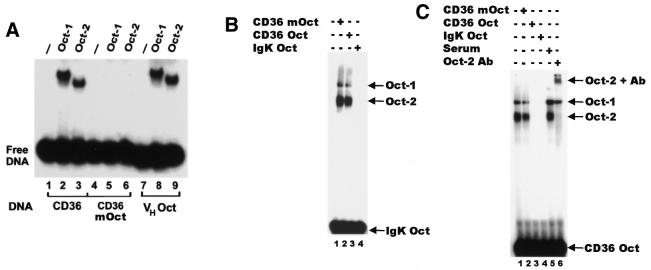
Gel retardation assays were used to establish if Oct-1 or Oct-2 is able to bind to the CD36 octamer element. A double-stranded oligonucleotide encompassing –58 to –26 bp of the mouse CD36 promoter (CD36) was used as a probe. When incubated with recombinant in vitro translated Oct-1 or Oct-2, specific retarded complexes were observed (Fig. 2A). Both Oct-1 and Oct-2 formed complexes with the CD36 probe (Fig. 2A, lanes 2 and 3). When Oct-1 or Oct-2 was incubated with the analogous probe containing a mutated octamer site (CD36mOct), no specific complexes were observed (Fig. 2A, lanes 5 and 6). Binding of Oct-1 and Oct-2 to the consensus octamer sequence (VHOct) is also shown (Fig. 2A, lanes 8 and 9). The results demonstrate that both Oct-1 and Oct-2 are able to bind specifically to the octamer site in the CD36 promoter.
Figure 2.
Specific binding of Oct-1 and Oct-2 to the CD36 octamer. (A) Gel retardation analysis of in vitro translated Oct-1 and Oct-2, demonstrating specific binding to the octamer site within the CD36 promoter. Reactions were performed with either the wild-type CD36 probe (CD36, lanes 1–3), the mutant octamer CD36 probe (CD36mOct, lanes 4–6) or a known octamer element (VHOct, lanes 7–9). Reticulocyte lysates were programmed with cDNAs for the proteins indicated above each lane. Lanes 1, 4 and 7 contain unprogrammed reticulocyte lysates. (B) Gel retardation analysis showing the CD36 octamer (CD36 Oct) specifically competes with the IgK octamer (IgK Oct). Nuclear extracts from WEHI231 cells were incubated with radiolabelled IgK Oct in the presence of 100-fold molar excess of competitor DNA as indicated above the lanes. The known Oct-1 and Oct-2 complexes are indicated. (C) Oct-2 specifically binds to the CD36 octamer. Nuclear extracts from WEHI231 cells were incubated with radiolabelled CD36 Oct in the presence of 100-fold molar excess of competitor DNA as indicated above the lanes (lanes 2–4). Lanes 5 and 6 contain serum and Oct-2-specific antibody, respectively. The identity of each of the complexes is indicated.
It is well established that when B cell nuclear extracts are incubated with an octamer element only complexes containing Oct-1 and Oct-2 are observed (1–6). We therefore performed a competition analysis to test the ability of the CD36 octamer (CD36 Oct) to abolish Oct-1- and Oct-2-specific complexes that form on the known octamer site from the IgK enhancer (Fig. 2B). When nuclear extracts from the B cell line WEHI231 were incubated with the IgK octamer in the absence of competitor DNA, both Oct-1- and Oct-2-specific complexes were observed (Fig. 2B, lane 1). In the presence of 100-fold molar excess of CD36 octamer both complexes were abolished. In contrast, both complexes were unaffected by the presence of mutated CD36 octamer DNA (Fig. 2B, lane 2). When the CD36 octamer DNA was used as a radiolabelled probe, two complexes were also observed that were completely abolished in the presence of excess IgK Oct competitor DNA (Fig. 2C, lanes 1 and 4). In contrast, competition with the mutated CD36 octamer DNA did not abolish binding. Taken together, the results clearly indicate that the octamer element in the CD36 promoter can be bound by endogenous Oct-1 and Oct-2.
