Abstract
This study investigated levels of eight polycyclic aromatic hydrocarbons (PAH8) compounds in both raw and processed marine products in South Korea. Katsuobushi exhibited the highest concentration of benzo[a]pyrene, at 14.22 µg/kg, exceeding the European Commission's regulation level of 5.0 µg/kg. The total PAH8 concentration in katsuobushi was 220.5 µg/kg. Among the product categories, shellfish had the highest detection rate (70%), followed by fish (19%) and crustacea (8%), with chrysene being the most prominent PAH8 congener in all marine products. Grilled fish predominantly contained pyrogenic PAHs from combustion byproducts, while shellfish primarily contained petrogenic ones from the aquatic environment. Grilling, smoking, and drying processes significantly contributed to the formation of PAH8 in these food products. Based on the results of a risk assessment using a margin of exposure approach through a total diet study, exposure to PAH8 from marine products is considered to pose low concern to the South Korean population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01491-y.
Keywords: Polycyclic aromatic hydrocarbon, Marine product, Total diet study, Risk assessment, Gas chromatography-mass spectrometry
Introduction
Polycyclic aromatic hydrocarbons (PAHs) can come from three sources: natural (biogenic), combustion (pyrogenic), and petroleum (petrogenic) (Abbas et al., 2018). Biogenic PAHs stem from living organisms, while pyrogenic PAHs result from the high-temperature processing of organic matter. Petrogenic PAHs enter the environment through natural processes like gas leaks and fossil fuel seepage (Ofosu et al, 2022).
Many PAHs are mutagenic and genotoxic, capable of causing DNA adduct formation in both laboratory and living organisms (Zaidi, et al., 2021). In addition to cancer, PAHs can lead to other adverse effects, including neurobehavioral changes in developing animals, decreased ovarian follicles and ovary weight, and altered thymus weight and serum immunoglobulin in rats (Kitts et al., 2012; Kroese et al., 2002).
The European Food Safety Authority (EFSA) indicated that utilizing the total PAH level of benzo[a]anthracene (B[a]A), chrysene (Chry), benzo[a]pyrene (B[a]P), and benzo[b]fluoranthene (B[b]F) represented for PAH4, or including B[a]A, chrysene, B[a]P, B[b]F, benzo[k]fluoranthene (B[k]F), indeno[1,2,3-cd]pyrene (I[c,d]P), dibenz[a,h]anthracene (D[a,h]A), and benzo[g,h,i]perylene (B[g,h,i]P) represented for PAH8 is more appropriate than using solely B[a]P level (EC, 2015). The primary human exposure factor is known to be dietary intake, and the risk of exposure to PAHs in aquatic products has increased due to higher seafood consumption and pollution of the aquatic environment by human activities (Alomirah et al., 2011; Llobet et al., 2006). Other studies also found that seafood is becoming the leading source of dietary PAH exposure (EFSA, 2008; Habibullah-Al-Mamun et al., 2019; Veyrand et al., 2013).
As a result, we employed a total diet study (TDS) approach to assess the levels of PAHs in marine products and investigate variations in PAH contents based on the preparation methods of these marine food items. TDS is one of the most effective and efficient methods for estimating dietary exposure and assessing health risk at the consumer level, considering the purchasing and eating habits of various populations (Ingenbleek et al., 2017). There are limited studies that monitor the levels of benzopyrene, the total of PAH4 (∑PAH4), and the total of PAH8 (∑PAH8) generated during the processing of marine products. In the case of barbecued salmon fillet and grilled anchovies, it was observed that they exhibited elevated levels of benzo[a]pyrene, the total of PAH4 (∑PAH4), and the total of PAH8 (∑PAH8), with concentrations of 0.52 µg/kg, 2.41 µg/kg, and 2.88 µg/kg for the former (Oz, 2020), and 0.73 µg/kg, 3.3 µg/kg, and 5.13 µg/kg for the latter (Sahin et al., 2020).
Because the toxicity of each PAH differs, the toxicity is assessed using a toxic equivalency factor (TEF) using benzo[a]pyrene as a reference molecule. EFSA adopted a margin of exposure (MOE) approach to estimate health risk to PAHs, based on benchmark dose lower-bound confidence limit 10% (BMDL10) (EFSA, 2008). However, EFSA has concluded the toxic equivalent quantity (TEQ) approach is only suitable for compounds with the same toxicological effect, such as dioxins. Given that several PAHs have carcinogenic properties and produce tumors through different mechanisms, the TEQ approach may not be appropriate for PAHs (EFSA, 2008). Using toxicological values, rather than the TEQ, may be necessary for assessing the risks associated with PAHs.
The primary objectives of this research are to: (1) validate methods for extracting PAHs to evaluate the PAH8 concentration, (2) identify the main contributors of PAH8 exposure in humans in terms of each PAH compound and the origin of PAHs associated with processing methods, (3) determine the types of marine products generating most PAH8, (4) assess the health risks associated with PAH8 from the consumption of marine products in South Korea, and (5) to compared the risks estimated using toxicological values and traditional TEF values. This study focused on examining the PAH8 levels in marine products in a table-ready form within the framework of a TDS.
Materials and methods
Sample selection and preparation
Marine product samples were selected based on the results of the 6th Korean National Health and Nutrition Examination Survey (KNHANES-VI) conducted by Korea Disease Control and Prevention Agency (KDCA) from 2013 to 2015 (MOHW, 2013). The raw data were obtained from the KNHANES website (https://knhanes.kdca.go.kr/knhanes/main.do).
Supplementary Table 1 contains a list of 109 products with high consumption rates (covering more than 95% of total accumulated consumption), high frequency levels (covering more than 1% of the consumption rate), and fat contribution (containing the cumulative rate of fat intake up to 95%). The food samples were gathered from 21 supermarkets in ten large cities with a population of over one million, including Seoul, Incheon, Suwon, Gwangju, Daejeon, Cheongju, Daegu, Busan, Ulsan, and Changwon in South Korea.
An identical quantity of food samples were gathered to create each composite sample, and they were prepared in a table-ready form according to the sample preparation methods guidebook for TDS provided by the Ministry of Food and Drug Safety (MFDS, 2019). The cooked samples (n = 287) are divided into eight groups: fish (139), shellfish (46), cephalopoda (25), crustacea (37), sea algae (30), echinodermata (5), tunicata (3), and cnidaria (2). A total of 287 subsamples were homogenized and kept in a polyethylene bottle at − 20 °C for PAH analysis.
Chemicals and materials
HPLC grade ethyl alcohol, methyl alcohol, n-hexane, and dichloromethane (DCM) were purchased from Burdick & Jackson (Muskegon, MI, USA). Younglin Instrument's AQUAMAX-Basic 363 water purification system (Dongan, Anyang, Republic of Korea) was used to produce distilled water. EPA 525 PAH Mix A, which contains 500 µg/mL of naphthalene (99% purity), acenaphthene (99% purity), fluoranthene (98% purity), acenaphthylene (99% purity), fluorene, phenanthrene, anthracene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene (98% purity), benzo[k]fluoranthene, benzo[a]pyrene (96% purity), indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene in dichloromethane was purchased from Sigma Aldrich (St. Louis, MO, USA). Benzo[a]pyrene-d12 (98% purity), benzo[a]pyrene-d12 (98% purity), benzo[b]fluoranthene-d12 (98% purity), and chrysene-d12 (98% purity) were purchased from Sigma Aldrich for internal standards. Potassium hydroxide was purchased from Showa Denko (Tokyo, Japan), and anhydrous sodium sulfate was from Yakuri Pure Chemicals (Kyoto, Japan). Filter paper was purchased from Whatman (Kent, UK) and Bond Elut SI for solid phase extraction (SPE) was obtained from Agilent Technologies (Santa Clara, CA, USA).
Extraction of PAHs
Extraction steps are essential for determining PAHs. Depending on the properties of the sample matrices, two distinct extraction procedures were employed. These methods were adapted in accordance with the Korea Food Code and previous studies (Kim et al., 2021; MFDS, 2019).
