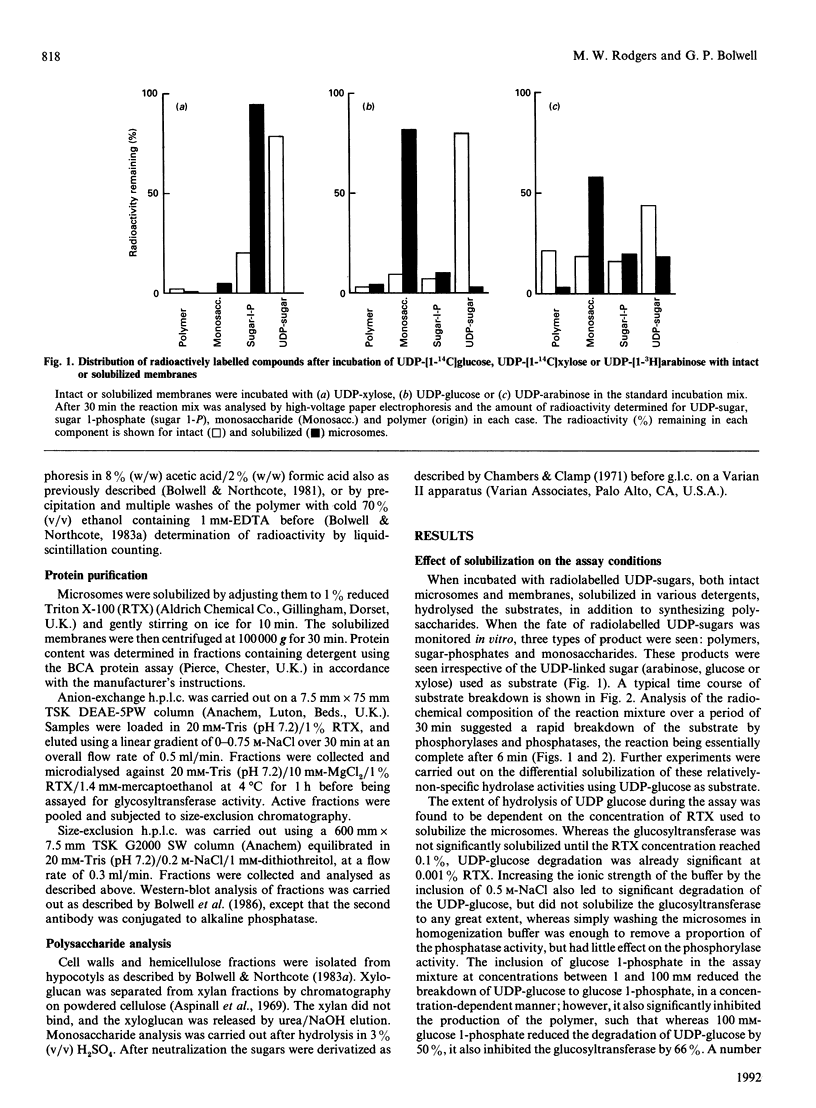
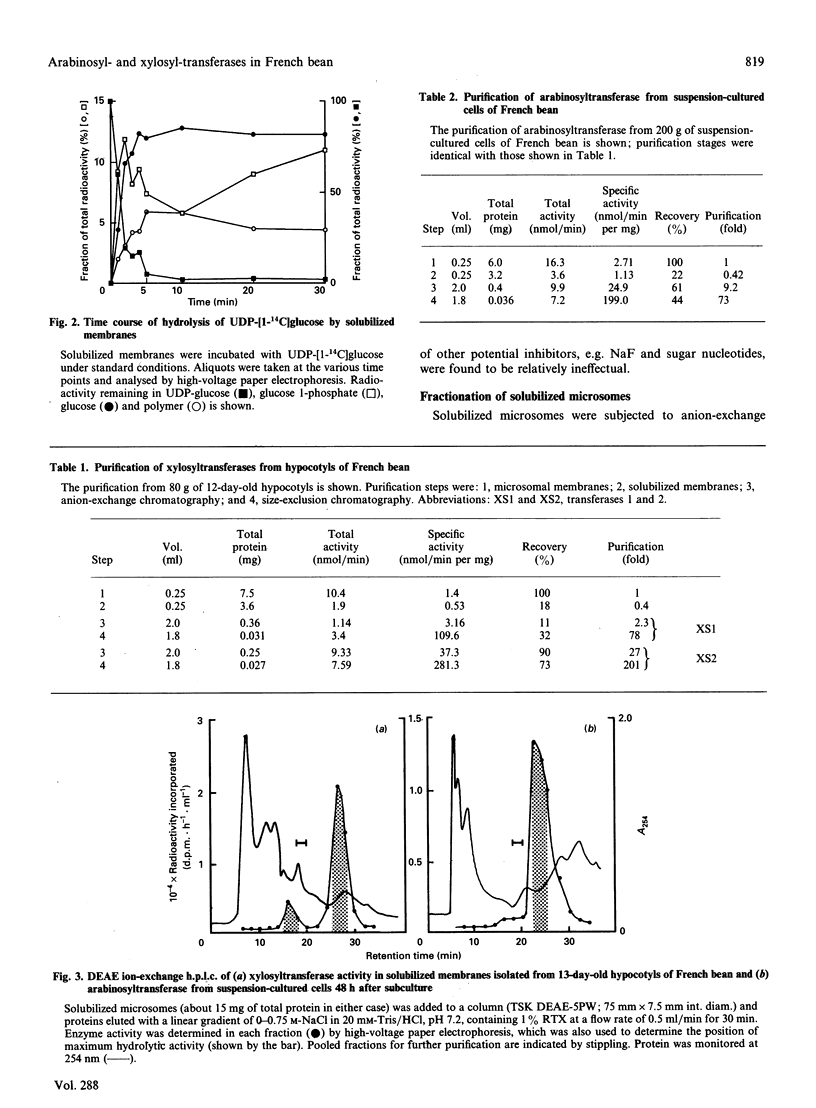
Abstract
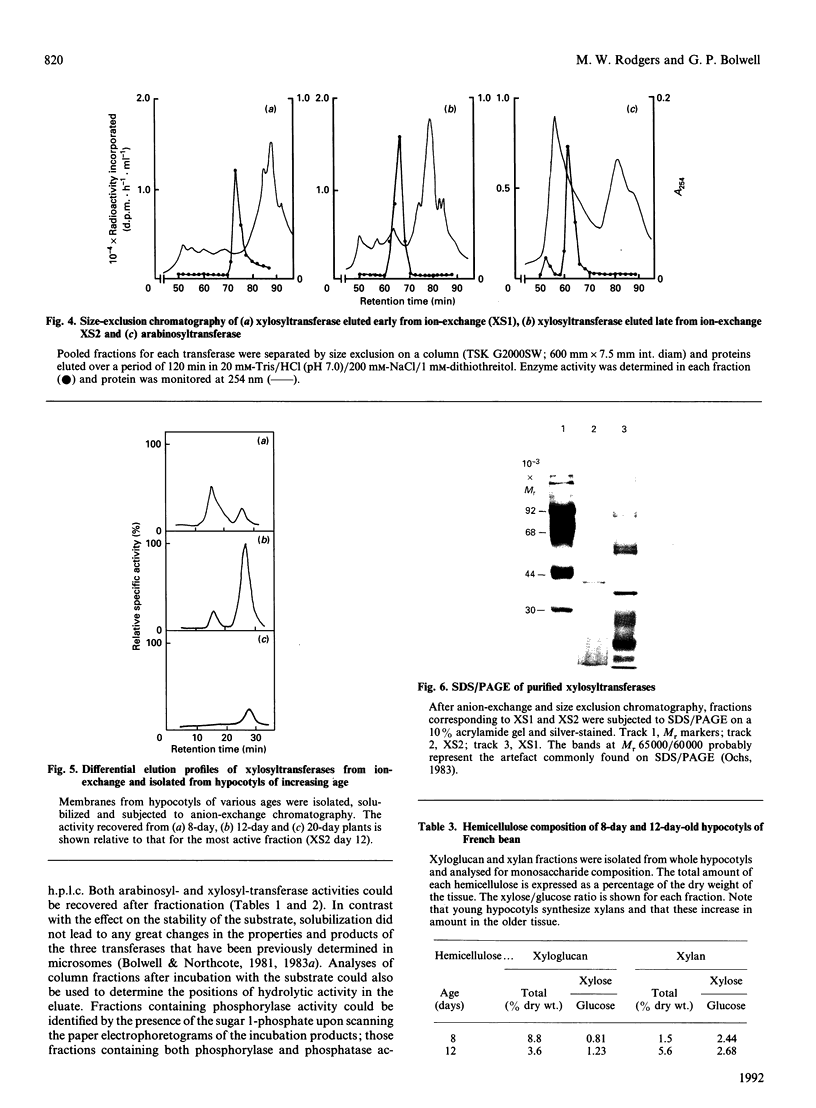
The purification of glycosyltransferases involved in wall matrix polysaccharide synthesis has been attempted. A number of activities readily demonstrated in isolated Golgi membranes are lost following detergent solubilization. However, solubilization releases pyrophosphorylases and phosphatases that hydrolyse the substrate in enzyme assays, whether UDP-glucose, -arabinose or -xylose is used. This hydrolysis, which cannot be completely inhibited, appears to be the major factor in the apparent loss of activity. Separation of this hydrolytic activity during further purification by ion-exchange and gel exclusion leads to recovery of glycosyltransferase activity. Thus two xylosyltransferases and one arabinosyltransferase could be partially purified. These appeared to be differentially expressed. The arabinosyltransferase of apparent M(r) 70,000 on size-exclusion chromatography was isolated from cells undergoing rapid growth and division. A xylosyltransferase of apparent M(r) 38,000 on size-exclusion chromatography was associated with cell expansion and primary wall synthesis. A second xylosyltransferase, which was purified to near homogeneity with M(r) 40,000, showed a peak of activity during the period of maximum secondary wall synthesis.