To establish unequivocally that endogenous Oct-2 is able to bind to the CD36 octamer, supershift analysis using an Oct-2-specific antibody was performed. In the presence of an Oct-2-specific antibody the lower mobility complex was abolished and an additional supershifted complex appeared (Fig. 2C, lane 6). Addition of serum alone did not abolish the Oct-2 complex nor produce a supershifted complex (Fig. 2C, lane 5). The results demonstrate clearly that endogenous Oct-2 can bind to the octamer element in the CD36 promoter.
Oct-2 activates the CD36 promoter in B cells
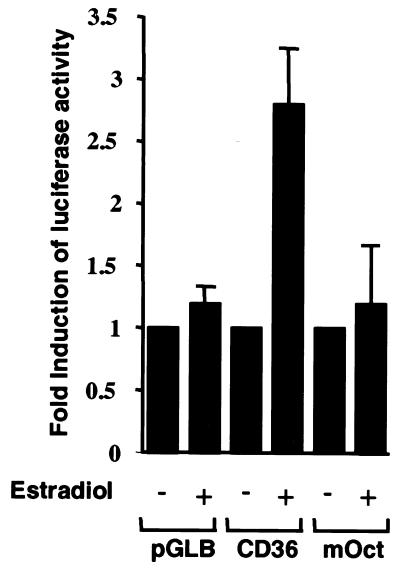
To investigate the ability of Oct-2 to activate CD36 transcription the CD36 promoter was ligated upstream of the luciferase cDNA in the reporter plasmid pGL2-B to produce pGLCD36. In order to examine the role of Oct-2 in regulating the B cell expression of CD36 we used the pre-B cell line Abl/Oct-2-ER (21). This cell line was derived from the mouse oct-2–/– pre-B cell line Abl1.1 by stable transfection of a construct expressing Oct-2 fused to the hormone-binding domain of the human estrogen receptor (21). The specific effect of Oct-2 in a B cell can therefore be determined by the addition of β-estradiol (21). Indeed, the endogenous transcription of the CD36 gene in this cell line is dependent upon β-estradiol activation of Oct-2 (Fig. 3A) (21). Abl1.1 and Abl1.1/Oct-2-ER cells were transfected with the CD36 reporter plasmid pGLCD36 (Fig. 3B). After transfection, the cells were divided into two separate cultures and one was stimulated by the addition of β-estradiol. In the oct-2–/– cells, addition of β-estradiol had no effect on the activity of the CD36 promoter (Fig. 3B, Abl1.1). In contrast, addition of β-estradiol to Abl/Oct-2-ER cells resulted in activation of the CD36 promoter (Fig. 3B, CD36). This is consistent with the activation of the endogenous CD36 gene as determined by northern blot analysis of mRNA (Fig. 3A). To determine that the activation was dependent upon the octamer site, we introduced specific mutations that abolish Oct-2 binding to the octamer. When the octamer sequence was mutated, addition of β-estradiol almost totally blocked activation in Abl1.1/Oct-2-ER cells (Fig. 4, mOct). The results demonstrate clearly that the octamer site in the CD36 promoter mediates its Oct-2-dependent activation.
Figure 3.
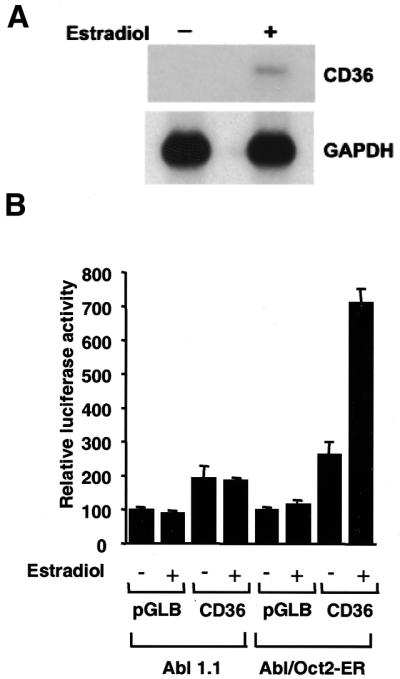
Transcriptional activation of the CD36 promoter in Abl1.1 and Abl/Oct-2-ER pre-B cells. (A) Northern blot of endogenous CD36 mRNA from Abl/Oct-2-ER cells before and after β-estradiol stimulation. Equal amounts of RNA were loaded; GAPDH mRNA is shown as a loading control. (B) Abl1.1 (oct-2–/–) or Abl/Oct-2-ER (Oct-2 inducible) cells were transfected with the luciferase reporter plasmid pGLCD36 or the control plasmid pGLB. Cells were left unstimulated (–) or stimulated by addition of 1 µM β-estradiol (+). Luciferase activities were determined from triplicate experiments (means ± standard errors) and are representative of several experiments performed in duplicate or triplicate.
