Abstract
EBUS-guided transbronchial mediastinal cryobiopsy (TBMC) has emerged as a promising biopsy tool for diagnosing hilar and mediastinal pathologies. However, several fundamental technical aspects of TBMC remain unexplored. This study aims to determine the optimal number of cryo-passes and freezing time of the ultrathin cryoprobe in EBUS-TBMC concerning specimen size and procedural diagnostic yield. We conducted a retrospective chart review of patients with mediastinal and hilar lesions who underwent EBUS-TBMC between January 2021 and April 2023 across three hospitals in Malaysia. A total of 129 EBUS-TBMC procedures were successfully completed, achieving an overall diagnostic yield of 88.4%. Conclusive TBMC procedures were associated with larger specimen sizes (7.0 vs. 5.0 mm, p < 0.01). Specimen size demonstrated a positive correlation with diagnostic yield (p < 0.01), plateauing at specimen size of 4.1–6.0 mm. A significant positive correlation was also observed between the number of cryo-passes and both specimen size (p < 0.01) and diagnostic yield (p < 0.05). Diagnostic yield plateaued after 2–3 cryo-passes. In contrast, longer freezing times trended towards smaller specimens and lower diagnostic yield, though not reaching statistical significance. The highest diagnostic yield was recorded at the 3.1–4.0 s freezing time. The safety profile of TBMC remains favourable, with one case (0.8%) of pneumothorax and nine cases (7%) of self-limiting bleeding. In our cohort, TBMC performance with 2–3 cryo-passes and a 3.1–4.0 s freezing time to achieve a total aggregate specimen size of 4.1–6.0 mm appeared optimal. Further prospective studies are needed to validate these findings.
Keywords: Endobronchial ultrasound, Mediastinal cryobiopsy, Freezing time, Cryo-pass, Transbronchial needle aspiration
Subject terms: Cancer imaging, Lung cancer, Cancer imaging
Introduction
Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) is a revolutionary diagnostic tool in the field of interventional pulmonology1,2. It allows precise access to mediastinal, hilar, and peribronchial structures—traditionally only reachable via invasive techniques such as mediastinoscopy1. These advantages, coupled with an excellent safety profile, solidifies EBUS-TBNA as the recommended tool for diagnosis and staging of lung cancer according to major guidelines3,4.
However, beyond its established role in lung cancer staging and in diagnosing common malignancies, the application of EBUS-TBNA in uncommon tumours such as lymphoproliferative disorders or benign entities such as sarcoidosis remains a subject of debate5. TBNA primarily provides cytology or cell block samples, which may be insufficient for pathologies with complex morphology which frequently requiring sophisticated immunohistochemical or molecular analyses6,7. Different techniques have been described to enhance the diagnostic capabilities of EBUS-TBNA, from using core biopsy needles to exploring different EBUS needle types and sizes as well as performing intra-nodal forceps (IFB) biopsies8–10. However, small sample sizes and crushed artefacts are among the main drawbacks of these supplementary techniques.
The idea of acquiring larger mediastinal samples with preserved tissue architecture has catapulted the idea of performing EBUS-guided transbronchial mediastinal cryobiopsy (EBUS-TBMC). EBUS-TBMC was made possible with concurrent miniaturization of flexible cryoprobes, allowing insertion of the 1.1 mm ultrathin cryoprobe via the EBUS working channel directly into mediastinal targets under real time ultrasound vision. The first description of EBUS-TBMC was back in 2020, followed by rapid emergence of multiple case reports and case series from various regions worldwide11–15. Subsequent randomized controlled trials have demonstrated the feasibility and safety of EBUS-TBMC, highlighting its superiority, particularly in the diagnosis of uncommon tumours and benign disorders16–18.
Nevertheless, a few fundamental technical aspects of EBUS-TBMC are still unanswered. To address these knowledge gaps, we performed an explorative analysis on the optimal number of cryo-passes and freezing time, and their impacts on the specimen sizes and diagnostic yield in EBUS-TBMC procedures. As a secondary endpoint, we also aimed to assess the efficacy and safety of EBUS-TBMC in a real-world setting.
Methods
Study design and setting
This retrospective, multi-centre study was conducted across three hospitals in Malaysia, including 2 in the island of Borneo: Sarawak General Hospital (SGH) in the region of Sarawak and Queen Elizabeth Hospital (QEH) in Sabah. The third hospital, University Malaya Medical Centre (UMMC), is in the state of Selangor, Peninsular Malaysia. The study spanned a total duration of 28 months, from January 1, 2021, to April 30, 2023. Adhering to the principles of the Declaration of Helsinki, the study obtained approval from the Medical Research and Ethics Committee, Ministry of Health Malaysia [NMRR-ID-23–00,577-6XP (IIR), dated June 2, 2023] in which individual consent for this study was waived according to the approved protocol.
EBUS guided transbronchial mediastinal cryobiopsy (EBUS/TBMC)
Pre-procedural planning and case selection
Patients selected for EBUS-TBNA were evaluated for any potential contraindications, such as bleeding diatheses. Medications such as anti-coagulants were suspended prior to procedure in accordance to recommended guidelines19.
All patients scheduled for EBUS-TBNA procedure were considered for additional TBMC procedure under the jurisdiction of the managing physician. Notably, there were no strict selection criteria for TBMC procedure in our study, TBMC were considered in the following situations, namely: when uncommon tumours or benign disorders were suspected prior to the procedure or when specific immune-histochemical or molecular analyses were considered necessary.