For solid food matrices such as salmon and oyster, alkali digestion was applied to extract PAHs from the products. In each flask, 10 g (wet weight) of samples or 1–2 g (dry weight) of sample were placed. Then, 100 mL of 1 M KOH solution in ethanol was added, along with 1 mL of 13C-labeled internal standard solution (100 µg/kg of each benzo[a]pyrene-d12 and chrysene-d12). The flask was connected to a reflux condenser and placed into a water bath, WB-22, (Daihan Scientific, Gangwon, Republic of Korea) at 80℃ for 3 h. After saponification, the flask was rapidly cooled down using cold water, and the reflux condensers were rinsed with n-hexane. The extract in the flask was transferred into a separatory funnel through a filter paper. The flask was washed with 50 mL of n-hexane: ethanol (1:1, v/v) solution, and the washed solution was added into the funnel. The funnel was thoroughly shaken using a funnel shaker (Changshin Science, Seoul, Republic of Korea) with 300 rpm for 10 min after adding 50 mL of distilled water. After shaking, the separated organic phase fraction from the organic solvent was collected in each Erlenmeyer flask. Then, 50 mL of n-hexane was added to distilled water in the separatory funnel and shaken to separate two immiscible liquid phases. This procedure was repeated twice. All organic solvent phases were collected in another separatory funnel, and distilled water was added to remove water-soluble compounds. The water layer was eliminated after vigorously shaking the funnel. The obtained extract was filtered through 10 g of anhydrous Na2SO4 (Yakuri Pure Chemicals, Kyoto, Japan) to remove any remaining water. Next, the extracts were concentrated using a rotary evaporator (EYELA, Tokyo, Japan) until the final volume was below 2 mL. The concentrate was applied to activate SPE (Bond Elut SI) cartridges (Agilent technologies) and eluted with n-hexane and DCM. The eluant was concentrated under N2 gas at 40 °C, and the residues were re-dissolved in 1 mL of DCM. The solution was filtered through 0.45 µm of PTFE membrane syringe filter for gas chromatography-mass spectrometry (GC–MS) analysis.
For liquid food matrices, such as fish sauce or seafood stock, an ultrasound-assisted extraction method was used to quantify PAHs. In each flask, 10 g of samples were placed, and 50 mL of n-hexane spiked with 1 mL of internal standard solution was added. The mixture was then subjected to 20 min of ultrasonication. Subsequently, 35 mL of n-hexane was added, and ultrasound-assisted extraction was carried out for another 20 min. The following extraction steps for liquid food samples were conducted in the same manner as previously described methods for solid food samples.
GC–MS analysis
PAH levels were determined using gas chromatography-mass spectrometry (GC–MS, 7890B/5977B, Agilent Technologies) with a HP-5MS Ultra Inert column (30 m × 0.25 mm × 0.25 μm) (Agilent Technologies). Helium was used as a carrier gas with a flow rate 1.0 mL/min. The GC oven temperature was programmed as follows: initially set at 80 °C and held for 1 min, then heated at a rate of 20 °C/min up to 220 °C and held for 10 min, followed by an increase to 280 °C at a rate of 2 °C/min and maintained for 10 min. Mass spectra were generated using an electron ionization ion source at 70 eV in scan mode to determine a quantitative ion and two qualitative ions (Table 1). PAH peaks were identified using the ion in the selected ion monitoring mode.
Table 1.
Summary of eight polycyclic aromatic hydrocarbons (PAH8) and validation results of two different methods (alkali digestion and ultrasound-assisted extraction) with linearity, limit of detection (LOD), limit of quantification (LOQ), and expanded uncertainty
| Compounds | Abbreviation | Quantitative ion (m/z) | Qualita-tive ion (m/z) | TEFa | Alkali digestion extraction method | Ultrasound assisted extraction method | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | LODb (µg/kg) | LOQc (µg/kg) | Expanded uncertaintyd (%) |
R2 | LOD (µg/kg) |
LOQ (µg/kg) |
Expanded uncertainty (%) |
|||||
| Benz[a]anthracene | B[a]A | 228 | 226, 229 | 0.1 | 0.9998 | 0.096 | 0.292 | 4.022 | 0.9991 | 0.079 | 0.241 | 4.463 |
| Chrysene | Chry | 228 | 226, 229 | 0.01 | 0.9996 | 0.082 | 0.248 | 6.145 | 0.9992 | 0.074 | 0.225 | 6.196 |
| Benzo[b]fluoranthene | B[b]F | 252 | 250, 253 | 0.1 | 0.9997 | 0.091 | 0.277 | 5.649 | 0.9999 | 0.083 | 0.252 | 5.603 |
| Benzo[k]fluoranthene | B[k]F | 252 | 250, 253 | 0.1 | 0.9999 | 0.120 | 0.365 | 6.485 | 0.9995 | 0.105 | 0.317 | 7.061 |
| Benzo[a]pyrene | B[a]P | 252 | 250, 253 | 1 | 0.9994 | 0.070 | 0.212 | 4.511 | 0.9998 | 0.115 | 0.348 | 4.495 |
| Indeno[1,2,3-cd]pyrene | I[c,d]P | 276 | 274, 277 | 0.1 | 0.9999 | 0.129 | 0.391 | 4.784 | 0.9997 | 0.108 | 0.327 | 4.995 |
| Dibenz[a,h]anthracene | D[a,h]A | 278 | 276, 279 | 5 | 0.9999 | 0.175 | 0.531 | 4.447 | 0.9998 | 0.097 | 0.294 | 4.615 |
| Benzo[g,h,i]perylene | B[g,h,i]P | 276 | 274, 277 | 0.01 | 0.9983 | 0.105 | 0.318 | 8.608 | 0.9984 | 0.104 | 0.315 | 8.529 |
aToxic equivalency factors (TEFs)
bMethod limit of detection, 3.3σ/s (σ = standard deviation of response; s = slope of regression equation)
cMethod limit of quantification, 10σ/s
dExpanded uncertainties in percentage at 10 μg/kg (confidence level about 95%, k = 2). If nominal concentration level is < 100 μg/kg, the typical expanded uncertainty range should be within 44% (Codex, 2011)
Method validation
Salmon (solid-type) and fish sauce (liquid-type) were utilized as representative samples for each alkali digestion method and ultrasound-assisted extraction method to validate the efficacy of these two distinct PAH extraction procedures. Linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, and measurement uncertainty were assessed to validate both PAH extraction methods. Additionally, proficiency testing using Food Analysis Performance Assessment Scheme (FAPAS) was also conducted.
Calibration curves for eight targets of PAHs (PAH8) were obtained through five replicate experiments with 6 data points spanning the range of 0 to 20 µg/kg range, each spiked with the 100 µg/kg of 13C-labeled internal standard solution. Linearity, LOD, and LOQ were determined by plotting the ratios of analyte compound peak area to their corresponding internal standards against nominal concentrations (0, 0.5, 1, 2, 5, 10, and 20 µg/kg). The linearities of each calibration curve were assessed as a coefficient of determination (R2). The LOD and LOQ were calculated using the following formula: LOD = 3.3σ/S and LOQ = 10σ/S, where σ represents the standard deviation of the response, and S is the slope of the calibration curve. Accuracy and precision were assessed at three different nominal concentration levels (5, 10, and 20 µg/kg), with intraday accuracy and precision determined from five replicates and interday accuracy and precision validated in triplicate across three days.
To ensure the reliability of our analysis data, the measurement uncertainty was calculated, following the EURACHEM/CITAC Guide (EURACHEM/CITAC, 2012). This involved evaluating the standard uncertainty of each factor affecting the measurement value, including balance, pipette, volume of mass flask, external standard solution, internal standard solution, calibration curve, matrix effects, and GC–MS. For determining the measurement uncertainty, 10 µg/kg of external standard solution and 100 µg/kg of internal standard solution were used. After assessing each standard uncertainty, they were integrated to obtain a combined standard uncertainty (u’). An expanded measurement uncertainty (U’) was calculated by multiplying u’ by a coverage factor (k = 2), representing a confidence level of approximately 95%.