Figure 4.
Mutation of the octamer element blocks Oct-2-dependent activation in Abl1.1/Oct-2-ER cells. Graph showing fold activation of the wild-type CD36 promoter reporter, CD36, and the mutant derivative containing a mutated octamer site (mOct). Transfected cells were left unstimulated (–) or stimulated with 1 µM β-estradiol (+). Luciferase activities (means ± standard errors; n = 3) are shown as fold activation relative to the unstimulated sample, which was set to 1.
Expression of CD36 in B cells requires Oct-2 but not OBF-1
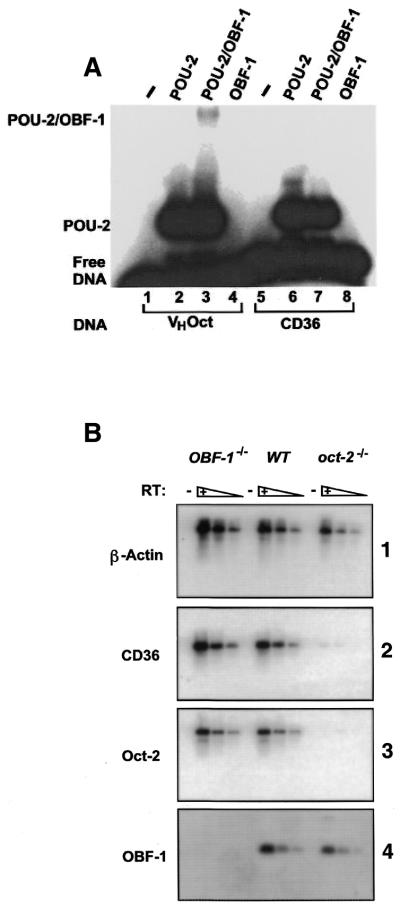
OBF-1 activates transcription of octamer-containing promoters by binding directly to either Oct-1 or Oct-2 (8–16). Previous studies have shown that OBF-1 recruitment is dependent upon the presence of adenosine at position five in the octamer sequence (ATGCAAAT) and that octamers with thymine at this position (ATGCTAAT) do not form a ternary complex with OBF-1 (13,14). The CD36 octamer is of the type ATGCTAAT, suggesting that it cannot support recruitment of OBF-1. We have demonstrated that both Oct-1 and Oct-2 can bind to the CD36 octamer element but that no significant transcriptional activity was detectable in oct-2–/– B cells despite the high level of Oct-1 in these cells. Oct-1 is a weak activator of mRNA genes and therefore requires the presence of a co-activator to achieve efficient transcriptional activation (reviewed in 2). We therefore tested the ability of the CD36 octamer to form a ternary complex with OBF-1 in a gel retardation assay (Fig. 5A). The Oct-2 POU domain was used in order to clearly resolve the ternary complex. OBF-1 forms a ternary complex with the Oct-2 POU domain on the VHOct site (ATGCTAAAT) (Fig. 5A, lane 3). In contrast, when the CD36 octamer was used as a probe, OBF-1 did not form a ternary complex (Fig. 5A, lane 7). This result demonstrates that OBF-1 is excluded from the CD36 promoter and is unlikely to participate in Oct-2 transcriptional activation of the CD36 gene. To confirm that CD36 expression in B cells is independent of OBF-1 expression, we investigated the expression of CD36 in mouse primary B cells. The requirement for OBF-1 was investigated by examining the expression of CD36 by RT–PCR using RNA from mature primary B cells derived from wild-type, oct-2–/– and obf-1–/– mice (Fig. 5B). In agreement with a previous report, comparison of CD36 expression in the wild-type and oct-2–/– cells clearly demonstrated the lack of CD36 expression in the oct-2–/– cells (Fig. 5B, panel 2) (21). In contrast, CD36 is expressed in obf-1–/– splenic B cells to equivalent levels seen in the wild-type cells. Expression of OBF-1 is unaffected in oct-2–/– B cells, suggesting that the lack of CD36 expression in oct-2–/– cells is not due to the absence of OBF-1 (Fig. 5B panel 2; compare OBF-1–/– with oct-2–/–). Taken together the data demonstrate that the Oct-2-dependent expression of CD36 in B cells is independent of the co-activator OBF-1.