TBMC was generally avoided on necrotic or cystic lymph nodes or when purulent materials were aspirated on TBNA, as they may suggest a higher pre-test probability of infection, especially tuberculosis in our region of practice. For TBMC, care was taken to avoid puncturing any target with abnormal overlying bronchial mucosa to mitigate the theoretical risk of poor wound healing, potentially leading to fistula formation.
Sedation and airway access
EBUS procedures were conducted by consultant pulmonologists with expertise in flexible and rigid bronchoscopy. Flexible bronchoscopy was first performed under conscious sedation or total intravenous anaesthesia in accordance with individual unit protocols. The decision for advanced airways (i.e., rigid bronchoscope, endotracheal intubation, laryngeal mask airway) was made at discretion of the bronchoscopist, considering the risks and benefits of the procedure for the patient20.
Various flexible echo-bronchoscopes (EB1970UK, Pentax Medical, Japan; BF-UC180F, BF-UC190F, Olympus Medical, Japan; EB530US, Fujifilm, Japan) were utilized for the procedures. Systematic examination of mediastinal and hilar lymph nodes was performed after endobronchial abnormalities were excluded during initial airway inspection by flexible bronchoscopy1,3. Each lymph node station was assessed, and their parameters (size, shape, border, central hilar structure, vascularity on Doppler assessment) were recorded.
EBUS-TBNA procedure
TBNA was conducted using needles of various sizes from 19 to 25 gauge (ViziShot 2, ViziShot FLEX, Olympus Medical, Japan; Echotip® Ultra HD™, ProCore™, Cook Medical, Inc. USA) determined by bronchoscopist discretion following standard procedural steps1. A minimum of three aspirations were performed with or without suction. Efforts were made to puncture through the same nodal entry point during each TBNA whenever possible to facilitate formation of a needle track for TBMC later. Pathologist-led rapid onsite cytology evaluation (ROSE) was routinely available only in QEH. The aspirated material was then transferred to a liquid-based cell-block for cytological analysis. In some cases, TBMC were performed upfront without TBNA.
EBUS-TBMC procedure
As mentioned, the target lesion was deliberately punctured with a TBNA needle multiple times at the exact same spot to facilitate creation of a needle tract, enabling easy insertion of a cryoprobe into the target lesion during TBMC later. An alternative approach was done using a 1.9mm high-frequency needle-knife (KD-31C-1, Olympus, Japan) to create a mucosal defect at the puncture site. Subsequently, the 1.1mm flexible ultrathin cryoprobe (ERBE Medizintechnik, Tübingen, Germany) was inserted through the working channel of the echobronchoscope, positioned at the mucosal defect, and directed into the core of the target lesion.
Once the cryoprobe was confirmed to be in the desired position on EBUS, Doppler ultrasound was activated to ensure that there were no significant surrounding vessels. The cryoprobe was then activated, and the echobronchoscope-cryoprobe assembly was removed en-bloc from the patient's natural or artificial airway. Upon successful specimen retrieval, the cryo-specimen was thawed in normal saline and immediately fixed in formalin for subsequent histological analysis. The freezing time and number of passes were not fixed and were adjusted based on the retrieved specimen. Generally, an incremental freezing time starting from 3–4 s was employed, and the procedure was repeated until the obtained specimen was deemed adequate by the bronchoscopist, barring any complications.
Post-procedural care
After the procedure, a routine chest radiograph was conducted to assess for the presence of pneumothorax and pneumomediastinum. Before discharging the patient, specific inquiries were made to detect potential signs and symptoms of vocal cord injury or mediastinitis. Prophylactic antibiotics were not routinely prescribed post-procedure. Patients were typically discharged 24 h after procedure with follow-up clinic appointments arranged in 2 weeks. We did not routinely perform repeat surveillance bronchoscopy unless clinically indicated or in the presence of suspected procedural complications, such as pneumothorax, pneumomediastinum, infection, etc.
Definition
Diagnostic yield
Overall EBUS procedure was deemed conclusive if the specimens provided a formal cytological or histopathological diagnosis (pathologically confirmed malignancy or specific benign disease). The presence of a preponderance of small mature lymphocytes in EBUS-TBNA smears or cell block and/or lymphoid tissue in EBUS-TBMC specimen were considered representative sampling and deemed diagnostic only after at least six months of radiological surveillance which confirmed clinical stability16–18,21.
Freezing time and specimen size
The total freezing time for each TBMC procedure was recorded. The average freezing time per cryo-pass was then calculated by dividing the total freezing time by the number of cryo-passes. A representative case and calculation were illustrated in Supplementary Material. The specimen size in total aggregate diameter was calculated summing each largest diameter of the any single sample measured under the microscope.
Common malignancy vs. uncommon tumours and benign disorders
For the purposes of the study, all non-small cell lung carcinoma, small cell carcinoma, and metastatic carcinoma, were classified as common malignancy. Any other diagnosis was classified as uncommon tumours (i.e., lymphoproliferative malignancy, germ cell tumour, thymic malignancy, etc.) or benign disorders (benign tumour or infective and inflammatory causes)16–18.
Statistical analysis
Data analysis was conducted using SPSS software (version 20; Chicago, IL, USA). The Shapiro–Wilk test was employed to assess normality. Normally distributed variables were presented as mean ± standard deviation (SD), while non-normally distributed variables were expressed as median and interquartile range (IQR). Categorical data were presented as absolute numbers and percentages, and comparisons were made using Pearson’s Chi-squared test or Fisher’s exact test. Independent sample t-tests and Mann–Whitney tests were utilized to compare normally and non-normally distributed variables between groups, respectively. A significance level of < 0.05 was applied.