Our laboratory’s performance for PAH quantitation was evaluated trough FAPAS proficiency testing, with results falling within the range of |z|< 2.
Exposure assessment and risk characterization
Various statistical treatments were employed to handle findings below the LOD depending on the detection rate. In accordance with JOINT GUIDANCE (FAO, 2011), it is typically recommended for risk assessment to use both a lower bound (LB) and an upper bound (UB). Results falling below LOD are substituted for zero at the LB and replaced by LOD at the UB (EFSA, 2008; WHO/IPCS, 2009).
To calculate total PAH8 concentration (TCPAH8 or ∑PAH8), the concentrations of individual congener were combined by using Eq. (1). Meanwhile, to determine the total B[a]P toxic equivalent quantity (TEQB[a]p) of PAH8, the concentrations of each PAH compound were multiplied by their toxic equivalency factors (TEFs) and then summed using Eq. (2). The TEFs used in this study are based on those reported by Nisbet and LaGoy (1992), which are presented in Table 1.
| 1 |
| 2 |
where, TCPAH8 is the total concentration of the ith individual congener of PAH8, TEQB[a]Pi is the total B[a]P toxic equivalent concentration of the ith individual congener of PAH8, Ci is the measured concentration for the ith individual congener of PAH8, and TEFi is the toxic equivalency factor of the ith individual congener.
The daily intakes of TCPAH8 and TEQB[a]P from marine food exposure were calculated using Eq. (3) and (4), respectively. These equations multiply the food intake rate (IRi) by the TCPAH8 or TEQB[a]P value and divide by the body weight (b.w.). The respective IRi and BW of total population and consumption group were obtained from the KNHANES published by the KDCA.
| 3 |
| 4 |
To determine the daily exposure to PAH8 through marine food, the MOE was recommended by a Scientific Committee (EFSA, 2005). The MOE was calculated based on the TEQ approach, where a Benchmark Dose Lower Limit (BMDL10) of PAH8 was divided by the daily dietary exposure expressed in total concentration of PAH8 (∑PAH8) or total B[a]P equivalent quantity (TEQB[a]P)) using the following Eq. (5). The BMD10 and BMDL10 values for PAH8 in the experimental animal diet ranged from 0.87 to 1.93 mg/kg b.w. per day and 0.49 to 1.35 mg/kg b.w./day, respectively. The CONTAM Panel used the lowest BMDL10 value of 0.49 mg/kg b.w. per day to derive an MOE, which measures how safe a chemical is at a given exposure level (EFSA, 2008). Thus, The BMDL10 of 0.49 mg/kg BW/d was chosen as the reference point of PAH8 in this study.
| 5 |
An MOE value lower than 10,000 is considered as a possible concern for risk management while an MOE of 10,000 or higher is regarded to indicate a low concern (EFSA, 2005, 2008).
Results and discussion
Method validation and quality control
The linearities (R2) of calibration curves after alkali digestion and ultrasound-assisted extraction, respectively were above 0.9983 (Table 1) satisfying the Codex guideline requirement which specifies that R2 should be 0.99 or above (Codex, 1993). While the LOD of the former pretreatment for PAH8 congeners ranged from 0.070 to 0.175 µg/kg, that of the latter method ranged from 0.074 to 0.115 µg/kg. The measurement uncertainty ranged from 4.022 to 8.608% and 4.463 to 8.529% in alkali digestion and ultrasound-assisted extraction methods, respectively. This result met the Codex criteria, which state that the expanded uncertainty should be less than 44% when the nominal concentration was 100 µg/kg or less (Codex, 2011). Additionally, standard uncertainty of calibration curve was found to be the most influential factor in determining the measurement uncertainty of PAH8. As shown in Table 2, the accuracy ranged from 91.83 to 111.8% and the precision were 0.07 to 8.75%. Based on these validation results, both extraction procedures were shown to be highly effective in determining the content of PAH8 in marine products.
Table 2.
Accuracy and precision of PAH8 using two different extraction methods
| Compounds | Nominal concentration (µg/kg) | Alkali digestion method | Ultrasonication method | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intra-daya (n = 5) | Inter-dayb (n = 3) | Intra-day (n = 5) | Inter-day (n = 3) | ||||||
| Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | Accuracy | Precision | ||
| (%) | (% RSDc) | (%) | (% RSD) | (%) | (% RSD) | (%) | (% RSD) | ||
| B[a]A | 5 | 97.35–100.00 | 1.92–3.42 | 99.74 | 1.09 | 98.43–101.39 | 0.53–2.27 | 100.70 | 0.84 |
| 10 | 98.80–101.02 | 1.07–3.41 | 99.79 | 0.62 | 99.54–103.56 | 1.87–2.17 | 100.45 | 0.80 | |
| 20 | 99.98–106.03 | 1.87–4.08 | 102.13 | 2.28 | 102.18–106.83 | 1.57–2.43 | 102.11 | 1.50 | |
| Chry | 5 | 98.30–100.27 | 0.76–0.99 | 99.85 | 0.68 | 98.38–100.72 | 1.05–2.26 | 99.77 | 0.53 |
| 10 | 96.75–99.73 | 1.72–3.13 | 99.52 | 0.81 | 98.29–100.83 | 0.82–2.21 | 99.93 | 0.26 | |
| 20 | 99.50–106.62 | 0.93–7.42 | 100.01 | 0.24 | 102.41–103.05 | 0.80–3.71 | 100.77 | 3.32 | |
| B[b]F | 5 | 97.92–98.47 | 1.20–2.59 | 99.93 | 0.47 | 96.82–98.15 | 0.55–1.20 | 98.60 | 0.66 |
| 10 | 97.23–97.76 | 2.66–4.86 | 99.58 | 0.42 | 97.78–98.06 | 0.60–0.76 | 98.65 | 0.10 | |
| 20 | 98.24–105.74 | 1.12–8.35 | 100.15 | 0.08 | 98.35–98.92 | 0.39–0.57 | 99.20 | 0.31 | |
| B[k]F | 5 | 99.07–100.30 | 0.87–2.49 | 99.99 | 0.07 | 96.29–100.39 | 1.32–4.22 | 98.82 | 0.60 |
| 10 | 98.49–99.64 | 2.32–3.22 | 100.24 | 0.13 | 89.93–96.94 | 2.83–6.72 | 97.81 | 2.43 | |
| 20 | 99.95–101.91 | 0.24–1.25 | 100.23 | 0.38 | 96.26–97.18 | 1.12–2.60 | 98.74 | 0.89 | |
| B[a]P | 5 | 98.80–100.52 | 1.03–1.87 | 99.96 | 0.25 | 98.79–101.00 | 1.20–3.76 | 100.60 | 0.68 |
| 10 | 98.31–100.29 | 1.31–3.92 | 100.48 | 0.91 | 96.86–98.77 | 0.75–2.95 | 99.60 | 0.64 | |
| 20 | 99.98–111.30 | 0.96–8.75 | 100.41 | 0.36 | 97.84–99.72 | 0.65–1.27 | 99.86 | 0.48 | |
| I[c,d]P | 5 | 98.92–102.63 | 1.13–4.87 | 99.76 | 0.75 | 91.83–93.76 | 0.64–1.77 | 94.44 | 1.71 |
| 10 | 98.25–101.87 | 1.94–4.27 | 100.27 | 0.38 | 94.07–96.76 | 2.29–2.85 | 98.19 | 1.47 | |
| 20 | 101.47–105.78 | 1.38–3.31 | 101.17 | 1.55 | 94.20–95.55 | 1.97–2.44 | 97.54 | 1.69 | |
| D[a,h]A | 5 | 99.85–105.53 | 0.78–7.00 | 101.37 | 0.71 | 94.62–96.16 | 1.53–7.36 | 98.88 | 1.65 |
| 10 | 100.32–107.50 | 0.51–5.29 | 100.92 | 0.63 | 98.53–104.07 | 1.98–3.86 | 99.82 | 0.89 | |
| 20 | 102.97–111.82 | 2.88–6.44 | 103.99 | 3.43 | 99.54–106.69 | 1.04–7.22 | 100.47 | 1.12 | |
| B[g,h,i]P | 5 | 99.23–105.51 | 1.22–7.10 | 101.03 | 1.41 | 92.71–93.11 | 0.61–1.36 | 94.01 | 0.69 |
| 10 | 104.06–109.97 | 2.77–4.49 | 102.13 | 1.93 | 95.60–96.21 | 1.15–2.50 | 97.70 | 1.10 | |
| 20 | 100.83–108.58 | 1.67–5.75 | 101.38 | 0.87 | 98.29–100.09 | 1.18–1.85 | 99.68 | 1.15 | |
Ranged from mean of 5 determinations performed daily for 3 days
Mean of 3 determinations
Relative standard deviation: 100× standard deviation/mean
Concentration of PAH8 in marine products
According to the results of the determination PAH8 in marine products prepared as ready-to-eat, the number of samples and the number of detected samples in the eight categories of marine samples were as follows: Fish (n = 26 out of 139; the number of detected samples.