Figure 5.
OBF-1 is not required for Oct-2-dependent CD36 gene expression. (A) Gel retardation analysis of the Oct-2 POU domain (POU-2) and OBF-1 on the CD36 promoter. Proteins were incubated with either the VHOct probe (lanes 1–4) or the CD36 probe (lanes 5–8). The identity of the proteins in the reaction is shown above each lane. Lanes 1 and 5 contain unprogrammed reticulocyte lysate. The positions of the free DNA, the binary complex (POU-2) and the ternary complex (POU-2/OBF-1) are indicated. (B) Expression of CD36 in OBF-1–/– and oct-2–/– splenic B cells. RT–PCR was performed using cDNAs derived from wild-type, OBF-1–/– or oct-2–/– splenic B cells. Each panel shows a titration of the RT–PCR products from each of the B cell samples. The RT–PCR product is indicated to the left of each panel and the genotype of the B cells is shown above each of the three lanes. The panel number referred to in the text is shown on the right.
DISCUSSION
Comparison of the mouse and human CD36 promoters
The conservation of transcription factor binding sites in the human and mouse CD36 promoters suggests that they are likely to be important in the regulation of CD36 gene expression in both mice and humans. The perfect conservation of the DR-1 element suggests that the PPARγ:RXRα complex regulates CD36 gene expression in mouse macrophages by the same mechanism as in humans (36). The AML1 site in the human gene is required for constitutive promoter activity in monocytes (24) and conservation of this sequence in the mouse promoter also suggests that it has a similar function in the mouse. The binding sites for the ubiquitous transcription factor NF1 (37) could also be important in regulation of CD36 gene expression.
The consensus octamer site in the mouse promoter is not conserved in the human promoter. This correlates with the fact that CD36 is expressed in mouse primary B cells but has not been reported in human primary B cells (21). However, it is possible that CD36 is expressed in human B cells under some circumstances and that an Oct-2 binding site exists elsewhere in the human CD36 gene, as CD36 expression in human lymphoma cells has been reported (38).
Oct-2-dependent transcription
The mouse CD36 gene was the first gene to be identified as an Oct-2 target gene (21) and our finding that the promoter contains a consensus octamer site provides a molecular explanation for the Oct-2-dependent expression of CD36 in B cells. In vitro, this octamer site can recruit either Oct-1 or Oct-2, but B cell-specific expression of CD36 in vivo is critically dependent upon Oct-2 (21; this study). Our analysis of the CD36 promoter activity demonstrated that octamer-dependent transcription requires the expression of Oct-2 and that the endogenous Oct-1 was insufficient.