Ethical approval
The study protocol was approved by the medical research and ethics committee, Ministry of Health Malaysia, NMRR-ID-23-00,577-6XP (IIR).
Results
Throughout the study period, a total of 222 EBUS procedures were performed across all sites. TBMC was attempted in 137 cases (61.7%). Among these cases, the cryoprobe failed to be inserted in 8 cases (5.8%), resulting in an overall technical feasibility of 94.2% (Supplementary Table 1). In these eight cases, the blunt cryoprobe failed to be inserted into the target despite using a high-frequency needle knife and TBNA needle due to the tough and hard capsular wall of the lymph nodes (Supplementary Table 2). Consequently, a total of 129 EBUS-TBMC procedures were available for analysis. A representative case was shown in Fig. 1.
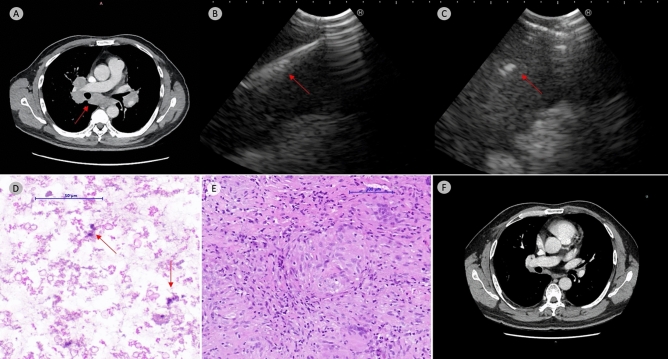
Figure 1.
Representative case. Contrast enhanced computed tomography show bilateral hilar and mediastinal lymphadenopathy (arrow, Panel A). EBUS-TBNA was performed with 22-gauge TBNA needle with a total of 5 passes (arrow, Panel B). 1.1 mm cryoprobe was then inserted after mucosal incision with high-frequency needle knife (arrow, Panel B); a total of 5 cryo-passes was performed with total freezing time of 22 s (average 4.4 s freezing time per cryo-pass). TBNA cell block revealed only cellular debris with scanty macrophages (arrow, hematoxylin & eosin stain, Panel D). Collectively, the TBMC specimen measured 11 mm in total aggregate diameter which demonstrated a well-formed non-caseating granuloma consistent with clinical suspicion of sarcoidosis. (hematoxylin & eosin stain, Panel E). Post treatment with steroid and methotrexate demonstrate complete resolution of hilar and mediastinal lymphadenopathy (Panel F).
Overall demographic and target lesions characteristic
The median age of our cohort was 63 (IQR 53–69) years, with two-thirds being male. Approximately half of the cases (51.2%) were performed on an outpatient basis. Concerning the target lesions, 87.6% were hilar or mediastinal lymph nodes, while 12.4% were peribronchial central lung masses. The median size for hilar or mediastinal lymph nodes was 17.0 (IQR 12.1–25.5) mm and for peribronchial central lung masses was 17.0 (IQR 11.0–37.3) mm. Lymph nodes were sampled in various stations, with the majority in station 4R (36.3%) and 7 (35.4%), followed by 11Rs/11Ri (15.9%). Complete characteristics were listed in Table 1.
Table 1.
Overall demographic, target lesion and procedural characteristic.
| Demographics & targets characteristic | |
|---|---|
| Median age (IQR), years | 63 (53–69) |
| Gender, n (%) | |
| Male | 78 (60.5) |
| Female | 51 (39.5) |
| Ethnicity, n (%) | |
| Malay | 35 (27.1) |
| Non-Malay Natives | 37 (28.7) |
| Chinese | 49 (38.0) |
| Indian | 8 (6.2) |
| Admission, n (%) | |
| In-patient | 63 (48.8) |
| Out-patient | 66 (51.2) |
| Indication, n (%) | |
| Diagnostic | 90 (69.8) |
| Staging | 39 (30.2) |
| Target lesion, n (%) | |
| Lymph node | 113 (87.6) |
| Central lung mass | 16 (12.4) |
| Median target size (IQR), mm | |
| Lymph node | 17.0 (12.1–25.5) |
| Central lung mass | 17.0 (11.0–37.3) |
| Lymph node station, n (%) | |
| 2R | 1 (0.9) |
| 4R | 41 (36.3) |
| 10R | 4 (3.5) |
| 11Rs/11Ri | 18 (15.9) |
| 2L | 1 (0.9) |
| 4L | 3 (2.7) |
| 11L | 5 (4.4) |
| 7 | 40 (35.4) |
| Procedural characteristic | |
| Median overall procedural time (IQR), min | 55.0 (43.0–66.5) |
| Echobronchoscope, n (%) | |
| Fujifilm EB-530US | 52 (40.3) |
| Pentax EB1970-UK | 46 (35.7) |
| Olympus BF-UC180F | 4 (3.1) |
| Olympus BF-UC190F | 27 (20.9) |
| Airway access, n (%) | |
| Trans-oral | 44 (34.1) |
| Laryngeal Mask Airway | 69 (53.5) |
| Endotracheal Tube | 11 (8.5) |
| Rigid Bronchoscopy | 5 (3.9) |
| Rapid on-site examination, n (%) | 5 (3.9) |
| Methods for initial cryobiopsy tract creation, n (%) | |
| Up-front high-frequency needle knife | 8 (6.2) |
| 19-gauge TBNA needle | 13 (10.1) |
| 21-gauge TBNA needle | 18 (14.0) |
| 22-gauge TBNA needle | 87 (67.4) |
| 25-gauge TBNA needle | 3 (2.3) |
| Median TBNA pass (IQR), passes | 4 (4–5) |
| Cryoprobe placement, n (%) | |
| After TBNA needle only | 66 (51.2) |
| Needle knife up-front or assistance | 63 (48.8) |
| Median cryo-pass (IQR), passes | 4 (3–4) |
| Median total freezing time (IQR), seconds | 24.0 (18.0–32.0) |
| Median average freezing time per pass (IQR), seconds | 7.0 (5.5–9.3) |
| Overall diagnostic yield for EBUS, n (%) | 114 (88.4) |
| Overall short-term complication, n (%) | 20 (15.5) |
| Sedation related hypoventilation, n (%) | 10 (7.8) |
| Pneumothorax, n (%) | 0 (0.0) |
| Pneumomediastinum, n (%) | 1 (0.8) |
| Bleeding, n (%) | 9 (7.0) |
| Bleeding intervention, n | |
| Prolonged suction | 5 |
| Adrenaline-saline instillation | 1 |
| Switch bronchoscope | 3 |
Overall procedural characteristic and outcome
The overall median procedural time was 55.0 (IQR 43.0–66.5) minutes, measured from the administration of sedative medication to the conclusion of procedures. Advanced airway (endotracheal intubation, rigid bronchoscopy) accounted for 12.4%, supraglottic airway (laryngeal mask airway) for 53.5%, and trans-oral route for 34.1%. (Table 1).