out of total samples), Shellfish (n = 32 out of 46), Cephalopods (n = 11 out of 25), Crustacea (n = 3 out of 37), Sea algae (n = 13 out of 30), Echinodermata (n = 5 out of 5), Tunicata (n = 2 out of 3), and Cnidaria (n = 1 out 2) (Supplementary Table 2). PAH8 levels were found to be above the limits of over the LODs in 93 of 287 subsamples. Among the tested samples, katsuobushi (dried and smoked bonito) exhibited the highest B[a]P levels, followed by dried sea cucumber (Supplementary Table 2). Katsuobushi showed the concentrations of B[a]P (14.22 μg/kg), B[a]A (70.95 μg/kg), Chry (90.63 μg/kg), B[b]F (24.62 μg/kg), B[k]F (8.482 μg/kg), I[c,d]P (4.924 μg/kg), D[a,h]A (1.277 μg/kg), and B[g,h,i]P (5.367 μg/kg) (data not shown).
However, both katsuobushi (smoked and dried bonito) and dried sea cucumber were found to contain levels of B[a]P, a known carcinogen (EFSA, 2008), that exceeded both EC (2015) and MFDS (2019) regulations. The EC regulation for B[a]P in smoked fish is 5 µg/kg, and the MFDS regulation is 5 µg/kg for smoked fish and 10 µg/kg for smoked and dried fish. Katsuobushi contained 14.22 µg/kg of B[a]P, and dried sea cucumber contained 11.35 µg/kg of B[a]P. Tsutsumi et al. (2019) demonstrated that 21 µg/kg B[a]P in dried bonito flakes demonstrated 1.5 times higher than the concentration in the present study. PAHs can form in the smoke that is produced during cooking, and they can also be formed on the surface of the food itself (Alomirah et al., 2011). Another study by Kafouris et al. (2020) found that smoked fish contained significantly higher levels of PAHs compared to fresh fish. The findings of these studies suggest that open flame cooking methods can elevate the B[a]P content of the smoked and dried katsuobushi. The sea cucumber samples in this study are dried. Dried sea cucumber is the most popular form of sea cucumber, accounting for 80% of the market. To reduce processing time, several drying methods have been developed, including hot-air drying at temperatures of 60–100 °C and vacuum cooking at 95 °C (Fan et al., 2022). The thermal processing of dried sea cucumber samples in this study may have increased the levels of B[a]P in the samples. For non-processed products other than smoked fish, the B[a]P level in the grilled salted mackerel was substantially higher (8.5 µg/kg). Open flame cooking methods, like grilling, are known to generate elevated levels of levels of PAHs in food, as PAHs are a class of organic compounds formed during the incomplete combustion of carbon-based materials such as wood, charcoal, and fat (Sampaio et al., 2017). How PAHs form in food during open flame cooking is a complex process that depends on a number of factors, such as the type of fuel used, the cooking temperature, and the cooking time. For instance, a study by Alomirah et al. (2011) found that grilled meat contained significantly higher levels of PAHs than baked meat. Consumers should be aware of the potential health risks associated with the consumption of PAHs and should consider alternative cooking methods, such as smoking, baking or roasting, whenever possible.
However, the shellfish category had the highest detection rate (70%) above LOD, while the fish and crustacea categories had low detection rates (19% and 8%, respectively). The different detection rates between shellfish and crustaceans were explained by their differing feeding habits. Shelfish feeds on suspended nutritional components in water, whereas crustaceans are scavengers (Veyrand et al., 2013). PAHs were found in all mussel subsamples, and the toxicants are thought to have come from contaminated sea water (EFSA, 2008). Although shellfish were frequently tested for contamination, none of the subsamples exceeded the regulatory limits set by EC and MFDS. According to the regulations from MFDS (2019), the maximum permissible levels of B[a]P in bivalvia and cephalopoda are 10.0 µg/kg and 5.0 µg/kg, respectively. Overall, there was a variation in PAH levels depending on cooking methods, with the smoke produced during the heating process appearing to be the primary cause of excessive PAH levels.
PAH8 profiling in marine products
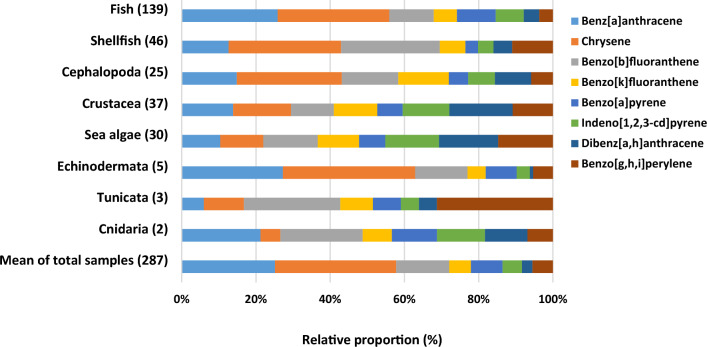
The relative proportions of PAH8 in the medium-bound state within eight categories are described in Fig. 1. Chrysene was a primary component accounting for 33% of an amount of PAH8 in total marine products, followed by B[a]A at 25%. In PAH8, D[a,h]A has the lowest percentage at 3%, followed by I[c,d]P at 5%. These distributions showed the similar tendency in which EFSA (2008) reported that chrysene was a dominant element showing 33% followed by B[a]A at 20%, while D[a,h]A made up the lowest rate at 2% followed by B[k]F at 6% (EFSA, 2008). Chrysene showed the highest contribution in French TDS as well, followed by B[b]F (Veyrand et al., 2013).
Fig. 1.