OBF-1 is not required for CD36 transcription
Our investigation of OBF-1 in the regulation of CD36 gene expression showed that Oct-2 is unable to recruit OBF-1 to the CD36 octamer in vitro and that expression of CD36 in primary B cells from obf-1–/– mice is unaffected. The regulation of CD36 transcription in B cells therefore appears to be dependent upon Oct-2 but not OBF-1, demonstrating that OBF-1 is not a prerequisite for transcriptional regulation by Oct-2. This is in contrast to the regulation of the blr1 gene whose promoter can be stimulated by OBF-1 and whose expression is >50% reduced in obf-1–/– B cells (26). The octamer sequence in the mouse blr1 gene is AAACAAAT and can presumably support Oct-2-dependent recruitment of OBF-1, although this has not been demonstrated. Other studies have shown that OBF-1 recruitment is dependent upon the presence of adenosine at position five in the octamer sequence (ATGCAAAT) and that mutation to a thymine (ATGCTAAT) prevents ternary complex formation (13,14). The CD36 octamer is of the type ATGCTAAT, suggesting that the presence of thymine at position 5 in the CD36 octamer discriminates between Oct-2 and an Oct factor/OBF-1 complex. It therefore appears that Oct-2 can specifically exclude OBF-1 from the regulation of some genes whilst actively recruiting it to regulate others, depending on the nature of the octamer. This is supported by a comparison of the phenotypes of the oct-2–/– and obf-1–/– mice, which both show severely reduced numbers of mature B cells and low levels of secondary antibody isotypes in serum. However, only B cells from the oct-2–/– mice are unable to proliferate normally when stimulated with anti-IgM or T cell-independent mitogens. Therefore, these overlapping, yet distinct, phenotypes support the idea that Oct-2 regulates gene expression by OBF-1-dependent and -independent mechanisms.
A possible mechanism to evade Oct-1 regulation
Members of a given transcription factor family often possess identical or overlapping DNA-binding specificities. Therefore, a complete understanding of transcription factor function must address the question of how transcription factors with the same DNA-binding specificity target different genes within the same cell. We have investigated how a single gene can be specifically regulated by the transcription factor Oct-2 but escapes the control of Oct-1, which has an identical DNA-binding specificity. Oct-1 is a weak activator of mRNA genes and therefore requires the presence of a co-activator to achieve efficient transcriptional activation (reviewed in 2). The fact that Oct-1 requires an additional co-activator such as VP16 or OBF-1 to stimulate transcription from mRNA promoters (reviewed in 2) suggests that exclusion of OBF-1 from the CD36 promoter by use of the ATGCTAAT sequence renders the CD36 gene refractory to Oct-1 transcriptional activation.
In summary, our analysis of the mouse CD36 promoter has revealed that Oct-2 regulates the transcription of the CD36 gene by directly binding to an octamer site located in the promoter. The octamer sequence is of a type that excludes the recruitment of OBF-1 whilst maintaining high affinity Oct-2 binding, thus providing a possible mechanism to enable CD36 expression to evade Oct-1 regulation.
Acknowledgments
ACKNOWLEDGEMENTS
We thank H. Singh and W. Schaffner for providing plasmids. We also thank P. Matthias for kindly providing the obf-1–/– mice. This work was supported by the Wellcome Trust (P.S.) and the Australian National Health and Medical Research Council (L.M.C.)