Full text
PDF


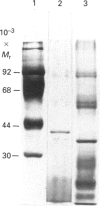
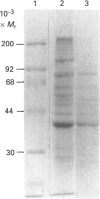
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor Y., Mayer R., Benziman M., Delmer D. Evidence for a cyclic diguanylic acid-dependent cellulose synthase in plants. Plant Cell. 1991 Sep;3(9):989–995. doi: 10.1105/tpc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Baydoun E. A., Waldron K. W., Brett C. T. The interaction of xylosyltransferase and glucuronyltransferase involved in glucuronoxylan synthesis in pea (Pisum sativum) epicotyls. Biochem J. 1989 Feb 1;257(3):853–858. doi: 10.1042/bj2570853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Arabinan synthase and xylan synthase activities of Phaseolus vulgaris. Subcellular localization and possible mechanism of action. Biochem J. 1983 Feb 15;210(2):497–507. doi: 10.1042/bj2100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Induction by growth factors of polysaccharide synthases in bean cell suspension cultures. Biochem J. 1983 Feb 15;210(2):509–515. doi: 10.1042/bj2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. E., Brett C. T., Hillman J. R. A xylosyltransferase involved in the synthesis of a protein-associated xyloglucan in suspension-cultured dwarf-French-bean (Phaseolus vulgaris) cells and its interaction with a glucosyltransferase. Biochem J. 1988 Aug 1;253(3):795–800. doi: 10.1042/bj2530795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Gordon R., Maclachlan G. Incorporation of UDP-[C]Glucose into Xyloglucan by Pea Membranes. Plant Physiol. 1989 Sep;91(1):373–378. doi: 10.1104/pp.91.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R., Brummell D. A., Camirand A., Hensel A., Russell E. F., Maclachlan G. A. Solubilization and properties of GDP-fucose: xyloglucan 1,2-alpha-L-fucosyltransferase from pea epicotyl membranes. Arch Biochem Biophys. 1991 Oct;290(1):7–13. doi: 10.1016/0003-9861(91)90584-6. [DOI] [PubMed] [Google Scholar]
- Hobbs M. C., Delarge M. H., Baydoun E. A., Brett C. T. Differential distribution of a glucuronyltransferase, involved in glucuronoxylan synthesis, within the Golgi apparatus of pea (Pisum sativum var. Alaska). Biochem J. 1991 Aug 1;277(Pt 3):653–658. doi: 10.1042/bj2770653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. J., Swords K. M., Lynch M. A., Staehelin L. A. Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J Cell Biol. 1991 Feb;112(4):589–602. doi: 10.1083/jcb.112.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983 Dec;135(2):470–474. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Roberts K. The plant extracellular matrix. Curr Opin Cell Biol. 1989 Oct;1(5):1020–1027. doi: 10.1016/0955-0674(89)90074-4. [DOI] [PubMed] [Google Scholar]
- Saugy M., Farkas V., Maclachlan G. Phosphatases and phosphodiesterases interfere with 1,3-beta-D-glucan synthase activity in pea epicotyl membrane preparations. Eur J Biochem. 1988 Oct 15;177(1):135–138. doi: 10.1111/j.1432-1033.1988.tb14353.x. [DOI] [PubMed] [Google Scholar]
- Waldron K. W., Baydoun E. A., Brett C. T. The solubilization of a glucuronyltransferase involved in pea (Pisum sativum var. Alaska) glucuronoxylan synthesis. Biochem J. 1989 Dec 15;264(3):643–649. doi: 10.1042/bj2640643. [DOI] [PMC free article] [PubMed] [Google Scholar]