Up-front TBMC were performed in 29/129 cases (22.5%) without prior TBNA. In these cases, cryo-biopsy tract was created using a high frequency needle knife and using the needle method in 8 and 21 cases respectively. Needle method was successful in all but 1 case, where cryo-biopsy tract was eventually created with a high frequency needle knife. For the remaining 100/129 cases, biopsy tract creation was attempted concurrently during TBNA, which was successful in 46% cases. The remaining 54 cases (54%) required needle knife for tract creation despite prior TBNA attempts. Overall, a conclusive diagnosis was established in 114 out of 129 cases (88.4%) in which TBMC was carried out. The histology of TBMC cases and their final outcome were listed in Supplementary Tables 3, 4 and5.
Remarkably, the cohort exhibited no recorded incidents of pneumothorax, with only one encounter of self-limiting pneumomediastinum (0.8%). Bleeding occurred in nine cases (7%) which was managed expectantly with prolonged suction (n = 5), changing to therapeutic bronchoscope for more effective suction (n = 3) and adrenaline saline instillation (n = 1).
Correlation of TBMC specimen size to overall diagnostic yield
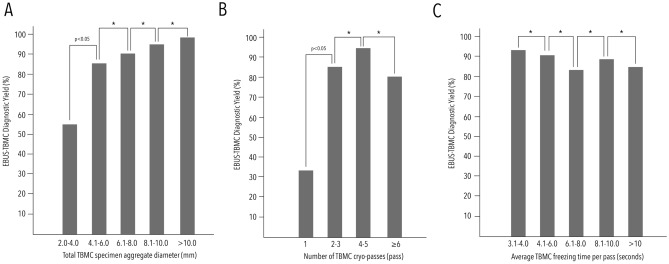
The overall median total aggregate diameter of TBMC specimens was 7.0 (IQR 5.0–9.5) mm. Conclusive TBMC procedures were associated with a larger specimen size [7.0 (IQR 5.0–10.0) vs. 5.0 (IQR 4.0–7.0) mm, p < 0.01]. TBMC demonstrated a significant increment in diagnostic yield (r = + 0.240, p < 0.01) up to a cut-off specimen size of 4.1–6.0 mm. Beyond this threshold, specimens exceeding 6 mm did not exhibit a discernible variance in the diagnostic yield. (Fig. 2, Table 2, Supplementary Table 6).
Figure 2.
Difference of EBUS-TBMC diagnostic yield across different TBMC specimen sizes in total aggregate diameter (Panel A), cryo-passes (Panel B) and average freezing time per cryo-pass (Panel C). * signify p > 0.05.
Table 2.
Diagnostic yield of EBUS-TBMC at different specimen size thresholds.
| Total aggregate diameter of specimen, mm | Diagnostic yield, n/N (%) | p-value* |
|---|---|---|
| 2.0–4.0 | 5/9 (55.6) | – |
| 4.1–6.0 | 45/52 (86.5) | 0.026 |
| 6.1–8.0 | 31/34 (91.2) | 0.512 |
| 8.1–10.0 | 24/25 (96.0) | 0.466 |
| > 10.1 | 9/9 (100.0) | 0.543 |
*p-value in comparison to previous specimen size threshold.
Correlation of TBMC cryo-passes to specimen size and overall diagnostic yield
Overall, a median of 4 (IQR 3–4) cryo-passes were performed in our cohort. A weak but significant positive correlation was observed between number of cryo-passes and the specimen size (r = + 0.240, p < 0.01), as well as the overall diagnostic yield (r = + 0.196, p < 0.05).
Conclusive TBMC procedures exhibited a higher median cryo-pass compared to inconclusive procedures [4 (IQR 3–4) vs. 3 (IQR 2–4) passes, p < 0.05]. Additionally, the diagnostic yield of 2–3 cryo-passes was significantly higher compared to a single pass of TBMC (85.0% vs. 33.3%, p < 0.05). Although 4–5 passes demonstrated a higher diagnostic yield at 95.1%, this did not reach statistical significance compared to 2–3 cryo-passes. (Fig. 2, Table 3, Supplementary Table 7).
Table 3.