REFERENCES
- 1.Staudt L.M. and Lenardo,M.J. (1991) Immunoglobulin gene transcription. Annu. Rev. Immunol., 9, 373–398. [DOI] [PubMed] [Google Scholar]
- 2.Herr W. and Cleary,M.A. (1995) The POU domain: versatility in transcriptional regulation by a flexible two-in-one DNA-binding domain. Genes Dev., 9, 1679–1693. [DOI] [PubMed] [Google Scholar]
- 3.Verrijzer C.P. and Van der Vliet,P.C. (1993) POU domain transcription factors. Biochim. Biophys. Acta, 1173, 1–21. [DOI] [PubMed] [Google Scholar]
- 4.Kemler I. and Schaffner,W. (1990) Octamer transcription factors and the cell type-specificity of immunoglobulin gene expression. FASEB J., 4, 1444–1449. [DOI] [PubMed] [Google Scholar]
- 5.Latchman D.S. (1996) The Oct-2 transcription factor. Int. J. Biochem. Cell Biol., 28, 1081–1083. [DOI] [PubMed] [Google Scholar]
- 6.Matthias P. (1998) Lymphoid-specific transcription mediated by the conserved octamer site: who is doing what? Semin. Immunol., 10, 155–163. [DOI] [PubMed] [Google Scholar]
- 7.Mittal V., Cleary,M.A., Herr,W. and Hernandez,N. (1996) The Oct-1 POU-specific domain can stimulate small nuclear RNA gene transcription by stabilizing the basal transcription complex SNAPc. Mol. Cell. Biol., 16, 1955–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y., Fujii,H., Gerster,T. and Roeder,R.G. (1992) A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell, 71, 231–241. [DOI] [PubMed] [Google Scholar]
- 9.Strubin M., Newell,J.W. and Matthias,P. (1995) OBF-1, a novel B cell-specific coactivator that stimulates immunoglobulin promoter activity through association with octamer-binding proteins. Cell, 80, 497–506. [DOI] [PubMed] [Google Scholar]
- 10.Gstaiger M., Knoepfel,L., Georgiev,O., Schaffner,W. and Hovens,C.M. (1995) A B-cell coactivator of octamer-binding transcription factors. Nature, 373, 360–362. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y. and Roeder,R.G. (1995) Cloning, functional characterization, and mechanism of action of the B-cell-specific transcriptional coactivator OCA-B. Mol. Cell. Biol., 15, 4115–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin X.F., Reichlin,A., Luo,Y., Roeder,R.G. and Nussenzweig,M.C. (1998) OCA-B integrates B cell antigen receptor-, CD40L- and IL 4-mediated signals for the germinal center pathway of B cell development. EMBO J., 17, 5066–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gstaiger M., Georgiev,O., van Leeuwen,H., van der Vliet,P. and Schaffner,W. (1996) The B cell coactivator Bob1 shows DNA sequence-dependent complex formation with Oct-1/Oct-2 factors, leading to differential promoter activation. EMBO J., 15, 2781–2790. [PMC free article] [PubMed] [Google Scholar]
- 14.Cepek K.L., Chasman,D.I. and Sharp,P.A. (1996) Sequence-specific DNA binding of the B-cell-specific coactivator OCA-B. Genes Dev., 10, 2079–2088. [DOI] [PubMed] [Google Scholar]
- 15.Sauter P. and Matthias,P. (1998) Coactivator OBF-1 makes selective contacts with both the POU-specific domain and the POU homeodomain and acts as a molecular clamp on DNA. Mol. Cell. Biol., 18, 7397–7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babb R., Cleary,M.A. and Herr,W. (1997) OCA-B is a functional analog of VP16 but targets a separate surface of the Oct-1 POU domain. Mol. Cell. Biol., 17, 7295–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U., Qin,X.F., Gong,S., Stevens,S., Luo,Y., Nussenzweig,M. and Roeder,R.G. (1996) The B-cell-specific transcription coactivator OCA-B/OBF-1/Bob-1 is essential for normal production of immunoglobulin isotypes. Nature, 383, 542–547. [DOI] [PubMed] [Google Scholar]
- 18.Schubart D.B., Rolink,A., Kosco-Vilbois,M.H., Botteri,F. and Matthias,P. (1996) B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature, 383, 538–542. [DOI] [PubMed] [Google Scholar]
- 19.Corcoran L.M., Karvelas,M., Nossal,G.J., Ye,Z.S., Jacks,T. and Baltimore,D. (1993) Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev., 7, 570–582. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran L.M. and Karvelas,M. (1994) Oct-2 is required early in T cell-independent B cell activation for G1 progression and for proliferation. Immunity, 1, 635–645. [DOI] [PubMed] [Google Scholar]