Relative proportions of mean concentrations of 8 polycyclic aromatic hydrocarbons (PAH8) in 287 marine product subsamples under medium-bound state
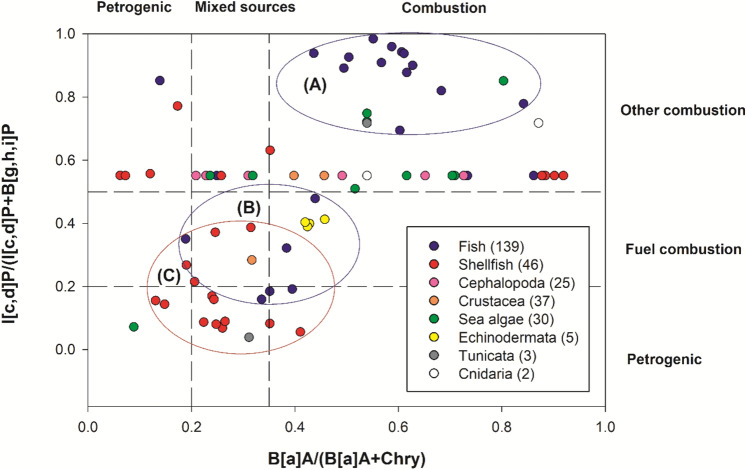
In Fig. 2, the ratios of B[a]A/(B[a]A + Chry) and I[c,d]P/(I[c,d]P + B[g,h,i]P were displayed. These ratios were employed as a marker to determine the origin of PAHs (Yan et al., 2005; Yunker et al., 2002). Medium bound was applied to obtain the ratios in the present study. When PAH8 were found in fish subsamples above LODs, they typically underwent heating processes like grilling or drying (Supplementary Table 2). Most of the dots in the plot's zone (A) are grilled fish, whereas those on the plot's zone (B) are often dried fish (Fig. 2). These patterns, specifically, demonstrate that whereas dried fish contains petrogenic PAHs from the environment, grilled fish contains PAHs produced by burning. In general, it has been noted that grilled food is one of the major contributors to PAH consumption (EFSA, 2008).
Fig. 2.
B[a]A/(B[a]A + Chry) ratio plotted against the I[c,d]P/(I[c,d]P + B[g,h,i]P ratio on a scatter plot for marine product subsamples. Reference ranges for diagnostic ratios were applied (Yan et al., 2005). B[a]A stands for Benz[a]pyrene, whereas Chry, Indeno[1,2,3-cd]pyrene, and B[g,h,i]P stand for Benzo[g,h,i]perylene
In the shellfish category, however, PAH8 concentrations were frequently above LODs in raw materials, and the variance of PAH8 levels depending on the heating methods was smaller than in the fish category (Supplementary Table 2). According to zone (C) in the scatter plot, the PAH8 in the shellfish samples might be derived from petrogenic origin rather than from incomplete combustion (Fig. 2). Fernando et al (2019) reported that shellfish products were contaminated from spilt crude oil in sea water (Fernando et al., 2019). In this research, cephalopods had a slightly larger B[a]A/(B[a]A + Chry) ratio (0.3) than the 0.2 from the Second French TDS (Veyrand et al., 2013) based on mean distributions of B[a]A and Chry.
Dietary exposure and risk assessment
To calculate daily dietary exposure, the daily food intake rate was multiplied by the total PAH8 concentration (∑PAH8) value or the TEQB[a]P value of PAH8 in the marine products using different cooking methods, and then divided the result by body weight. The outcomes are represented in Table 3 with both LB and UB values. On average, South Koreans are exposed to 1.278 ng per kg of body weight per day (ng/kg bw/d) of ∑PAH8 and 0.399 ng/kg bw/d of TEQB[a]P from total marine products under the LB scenario. Under the UB scenario, the average exposure is 2.164 ng/kg bw/d for ∑PAH8 and 1.347 ng/kg bw/d for TEQB[a]P. The 1–2 age group's mean dietary exposure to PAH8 from total marine products utilizing the UB scenario had the greatest mean daily exposure (3.212 ng/kg bw/d for ∑PAH8 and 2.071 ng/kg bw/d for TEQB[a]P) due to their lowest body weights across all age groups.
Table 3.
Mean dietary exposure to (a) total PAH8 concentration (∑PAH8) and (b) total B[a]P toxic equivalent quantity (TEQB[a]p) of PAH8, with contributions from marine products, for the total consumer population and different age groups, with lower bound (LB) and upper bound (UB) estimates
| 1–2 years (n = 519) | 3–6 years (n = 1,062) | 7–12 years (n = 1,601) | 13–19 years (n = 1,629) | 20–64 years (n = 11,592) | over 64 years (n = 4,268) | Total population (n = 20,671) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | Mean dietary exposure | Contribution | |||||||||||||||
| (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | (ng/kg bw/day) | (%) | |||||||||||||||
| a) | ||||||||||||||||||||||||||||
| Category | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB |
| (Number of composite samples) | ||||||||||||||||||||||||||||
| Fish (55) | 1.467 | 1.905 | 84.7 | 59.3 | 1.486 | 1.857 | 88.8 | 65.6 | 0.791 | 1.089 | 73.9 | 56.5 | 0.466 | 0.659 | 74.8 | 57.2 | 0.866 | 1.235 | 60.1 | 52.3 | 0.562 | 0.873 | 77.6 | 55.3 | 0.805 | 1.152 | 63.0 | 53.2 |
| Shellfish (17) | 0.117 | 0.166 | 6.8 | 5.2 | 0.066 | 0.104 | 3.9 | 3.7 | 0.088 | 0.128 | 8.2 | 6.7 | 0.060 | 0.089 | 9.6 | 7.7 | 0.123 | 0.179 | 8.5 | 7.6 | 0.084 | 0.117 | 11.6 | 7.4 | 0.111 | 0.162 | 8.7 | 7.5 |
| Cephalopoda (8) | 0.129 | 0.187 | 7.5 | 5.8 | 0.093 | 0.222 | 5.5 | 7.8 | 0.096 | 0.184 | 9.0 | 9.6 | 0.055 | 0.134 | 8.8 | 11.6 | 0.078 | 0.181 | 5.4 | 7.7 | 0.014 | 0.066 | 1.9 | 4.2 | 0.069 | 0.164 | 5.4 | 7.6 |
| Crustacea (8) | 0.005 | 0.103 | 0.3 | 3.2 | 0.006 | 0.116 | 0.4 | 4.1 | 0.006 | 0.088 | 0.5 | 4.6 | 0.002 | 0.060 | 0.3 | 5.2 | 0.004 | 0.067 | 0.3 | 2.8 | 0.004 | 0.041 | 0.5 | 2.6 | 0.004 | 0.065 | 0.3 | 3.0 |
| Sea algae (16) | 0.012 | 0.850 | 0.7 | 26.5 | 0.014 | 0.523 | 0.9 | 18.5 | 0.009 | 0.354 | 0.8 | 18.4 | 0.004 | 0.170 | 0.6 | 14.8 | 0.005 | 0.327 | 0.4 | 13.9 | 0.004 | 0.421 | 0.6 | 26.7 | 0.005 | 0.333 | 0.4 | 15.4 |
| Echinodermata (2) | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.076 | 0.076 | 7.1 | 4.0 | 0.021 | 0.022 | 3.4 | 1.9 | 0.350 | 0.350 | 24.3 | 14.8 | 0.048 | 0.048 | 6.6 | 3.1 | 0.269 | 0.270 | 21.1 | 12.5 |
| Tunicata (2) | 0.000 | 0.001 | 0.0 | 0.0 | 0.009 | 0.010 | 0.5 | 0.4 | 0.005 | 0.007 | 0.5 | 0.3 | 0.015 | 0.018 | 2.5 | 1.6 | 0.015 | 0.019 | 1.0 | 0.8 | 0.008 | 0.011 | 1.2 | 0.7 | 0.014 | 0.017 | 1.1 | 0.8 |
| Cnidaria (1) | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.001 | 0.002 | 0.1 | 0.1 | 0.000 | 0.001 | 0.0 | 0.1 | 0.001 | 0.002 | 0.0 | 0.1 |
| Total marine products (109) | 1.731 | 3.212 | 100.0 | 100.0 | 1.673 | 2.833 | 100.0 | 100.0 | 1.071 | 1.926 | 100.0 | 100.0 | 0.623 | 1.151 | 100.0 | 100.0 | 1.440 | 2.360 | 100.0 | 100.0 | 0.724 | 1.578 | 100.0 | 100.0 | 1.278 | 2.164 | 100.0 | 100.0 |