Diagnostic yield of EBUS-TBMC at different cryo-passes ranges.
| Cryo-passes, pass | Diagnostic yield, n/N (%) | p-value* |
|---|---|---|
| 1 pass | 1/3 (33.3) | – |
| 2–3 pass | 51/60 (85.0) | 0.021 |
| 4–5 pass | 58/61 (95.1) | 0.065 |
| 6 pass or more | 4/5 (80.0) | 0.174 |
*p-value in comparison to previous cryo-passes range.
Correlation of TBMC freezing time to specimen size to overall diagnostic yield
The median total freezing time was 24.0 (IQR 18.0–32.0) seconds per case, with an average freezing time per cryo-pass at 7.0 (IQR 5.5–9.3) seconds per case. Interestingly, a non-statistically significant negative correlation was observed between specimen size (r = − 0.040, p > 0.05) and diagnostic yield (r = − 0.052, p > 0.05) with longer freezing times. (Supplementary Table 8).
The median freezing time did not show a significant difference between conclusive and inconclusive TBMC cases [7.0 (IQR 5.5–8.8) vs. 8.0 (IQR 6.0–10.0) seconds, p > 0.05]. The highest diagnostic yield were recorded in the 3.1 to 4.0 s freezing time group. Exploration of various freezing time thresholds revealed no significant differences in diagnostic yield (Table 4).
Table 4.
Diagnostic yield EBUS-TBMC at different freezing time ranges.
| Freezing time, seconds | Diagnostic yield, n/N (%) | p-value* |
|---|---|---|
| 3.1–4.0 | 94.4, 17/18 | – |
| 4.1–6.0 | 91.2, 31/34 | 0.674 |
| 6.1–8.0 | 83.3, 30/36 | 0.327 |
| 8.1–10.0 | 89.3, 25/28 | 0.497 |
| > 10.1 | 84.6, 11/13 | 0.671 |
*p-value in comparison to previous freezing time range.
Subgroup analysis comparing the diagnostic yield of TBNA to TBMC in different diagnosis groups
Overall, the diagnostic yield of TBMC was 88.4% (114/129) compared to 53% (53/100) for TBNA. EBUS exhibited a significantly higher diagnostic yield in cases of common malignancies compared to uncommon tumors and benign disorders (96.1% vs. 85.4%, p < 0.05). Within the realm of common malignancies, the diagnostic yields of TBNA and TBMC were statistically comparable (60.0% vs. 96.4%, p = 0.078). However, a notable disparity emerged in cases of uncommon tumors and benign disorders, where TBMC outperformed TBNA (48.8% vs. 82.9%, p < 0.01). (Supplementary Table 9, 10, 11 and 12).
Discussion
Cryobiopsy in respiratory endoscopy has been in practice for almost a decade, experiencing a resurgence in popularity, particularly in the diagnosis of interstitial lung disease using TBLC (ILD-TBLC)22,23. The technical intricacies of ILD-TBLC have been extensively explored and tested in animal studies, covering aspects such as the number of passes, freezing time, and their correlation with specimen size and quality; including the utilization of newer-generation single-use ultrathin flexible cryoprobes24–26. Thus, specific expert recommendations on the number of passes and freezing time for ILD-TBLC are well-established27. Similarly, recommendations exist for transbronchial cryobiopsy of peripheral pulmonary lesions (PPL-TBLC)28,29. On the other hand, technical data for EBUS-TBMC is currently lacking, potentially due to the challenges of creating sizeable mediastinal lymph nodes in animal models for testing30. Given the initial scarcity of technical details, various number of cryo-passes and freezing times had been explored in our cohort which enables the exploratory analysis of this novel procedure.
Firstly, in the context of ILD-TBLC, a gross specimen size of at least 5 mm is suggested to be optimal for histopathologic analysis27. However, the optimum specimen size for TBMC remains unknown. Existing literature reports a range of specimen sizes from 3.8 to 4.6 mm with variations in cryo-passes and freezing time16,17,31. In our study, we observed a positive correlation between specimen size and diagnostic yield, which plateaued at 4.1 to 6.0 mm of total aggregate specimen size. We hypothesized that the plateau of diagnostic yield is likely due to the intranodal heterogeneity frequently observed in pathological lymph nodes32. As TBMC often samples only a limited region of the target lesion, the actual biopsy site may not be wholly representative despite providing a larger specimen size. Similarly, in ILD-TBLC using the ultrathin cryoprobe in an animal model, an increase in specimen size did not consistently impact the representativeness of specimens25. Further studies exploring this intriguing finding in the future could provide valuable insights into optimizing the diagnostic process in TBMC procedures, especially in utilizing advanced imaging tools such as elastography to guide the biopsy site.
Secondly, the optimal number of TBMC cryo-passes remains unknown. In TBNA, it is recommended to perform at least three needle passes and, even more passes if molecular analysis is required33. In the TBMC literature, 2–4 passes were generally shown to achieve a good diagnostic yield14,16,31,34. Fan et al.17 had also demonstrated that just one additional TBMC pass can significantly complement the diagnostic performance of TBNA. Importantly, our study had revealed a significant positive correlation between the number of cryo-passes and specimen size. Our exploratory data further indicate that diagnostic yield plateaued after 2–3 cryo-passes. This finding is crucial, especially in cases performed through the trans-oral route or supra-glottic airway, where unnecessary cryo-passes should be avoided to limit potential vocal cord irritation and patient discomfort.