- 21.Konig H., Pfisterer,P., Corcoran,L.M. and Wirth,T. (1995) Identification of CD36 as the first gene dependent on the B-cell differentiation factor Oct-2. Genes Dev., 9, 1598–1607. [DOI] [PubMed] [Google Scholar]
- 22.Daviet L. and McGregor,J.L. (1997) Vascular biology of CD36: roles of this new adhesion molecule family in different disease states. Thromb. Haemost., 78, 65–69. [PubMed] [Google Scholar]
- 23.Armesilla A.L. and Vega,M.A. (1994) Structural organization of the gene for human CD36 glycoprotein. J. Biol. Chem., 269, 18985–18991. [PubMed] [Google Scholar]
- 24.Armesilla A.L., Calvo,D. and Vega,M.A. (1996) Structural and functional characterization of the human CD36 gene promoter: identification of a proximal PEBP2/CBF site. J. Biol. Chem., 271, 7781–7787. [DOI] [PubMed] [Google Scholar]
- 25.Pfisterer P., Konig,H., Hess,J., Lipowsky,G., Haendler,B., Schleuning,W.D. and Wirth,T. (1996) CRISP-3, a protein with homology to plant defense proteins, is expressed in mouse B cells under the control of Oct2. Mol. Cell. Biol., 16, 6160–6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf I., Pevzner,V., Kaiser,E., Bernhardt,G., Claudio,E., Siebenlist,U., Forster,R. and Lipp,M. (1998) Downstream activation of a TATA-less promoter by Oct-2, Bob1, and NF-kappaB directs expression of the homing receptor BLR1 to mature B cells. J. Biol. Chem., 273, 28831–28836. [DOI] [PubMed] [Google Scholar]
- 27.Endemann G., Stanton,L.W., Madden,K.S., Bryant,C.M., White,R.T. and Protter,A.A. (1993) CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem., 268, 11811–11816. [PubMed] [Google Scholar]
- 28.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Shore P., Whitmarsh,A.J., Bhaskaran,R., Davis,R.J., Waltho,J.P. and Sharrocks,A.D. (1996) Determinants of DNA-binding specificity of ETS-domain transcription factors. Mol. Cell. Biol., 16, 3338–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth T., Priess,A., Annweiler,A., Zwilling,S. and Oeler,B. (1991) Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res., 19, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shore P., Bisset,L., Lakey,J., Waltho,J.P., Virden,R. and Sharrocks,A.D. (1995) Characterization of the Elk-1 ETS DNA-binding domain. J. Biol. Chem., 270, 5805–5811. [DOI] [PubMed] [Google Scholar]
- 32.Shah P.C., Bertolino,E. and Singh,H. (1997) Using altered specificity Oct-1 and Oct-2 mutants to analyze the regulation of immunoglobulin gene transcription. EMBO J., 16, 7105–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber E., Matthias,P., Muller,M.M. and Schaffner,W. (1989) Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res., 17, 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grumont R.J., Richardson,I.B., Gaff,C. and Gerondakis,S. (1993) rel/NF-kappa B nuclear complexes that bind kB sites in the murine c-rel promoter are required for constitutive c-rel transcription in B-cells. Cell Growth Differ., 4, 731–743. [PubMed] [Google Scholar]
- 35.Grosschedl R. and Baltimore,D. (1985) Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell, 41, 885–897. [DOI] [PubMed] [Google Scholar]
- 36.Tontonoz P., Nagy,L., Alvarez,J.G., Thomazy,V.A. and Evans,R.M. (1998) PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell, 93, 241–252. [DOI] [PubMed] [Google Scholar]
- 37.Courtois S.J., Lafontaine,D.A., Lemaigre,F.P., Durviaux,S.M. and Rousseau,G.G. (1990) Nuclear factor-I and activator protein-2 bind in a mutually exclusive way to overlapping promoter sequences and trans-activate the human growth hormone gene. Nucleic Acids Res., 18, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muroi K., Toya,K., Suzuki,T., Suda,T., Sakamoto,S. and Miura,Y. (1992) Expression of CD11B, CD14 and CD36 antigens by B-cell lymphoma. Br. J. Haematol., 80, 126–127. [DOI] [PubMed] [Google Scholar]