| b) | ||||||||||||||||||||||||||||
| Category | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB |
| (Number of composite samples) | ||||||||||||||||||||||||||||
| Fish (55) | 0.538 | 1.098 | 96.6 | 53.0 | 0.580 | 1.033 | 97.3 | 55.9 | 0.294 | 0.652 | 91.0 | 52.2 | 0.294 | 0.652 | 91.0 | 52.2 | 0.362 | 0.787 | 81.7 | 54.7 | 0.243 | 0.603 | 93.2 | 53.9 | 0.334 | 0.735 | 83.7 | 54.6 |
| Shellfish (17) | 0.006 | 0.083 | 1.2 | 4.0 | 0.005 | 0.062 | 0.8 | 3.4 | 0.006 | 0.069 | 1.9 | 5.5 | 0.006 | 0.069 | 1.9 | 5.5 | 0.009 | 0.100 | 2.1 | 7.0 | 0.006 | 0.058 | 2.2 | 5.2 | 0.008 | 0.085 | 2.1 | 6.3 |
| Cephalopoda (8) | 0.009 | 0.099 | 1.7 | 4.8 | 0.007 | 0.183 | 1.1 | 9.9 | 0.007 | 0.130 | 2.2 | 10.4 | 0.007 | 0.130 | 2.2 | 10.4 | 0.006 | 0.143 | 1.3 | 9.9 | 0.001 | 0.068 | 0.4 | 6.1 | 0.005 | 0.131 | 1.2 | 9.7 |
| Crustacea (8) | 0.000 | 0.113 | 0.0 | 5.5 | 0.000 | 0.127 | 0.1 | 6.9 | 0.000 | 0.096 | 0.1 | 7.7 | 0.000 | 0.096 | 0.1 | 7.7 | 0.000 | 0.073 | 0.0 | 5.1 | 0.000 | 0.044 | 0.1 | 3.9 | 0.000 | 0.071 | 0.0 | 5.3 |
| Sea algae (16) | 0.003 | 0.677 | 0.5 | 32.7 | 0.003 | 0.437 | 0.5 | 23.7 | 0.001 | 0.284 | 0.5 | 22.8 | 0.001 | 0.284 | 0.5 | 22.8 | 0.001 | 0.262 | 0.2 | 18.2 | 0.001 | 0.331 | 0.4 | 29.6 | 0.001 | 0.266 | 0.3 | 19.8 |
| Echinodermata (2) | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.014 | 0.014 | 4.2 | 1.1 | 0.014 | 0.014 | 4.2 | 1.1 | 0.063 | 0.064 | 14.2 | 4.4 | 0.009 | 0.009 | 3.3 | 0.8 | 0.048 | 0.049 | 12.2 | 3.6 |
| Tunicata (2) | 0.000 | 0.001 | 0.0 | 0.1 | 0.001 | 0.005 | 0.2 | 0.2 | 0.001 | 0.003 | 0.2 | 0.3 | 0.001 | 0.003 | 0.2 | 0.3 | 0.002 | 0.009 | 0.4 | 0.6 | 0.001 | 0.005 | 0.4 | 0.4 | 0.002 | 0.008 | 0.4 | 0.6 |
| Cnidaria (1) | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.000 | 0.0 | 0.0 | 0.000 | 0.002 | 0.0 | 0.1 | 0.000 | 0.001 | 0.0 | 0.1 | 0.000 | 0.002 | 0.0 | 0.1 |
| Total marine products (109) | 0.557 | 2.071 | 100.0 | 100.0 | 0.596 | 1.848 | 100.0 | 100.0 | 0.323 | 1.248 | 100.0 | 100.0 | 0.323 | 1.248 | 100.0 | 100.0 | 0.443 | 1.438 | 100.0 | 100.0 | 0.261 | 1.119 | 100.0 | 100.0 | 0.399 | 1.347 | 100.0 | 100.0 |
The results of below LOD are substituted for zero at the LB, while the results are replaced by LOD at the UB (EFSA, 2010; WHO/IPCS, 2009)
In this study, dietary exposure to PAHs from fish is higher than from shellfish, cephalopods and crustaceans. The average dietary intake for the fish category was found to be 0.805 ng/kg bw/d for ∑PAH8 and 0.334 ng/kg bw/d for TEQB[a]P in the LB scenario, while in the UB scenario, it was 1.152 ng/kg bw/d for ∑PAH8 and 0.735 ng/kg bw/d for TEQB[a]P for the total population, whereas the overall dietary exposure for the shellfish, cephalopods, and crustaceans was 0.184 ng/kg bw/d for ∑PAH8 and 0.013 ng/kg bw/d for TEQB[a]P under the LB scenario, and 0.391 ng/kg bw/d for ∑PAH8 and 0.287 ng/kg bw/d for TEQB[a]P under the UB scenario for total population (Table 3). However, it is important to note that shellfish, cephalopods, and crustaceans constituted the primary contributors to PAH exposure during the Second French TDS (Veyrand et al., 2013), which is believed to be due to varying consumption habits among country. In their investigation, the average dietary exposure from fish was 0.03 ng/kg bw/d for adults and 0.07 ng/kg bw/d for children, compared to 0.193 ng/kg bw/d for adults and 0.098 ng/kg bw/d for children from mollusks and crustaceans. As seen in Table 3, fish products were the highest contributor to dietary exposure in this study, ranging from 63.0% for ∑PAH8 (83.7% for TEQB[a]P) in the total population to 88.8% for ∑PAH8 (97.3% for TEQB[a]P) in the 3–6 year old population using the LB value, and from 53.2% for ∑PAH8 (54.6% for TEQB[a]P) in the total population to 65.6% for ∑PAH8 (55.9% for TEQB[a]P) in the 3–6 year old population using the UB value. Sea algae was the next major contributor, ranging from 15.4% for ∑PAH8 (19.8% for TEQB[a]P) in the total population to for 18.5% ∑PAH8 (23.7% for TEQB[a]P) in 3–6 years population under the UB scenario, while its contribution was smaller under the LB scenario. The high consumption level of sea algae appears to result from its significant contribution, despite its low contamination level. Particularly in the 20–64 age group under the LB scenario, Echinodermata, including dried sea cucumber, also made a considerable contribution of 24.3% for ∑PAH8 (14.2% for TEQB[a]P). The marine subsample in Supplementary Table 1 and 2 with the highest dietary exposure level was dried sea cucumber, which the adult group consumed more than the other groups.
The MOE approach was used for the risk assessment. According to the findings of this study, independent of age groups or marine products, all MOEs were above 10,000 (Table 4). The calculated MOEs were about 1,055,476,358 for ∑PAH8 (6,469,460,509 for TEQB[a]P) at the LB scenario and 346,669,319 for ∑PAH8 (407,307,792) for TEQB[a]P) at the UB scenario for the total population. It's important to note that the MOEs for the calculated equivalent B[a]P, as determined by TEQ (TEQB[a]P), in the case of the other 7 PAHs, ranged from 1.2 times higher in the UB scenario to 6.1 times higher in the LB scenario compared to those of ∑PAH8. All MOEs exceeded 1.0 × 104, indicating that the risk assessment results indicate a low level of concern from a public perspective regarding health risks associated with dietary exposure to PAH8 from marine products. In the 1–2 age group under the UB scenario, the risk of PAH8 from fish diet had the lowest MOE value (257,240 for ∑PAH8 and 446,434 for TEQB[a]P), followed by the risk of PAH8 through sea algae (MOE value: 576,548 for ∑PAH8 and 724,207 for TEQB[a]P).
Table 4.