Thirdly, while freezing times have been extensively explored in ILD-TBLC and PPL, this aspect has not received thorough investigation in TBMC23,27,29. The first TBMC report in humans initiated with a freezing time of 15 s, later reduced to 7 s in a randomized controlled trial by the same group11,16. Subsequent studies have reported various freezing times ranging from 3 to 7 s 14,16–18,31,34. One might intuitively assume that longer freezing times would yield larger specimens, akin to the observations in ILD-TBLC. However, the fundamental difference is that TBLC specimens are retrieved through normal airway conduit, while TBMC specimens are obtained through an artificially created airway mucosal defect. In an animal study, utilizing an ultrathin cryoprobe with an outer sheath in ILD-TBLC, a maximum freezing time of 6–10 s was demonstrated. Beyond this range, specimens failed to be retrieved due to sheath compression24,25. Aligning with this, our data showed a non-statistically significant negative correlation between freezing time and specimen size. This suggested that the artificially created mucosal defect and track might not be large enough to retrieve larger TBMC samples, especially with longer freezing times. However, it is crucial to note that the freezing time in our study was not executed in a pre-defined incremental or decremental protocol; instead, an average freezing time per pass was calculated from the total activation time and cryo-passes. Therefore, this finding should be interpreted with caution. Nonetheless, we believe this pave the way for future studies to confirm and further explore this important aspect of TBMC procedures.
As a secondary endpoint of this explorative analysis, the overall diagnostic yield of TBMC was 88.4% in our cohort, in line with various other recent studies quoting diagnostic yields of 71.7–96.0%13,16–18,34. Importantly, our findings reaffirmed the superior role of TBMC in the diagnosis of uncommon tumours and benign disorders compared to TBNA in a real-world setting16–18. Our study also reinforced TBMC as a safe procedure, with only 0.7% incidence of self-limiting pneumomediastinum, consistent with literature at 0.5–1.0%16,17. The clinically significant bleeding rate in our cohort was 7%, which was consistent with TBMC literature, ranging from 6.4 to 14.0%, despite our various cryo-passes and freezing times16–18. Overall, the bleeding rate of TBMC in literature seemed to be lower compared to the bleeding rate of transbronchial lung cryobiopsy (TBLC), which was around 29.3% in meta-analysis. Moreover, no severe life-threatening bleeding complications have been reported yet in the TBMC literature compared to the TBLC literature28,35. Thus, more data on the TBMC safety profile is essential to ascertain the actual bleeding risk of this novel procedure and to determine the need for additional bleeding mitigation methods as in TBLC. Interestingly, we also demonstrated that TBMC is not always feasible in all cases in a real-world setting, as the cryoprobe failed to be inserted in eight cases due to a tough and dense lymph node capsule in our cohort. This is likely due to the blunt nature of the cryoprobe, which does not allow effective penetration of an occasionally stony-hard metastatic lymph node.
This study had several important limitations.
Firstly, the retrospective design and the absence of a standardized procedural protocol indicate that the actual causal relationship between the number of cryo-passes and freezing time to diagnostic yield cannot be determined confidently. However, we believed that the heterogeneity in procedural characteristics mirrors real-world clinical practice, making the findings relevant for clinicians in the region. Moreover, the unblinded study design may have introduced review bias favoring TBMC over TBNA from the pathologist's perspective, resulting in a significantly lower TBNA yield (53%) in our cohort. This bias was also demonstrated by Poletti et al.15, who found that in an unblinded cohort, pathologists tended to prefer TBMC material over TBNA specimens, especially for molecular analysis. We also believed that this bias was more apparent in our region, where cytopathology services were less developed6.
Another limitation of our study was the absence of ROSE in the majority of our cases. Although ROSE had been shown to complement bronchoscopic procedures by reducing procedural time and the need for additional procedures; its role in increasing the diagnostic yield of EBUS-TBNA has not been proven33,36. Nevertheless, a recent study demonstrated that using ROSE to stratify and select patients undergoing EBUS-TBNA for additional TBMC procedures resulted in an additional 43.7% diagnostic yield to overall EBUS procedures34. Thus, implementing an algorithm for case selection in TBMC based on intra-procedural ROSE during TBNA may prove to be a valuable strategy.
Thirdly, our study recorded a prolonged procedural time due to the inclusion of time required for anesthetic initiation, placement of the artificial airway, and initial airway examination. Additionally, we did not separately record the timings for TBNA and TBMC. Previous studies had indicated that overall EBUS procedural duration typically range from 31.9 to 33.9 min, with TBMC specifically requiring an additional 2.3–5.3 min16,17,31. Future studies that provide a more detailed breakdown of procedure times for various cryo-passes and freezing times would offer a more precise evaluation of procedural efficiency.
Next, the follow-up duration in our study may not be optimal. As of the time of writing, the current CT surveillance period for the 15 conclusive TBMC cases with lymphoid tissue was less than 12 months. Consequently, there is a possibility that the overall diagnostic yield could be overestimated. Reassuringly, most literature established a 6-month radiological surveillance period to define the stability of hilar and mediastinal lymphadenopathy16–18,21. The limited follow-up duration also meant that the long-term complications remained unknown. Notably, as of the 6-month follow-up for this cohort of patients, no delayed complications were encountered.
Lastly, our study only exclusively explored the technical details of the 1.1 mm cryoprobe in TBMC procedures. Recent reports using the larger 1.7 mm cryoprobe for TBMC had shown promising results with no increments in complication rates37,38. Interestingly, a recent report on EBUS-IFB using conventional 1.8 mm forceps also demonstrated procedural feasibility, achieving a high diagnostic yield of 99.5%39. However, a recent randomized controlled trial comparing EBUS-TBNA with supplementary IFBs and TBMC showed that although both biopsy techniques enhanced the diagnostic yield of EBUS-TBNA, TBMC was able to retrieve larger specimens with more preserved cellular architecture and fewer crushed artifacts, making it more suitable for lung cancer molecular testing18. Thus, further comparative studies using different sizes of cryoprobes and biopsy techniques regarding diagnostic yield, suitability for molecular analysis, and cost-effectiveness are eagerly anticipated.