Margin of exposure (MOE) of a) total PAH8 concentration (∑PAH8) and b) total B[a]P toxic equivalent quantity (TEQB[a]p) of PAH8, with contributions from marine products, for the total consumer population and different age groups, with lower bound (LB) and upper bound (UB) estimates
| Category | 1–2 years (n = 519) | 3–6 years (n = 1062) | 7–12 years (n = 1601) | 13–19 years (n = 1629) | 20–64 years (n = 11,592) | over 64 years (n = 4268) | Total population (n = 20,671) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Number of composite samples) | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB |
| a) | ||||||||||||||
| Fish (55) | 334,126 | 257,240 | 329,672 | 263,828 | 619,093 | 449,885 | 1,051,318 | 744,090 | 566,051 | 396,746 | 872,216 | 561,154 | 608,627 | 425,384 |
| Shellfish (17) | 4,173,301 | 2,948,542 | 7,478,775 | 4,722,478 | 5,589,635 | 3,822,012 | 8,217,150 | 5,511,352 | 3,998,845 | 2,730,755 | 5,835,694 | 4,201,324 | 4,413,599 | 3,025,218 |
| Cephalopoda (8) | 3,794,026 | 2,616,220 | 5,293,790 | 2,205,153 | 5,108,335 | 2,656,666 | 8,889,355 | 3,656,622 | 6,277,911 | 2,703,296 | 35,798,002 | 7,379,119 | 7,054,954 | 2,978,939 |
| Crustacea (8) | 94,098,922 | 4,771,464 | 77,683,847 | 4,219,418 | 84,572,057 | 5,571,220 | 313,668,411 | 8,208,975 | 135,702,943 | 7,360,908 | 123,900,514 | 11,871,437 | 135,341,634 | 7,565,106 |
| Sea algae (16) | 39,768,295 | 576,548 | 34,393,293 | 936,354 | 54,995,892 | 1,386,110 | 130,885,860 | 2,881,249 | 97,036,196 | 1,498,867 | 118,496,348 | 1,163,862 | 95,388,260 | 1,472,543 |
| Echinodermata (2) | – | – | – | – | 6,445,122 | 6,433,294 | 22,903,645 | 22,647,957 | 1,401,658 | 1,399,306 | 10,230,093 | 10,164,192 | 1,821,130 | 1,817,747 |
| Tunicata (2) | 1,649,994,173 | 347,735,179 | 57,460,091 | 46,883,430 | 99,486,485 | 75,014,965 | 31,639,822 | 27,095,451 | 33,139,024 | 26,196,820 | 58,251,076 | 45,630,811 | 35,877,873 | 28,543,013 |
| Cnidaria (1) | – | – | – | – | 9,316,679,023 | 3,616,709,611 | 4,707,911,175 | 1,827,598,391 | 624,141,042 | 242,289,865 | 1,542,127,897 | 598,649,881 | 774,970,282 | 300,841,368 |
| Total marine products (109) | 1,792,162,843 | 358,905,194 | 182,639,467 | 59,230,660 | 9,573,495,642 | 3,712,043,762 | 5,225,166,737 | 1,898,344,087 | 902,263,669 | 284,576,563 | 1,895,511,840 | 679,621,779 | 1,055,476,358 | 346,669,319 |
| b) | ||||||||||||||
| Fish (55) | 910,244 | 446,434 | 844,739 | 474,122 | 1,667,605 | 751,356 | 2,766,079 | 1,206,274 | 1,354,396 | 622,877 | 2,016,766 | 812,546 | 1,467,855 | 666,886 |
| Shellfish (17) | 76,293,931 | 5,884,162 | 100,892,860 | 7,858,807 | 80,115,752 | 7,115,484 | 125,170,607 | 9,945,102 | 51,909,404 | 4,900,896 | 83,707,628 | 8,376,380 | 58,483,416 | 5,755,430 |
| Cephalopoda (8) | 53,148,375 | 4,951,244 | 73,248,030 | 2,678,672 | 69,919,626 | 3,767,841 | 125,452,128 | 4,489,832 | 88,277,852 | 3,436,359 | 446,805,458 | 7,235,757 | 98,751,030 | 3,738,716 |
| Crustacea (8) | 1,770,251,200 | 4,339,809 | 1,461,439,931 | 3,848,072 | 1,591,025,493 | 5,125,266 | 5,900,937,719 | 7,332,007 | 2,552,933,572 | 6,712,720 | 2,330,898,461 | 11,173,842 | 2,546,136,377 | 6,907,488 |
| Sea algae (16) | 179,469,856 | 724,207 | 156,997,074 | 1,120,714 | 336,997,268 | 1,723,959 | 693,764,875 | 3,533,473 | 491,833,900 | 1,871,167 | 536,103,140 | 1,480,177 | 482,062,531 | 1,840,611 |
| Echinodermata (2) | – | – | – | – | 35,763,094 | 35,292,123 | 127,177,853 | 117,539,177 | 7,777,516 | 7,683,768 | 56,784,840 | 54,231,720 | 10,105,218 | 9,970,520 |
| Tunicata (2) | 16,499,941,725 | 334,063,204 | 451,146,740 | 107,479,626 | 785,944,268 | 150,611,606 | 247,544,766 | 68,457,950 | 260,817,831 | 56,751,502 | 458,784,952 | 97,342,423 | 282,235,083 | 62,517,277 |
| Cnidaria (1) | – | – | – | – | 35,948,359,927 | 3,797,862,795 | 18,165,451,982 | 1,919,138,853 | 2,408,245,125 | 254,425,642 | 5,950,292,862 | 628,634,962 | 2,990,218,999 | 315,909,864 |
| Total marine products (109) | 18,580,015,331 | 350,409,060 | 2,244,569,374 | 123,460,013 | 38,849,793,033 | 4,002,250,430 | 25,388,266,009 | 2,131,642,668 | 5,863,149,596 | 336,404,931 | 9,865,394,107 | 809,287,807 | 6,469,460,509 | 407,306,792 |
Individual MOE values for composite samples are further included in Supplementary Tables 1 and 2. Considering the total population based on KNHANES (MOHW, 2013), the MOE for all individual samples was above 10,000. When considering only those people in the consumption group who consumed the specific food samples listed in the KNHANES under the UB scenario, dried sea cucumber had the lowest MOE value, with 6,201 for ∑PAH8 and 38,651 for TEQB[a]P. Salted mackerel had the second lowest MOE values, with 36,481 for ∑PAH8 and 87,244 for TEQB[a]P. This is attributed to their elevated levels of PAH8 and substantial consumption rates. On the other hand, katsuobushi showed relatively high MOE value (758,956 for ∑PAH8 and 4,934,786 for TEQB[a]P), even though it contained high PAH8 level. Katsuobushi is typically used as a stock ingredient or spice in Korean cuisine, resulting in modest daily intakes. Another study also reported that Katsuobushi had comparably low daily exposure to PAHs because to its low consumption rate (Lee et al., 2018).
In conclusion, this study found that fish is the primary source of PAH exposure in marine products, and that grilling, smoking, and drying processes increase PAH levels in food. The estimated risk of exposure to eight PAHs using TEFs may be an overestimation. It is advisable for many studies using TEFs to consider using the total concentration and the toxicological values for PAH mixtures. Following a risk assessment using a MOE approach in a TDS, PAH8 exposure from marine products is considered low for the South Korean population. The findings of this study can serve as a foundation for developing strategies to reduce PAH levels in food and to identify the most effective ways for consuming marine products.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by a grant (18162MFDS053) from the Ministry of Food and Drug Safety and a Korea University Grant (K2202721). and the School of Life Sciences & Biotechnology of Korea University for BK21PLUS. The authors are grateful to the Korea University-CJ Food Safety Center (Seoul, Republic of Korea) for allowing access to their equipment and facilities.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas I, Badran G, Verdin A, Ledoux F, Roumié M, Courcot D, Garçon G. Polycyclic aromatic hydrocarbon derivatives in airborne particulate matter: sources, analysis and toxicity. Environ Chem Lett 16: 439-75 (2018) 10.1007/s10311-017-0697-0 [DOI] [Google Scholar]
- Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, Ahmed N, Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 22: 2028-2035 (2011) 10.1016/j.foodcont.2011.05.024 [DOI] [Google Scholar]
- Codex. Guidelines on good laboratory practice in pesticide residue analysis CAC/GL 40 (1993).
- Codex. Guidelines on Measurement Uncertainty, CAC/GL 54–2004 (2011).
- EC. Commission Regulation (EU) 2020/1255 of 7 September 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Polycyclic Aromatic Hydrocarbons (PAHs) in Traditionally Smoked Meat and Smoked Meat Products and Traditionally Smoked Fish and Smoked Fishery Products and Establishing a Maximum Level of PAHs in Powders of Food of Plant Origin Used for the Preparation of Beverages. Off J Eur Union 293: 1-4 (2020)
- EC. Commission Regulation (EU) 2020/1255 of 7 September 2020 amending Regulation (EC) No 1881/2006. Off J Eur Union 2016: 48–119 (2018).
- EC. Commission Regulation (EU) 2015/1125 of 10 July 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in Katsuobushi (dried bonito) and certain smoked Baltic herring (Text with EEA relevance). Off J Eur Union 184: 7-10 (2015)
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to a harmonized approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 3: 1-31 (2005) [Google Scholar]
- EFSA. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on Polycyclic Aromatic Hydrocarbons in Food. EFSA Journal 724: 1-114 (2008) [Google Scholar]
- EFSA. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3): 1557 (2010) [Google Scholar]
- EURACHEM/CITAC. Quantifying Uncertainty in Analytical Measurements (2012).
- Fan X, Ma Y, Li M, Li Y, Sang X, Zhao Q. Thermal treatments and their influence on physicochemical properties of sea cucumbers: a comprehensive review. Int J Food Sci Tech 57(9): 5790-5800 (2022) 10.1111/ijfs.15922 [DOI] [Google Scholar]
- FAO. Towards a harmonised Total Diet Study approach: a guidance document. EFSA Journal 9(11): 2450 (2011) [Google Scholar]
- Fernando H, Ju H, Kakumanu R, Bhopale KK, Croisant S, Elferink C, Kaphalia BS, Ansari GAS. Distribution of petrogenic polycyclic aromatic hydrocarbons (PAHs) in seafood following Deepwater Horizon oil spill. Mar Pollut Bull 145: 200-207 (2019) 10.1016/j.marpolbul.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibullah-Al-Mamun M, Ahmed MK, Islam MS, Tokumura M, Masunaga S. Distribution of polycyclic aromatic hydrocarbons (PAHs) in commonly consumed seafood from coastal areas of Bangladesh and associated human health implications. Environ Geochem Health 41: 1105-1121 (2019) 10.1007/s10653-018-0202-0 [DOI] [PubMed] [Google Scholar]
- Ingenbleek L, Jazet E, Dzossa AD, Adebayo SB, Ogungbangbe J, Dansou S, Diallo ZJ, Kouebou C, Adegboye A, Hossou E, Coulibaly S, Eyangoh S, Le Bizec B, Verger P, Kamanzi J, Merten C, Leblanc JC. Methodology design of the regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria. Food Chem Toxicol 109: 155-169 (2017) 10.1016/j.fct.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Kafouris D, Koukkidou A, Christou E, Hadjigeorgiou M, Yiannopoulos S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Science 164: 108088 (2020) 10.1016/j.meatsci.2020.108088 [DOI] [PubMed] [Google Scholar]
- Kim H-S, Kim J, Choi J, Paik Y, Moon B, Joo Y-S, Lee K-W. Polycyclic aromatic hydrocarbons in beverage and dairy products in South Korea: a risk characterization using the total diet study. Food Science and Biotechnology 30: 989-1002 (2021) 10.1007/s10068-021-00927-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts DD, Chen XM, Broda P. Polyaromatic hydrocarbons of smoked cured muscle foods prepared by Canadian Tl’azt’en and Llheidli T’enneh first nation communities. J Toxicol Environ Heal - Part A Curr Issues; 75: 1249-52 (2012) 10.1080/15287394.2012.709410 [DOI] [PubMed] [Google Scholar]
- Kroese ED, Muller JJ, Mohn GR, Dortant PM, Wester PW. Tumorigenic effects in Wistar rats orally administered benzo [a] pyrene for two years (gavage studies). Implications for human cancer risks associated with oral exposure to polycyclic aromatic hydrocarbons (2002)=
- Lee J, Jeong J-H, Park S, Lee K-G. Monitoring and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in processed foods and their raw materials. Food Control 92: 286-292 (2018) 10.1016/j.foodcont.2018.05.012 [DOI] [Google Scholar]
- Llobet JM, Falcó G, Bocio A, Domingo JL. Exposure to polycyclic aromatic hydrocarbons through consumption of edible marine species in Catalonia, Spain. J Food Prot 69: 2493-9 (2006).= 10.4315/0362-028X-69.10.2493 [DOI] [PubMed] [Google Scholar]
- MFDS. Sample Preparation Methods Guidebook for Total Diet Study. Ministry of Food and Drug Safety of South Korea (2017)
- MFDS. Evaluation Report of Standards and Specifications of Benzoapyrene in Foods. Ministry of Food and Drug Safety of South Korea: 23 (2019) 10.1007/s10068-021-00943-7 [DOI] [Google Scholar]
- MFDS. Food Code. Ministry of Food and Drug Safety of South Korea (2020).
- MOHW. Korea health statistics 2013 : Korea national health and nutrition examination survey (KNHANES VI-1). Ministry of Health and Welfare of South Korea (2014)
- Nisbet IC, LaGoy PK. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16: 290-300 (1992) 10.1016/0273-2300(92)90009-X [DOI] [PubMed] [Google Scholar]
- Ofosu IW, Larbi EA, Alale D, Ankar-Brewoo GM, Lutterodt HE. Cooked rice products (Kwenkwen, Jollof, Fried-rice, Angwamo and Kanzo) as sources of polyaromatic hydrocarbons and a potential public health concern. J Food Nutr Res 10(7): 467-475 (2022) 10.12691/jfnr-10-7-4 [DOI] [Google Scholar]
- Oz E. Effects of smoke flavoring using different wood chips and barbecuing on the formation of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in salmon fillets. PLoS One 14;15(1): e0227508 (2020) 10.1371/journal.pone.0227508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res 443: 139-47 (1999). 10.1016/S1383-5742(99)00016-2 [DOI] [PubMed] [Google Scholar]
- Sahin S, Ulusoy HI, Alemdar S, Erdogan S, Agaoglu S. The presence of polycyclic aromatic hydrocarbons (PAHs) in grilled beef, chicken and fish by considering dietary exposure and risk assessment. Food Sci Anim Resour 40(5): 675 (2020) 10.5851/kosfa.2020.e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi T, Adachi R, Matsuda R, Watanabe T, Teshima R, Akiyama H. Concentrations of Polycyclic Aromatic Hydrocarbons in Smoked Foods in Japan. Journal of Food Protection 83: 692-701 (2019) 10.4315/JFP-19-486 [DOI] [PubMed] [Google Scholar]
- Veyrand B, Sirot V, Durand S, Pollono C, Marchand P, Dervilly-Pinel G, Tard A, Leblanc JC, Le Bizec B. Human dietary exposure to polycyclic aromatic hydrocarbons: results of the second French Total Diet Study. Environ Int 54: 11-7 (2013) 10.1016/j.envint.2012.12.011 [DOI] [PubMed] [Google Scholar]
- WHO/IPCS. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety. Environmental Health Criteria 240 (2009)
- Yan B, Abrajano TA, Bopp RF, Chaky DA, Benedict LA, Chillrud SN. Molecular tracers of saturated and polycyclic aromatic hydrocarbon inputs into Central Park Lake, New York City. Environ Sci Technol 39: 7012-7019 (2005) 10.1021/es0506105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Sylvestre S, Mitchell RH, Goyette D. PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry 33: 489-515 (2002) 10.1016/S0146-6380(02)00002-5 [DOI] [Google Scholar]
- Zaidi AJ, Ahsan H, Munshi AB. A review on cancer probability in human beings due to environmental impact of polycyclic aromatic hydrocarbons (PAHs) and remediation. Pakistan J Sci Ind R Series A: Phy Sci 64(3):275-86 (2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.