Conclusion
EBUS-TBMC using the ultrathin cryoprobe is useful and safe in the diagnosis of mediastinal and hilar pathologies. The diagnostic yield plateaued once the total aggregate specimen size reached 4.1–6.0 mm. Optimal performance is linked to 2–3 cryo-passes and a freezing time of 3.1–4.0 s. Prospective studies are necessary to validate these findings.
Supplementary Information
Acknowledgements
We would like to acknowledge the Director General of Health of the Ministry of Health Malaysia for granting permission to publish this research paper.
Abbreviations
- EBUS
Endobronchial ultrasound
- IFB
Intranodal forceps biopsy
- ILD
Interstitial lung disease
- IQR
Interquartile range
- PPL
Peripheral pulmonary lesion
- SD
Standard deviation
- TBLC
Transbronchial lung cryobiopsy
- TBMC
Transbronchial mediastinal cryobiopsy
- TBNA
Transbronchial needle aspiration
Author contributions
Conceptualization: SSK, CIS, HYDR, NCH, KLN, VP, YM, STT. Data Curation: SSK, SHT, CIS, HYDR, NCH, KLN. Formal Analysis: SSK, SHT, CIS, HYDR, NCH. Investigation: SSK, CCS, CIS, HYDR, NCH, STT. Methodology: SSK, SHT, CIS, CCS, STT, HYDR, NCH. Supervision: SSK, STT, CIS, HYDR, VP. Visualization: SSK, CIS, HYDR, VP. Writing Original Draft: SSK, CIS, SHT, NCH. Writing Review & Editing: HYDR, STT, CCS, YM, VM. All authors had read and approved the final version of this manuscript.
Funding
The authors declare that no funding was received for the publication of this study.
Data availability
The data that support the findings of this study are available from the corresponding author, SSK, upon reasonable request.
Competing interests
SSK and VP received lecture honorarium from ERBE Elektromedizin GmbH. The authors declare that they have no competing interests in regards to this research.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69702-y.
References
- 1.Yasufuku, K. et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J. Thorac. Cardiovasc. Surg.142(6), 1393–400.e1 (2011). 10.1016/j.jtcvs.2011.08.037 [DOI] [PubMed] [Google Scholar]
- 2.Gompelmann, D., Eberhardt, R. & Herth, F. J. Endobronchial ultrasound. Endosc. Ultrasound1(2), 69–74 (2012). 10.7178/eus.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilmann, P. et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur. Respir. J.46(1), 40–60 (2015). 10.1183/09031936.00064515 [DOI] [PubMed] [Google Scholar]
- 4.Silvestri, G. A. et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest143(5 Suppl), e211S-e250S (2013). 10.1378/chest.12-2355 [DOI] [PubMed] [Google Scholar]
- 5.Labarca, G. et al. Diagnostic accuracy of endobronchial ultrasound transbronchial needle aspiration in lymphoma. A systematic review and meta-analysis. Ann. Am. Thorac. Soc.16(11), 1432–1439 (2019). 10.1513/AnnalsATS.201902-175OC [DOI] [PubMed] [Google Scholar]
- 6.Kho, S. S., Chan, S. K., Ismail, A. M. & Tie, S. T. Tissue-clot-coagulum cell block in EBUS/TBNA specimen adequacy: A real world experience. Diagn Cytopathol.50(12), 583–585 (2022). 10.1002/dc.25056 [DOI] [PubMed] [Google Scholar]
- 7.Lindeman, N. I. et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. J. Mol. Diagn.20(2), 129–159 (2018). 10.1016/j.jmoldx.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 8.Minami, D. et al. Comparing the clinical performance of the new 19-G ViziShot FLEX and 21- or 22-G ViziShot 2 endobronchial ultrasound-guided transbronchial needle aspiration needles. Intern. Med.57(24), 3515–3520 (2018). 10.2169/internalmedicine.0967-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herth, F. J., Morgan, R. K., Eberhardt, R. & Ernst, A. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann. Thorac. Surg.85(6), 1874–1878 (2008). 10.1016/j.athoracsur.2008.02.031 [DOI] [PubMed] [Google Scholar]
- 10.Chrissian, A., Misselhorn, D. & Chen, A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann. Thorac. Surg.92(1), 284–288 (2011). 10.1016/j.athoracsur.2011.03.069 [DOI] [PubMed] [Google Scholar]
- 11.Zhang, J. et al. Primary mediastinal seminoma achieved by transbronchial mediastinal cryobiopsy. Respiration99(5), 426–430 (2020). 10.1159/000505936 [DOI] [PubMed] [Google Scholar]
- 12.Genova, C. et al. Potential application of cryobiopsy for histo-molecular characterization of mediastinal lymph nodes in patients with thoracic malignancies: A case presentation series and implications for future developments. BMC Pulm. Med.22(1), 5 (2022). 10.1186/s12890-021-01814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariza-Prota, M. A. et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lymph nodes: A case series: How to do it. Arch Bronconeumol58(10), 718–721 (2022). 10.1016/j.arbres.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Gonuguntla, H. K. et al. Endobronchial ultrasound-guided transbronchial cryo-nodal biopsy: A novel approach for mediastinal lymph node sampling. Respirol. Case Rep.9(8), e00808 (2021). 10.1002/rcr2.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poletti, V. et al. EBUS-guided cryobiopsy in the diagnosis of thoracic disorders. PulmonologyS2531–0437(23), 00223–00224 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Zhang, J. et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: A randomised trial. Eur. Respir. J.58(6), 2100055 (2021). 10.1183/13993003.00055-2021 [DOI] [PubMed] [Google Scholar]
- 17.Fan, Y. et al. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: A multicentre, open-label, randomised trial. Lancet Respir. Med.S2213–2600(22), 00392–00397 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Cheng, T. L. et al. Comparison of cryobiopsy and forceps biopsy for the diagnosis of mediastinal lesions: A randomised clinical trial. PulmonologyS2531–0437(23), 00240–00244 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Abuqayyas, S., Raju, S., Bartholomew, J. R., Abu Hweij, R. & Mehta, A. C. Management of antithrombotic agents in patients undergoing flexible bronchoscopy. Eur. Respir. Rev.26(145), 170001 (2017). 10.1183/16000617.0001-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kho, S. S., Soo, C. I., Nasaruddin, M. Z., Ngan, K. W. & Abdul Rahaman, J. A. Multimodal linear endobronchial ultrasound guided mediastinal lymph node biopsy in the diagnosis of isolated mediastinal lymphadenopathy. Proc. Singap. Healthc.31, 20101058221111656 (2022). 10.1177/20101058221111655 [DOI] [Google Scholar]
- 21.Munden, R. F. et al. Managing incidental findings on thoracic CT: Mediastinal and cardiovascular findings. A white paper of the ACR incidental findings committee. J. Am. Coll. Radiol.15(8), 1087–96 (2018). 10.1016/j.jacr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 22.Babiak, A. et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration78(2), 203–208 (2009). 10.1159/000203987 [DOI] [PubMed] [Google Scholar]
- 23.Ravaglia, C. et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: A large cohort of 699 patients. BMC Pulm Med.19(1), 16 (2019). 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hetzel, J., Linzenbold, W., Boesmueller, H., Enderle, M. & Poletti, V. Evaluation of efficacy of a new cryoprobe for transbronchial cryobiopsy: A randomized. Controll. Vivo Anim. Study Respir.99(3), 248–256 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Yarmus, L. B. et al. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest150(2), 329–336 (2016). 10.1016/j.chest.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 26.Franke, K. J. et al. A new tool for transbronchial cryobiopsies in the lung: An experimental feasibility ex vivo study. Respiration91(3), 228–234 (2016). 10.1159/000443990 [DOI] [PubMed] [Google Scholar]
- 27.Hetzel, J. et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: Expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration95(3), 188–200 (2018). 10.1159/000484055 [DOI] [PubMed] [Google Scholar]
- 28.Tang, Y. et al. Transbronchial lung cryobiopsy for peripheral pulmonary lesions. A Narrat. Rev. Pulmonol.S2531–0437(23), 00163 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Kho, S. S. et al. Exploring the optimal freeze time and passes of the ultrathin cryoprobe in transbronchial cryobiopsy of peripheral pulmonary lesions. ERJ Open Res.10(1), 00506–02023 (2024). 10.1183/23120541.00506-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke, K. J. et al. The cryo-needle: A new tool for histological biopsies. A Feasibility Study Lung191(6), 611–617 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Ariza-Prota, M. et al. Endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: safety, feasibility and diagnostic yield: Experience in 50 cases. ERJ Open Res.9(2), 00448–02022 (2023). 10.1183/23120541.00448-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurimoto, N. et al. Targeting area in metastatic lymph nodes in lung cancer for endobronchial ultrasonography guided transbronchial needle aspiration. J. Bronchol.15, 134–213 (2008). 10.1097/LBR.0b013e31817ec366 [DOI] [Google Scholar]
- 33.Wahidi, M. M. et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST guideline and expert panel report. Chest149(3), 816–835 (2016). 10.1378/chest.15-1216 [DOI] [PubMed] [Google Scholar]
- 34.Maturu, V. N., Prasad, V. P., Vaddepally, C. R., Dommata, R. R. & Sethi, S. Endobronchial ultrasound-guided mediastinal lymph nodal cryobiopsy in patients with nondiagnostic/inadequate rapid on-site evaluation: A new step in the diagnostic algorithm. J. Bronchol. Interv. Pulmonol.31(1), 2–12 (2024). 10.1097/LBR.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 35.Sryma, P. B. et al. Efficacy of radial endobronchial ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL): A systematic review and meta-analysis. Pulmonology29(1), 50–64 (2023). 10.1016/j.pulmoe.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 36.Huang, H. et al. Application of bronchoscopy in the diagnosis and treatment of peripheral pulmonary lesions in China: A national cross-sectional study. J. Cancer14(8), 1398–1406 (2023). 10.7150/jca.84220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gershman, E., Amram Ikan, A., Pertzov, B., Rosengarten, D. & Kramer, M. R. Mediastinal “deep freeze”-transcarinal lymph node cryobiopsy. Thorac. Cancer13(11), 1592–1596 (2022). 10.1111/1759-7714.14422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemura, C. et al. Thoracic SMARCA4-deficient undifferentiated tumor diagnosed by transbronchial mediastinal cryobiopsy: A case report. Thorac. Cancer14(10), 953–957 (2023). 10.1111/1759-7714.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konno-Yamamoto, A. et al. Feasibility of modified endobronchial ultrasound-guided intranodal forceps biopsy: A retrospective analysis. Respiration102(2), 143–153 (2023). 10.1159/000528644 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SSK, upon reasonable request.